Abstract
Background and Aims:
Patients with cirrhosis and males have been under-represented in most studies examining the clinical benefit of response to Ursodeoxycholic Acid (UDCA) in primary biliary cholangitis (PBC). The aim of this study was to study the association of UDCA response and liver-related death or transplantation, hepatic decompensation and hepatocellular carcinoma (HCC) in patients with PBC cirrhosis.
Methods:
We conducted a retrospective cohort study of Veterans, predominantly males, with PBC and compensated cirrhosis, to assess the association of UDCA response, with the development of all-cause and liver-related mortality or transplantation, hepatic decompensation and hepatocellular carcinoma (HCC), using competing risk time-updating Cox proportional hazards models.
Results:
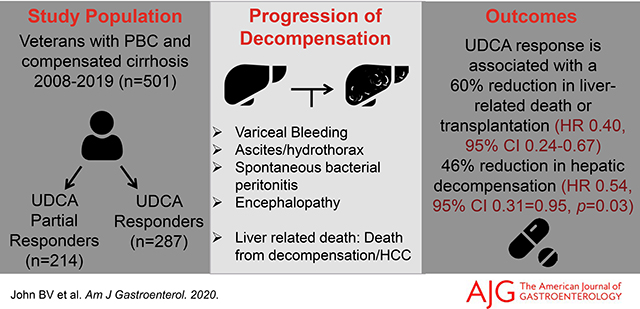
We identified 501 subjects with PBC and compensated cirrhosis, including 287 UDCA responders (1692.8 patient-years (PY) of follow up) and 214 partial responders (838.9 PY of follow up). The unadjusted rates of hepatic decompensation (3.8 vs. 7.9 per 100 PY, p<0.0001) and liver-related death or transplantation (3.7 vs. 6.2 per 100 PY, p <0.0001) were lower in UDCA responders compared to partial responders. UDCA response was associated with a lower risk of hepatic decompensation (sub-Hazard Ratio [sHR] 0.54, 95% CI 0.31–0.95, p=0.03), death from any cause or transplantation (adjusted Hazard Ratio [aHR] 0.49, 95%CI 0.33–0.72, p=0.0002), liver-related death or transplantation (sHR 0.40, 95% CI 0.24–0.67, p=0.0004), but not HCC (sHR 0.39, 95% CI 0.60–2.55, p=0.32). In a sensitivity analysis, the presence of portal hypertension was associated with the highest UDCA-associated effect.
Conclusion:
UDCA response is associated with a reduction in decompensation, all-cause, and liver-related death or transplantation in a cohort of predominantly male patients with cirrhosis, with the highest benefit in patients with portal hypertension.
Keywords: UDCA, partial responder, liver-related death, Hepatocellular carcinoma
Graphical Abstract
INTRODUCTION
Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease characterized by the destruction of small intrahepatic bile ducts.1 The long-term outcome is determined by the presence of portal hypertension and the development of hepatic decompensation, with a median survival of approximately nine years from the time of diagnosis.2,3
Both the American Association of the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommend UDCA as the treatment of choice for PBC.4,5 However, a Cochrane systematic review that examined 16 clinical trials with 1447 subjects found that UDCA did not demonstrate benefits on overall mortality, liver transplantation, pruritus, or fatigue.6 By contrast, retrospective data from the multicenter Global PBC study group has shown that UDCA is associated with a reduction in death or need for transplantation, with a number needed to treat of 11 over 5 years.7
Approximately 20–40% of subjects (depending on the UDCA response criteria used and study population) are partial responders to UDCA, which is the preferred term for what was previously described as non-responders.8,9,10,11 Although newer agents such as obeticholic acid (OCA) and bezafibrate are now available for patients who do not have a complete response to UDCA, there is limited data on the safety or efficacy of either agent on hepatic decompensation or mortality.12,13,14 Therefore, UDCA will remain the mainstay of therapy in patients with PBC cirrhosis.
Large studies addressing the association of UDCA response with hepatic decompensation and overall mortality have primarily included limited percentages (~10%) of patients with cirrhosis 8,13 Prior studies have shown that the degree of fibrosis is a pre-treatment predictor of inadequate response to UDCA.15 Regardless of response to UDCA, advanced fibrosis is an independent predictor of clinical outcomes, as demonstrated by studies using the Nakanuma histological scoring system.12,16 Both commonly used UDCA response prediction models, the GLOBE PBC and UK PBC scores, incorporate platelet count as a surrogate for advanced fibrosis.17,18 However, in prior studies that included PBC patients in varying stages of fibrosis, it has been challenging to differentiate the relative effects of the degree of fibrosis and UDCA response on clinical events of decompensation, death or transplantation. The effect of UDCA after cirrhosis or portal hypertension has already developed is controversial. Many publications have demonstrated histological and clinical benefits with UDCA when initiated in the early stages of the disease and have questioned its efficacy when initiated in patients with advanced fibrosis.4,19,20 The presence of cirrhosis might impact the benefit associated with UDCA in many ways. Theoretically, due to the higher baseline risk of hepatic decompensation and death in this population, the clinical impact might be more significant. Alternatively, cirrhosis alters the bioavailability of UDCA, which can decrease the benefit of the drug.21
Another gap in the existing literature is the exploration of the impact of UDCA on liver-related rather than all-cause mortality.
Therefore, the aim of our study was to study the association of UDCA response and liver-related death or transplantation, hepatic decompensation and hepatocellular carcinoma (HCC) in patients with PBC cirrhosis.
METHODS
Study Design
This was a retrospective cohort study using national VA data on Veterans who received care at any of the Veterans Affairs Medical Centers (VAMCs) in the United States. These data were assembled by first screening using the VA Corporate Data warehouse (CDW), a clinical data repository that contains patient demographics, International Classification of Diseases Revision Clinical Modification (ICD-CM) diagnosis codes, laboratory, imaging, elastography, pathology, endoscopy and prescription records on all patients receiving care within the VA medical system. Findings were confirmed using manual extraction of data by review of individual charts of potential recipients.22 All institutional review boards at participating VA medical centers approved the study.
Subject Identification
Unique cirrhotic patients with PBC were initially identified by querying the CDW using ICD9-CM or ICD10-CM primary or secondary two outpatient or one inpatient diagnosis codes for primary biliary cholangitis (ICD9-CM: 571.6, ICD10-CM: K74.3) and cirrhosis (ICD9-CM 571.5, ICD10-CM: K70.3x) from January 2008 to December 2016, with follow up to December 31st 2019. Once these potential subjects were identified, manual chart review was performed to confirm the diagnosis of PBC: PBC was diagnosed by the presence of two out of three clinical features: alkaline phosphatase (ALP) of greater than 1.5 times the upper limit of normal, a positive anti-mitochondrial antibody (AMA), and a liver biopsy consistent with PBC. Patients were included if PBC was diagnosed before or at the same time as cirrhosis. Patients with evidence of overlap syndrome on the liver biopsy were excluded. The diagnosis of cirrhosis was confirmed by either a liver biopsy, a Vibration Controlled Transient Elastography with liver stiffness >16.9 KPa,13,23or a nodular appearing liver on ultrasound with or without the presence of portal hypertension. Portal hypertension was defined as the presence of thrombocytopenia (<150×109/ml) in the absence of alternative explanations, varices on upper endoscopy or the presence of splenomegaly (spleen size ≥14 cm), or collaterals on abdominal imaging.
