Abstract
Purpose
The tumor-infiltrating lymphocytes (TILs) expression in breast cancer is a positive prognostic marker for certain breast cancer subtypes. We evaluated the efficacy of dual anti-human epidermal growth factor receptor 2 (HER2) blockade in HER2-positive breast cancer and hypothesized that high TILs tumors are associated with better outcomes.
Methods
A total of 176 patients who were treated with neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) between December 2015 and December 2018 were reviewed. They were grouped based on a cut-off value of the stromal TILs grade (≤ 20% TILs, > 20% TILs).
Results
In total, 107 patients (60.8%) achieved pathological complete response (pCR). Hormone receptor (HR)-negativity (p = 0.001) and a high TILs grade (p = 0.022) were independent predictors of pCR. Among the HR-negative patients, high TILs tumors were significantly associated with pCR (p = 0.035).
Conclusion
HR status and the TILs grade are significantly correlated with pCR in dual anti-HER2 neoadjuvant therapy. The evaluation of the TILs at baseline may be beneficial for predicting pCR in HER2-positive breast cancer.
Keywords: Receptor, ErbB-2; Neoadjuvant therapy; Lymphocytes; Tumor microenvironment
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer is associated with an increased risk of disease recurrence and poor prognosis. However, in the CLEOPATRA trial, dual HER2 blockade demonstrated unprecedented overall survival benefits with long-term follow-up [1]. In the neoadjuvant setting, patients with HER2-positive early breast cancer showed that the combination of pertuzumab- and trastuzumab-based treatments, compared with the trastuzumab-based therapy alone, significantly improved the pathological complete response (pCR) rate, which has been linked to long-term benefits [2,3]. Therefore, docetaxel, trastuzumab, and pertuzumab were approved for metastatic and early-stage HER2-positive breast cancer in 2012 and 2013, respectively. Moreover, the TRYPHAENA study showed that the THP and carboplatin regimens achieved the best pCR rate among the other dual HER2 blockade regimens [3]. However, the pCR rate remained at 57%–66%; however, the indications of dual HER2 blockade are unclear [4]. Previous studies have shown that immunological markers are closely related to neoadjuvant chemotherapy outcomes in breast cancer [5]. Tumor-infiltrating lymphocytes (TILs) have been prognostic markers for certain subtypes of breast cancers [6,7]. Moreover, the TILs at diagnosis is a positive prognostic marker in HER2-positive early breast cancer treated with neoadjuvant anti-HER2 agents [8]. In this study, we retrospectively evaluated the efficacy of the docetaxel, carboplatin, trastuzumab, and pertuzumab (TCHP) regimen and hypothesized that higher levels of TILs would be associated with better clinical outcomes in patients treated with TCHP.
METHODS
Study population and treatment
The data of 176 patients who were pathologically confirmed as HER2-positive based on preoperative breast biopsy between December 2015 and December 2018 were reviewed retrospectively. The clinical stages were determined based on the outcomes of physical examination, radiological studies (mammography, breast sonography, breast magnetic resonance imaging), and biopsy findings before any surgical intervention. These patients received TCHP every 3 weeks for 6 cycles. Trastuzumab was administered every 3 weeks at a dose of 8 mg/kg (cycle 1), which was reduced to 6 mg/kg. The pertuzumab loading dose was 840 mg, followed by 420 mg every 3 weeks. Carboplatin was administered at a dose of area under the plasma concentration-time curve (area under the receiver operating characteristic [ROC] curve [AUC] 6), and docetaxel was administered at 75 mg/m2 [3]. After the neoadjuvant therapy, the patients underwent surgery and resumed the 1-year of adjuvant trastuzumab therapy. They received further adjuvant therapy (radiotherapy, chemotherapy, and hormonal treatment) according to local practice guidelines. This retrospective study was approved by the Institutional Review Board of Asan Medical Center (IRB number: 2019-1061), and the requirement for informed patient consent for the use of data was waived.
