Abstract
Background:
Choline is a dietary precursor to the gut microbial generation of the pro-thrombotic and pro-atherogenic metabolite trimethylamine-N-oxide (TMAO). Eggs are rich in choline, yet the impact of habitual egg consumption on TMAO levels and platelet function in human subjects remains unclear.
Methods:
Healthy volunteers (42% male, 82% Caucasian, median age 28) with normal renal function (eGFR>60) were recruited and assigned to one of five daily interventions for four weeks: (i) hardboiled eggs (N=18); (ii) choline bitartrate supplements (N=20); (iii) hardboiled eggs + choline bitartrate supplements (N=16); (iv) egg whites + choline bitartrate supplements (N=18); (v) phosphatidylcholine supplements (N=10). Fasting blood and urine samples were collected for quantification of TMAO, its precursors, and platelet aggregometry.
Results:
Participant’s plasma TMAO levels increased significantly in all three intervention arms containing choline bitartrate (all P<0.0001), but daily ingestion of four large eggs p=0.20 or phosphatidylcholine supplements (P=0.27) failed to increase plasma TMAO levels. Platelet reactivity also significantly increased in the three intervention arms containing choline bitartrate (all P<0.01), but not with eggs (P=0.10) or phosphatidylcholine supplements (P=0.79).
Conclusions:
Despite high choline content in egg yolks, healthy participants consuming four eggs daily showed no significant increase in TMAO or platelet reactivity. However, choline bitartrate supplements providing comparable total choline raised both TMAO and platelet reactivity, demonstrating that the form and source of dietary choline differentially contributes to systemic TMAO levels and platelet responsiveness.
Keywords: Eggs, TMAO, Choline
INTRODUCTION
Recent studies have shown that circulating levels of trimethylamine N-oxide (TMAO) are associated with atherosclerosis development and incident risk of major adverse cardiovascular events like heart attack, stroke, and death.1–3 Meta-analyses have confirmed that heightened levels of circulating TMAO are associated with significantly increased risk for both cardiovascular disease and mortality.4–6 TMAO production requires interactions between environmental exposures (i.e. dietary intake), the gut microbiota-dependent generation of trimethylamine (TMA), and the conversion of TMA to TMAO by host hepatic flavin monooxygenases.3, 7–9 TMAO generation has been mechanistically linked to both pro-atherosclerotic and pro-thrombotic effects, including alterations in platelet responsiveness and calcium signaling.2, 3, 10
Choline, a common dietary supplement, contains a trimethylamine moiety and is therefore a precursor to TMAO. Animal studies have shown that choline supplementation increases circulating TMAO levels and in vivo thrombosis potential, and that interventions blocking microbial production of TMAO attenuate platelet hyperactivity and thrombosis potential in vivo.8, 10–12 A recent epidemiological study also reported a strong association between dietary intake of choline as phosphatidylcholine and cardiovascular disease risk.13
Consumption of choline is common in the Western world. Over-the-counter choline-containing supplements and multivitamins are frequently used to promote brain and heart health or prevent liver damage, and choline supplementation is often advised during pregnancy to aid fetal neural development.14–16 Additionally, foods with high choline content, like eggs, are regularly consumed in the Western diet.17, 18 Eggs are of special interest because, despite an increasing number of studies investigating the risks and benefits of the dietary consumption of eggs, mixed study results prohibit a consensus recommendation.19–24
On the one hand, several studies have linked egg consumption with increased cardiovascular disease risk. A recent study by Zhong, et al. suggested that consuming an additional half egg per day increases incident cardiovascular disease and all-cause mortality,20 while in prospective observational studies by Spence and colleagues, egg consumption was strongly associated with carotid artery plaque generation.24 On the other hand, a recently updated meta-analysis of three large cohorts22 found that consumption of one egg per day was not associated with cardiovascular disease risk and another meta-analysis of 23 prospective studies23 found that increased egg consumption was not associated with cardiovascular disease. There are similarly mixed results on the specific relationship between egg/choline consumption and TMAO. Previous studies by our group showed a 10-fold increase in plasma TMAO levels following choline supplementation over a one month period with an accompanying increase in platelet reactivity,25 and a randomized, dose-response study by Miller et al. showed elevated TMAO concentrations 24 hours after ingesting two eggs.26 However, other studies failed to find a similar increase in plasma TMAO after consumption of up to three whole eggs per day over four weeks,27 and a recent study by Lemos et al. also failed to see elevated plasma TMAO concentrations after four weeks of daily egg consumption.28 In the present study, we investigated these observed discrepancies by examining plasma and urinary levels of choline metabolites, including TMAO, and platelet reactivity during a head-to-head comparison of daily supplementary dietary ingestion of a roughly equivalent mass of total choline in the form of hardboiled eggs, choline bitartrate, or phosphatidylcholine.