Patients were excluded if they had decompensation at the time of initial diagnosis of cirrhosis, as documented by the presence of variceal bleeding, ascites, hepatic encephalopathy, hepatic hydrothorax or Child- Turcotte-Pugh (CTP) B or C. We excluded patients who did not meet criteria for cirrhosis or PBC, those who developed cirrhosis after liver transplantation, were not treated with UDCA and those subjects where there was inadequate data within the VA system.
Study Time points
The study entry date or baseline was defined as the first date of documentation of the diagnosis of cirrhosis. A diagnosis of PBC was made either at the time of, or prior to study entry. Patients were classified as responders or partial responders 24 months after the initiation of UDCA, which in many cases, but not always, preceded the cohort entry date. From study entry, all patients were followed until death, transplantation or December 31st, 2019 (whichever was earliest). The outcome of hepatic decompensation was defined as the development of variceal bleeding, ascites, hepatic hydrothorax, spontaneous bacterial peritonitis, or hepatic encephalopathy.
Chart review was performed to identify the cause of death, which was considered liver related if it was attributable to hepatic decompensation or progression of hepatocellular carcinoma. Patients were censored if they died or received a liver transplant.
Covariates
Endoscopy findings, imaging, transient elastography, liver biopsy, clinical details of decompensation, mortality data and cause of death were obtained from a direct review of the chart. Data from manual chart review were combined with laboratory values obtained from the CDW. UDCA response was defined as ALP less than 1.67 times the upper limit of normal 24 months after initiation of UDCA, based on the Toronto criteria, which was chosen because of its derivation from prospective data and widespread use and validation.24 A sensitivity analysis was performed using a definition of UDCA response as ALP and total bilirubin less than the upper limit of normal at 12 months, as defined by Harms et al.10 In addition, the following laboratory values were obtained at the time of cirrhosis diagnosis and throughout follow-up: serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), ALP, platelet count, as well as international normalized ratio (INR), creatinine, total bilirubin and serum sodium to calculate MELD and MELD-Na. Baseline laboratory values were the closest to the index date of cirrhosis obtained from 180 days before to 30 days after diagnosis. Tobacco use was characterized as current use, former use, or lifetime non-use. 25 Alcohol use was characterized by ICD codes and review of the chart, and standardized using Alcohol Use Disorders Identification Test (AUDIT-C) scores; AUDIT-C scores ≥4 for males and ≥3 among females at the time of diagnosis of cirrhosis was considered alcohol misuse.26
Outcomes
The primary outcome was a composite of liver-related mortality (death attributable to hepatic decompensation or HCC) and transplantation. The secondary outcomes were all-cause mortality or transplantation, hepatic decompensation and HCC.
Statistical Analysis
The associations of UDCA response and all-cause mortality or transplantation, liver-related mortality or transplantation (with non-liver-related death as competing risk), decompensation and HCC (with death or transplantation as competing risk) were estimated using time-updating Cox proportional hazards models (with diagnosis of cirrhosis as the baseline), adjusted for the following covariates that were defined a priori, based on previously published data: Age, gender, 27 race-ethnicity,28 baseline tobacco use,29 BMI, 30 diabetes,30 CTP score,31 and time updating ALP,32 total bilirubin,32 platelet count,17 AUDIT-C score,33 and MELD-Na.34 A sensitivity analysis was performed using a definition of UDCA response as ALP and total bilirubin less than the upper limit of normal at 12 months.10 We also did a sensitivity analysis examining the same clinical outcomes in patients with portal hypertension.
RESULTS
Patient Characteristics
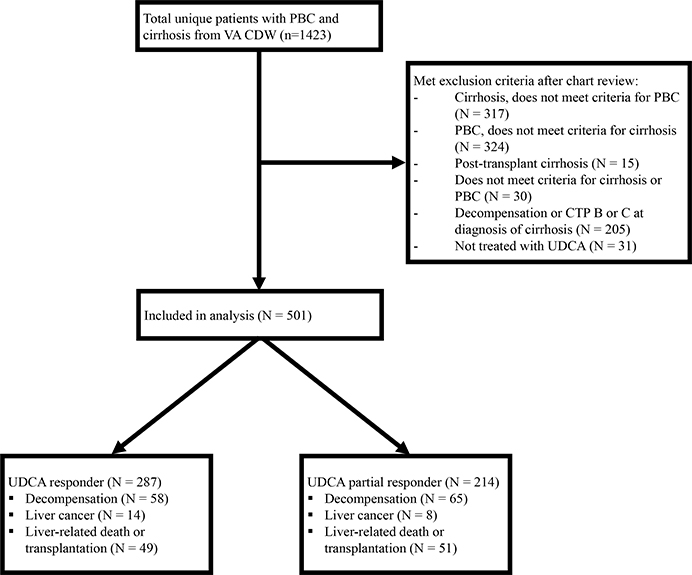
Out of a total of 1493 subjects identified from the VA CDW, after excluding ineligible subjects, the final study population consisted of 501 subjects with PBC and compensated cirrhosis, of whom 287 were UDCA responders and 214 partial responders (Figure 1).
Figure.1.
Study flow diagram
Table 1 summarizes the baseline characteristics at diagnosis of cirrhosis. Consistent with a Veteran population, the cohort was predominantly male (77.8%) and white (73.2%). The UDCA responders and partial responders were similar with respect to other baseline characteristics, including age, gender, race/ethnicity, diabetes mellitus, tobacco use, and AUDIT-C score. A total of 410 out of the 464 subjects (88.4%) who had available anti-mitochondrial antibodies in the VA system were positive. A liver biopsy confirming the diagnosis of PBC was available in 68% of subjects. UDCA responders had a higher BMI (28.2 vs 25.7, p<0.001), were more likely to be CTP A6 (vs A5, 14.5% vs. 7.3%, p=0.009), had a greater duration of exposure to UDCA (103.4 vs. 71.3 months, p<0.0001), a greater duration of UDCA exposure after diagnosis of cirrhosis (79.0 vs. 51.0 months, p<0.0001), but lower baseline values of AST (33.0 vs. 62.0 IU/ml, p<0.0001), ALT (35.0 vs. 59.0 IU/ml, p<0.0001), ALP (131.0 vs. 338.5, p<0.0001), total bilirubin (0.9 vs. 1.0 mg/dl, p<0.0001) and platelet count (178.0 vs 189.0 ×10E9/L, p=0.02), as well as lower dose of UDCA (13.1 vs. 13.8 mg/kg, p=0.02) and MELD-Na (6.0 vs. 7.0, p=0.0004) compared to partial responders.