Histological evaluation and immunohistochemistry
HER2-positive breast cancer patients who underwent pre-treatment biopsy for breast cancer and formalin-fixed paraffin-embedded tissue samples for analysis were included in this study. The histological subtype of HER2-positive breast cancer was defined according to the 2012 World Health Organization criteria and graded based on the modified Bloom–Richardson classification [9]. The Ki-67 index was assessed, and a cut-off value of 20%, proposed by a recent meta-analysis, was used [10]. Therefore, a Ki-67 index of > 20% was considered high. Standard biomarkers, estrogen receptor (ER), progesterone receptor (PR), and HER2 were reviewed at the time of diagnosis. All tumors were histopathologically reviewed for the presence of TILs, and the TILs were counted according to the International Immuno-Oncology Biomarker Working Group guidelines [11]. The TILs count was defined as the percentage of the stroma of the invasive carcinoma infiltrated by lymphocytes, and it was determined in 10% increments. Since there is no clear standard for the optimal cut-off value for the TILs, we analyzed it based on our data. The correlation between the 10% and 20% TILs cut-off groups with pCR was plotted as an ROC curve (Supplementary Figure 1). The AUC was calculated. The AUC of the 20% TILs cut-off group was higher than that of the 10% TILs cut-off group (AUC, 0.627 vs. 0.561). According to these results, we classified the TILs grade with a cut-off value of stromal TILs (≤ 20% TILs vs. > 20% TILs). Tumors with TILs less than 20% were allocated to the low TILs grade, and those with TILs levels of 20% or more were allocated to the high TILs grade. The pathological response after neoadjuvant therapy was determined from the surgical specimen. We defined pCR as the absence of any residual invasive tumor cells in the breast and lymph nodes (ypT0/is, ypN0).
Statistical analysis
The patient demographics, clinicopathological characteristics including hormone receptor (HR) status, and treatment outcomes were analyzed using χ2 or Fisher's exact tests. Univariate and multivariate analyses were performed to determine the factors significantly associated with pCR. The grading of TILs was categorized into two groups according to the percentage of stromal TILs (cut off label; 20%) based on ROC curve analysis. Correlations between TILs grade, clinicopathological factors, and tumor response were evaluated using the χ2 or Fisher's exact tests, and the Cochran-Mantel-Haenszel χ2 test was performed to verify the significant independent factors associated with pCR. Univariate and multivariate analyses were performed using a binary logistic regression model. The odds ratios (ORs) and 95% confidence intervals (CIs) with two-sided p-values were used. All the statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corporation, New York, USA). The p < 0.05 denoted statistical significance.
RESULTS
Patients
A total of 176 patients with HER2-positive breast cancer were treated with neoadjuvant TCHP therapy. All of them completed six cycles of neoadjuvant chemotherapy followed by surgery. The median follow-up duration was 20.5 months (range, 9.3–47.0 months), and the baseline demographics of the 176 patients are presented in Table 1. The median age at diagnosis was 50 years (range, 25–74 years). Ninety-six (54.5%) patients had stage II disease, and 80 (45.5%) had stage III disease according to the seventh edition of American Joint Committee on Cancer. Ninety-three patients (47.2%) had ER- or PR-positive tumors, and most of the patients (172, 97.7%) showed a high Ki-67. The median TILs level was 15.0% (range, 0%–80%), 125 (71.0%) patients had low TILs grade (< 20%), and 48 (27.3%) had high TILs grade (≥ 20%).
Table 1. Baseline characteristics of the study cohort stratified by pathologic complete response (n = 176).
| Characteristics | Total patients | Non-pCR | pCR | p-value | |
|---|---|---|---|---|---|
| No. of patients | 176 (100) | 69 (39.2) | 107 (60.8) | ||
| Age at diagnosis | 50 (25–74) | 49 (25–74) | 51 (26–73) | 0.117 | |
| < 50 | 84 (47.7) | 38 (45.2) | 46 (54.8) | ||
| ≥ 50 | 92 (43.2) | 31 (33.7) | 61 (66.3) | ||
| Menopausal state | 0.384 | ||||
| Premenopausal | 100 (56.8) | 42 (42.0) | 58 (58.0) | ||
| Postmenopausal | 76 (43.2) | 27 (35.5) | 49 (64.5) | ||
| Clinical stage at diagnosed (AJCC 7th) | 0.260 | ||||
| Stage II | 96 (54.5) | 34 (35.8) | 62 (64.6) | ||
| Stage III | 80 (45.5) | 35 (43.8) | 45 (56.3) | ||
| Clinical nodal status | 0.052 | ||||
| LN negative | 53 (30.1) | 15 (28.3) | 38 (71.7) | ||
| LN positive | 123 (69.9) | 54 (43.9) | 69 (56.1) | ||
| HR status | 0.001 | ||||
| HR (−) | 83 (47.2) | 22 (26.5) | 61 (73.5) | ||
| HR (+) | 93 (52.8) | 47 (50.5) | 46 (49.5) | ||
| Ki-67 | 0.655 | ||||
| Low (< 20%) | 4 (2.3) | 2 (50.0) | 2 (50.0) | ||
| High (≥ 20%) | 172 (97.7) | 67 (39.0) | 105 (61.0) | ||
| Histologic grade | 0.475 | ||||
| I, II | 130 (73.9) | 53 (40.8) | 77 (59.2) | ||
| III | 46 (26.1) | 16 (34.8) | 30 (65.2) | ||
| TILs | 15.0 (0–75) | 5.0 (0–75) | 15.0 (0–75) | 0.035 | |
| < 20% | 125 (71.0) | 56 (44.8) | 69 (55.2) | ||
| ≥ 20% | 48 (27.3) | 13 (27.1) | 35 (72.9) | ||
| Unknown | 3 (1.7) | 0 | 3 (100) | ||
Values are presented as median (range) or number (%).
pCR = pathological complete response; AJCC = American Joint Committee on Cancer; LN = lymph node; HR = hormone receptor; TIL = tumor-infiltrating lymphocyte.
Treatment responses
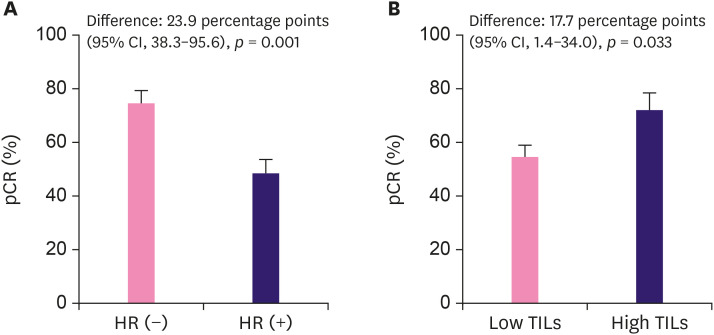
Of the 176 patients, 107 (60.8%) achieved pCR after dual HER2 blockade. Correlation analysis showed that HR status and TILs grade are significantly correlated with pCR (p = 0.001, p = 0.038, χ2 test, respectively) (Table 1). In a univariable analysis, HR-negativity (OR, 2.83; 95% CI, 1.50–5.34; p = 0.001) and high TILs grade (OR, 2.19; 95% CI, 1.06–4.52; p=0.035) were significantly correlated with pCR. No significant associations between pCR and the other factors were found (Table 2). In a multivariable analysis adjusted for clinicopathologic factors, HR-negativity (OR, 2.98; 95% CI, 1.55–5.72; p = 0.001) and a high TILs grade (OR, 2.41; 95% CI, 1.13–5.12; p = 0.022) were independent predictors of pCR. The difference in pCR rates between the HR-negative and HR-positive patients was 23.9% (95% CI, 38.3–95.6; p = 0.001) (Figure 1A). The difference in the pCR rate between the high and low TILs grade was 17.7% (95% CI, 1.4–34.0; p = 0.033) (Figure 1B).