Table 1:
Descriptive statistics for PBC patients by UDCA response
| All | UDCA Responder | UDCA Partial Responder | P Value | |
|---|---|---|---|---|
|
| ||||
| N of Patients | 501 | 287 | 214 | - |
|
| ||||
| Gender, N (%) | 0.7586 | |||
| Male | 390 | 222 (77.4%) | 168 (78.5%) | |
| Female | 111 | 65 (22.6%) | 46 (21.5%) | |
|
| ||||
| Age, Median (IQR) | 65.0 (13.0) | 66.0 (12.0) | 64.0 (15.0) | 0.0596 |
|
| ||||
| Race / Ethnicity, N (%) | 0.1763 | |||
| White | 363 | 218 (76%) | 145 (67.8%) | |
| Black | 30 | 12 (4.2%) | 18 (8.4%) | |
| Other | 58 | 32 (11.2%) | 26 (12.2%) | |
| Hispanic/Latino | 22 | 10 (3.5%) | 12 (5.6%) | |
| Unknown | 28 | 15 (5.2%) | 13 (6.1%) | |
|
| ||||
| BMI, Median (IQR) | 27.1 (6.1) | 28.2 (5.7) | 25.7 (5.5) | <.0001 |
|
| ||||
| BMI Class, N (%) | <.0001 | |||
| Underweight (less than 18.5) | 13 | 4 (1.4%) | 9 (4.2%) | |
| Normal weight (18.5 to 25) | 139 | 60 (21.2%) | 79 (36.9%) | |
| Overweight (25 to 30) | 213 | 128 (45.2%) | 85 (39.7%) | |
| Obese (more than 30) | 132 | 91 (32.2%) | 41 (19.2%) | |
|
| ||||
| Diabetes, N (%) | 0.2269 | |||
| No | 324 | 192 (66.9%) | 132 (61.7%) | |
| Yes | 177 | 95 (33.1%) | 82 (38.3%) | |
|
| ||||
| Tobacco Use, N (%) | 0.1583 | |||
| Current smoker | 133 | 68 (23.7%) | 65 (30.4%) | |
| Former smoker | 188 | 119 (41.5%) | 69 (32.2%) | |
| Never smoker | 170 | 95 (33.1%) | 75 (35.1%) | |
| Unknown | 10 | 5 (1.7%) | 5 (2.3%) | |
|
| ||||
| AUDIT-C Score, N (%) | 0.6300 | |||
| Low | 465 | 265 (92.3%) | 200 (93.5%) | |
| High | 36 | 22 (7.7%) | 14 (6.5%) | |
|
| ||||
| CTP A5, N (%) | 449 | 266 (92.7%) | 183 (85.5%) | 0.0093 |
| CTP A6, N (%) | 52 | 21 (7.3%) | 31 (14.5%) | |
|
| ||||
| Lab Results, Median (IQR) | ||||
| Aspartate Aminotransferase (IU/ml) | 40.0 (35.0) | 33.0 (16.0) | 62.0 (43.0) | <0.0001 |
| Alanine Aminotransferase (IU/ml) | 40.0 (36.0) | 35.0 (21.0) | 59.0 (52.0) | <0.0001 |
| Alkaline Phosphatase (IU/ml) | 180.0 (166.0) | 131.0 (60.0) | 338.5 (228.0) | <0.0001 |
| Albumin (g/dL) | 3.8 (0.7) | 3.8 (0.5) | 3.6 (0.8) | <0.0001 |
| Platelet Count (x10E9/L) | 184.0 (113.0) | 178.0 (109.0) | 189.0 (124.0) | 0.0229 |
| Serum Sodium (mEq/L) | 139.0 (4.0) | 139.0 (4.0) | 138.0 (5.0) | 0.0146 |
| Creatinine (mg/dL) | 1.0 (0.4) | 1.0 (0.4) | 1.0 (0.4) | 0.3033 |
| Total Bilirubin (mg/dL) | 0.8 (0.7) | 0.8 (0.6) | 1.0 (0.8) | <.0001 |
| International Normalized Ratio | 1.0 (0.2) | 1.0 (0.2) | 1.0 (0.1) | 0.0068 |
| MELD Score | 6.0 (0.0) | 6.0 (0.0) | 6.0 (0.0) | 0.0521 |
| MELD-Na Score | 6.0 (3.0) | 6.0 (3.0) | 7.0 (4.0) | 0.0004 |
|
| ||||
| Globe Score after 12 months of UDCA, Median (IQR) | 1 (13) | 0.9 (1.2) | 1.4 (1.3) | <0.0001 |
|
| ||||
| UDCA, Median (IQR) | ||||
| UDCA Dose / Weight (mg/Kg) | 13.3 (4.0) | 13.1 (4.0) | 13.8 (3.7) | 0.0235 |
| UDCA use total time in months | 88.0 (90.9) | 103.4 (93.4) | 71.3 (83.6) | <0.0001 |
| UDCA use time after cirrhosis in months | 68.0 (81.0) | 79.0 (75.0) | 51.0 (67.0) | <0.0001 |
|
| ||||
| Positive AMA, N (%) | 0.0051 | |||
| Yes | 410 | 231 (89.9%) | 179 (86.5%) | |
| No* | 54 | 26 (10.1%) | 28 (13.5%) | |
|
| ||||
| Liver Biopsy at PBC Diagnosis, N (%) | 0.0743 | |||
| Yes | 338 | 198 (68.9%) | 140 (65.4%) | |
| No | 163 | 89 (31.1%) | 74 (34.6%) | |
|
| ||||
| Varices, N (%) | 0.3094 | |||
| Yes | 128 | 69 (24.1%) | 59 (27.5%) | |
| No | 367 | 213 (74.2%) | 154 (72.0%) | |
| Unknown | 6 | 5 (1.7%) | 1 (0.5%) | |
|
| ||||
| Method of Cirrhosis Diagnosis, N (%) | 0.6305 | |||
| Biopsy | 65 | 39 (13.6%) | 26 (12.2%) | |
| Elastography | 17 | 8 (2.8%) | 9 (4.2%) | |
| Nodular liver with portal hypertension | 338 | 195 (67.9%) | 143 (66.8%) | |
| Nodular liver without portal hypertension | 81 | 45 (15.7%) | 36 (16.8%) | |
|
| ||||
| Follow-up in months, Median (IQR) | ||||
| Follow-up time after diagnosis of PBC | 93.0 (95.5) | 108.9 (102.0) | 81.4 (87.6) | <0.0001 |
| Follow-up time after diagnosis of cirrhosis | 68.0 (81.0) | 79.0 (75.0) | 51.0 (67.0) | <0.0001 |
❖ UDCA: Ursodeoxycholic Acid; BMI: Body Mass Index; AUDIT-C: Alcohol Use Disorders Identification Test – Concise; CTP: Child-Turcotte-Pugh; MELD: Model for End-stage Liver Disease; MELD-Na: Model for End-stage Liver Disease-Sodium Score; AMA: Antimitochondrial Antibody; IQR: Inter Quartile Range
❖ AMA was unavailable for 37 subjects.
Clinical Event-Decompensation, HCC and Death
A total of 202 subjects died during the study period, of which 96 were liver-related (Supplemental Table 1). The unadjusted rate of liver-related death or transplantation (3.7 vs. 6.2 per 100 PY, p<0.0001), and decompensation (3.7 vs. 6.2 per 100 PY, p<0.0001) were both lower in UDCA responders compared to partial responders (Table 2). The first documented decompensating event was ascites in 90 subjects, followed by encephalopathy (n=21), and variceal bleed (n=12).
Table 2:
Rates of decompensation and liver-related death or transplantation in compensated cirrhosis by UDCA response
| Event | Number of Patients | Patient-Years | Event per 100 Person-Years | P value |
|---|---|---|---|---|
|
| ||||
| Decompensation | <.0001 | |||
| UDCA responder | 287 | 1692.8 | 3.8 | |
| UDCA partial responder | 214 | 838.9 | 7.9 | |
|
| ||||
| Liver–related Death or Transplantation | <.0001 | |||
| UDCA responder | 287 | 1688.4 | 3.7 | |
| UDCA partial responder | 214 | 996.4 | 6.2 | |
|
| ||||
| Liver cancer | 0.0352 | |||
| UDCA responder | 287 | 1684.7 | 0.6 | |
| UDCA partial responder | 214 | 1068.3 | 0.7 | |
❖ UDCA: Ursodeoxycholic Acid
Factors Associated with Mortality or Transplantation
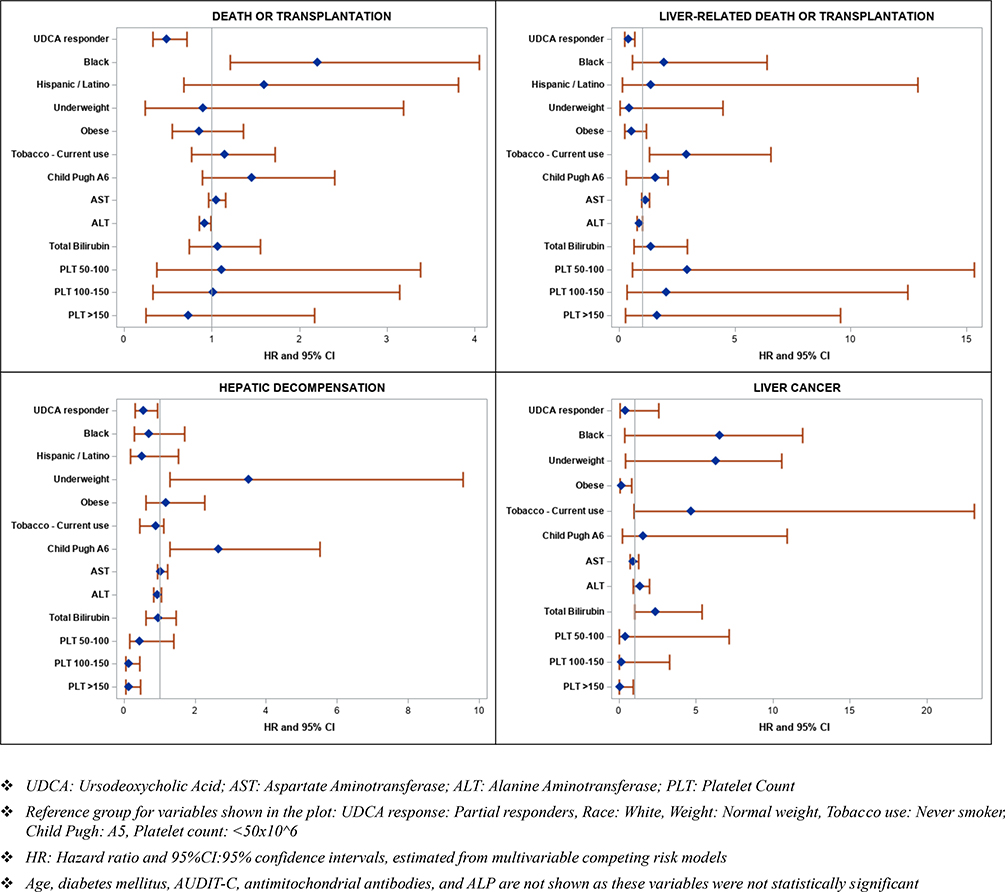
The risk factors associated with death from any cause or transplantation included black race (aHR 2.21, 95% CI 1.21–4.05, p=0.01) and ALT levels (per 10 IU/ml, aHR 0.92, 95% CI 0.86–0.99, p=0.03) (Table 3 and Figure 3). After adjusting for potential confounders, UDCA response was associated with a significant reduction in all-cause mortality or transplantation (aHR 0.49, 95% CI 0.33–0.72, p=0.0002) (Table 3 and Figures 2 and 3).