Table 2. Univariable and multivariable analysis for the prediction of pathologic complete response.
| Characteristics | Univariable analysis | Multivariable analysis* | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age ≥ 50 | 1.63 | 0.88–2.99 | 0.118 | |||
| Postmenopausal | 1.31 | 0.71–2.43 | 0.384 | |||
| Stage III | 0.71 | 0.38–1.30 | 0.260 | |||
| LN negative | 1.98 | 0.99–3.98 | 0.052 | |||
| Ki-67 ≥ 20% | 0.64 | 0.09–4.64 | 0.657 | |||
| HR (−) | 2.83 | 1.50–5.34 | 0.001 | 2.98 | 1.55–5.72 | 0.001 |
| Histologic grade III | 1.29 | 0.64–2.60 | 0.475 | |||
| TILs ≥ 20% | 2.19 | 1.06–4.52 | 0.035 | 2.41 | 1.13–5.12 | 0.022 |
LN = lymph node; OR = odds ratio; CI = confidence interval; HR = hormone receptor; TIL = tumor-infiltrating lymphocyte.
*Adjusted for LN involvement, HR, TILs grade.
Figure 1. The pCR rates stratified by HR status (A) and TIL grade (B).
HR = hormone receptor; TIL = tumor-infiltrating lymphocyte; CI = confidence interval; pCR = pathological complete response.
Associations between pCR and TILs grade
A comparison of the clinicopathological features according to the TILs grade is shown in Table 3. Tumors with a high TILs grade had a pCR rate of 72.9%, compared with 55.2% for those with low TILs grade (p = 0.033). The less advanced stages were associated with high TILs grade (p = 0.012). No other factors were correlated with the TILs grade.
Table 3. Clinicopathologic features stratified by the TILs grade.
| Characteristics | Low TILs (n = 125) | High TILs (n = 48) | p-value | |
|---|---|---|---|---|
| Age at diagnosis | 50.0 (27–74) | 49.5 (25–67) | 0.814 | |
| < 50 | 60 (71.4) | 24 (28.6) | ||
| ≥ 50 | 65 (73.0) | 24 (27.0) | ||
| Menopausal state | 0.683 | |||
| Premenopausal | 72 (73.5) | 26 (26.5) | ||
| Postmenopausal | 53 (70.7) | 22 (29.3) | ||
| Clinical stage at diagnosed (AJCC 7th) | 0.012 | |||
| Stage II | 61 (64.2) | 34 (35.8) | ||
| Stage III | 64 (82.1) | 14 (17.9) | ||
| Clinical nodal status | 0.225 | |||
| LN negative | 35 (66.0) | 18 (34.0) | ||
| LN positive | 90 (75.0) | 30 (25.0) | ||
| HR status | 0.616 | |||
| HR (−) | 60 (74.1) | 21 (25.9) | ||
| HR (+) | 65 (70.7) | 27 (29.3) | ||
| Ki-67 | 0.901 | |||
| Low (< 20%) | 3 (75.0) | 1 (25.0) | ||
| High (≥ 20%) | 122 (72.2) | 47 (27.8) | ||
| Treatment responses | 0.033 | |||
| Non-pCR | 56 (81.2) | 13 (18.8) | ||
| pCR | 69 (66.3) | 35 (33.7) | ||
Values are presented as median (range) or number (%).
TIL = tumor-infiltrating lymphocyte; pCR = pathological complete response; AJCC = American Joint Committee on Cancer; HR = hormone receptor.
Among the HR-negative patients, high TILs grade was significantly associated with pCR (OR, 4.75; 95% CI, 1.01–22.44; p = 0.035). On the contrary, in HR-positive patients, the association between the TILs grade and pCR was not statistically significant (OR, 1.81; 95% CI, 0.73–4.49; p = 0.208) (Table 4). When stratified by HR status, high TILs grade was significantly associated with pCR (Cochran-Mantel-Haenszel χ2 test, p = 0.021).
Table 4. The pCR stratified by TILs grade and HR status.
| HR status | TILs grade | Non-pCR | pCR | p-value |
|---|---|---|---|---|
| HR (−) | Low TILs | 20 (33.3) | 40 (66.7) | 0.035 |
| High TILs | 2 (9.5) | 19 (90.5) | ||
| HR (+) | Low TILs | 36 (55.4) | 29 (44.6) | 0.208 |
| High TILs | 11 (40.7) | 16 (59.3) |
Values are presented as number (%).
pCR = pathological complete response; HR = hormone receptor; TILs = tumor-infiltrating lymphocytes.