Table 3:
Univariable and multivariable hazard ratios for the risk of death or transplantation and liver-related death or transplantation
| Death or Transplantation | Liver-related Death or Transplantation | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Number of events | 206 | 100 | ||||||
|
|
||||||||
| Univariable | Multivariable | Univariable | Multivariable | |||||
|
| ||||||||
| Variable | HR (95%CI) | P Value | aHR (95%CI) | P Value | HR (95%CI) | P Value | aHR (95%CI) | P Value |
|
| ||||||||
| UDCA Responders | ||||||||
| No | REF | REF | REF | REF | ||||
| Yes | 0.60 (0.45,0.80) | 0.0005 | 0.49 (0.33,0.72) | 0.0002 | 0.54 (0.35,0.82) | 0.0040 | 0.40 (0.24,0.67) | 0.0004 |
|
| ||||||||
| Gender | ||||||||
| Female | REF | REF | REF | REF | ||||
| Male | 1.24 (0.84,1.84) | 0.2814 | 1.47 (0.85,2.54) | 0.1650 | 2.48 (1.46,4.24) | 0.0008 | 1.27 (0.22,1.93) | 0.0580 |
|
| ||||||||
| Age at Cirrhosis | 1.01 (0.99,1.02) | 0.9812 | 1.01 (0.99,1.03) | 0.3307 | 1.01 (0.98,1.03) | 0.6954 | 1.04 (0.99,1.08) | 0.1157 |
|
| ||||||||
| Race / Ethnicity | ||||||||
| White | REF | REF | REF | REF | ||||
| Black | 1.62 (0.92,2.87) | 0.0953 | 2.21 (1.21,4.05) | 0.0102 | 1.46 (0.80,2.68) | 0.2181 | 1.93 (0.58,6.40) | 0.2801 |
| Other | 2.01 (1.23,3.26) | 0.0050 | 2.11 (1.27,3.50) | 0.0038 | 1.92 (1.01,3.65) | 0.0479 | 2.24 (1.04,4.84) | 0.0402 |
| Hispanic/Latino | 1.42 (0.58,3.45) | 0.4410 | 1.60 (0.68,3.81) | 0.2856 | 2.18 (1.12,4.25) | 0.0226 | 1.37 (0.15,12.9) | 0.7814 |
| Unknown | 1.30 (0.67,2.52) | 0.4432 | 0.91 (0.49,1.67) | 0.7493 | 2.12 (1.06,4.24) | 0.0340 | 0.69 (0.27,1.78) | 0.4431 |
|
| ||||||||
| BMI | ||||||||
| Normal Weight (18.5 to 25) | REF | REF | REF | REF | ||||
| Underweight (less than 18.5) | 1.27 (0.50,3.22) | 0.6173 | 0.90 (0.25,3.19) | 0.8657 | 0.65 (0.22,1.92) | 0.4377 | 0.44 (0.04,4.48) | 0.4903 |
| Overweight (25 to 30) | 0.82 (0.59,1.15) | 0.2489 | 0.73 (0.52,1.01) | 0.0605 | 0.69 (0.44,1.09) | 0.1081 | 0.47 (0.24,0.93) | 0.0310 |
| Obese (more than 30) | 0.79 (0.54,1.16) | 0.2233 | 0.86 (0.55,1.36) | 0.5168 | 0.49 (0.29,0.84) | 0.0092 | 0.53 (0.24,1.16) | 0.1115 |
|
| ||||||||
| Diabetes | ||||||||
| No | REF | REF | REF | REF | ||||
| Yes | 1.11 (0.84,1.46) | 0.4542 | 1.16 (0.85,1.57) | 0.3521 | 1.26 (0.84,1.90) | 0.2687 | 1.33 (0.75,2.34) | 0.3282 |
|
| ||||||||
| Tobacco Use | ||||||||
| Never Smoker | REF | REF | REF | REF | ||||
| Current Smoker | 1.21 (0.86,1.70) | 0.2792 | 1.15 (0.77,1.72) | 0.4960 | 1.32 (0.82,2.14) | 0.2575 | 2.92 (1.31,6.55) | 0.0091 |
| Former Smoker | 0.85 (0.62,1.17) | 0.3101 | 0.84 (0.58,1.20) | 0.3286 | 0.93 (0.60,1.46) | 0.7632 | 1.62 (0.78,3.39) | 0.1991 |
| Unknown | 5.05 (2.70,9.44) | <.0001 | 3.32 (1.32,8.35) | 0.0106 | 5.79 (3.35,10.00) | <.0001 | 24.67 (7.60,80.09) | <.0001 |
|
| ||||||||
| AUDIT-C Score | ||||||||
| Low | REF | REF | REF | REF | ||||
| High | 1.31 (0.86,1.99) | 0.2109 | 1.46 (0.89,2.40) | 0.1391 | 1.17 (0.60,2.28) | 0.6465 | 1.64 (0.33,2.25) | 0.1912 |
|
| ||||||||
| CTP Score | ||||||||
| CTP A5 | REF | REF | REF | REF | ||||
| CTP A6 | 1.59 (0.96,2.62) | 0.0717 | 1.58 (0.79,3.14) | 0.1953 | 1.47 (0.82,2.62) | 0.1936 | 1.59 (0.32,2.10) | 0.0958 |
|
| ||||||||
| AMA | ||||||||
| Yes | REF | REF | REF | REF | ||||
| No | 1.36 (0.90,2.07) | 0.1473 | 1.31 (0.82,2.11) | 0.2629 | 2.26 (1.39,3.67) | 0.0010 | 1.55 (0.68,3.55) | 0.2953 |
|
| ||||||||
| Aspartate Aminotransferase per 10 unites change | 1.03 (0.98,1.08) | 0.2447 | 1.05 (0.96,1.16) | 0.2966 | 1.03 (0.96,1.09) | 0.4162 | 1.13 (0.97,1.32) | 0.1195 |
|
| ||||||||
| Alanine Aminotransferase per 10 unites change | 0.98 (0.95,1.02) | 0.2277 | 0.92 (0.86,0.99) | 0.0315 | 0.97 (0.93,1.09) | 0.1194 | 0.88 (0.76,1.02) | 0.0785 |
|
| ||||||||
| Alkaline Phosphatase per 10 unites change | 1.04 (0.98,1.09) | 0.1794 | 0.97 (0.95,1.03) | 0.6041 | 1.01 (0.99,1.08) | 0.8403 | 0.99 (0.96,1.02) | 0.5717 |
|
| ||||||||
| Total Bilirubin | 1.36 (1.09,1.69) | 0.0063 | 1.07 (0.74,1.55) | 0.7053 | 1.43 (1.09,1.89) | 0.0108 | 1.38 (0.64,2.93) | 0.4102 |
|
| ||||||||
| Platelet Count | ||||||||
| <=50 | REF | REF | REF | REF | ||||
| 50 –100 | 0.97 (0.38,2.47) | 0.9479 | 1.11 (0.37,3.38) | 0.8510 | 1.46 (0.58,3.68) | 0.4281 | 2.95 (0.57,15.33) | 0.1993 |