DISCUSSION
The development of multiple highly effective HER2-targeting drugs has transformed treatments and remarkably improved survival outcomes. Without more sensitive and specific biomarkers to tailor therapy based on the risk of recurrence and the likelihood of treatment response, over-treatment or under-treatment remains a concern in early breast cancer.
This study highlights the prognostic impact of HR status and TILs grade in HER2-positive breast cancer patients receiving neoadjuvant therapy with dual HER2 blockade with pertuzumab and trastuzumab. HR-negativity with high TILs grade was significantly associated with pCR.
These results corroborate the outcomes of a previous study by Salgado et al. [8] that reported that the pCR rate was associated with TILs grade in HER2-positive breast cancers after neoadjuvant therapy. Specifically, the pCR rate was significantly higher in the high TILs group than in the low TILs group in HER2-positive patients treated with neoadjuvant anti-HER2 blockade using trastuzumab and lapatinib. Thus, we believe that these results strengthen the positive prognostic impact of stromal TILs in HER2-positive breast cancer patients treated with dual HER2 blockade using pertuzumab and chemotherapy.
However, the predictive value of TILs with anti-HER2 therapy is controversial. In the FINHER trial, HER2-positive breast cancer patients were randomly treated with trastuzumab for nine weeks in addition to adjuvant chemotherapy [7]. In this study, a significant interaction between TILs and trastuzumab related to the distant recurrence rate was observed, suggesting that trastuzumab may be more beneficial in the presence of TILs. The NSABP-31 adjuvant trastuzumab trial reported similar results: high expression of TILs-associated genes correlated with greater efficacy of trastuzumab. However, it did not confirm the correlations between TILs and the beneficial effects of trastuzumab [12,13]. In addition, a retrospective analysis of data from the N9831 trial showed discordant results, as the benefit of trastuzumab was observed in non-lymphocyte predominant breast cancer (LPBC), but not in LPBCs [14]. Our study demonstrated a discordant association between pCR and TILs grade in HR-positive tumors. Denkert et al. [15] also showed that an increase in TILs was an adverse prognostic factor for the survival of HR-positive HER2-negative breast cancer patients, in contrast with HER2-positive and triple-negative breast cancer patients, suggesting a different biology of the immunological infiltrate in this subtype. Moreover, Svoronos et al. [16] reported that estrogen signaling through ERα drives the mobilization of myeloid-derived suppressor cells and enhances their intrinsic immunosuppressive activity. Thus, the HR status and TILs interaction may explain these discordant results and require further validation.
In this study, HR status and the TILs were prognostic. Hence, as we move forward into an era of adjuvant trastuzumab emtansine (T-DM1) [17], it is possible to distinguish the high-risk subgroup of patients based on their HR status and TILs at baseline to optimize the current adjuvant standard of T-DM1 and chemotherapy.
TILs have been associated with a response to pembrolizumab, an immune checkpoint inhibitor, in metastatic TNBC [18]. It has also been suggested that mutational load, the number of neoantigens, and the clonal and subclonal neoantigens are associated with the response to immune checkpoint inhibitors [11,14,19]. T-cell population analysis by sequencing the T-cell receptor repertoire may be predictive of anti-HER2 therapy response [20]. This information may provide a more comprehensive interpretation of our biological findings, especially to link the benefits of HER2-targeted agents and immune checkpoint inhibitors.
Our study has several limitations. First, it was retrospective, and it was performed in a single center. Second, we were unable to evaluate the relapse-free interval and survival because of the short follow-up duration. Further follow-up studies are required to verify these results. Lastly, all patients received dual anti-HER2 blockades; thus, we could not evaluate the difference between the single and dual anti-HER2 blockades. Despite these limitations, our study established correlations between HR status, baseline TILs counts, and pCR in patients receiving a homogenous dual anti-HER2 neoadjuvant therapy in HER2 breast cancer. It is likely that future strategies to escalate and de-escalate treatment with fewer chemotherapy treatments and anti-HER2 drugs or facilitate shorter durations of treatment will depend on integrated clinical and specific biomarkers.