| 100 – 150 | 0.91 (0.37,2.23) | 0.8382 | 1.02 (0.33,3.14) | 0.9684 | 1.08 (0.41,2.81) | 0.8823 | 2.04 (0.34,12.45) | 0.4379 |
| >150 | 0.69 (0.29,1.63) | 0.3988 | 0.73 (0.25,2.17) | 0.5748 | 0.85 (0.34,2.11) | 0.7179 | 1.65 (0.29,9.55) | 0.5736 |
|
| ||||||||
| MELD-Na Score | 1.05 (0.99,1.11) | 0.0504 | 1.05 (0.99,1.11) | 0.0504 | 1.07 (0.99,1.15) | 0.0546 | 1.11 (0.99,1.25) | 0.0880 |
❖ UDCA: Ursodeoxycholic Acid; BMI: Body Mass Index; AUDIT-C: Alcohol Use Disorders Identification Test – Concise; CTP: Child-Turcotte-Pugh; MELD: Model for End-stage Liver Disease; MELD-Na: Model for End-stage Liver Disease-Sodium Score; AMA: Antimitochondrial Antibody; IQR: Inter Quartile Range
Figure 3:
Hazard ratio and 95% confidence intervals, estimated from multivariable competing risk models for association of UDCA response with death or transplantation, liver-related death or transplantation, hepatic decompensation, and liver cancer
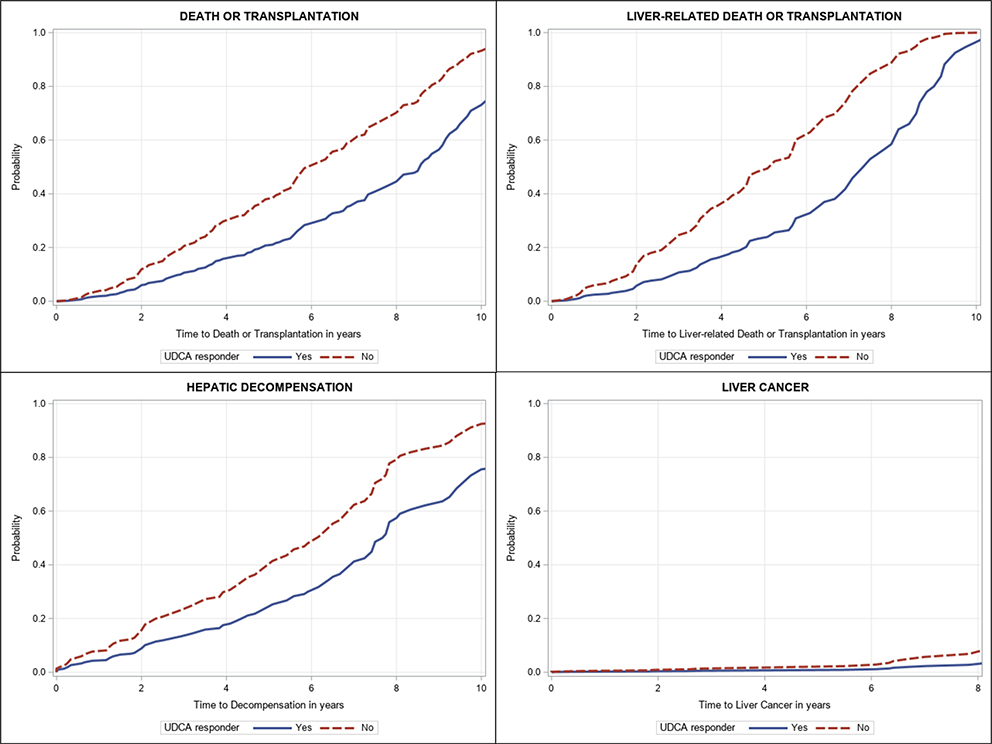
Figure.2.
Adjusted time from diagnosis of cirrhosis to death or transplantation, liver-related death or transplantation, hepatic decompensation, and liver cancer, by UDCA response
The risk factors associated with liver-related-death or transplantation included current smoking status (sub-Hazard ratio [sHR] 2.92, 95% CI 1.31–6.55, p=0.009) and being overweight (sHR 0.47, 95% CI 0.24–0.93, p=0.03) (Table 3 and Figure 3). After adjusting for potential confounders, UDCA response was associated with a significant reduction in liver-related-mortality or transplantation (sHR 0.40, 95% CI 0.24–0.67, p=0.0004) (Table 3 and Figures 2 and 3).
Factors Associated with Hepatic Decompensation and Hepatocellular Carcinoma
On multivariable analysis, hepatic decompensation was associated with being underweight with BMI<18.5 (sHR 3.51, 95% CI 1.29–9.55, p=0.01), diabetes mellitus (sHR 2.47, 95% CI 1.54–3.95, p=0.0002), CTP Class A6 (vs. A5, sHR 2.67, 95% CI 1.29–5.52, p=0.008) and MELD-Na (sHR 1.12, 95% CI 1.02–1.22, p=0.02). (Supplementary Table 2 and Figure 3). After adjusting for potential confounders, UDCA response was associated with a significant reduction in hepatic decompensation (aHR 0.54, 95% CI 0.31–0.95, p=0.03, Supplementary Table 2 and Figures 2 and 3).
Twenty-two patients developed HCC during the study period. On multivariable analysis, HCC was associated with older age (aHR 1.09, 95% CI 1.02–1.17, p=0.008), absence of obesity (sHR 0.17, 95% CI 0.04–0.82, p=0.03) and total bilirubin (sHR 2.37, 95% CI 1.04–5.40, p=0.04) (Supplementary Table 2). After adjusting for potential confounders, response to UDCA was not associated with HCC (sHR 0.39, 95% CI 0.06–2.55, p=0.33).
Sensitivity Analysis
We performed a sensitivity analysis defining UDCA response as ALP and total bilirubin below the upper limit of normal at 12 months. This indicated that UDCA response was associated with a lower rate of hepatic decompensation (sHR 0.37, 95% CI 0.16–0.86, p=0.02) but association with liver-related death or transplantation (sHR 0.47, 95% CI 0.23–1.04, p=0.06) and HCC (sHR 0.65, 95% CI 0.06–6.81, p=0.72) were not statistically significant.
We also performed a sensitivity analysis restricting analysis to patients with portal hypertension. After adjusting for potential confounders, UDCA response was associated with greater reductions compared to the overall cohort in both death due to any cause or transplantation (aHR 0.49, 95% CI 0.29–0.84, p=0.009) and liver-related-mortality or transplantation (sHR 0.21, 95% CI 0.09–0.49, p=0.0003) (Supplementary Table 3 and Supplementary Figure 1), but not with decompensation (sHR 0.95, 95% CI 0.46–1.93, p=0.88) or HCC (sHR 0.28, 95% CI 0.02–4.15, p=0.36). (Supplementary Table 4 and Supplementary Figure 1).
DISCUSSION
Despite widespread use of UDCA in PBC, the impact of the drug on hepatic decompensation and overall mortality in patients who have developed cirrhosis is unclear. Because most studies in PBC have relatively low numbers of men and patients with cirrhosis, we chose to assemble this cohort of subjects with compensated PBC cirrhosis, to better understand the disease progression in this sub-group of subjects at higher risk for clinical events.
Our data shows that UDCA response is associated with a 51% reduction in death or transplantation, and a greater magnitude (60%) reduction in liver-related death or transplantation, an outcome that has not previously been reported. Our data also suggests that the benefit associated with UDCA is greatest in patients with portal hypertension. These data support recent recommendations that UDCA should be continued even after the development of advanced fibrosis.35
This study is unable to answer the question whether the benefit of UDCA is maintained in the absence of complete response, because the population of non-UDCA users was small, and excluded from this study. We also found that black race was associated with all-cause mortality or transplantation, but the association between black race and liver-related-death or transplantation was numerically higher but not statistically significant. Blacks formed only 6% of the overall cohort, and therefore, this finding need to be corroborated in future studies. We also identified unique risk factors for hepatic decompensation. Though there were no differences in diabetes between UDCA responder and non-responders, diabetes was associated with increased hepatic decompensation. This validates prior data that shows the association between metabolic syndrome (including diabetes) and fibrosis. 30 As the prevalence of metabolic syndrome increases in the population, the presence of concomitant NAFLD will likely have a greater impact on disease progression in PBC. Our data also demonstrated an association between being underweight (BMI<18.5) and decompensation. Because we used the baseline BMI at the time of diagnosis of cirrhosis to define BMI sub-classes, the presence of concomitant sarcopenia at the time of cirrhosis diagnosis may be a marker of advanced disease. We also found that being overweight was associated with lower liver-related death or transplantation, while obesity was associated with lower rates of HCC. These analyses should be interpreted with caution in light of the relatively small number of subjects in the sub-groups that developed events. In particular, the finding that obesity was associated with a decreased hazard of developing HCC may have been driven by the fact that only two subjects with obesity developed HCC in the cohort.
Prior studies have shown that complications of cirrhosis such as decompensation and mortality are associated with low BMI and negatively associated with obesity, in patients with cirrhosis,36 including those with PBC.37 Therefore, we hypothesize that patients with cirrhosis with sarcopenia had more advanced disease and more likely to develop complications, while patients with obesity were “protected”.
Our data contrasts from that reported by Trivedi et al. that showed a protective association between UDCA response and HCC, with the relative benefit of UDCA response being greater among non-cirrhotics than cirrhotics.38 This may represent a Type II error as our study was under-powered to detect differences in HCC. The computed power on post hoc analysis for the hazard model for HCC (assuming alpha of 0.05) was only 6.9%. Also, male gender is associated with increased HCC in subjects with PBC cirrhosis.39 It is possible that the benefit of UDCA response may be diminished in our male predominant cohorts, who are at elevated baseline risk of HCC.
Although this is a large sample size for a cohort of patients with PBC cirrhosis, we acknowledge the following limitations: First, the retrospective nature of the study precluded a standardized evaluation in every patient. We included only patients who met the diagnostic criteria for PBC, with 68% had biopsy confirmation. Though guidelines do not recommend liver biopsies to confirm the diagnosis of PBC in most subjects, we believe that the high biopsy rate strengthens the validity of the findings. Second, the cohort had significantly more males than traditionally described in PBC, consistent with distribution among a Veteran population. Males are under-represented in PBC studies, and therefore, this cohort gives valuable information of the outcomes among males with PBC. However, this limits direct comparison to data described by other multi-center groups describing PBC outcomes. Third, the population had higher rates of obesity and alcohol use, which may impact the natural history of this population. We attempted to mitigate this by adjusting for time updated AUDIT-C scores and metabolic syndrome (BMI and diabetes) but there remains the possibility of residual confounding. However, this affords us an opportunity to study the association between metabolic syndrome and clinical progression of HCC in patients with PBC, which is important with the rising prevalence of metabolic syndrome and obesity in the population. Fourthly, while the diagnosis of cirrhosis was established based on biopsy, elastography or abnormal liver imaging and portal hypertension in 80% of subjects, 81 of the 501 subjects had a diagnosis of cirrhosis based on abnormal liver imaging without portal hypertension. However, we found similar associations as those noted in the primary analysis, after excluding these 81 subjects, as well as while restricting analysis among patients with portal hypertension. The limitations are outweighed by relative strengths including a large sample size of patients with PBC and compensated cirrhosis with a relatively long follow up and high number of clinical events. This cohort represents patients in a real world setting without a tertiary center bias. The data were obtained from patients receiving care from a national system which offers access to care for all eligible Veterans. We were able to identify the cause of death and report liver-related-death or transplantation as an outcome, than all-cause mortality, which is novel.
In summary, our data suggests that UDCA response in patients with PBC cirrhosis is associated with lower risk of hepatic decompensation, overall and liver-related mortality or transplantation than previously described in PBC without cirrhosis. The benefit of UDCA response appears to be the highest in subjects with portal hypertension. Future studies are needed to evaluate if newer agents, such as obeticholic acid and the fibrates, can improve the outcomes of UDCA partial responders with compensated cirrhosis.
Supplementary Material
Study highlights:
What is Known:
UDCA response is associated with reduced mortality in patients with Primary Biliary Cholangitis (PBC). However, it is unclear if this relationship holds true in patients with cirrhosis, as most studies that have explored this have limited number of patients with cirrhosis (~10%). Also, males are under-represented in most PBC studies.
What is New Here:
In a cohort of primarily male patients with PBC cirrhosis, UDCA response is associated with a significant reduction in hepatic decompensation as well as all-cause mortality or transplantation, with the highest effect in patients with portal hypertension.
UDCA response is associated with a greater reduction in liver-related-death or transplantation, compared to all-cause mortality or transplantation that has been traditionally used in prior PBC studies.
Acknowledgments
Financial support: Services supporting this research project were generated by the VCU Massey Cancer Center Biostatistics Shared Resource, supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Abbreviations:
- ALP
alkaline phosphatase
- AMA
anti-mitochondrial antibody
- AUDIT-C
Alcohol Use Disorder Identification Test
- BMI
body mass index
- CDW
Corporate Data Warehouse
- CTP
Child-Turcotte-Pugh
- FDA
Food and Drug Administration
- ICD
international classification of disease
- INR
international normalized ratio
- IQR
interquartile range
- MELD
model for end stage liver disease
- PBC
primary biliary cholangitis
Footnotes
Conflict of interest statement: None of the authors report financial conflicts of interest directly related to this publication
Disclaimer: The authors prepared this work in their personal capacity. The opinions expressed in this article are the author’s own and do not reflect the view of the Department of Veterans Affairs or the United States government.