In conclusion, we suggest that HR status and the TILs grade are significantly correlated with pCR in dual anti-HER2 neoadjuvant therapy. Among the HR-negative patients, high TILs grade in primary tumors served as a better predictor than those with low TILs grades in HR-positive tumors. Evaluation of TILs at baseline may be beneficial for predicting patients who will achieve pCR.
Footnotes
Conflict of Interest: Dr. Kim SB has received research funding from Novartis, Sanofi-Aventis, Kyowa-Kirin Inc., and DongKook Pharm Co. and has participated as a consultant in advisory boards of Novartis, AstraZeneca, Lilly, Dae Hwa Pharmaceutical Co. Ltd, ISU Abxis, and Daiichi-Sankyo. Dr. Jung KH has participated as a consultant in advisory boards of Roche, AstraZeneca, Novartis, Takeda, and MSD.
- Conceptualization: Kim JE, Lee HJ, Jeong JH, Ahn JH, Jung KH.
- Data curation: Ha JY, Kim SB.
- Formal analysis: Ha JY.
- Investigation: Kim JE.
- Project administration: Gong G, Chae EY, Kim SB.
- Resources: Lee HJ, Jeong JH, Ahn JH, Jung KH, Gong G, Chae EY, Kim HH, Chung IY, Ko BS.
- Software: Kim HH, Chung IY, Ko BS.
- Supervision: Kim JE, Jung KH, Kim SB.
- Validation: Lee HJ.
- Writing - original draft: Ha JY.
- Writing - review & editing: Kim SB.
SUPPLEMENTARY MATERIAL
ROC curve analysis for TILs cut-off levels.
References
- 1.Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 2.Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 3.Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi R, Tanaka M, Yano A, Tse GM, Yamaguchi M, Koura K, et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol. 2012;43:1688–1694. doi: 10.1016/j.humpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PLoS One. 2013;8:e79775. doi: 10.1371/journal.pone.0079775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 7.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 8.Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1:448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO Classification of Tumours of the Breast: WHO Classification of Tumours. 4th ed. Lyon: IARC Publications; 2012. [Google Scholar]
- 10.Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathology. 2017;49:166–171. doi: 10.1016/j.pathol.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim RS, Song N, Gavin PG, Salgado R, Bandos H, Kos Z, et al. NRG Oncology/NSABP B-31: stromal tumor infiltrating lymphocytes (sTILs) and outcomes in early-stage HER2-positive breast cancer (BC) J Clin Oncol. 2018;36:12010. [Google Scholar]
- 13.Kim RS, Song N, Gavin PG, Salgado R, Bandos H, Kos Z, et al. Stromal tumor-infiltrating lymphocytes in NRG oncology/NSABP B-31 adjuvant trial for early-stage HER2-positive breast cancer. J Natl Cancer Inst. 2019;111:867–871. doi: 10.1093/jnci/djz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez EA, Thompson EA, Ballman KV, Anderson SK, Asmann YW, Kalari KR, et al. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J Clin Oncol. 2015;33:701–708. doi: 10.1200/JCO.2014.57.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 16.Svoronos N, Perales-Puchalt A, Allegrezza MJ, Rutkowski MR, Payne KK, Tesone AJ, et al. Tumor cell–independent estrogen signaling drives disease progression through mobilization of myeloid-derived suppressor cells. Cancer Discov. 2017;7:72–85. doi: 10.1158/2159-8290.CD-16-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 18.Loi S, Adams S, Schmid P, Cortés J, Cescon DW, Winer EP, et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann Oncol. 2017;28:V608. [Google Scholar]
- 19.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, et al. Assessing tumor-infiltrating lymphocytes in solid tumors: a practical review for pathologists and proposal for a standardized method from the International Immunooncology Biomarkers Working Group: part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv Anat Pathol. 2017;24:235–251. doi: 10.1097/PAP.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powles RL, Redmond D, Sotiriou C, Loi S, Fumagalli D, Nuciforo P, et al. Association of T-cell receptor repertoire use with response to combined trastuzumab-lapatinib treatment of HER2-positive breast cancer: secondary analysis of the NeoALTTO randomized clinical trial. JAMA Oncol. 2018;4:e181564. doi: 10.1001/jamaoncol.2018.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ROC curve analysis for TILs cut-off levels.