REFERENCES
- 1.Goel A, Kim WR. Natural History of Primary Biliary Cholangitis in the Ursodeoxycholic Acid Era: Role of Scoring Systems. Clin Liver Dis. 2018;22(3):563–578. [DOI] [PubMed] [Google Scholar]
- 2.Price M, Chetwynd A, Newman W, Metcalf JV, James OF. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: follow-up for up to 28 years Gastroenterology. 2002October;123(4):1044–51. [DOI] [PubMed] [Google Scholar]
- 3.Imam MH, Lindor KD. The natural history of primary biliary cirrhosis. Semin Liver Dis. 2014;34(3):329–333. [DOI] [PubMed] [Google Scholar]
- 4.Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Hepatology. 2019January;69(1):394–419. Primary Biliary Cholangitis: 2018 Practice Guidance From theAmerican Association for the Study of Liver Diseases [DOI] [PubMed] [Google Scholar]
- 5.EASL Clinical Practice Guidelines: The Diagnosis and Management of Patients With Primary Biliary Cholangitis. J Hepatol. 2017; 67(1):145–172. [DOI] [PubMed] [Google Scholar]
- 6.Rudic JS, Poropat G, Krstic MN, Bjelakovic G, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2012, Issue 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harms MH, de Veer RC, Lammers WJ, et al. Number needed to treat with ursodeoxycholic acid therapy to prevent liver transplantation or death in primary biliary cholangitis. Gut 2020;69: 1502–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130(3):715–720 [DOI] [PubMed] [Google Scholar]
- 9.Murillo Perez CF, Hirschfield GM, Corpechot C, et al. Fibrosis stage is an independent predictor of outcome in primary biliary cholangitis despite biochemical treatment response. Aliment Pharmacol Ther. 2019;50(10):1127–1136. [DOI] [PubMed] [Google Scholar]
- 10.Harms MH, van Buuren HR, Corpechot C, et al. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71(2):357–365. [DOI] [PubMed] [Google Scholar]
- 11.Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR; Dutch PBC Study Group. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology 2009; 136(4): 1281–7 [DOI] [PubMed] [Google Scholar]
- 12.Rudic JS Poropat G, Krstic MN, Bjelakovic G, Gluud C. Bezafibrate for primary biliary cirrhosis. Cochrane Database of Systematic Reviews 2012, Issue 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nevens F, Andreone P, Mazzella G, et al. POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016; 375(7):631–43. [DOI] [PubMed] [Google Scholar]
- 14.Eaton JE, Vuppalanchi R, Reddy R, et al. Liver Injury in Patients with Cholestatic Liver Disease Treated with Obeticholic Acid. Hepatology 2020; 71(4): 1511–4. [DOI] [PubMed] [Google Scholar]
- 15.Carbone M, Nardi A, Flack S, et al. Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: development and validation of the UDCA Response Score. Lancet Gastroenterol Hepatol. 2018;3(9):626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada K, Hsu M, Ikeda H, Zeniya M, Nakanuma Y. Application and validation of a new histologic staging and grading system for primary biliary cirrhosis. J Clin Gastroenterol. 2013February;47(2):174–81. [DOI] [PubMed] [Google Scholar]
- 17.Lammers WJ, Hirschfield GM, Corpechot C et al. Development and Validation of a Scoring System to Predict Outcomes of Patients With Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology. 2015December; 149(7):1804–1812.e4. [DOI] [PubMed] [Google Scholar]
- 18.Carbone M, Sharp SJ, Flack S, et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology. 2016;63(3):930–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poupon RE, Lindor KD, Parés A, Chazouillères O, Poupon R, Heathcote EJ. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39(1):12–16 [DOI] [PubMed] [Google Scholar]
- 20.Paumgartner G Ursodeoxycholic acid for primary biliary cirrhosis: treat early to slow progression. J Hepatol. 2003;39(1):112–114. [DOI] [PubMed] [Google Scholar]
- 21.Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J Hepatol. 2001;35(1):134–146. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan DE, Serper M, John BV, et al. Effects of Metformin Exposure on Survival in a Large National Cohort of Patients with Diabetes and Cirrhosis [published online ahead of print, 2020 Aug 13]. Clin Gastroenterol Hepatol. 2020;S1542–3565(20)31135–6. [DOI] [PubMed] [Google Scholar]
- 23.Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012;56(1):198–208. [DOI] [PubMed] [Google Scholar]
- 24.Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C, Heathcote EJ, Hirschfield GM. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol 2010October; 105(10):2186–94. [DOI] [PubMed] [Google Scholar]
- 25.Calhoun PS, Wilson SM, Hertzberg JS, Kirby AC, McDonald SD, Dennis PA, et al. Validation of Veterans Affairs electronic medical record smoking data among Iraq- and Afghanistan-era veterans. J Gen Intern Med 2017; 32:1228–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007; 31: 1208–1217 [DOI] [PubMed] [Google Scholar]
- 27.John BV, Khakoo NS, Aitcheson G et al. Male gender is associated with a high rate of decompensation and mortality in Primary biliary cholangitis with well compensated cirrhosis. Gastroenterology 2020158(6):S-1372 [Google Scholar]
- 28.Peters MG, Di Bisceglie AM, Kowdley KV et al. Differences between Caucasian, African American, and Hispanic patients with primary biliary cirrhosis in the United States. Hepatology. 2007;46(3):769–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zein CO, Beatty K, Post AB, Logan L, Debanne S, McCullough AJ. Smoking and increased severity of hepatic fibrosis in primary biliary cirrhosis: A cross validated retrospective assessment. Hepatology. 2006;44(6):1564–1571 [DOI] [PubMed] [Google Scholar]
- 30.Híndi M, Levy C, Couto CA, Bejarano P, Mendes F. Primary biliary cirrhosis is more severe in overweight patients. J Clin Gastroenterol. 2013;47(3): e28–e32 [DOI] [PubMed] [Google Scholar]
- 31.Reisman Y, van Dam GM, Gips CH, Lavelle SM, Euricterus PM. Survival probabilities of Pugh-Child-PBC classified patients in the euricterus primary biliary cirrhosis population, based on the Mayo clinic prognostic model. Hepatogastroenterology. 1997;44(16):982–989. [PubMed] [Google Scholar]
- 32.Lammers WJ, Van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology. 2014;147(6):1338–1349 [DOI] [PubMed] [Google Scholar]
- 33.Hagström H Alcohol Consumption in Concomitant Liver Disease: How Much is Too Much? Curr Hepatol Rep. 2017;16(2):152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khungar V, Goldberg DS. Liver Transplantation for Cholestatic Liver Diseases in Adults. Clin Liver Dis. 2016;20(1):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montano-Loza AJ, Corpechot C. Definition and Management of Patients With Primary Biliary Cholangitis and an Incomplete Response to Therapy [published online ahead of print, 2020 Jul 3]. Clin Gastroenterol Hepatol. 2020;S1542–3565(20)30926–5. [DOI] [PubMed] [Google Scholar]
- 36.Karagozian R, Bhardwaj G, Wakefield DB, Baffy G. Obesity paradox in advanced liver disease: obesity is associated with lower mortality in hospitalized patients with cirrhosis. Liver Int. 2016; 36(10):1450–6. [DOI] [PubMed] [Google Scholar]
- 37.Dalapathi V, Kroner P, Mankal P, Monkemuller K. The Obesity Paradox in Primary Biliary Cirrhosis: A Nationwide Analysis. American Journal of Gastroenterology 2016; 111: S427 [Google Scholar]
- 38.Trivedi PJ, Lammers WJ, van Buuren HR, et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicenter international study. Gut. 2016;65(2):321–329 [DOI] [PubMed] [Google Scholar]
- 39.Suzuki A, Lymp J, Donlinger J, Mendes F, Angulo P, Lindor K. Clinical predictors for hepatocellular carcinoma in patients with primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2007;5(2):259–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.