We also included an intervention of consuming egg whites with a source of choline. Egg whites contain antimicrobial peptides called ovodefensins,29 the presence of which could partially explain the discrepant outcomes observed in prior studies examining whole eggs.
METHODS
Authentication of Biological Sources of Choline Content.
To authenticate the equivalency in supplemental total choline content provided by the different interventions used in this study, their total choline content were independently quantified following base hydrolysis by stable isotope dilution LC/MS/MS, as previously described.3, 30 Additional details are provided in the supplementary material online.
Study Population.
Healthy men and women with normal renal function (eGFR > 60, normal serum creatinine, and no evidence of microalbuminuria) 18 years of age or older were recruited from the Cleveland, Ohio area. Participants were excluded if they had a known history of chronic illness, inflammatory bowel disease, or any bariatric surgeries or procedures, or if they had taken any antibiotics or probiotic supplements in the month prior to enrollment. This study received approval from the Cleveland Clinic Institutional Review Board and all participants provided written informed consent before enrollment.
Study Protocol.
Participants were randomized to one of four arms and asked to consume one of the following interventions daily for 28 days: four whole, large, hardboiled eggs; two 500 mg choline bitartrate tablets (choline tablets); four whole, hardboiled eggs and two 500 mg choline tablets; or the whites of four hardboiled eggs (discarding the yolks) and two 500 mg choline tablets. We later enrolled a fifth arm to investigate if choline ingestion in the form of phosphatidylcholine, delivered as six 420 mg phosphatidylcholine capsules daily, more closely mirrored the effects of free choline or whole eggs. Additional details of the randomization process are available in the online supplement. Participants were instructed to ingest their assigned intervention as a divided dose (½ in the AM and ½ in the PM) on top of their regular diet. The hardboiled eggs and dietary supplements were obtained in bulk from a commercial source and provided to participants on a weekly basis. A subset of study participants (N=50) consented to larger volume blood collections, enabling platelet rich plasma isolation and platelet reactivity monitoring via platelet aggregometry.25
Sample Collection.
Blood and spot urine samples were collected at weekly study visits. Blood was obtained through venipuncture using standard procedures following an 8+ hour fast and spot urine samples were collected in sterile containers. Three 24-hour urine collections were also obtained during the study period in opaque jugs that were kept chilled during the collection period.
Sample Analysis.
All analyses were performed by investigators blinded from study group assignments. Plasma TMAO, choline, betaine, carnitine, and creatinine levels were determined by stable isotope dilution high-performance liquid chromatography with online electrospray ionization tandem mass spectrometry on a Shimadzu LCMS 8050 triple quadrupole mass spectrometer with UHPLC interface (Shimadzu, Columbia, MD) using d9-(trimethyl)-labeled TMAO, choline, and betaine, and d3-(methyl)-labeled carnitine and creatinine as internal standards, respectively, as described previously.3, 31, 32 Fasting cholesterol panels, urine albumin/creatinine ratios, and creatinine levels were analyzed on a Roche Cobas platform (Roche Diagnostics, Indianapolis IN).
Platelet rich plasma was isolated for tracking platelet reactivity by aggregometry. Platelets were counted using an Advia 120 Hematology System Analyzer (Siemens) with concentrations adjusted to 2 x 108 cells/ml by dilution with platelet poor plasma, as previously described.25 CCl2 and MgCl2 (both 1 mM final concentration) were added immediately before platelet aggregation studies. Platelet aggregation in response to submaximal (1 μM) ADP was assessed in triplicate at 37°C in a dual channel Type 500 VS aggregometer (Chrono-log Corporation, Havertown, PA) with stirring at 1000 rpm, as previously described.10, 11, 25
Statistical Analysis.
Normality of all data was assessed with the Shapiro-Wilk test. Comparisons between-arms were examined with the Kruskal Wallis test and within-arm comparisons with the Wilcoxon Signed Rank test. Spearman correlations for non-normally-distributed data were used to analyze associations between quantitative variables. Statistical analyses were done with Graphpad Prism Version 7 (San Diego, CA) and R version 3.4.4 (Vienna, Austria). A p-value <0.05 was considered significant.
RESULTS
Roughly similar total choline content was observed in four large, whole, hardboiled eggs (467 mg total choline, virtually all esterified with negligible free choline), two 500 mg choline bitartrate tablets (411 mg free choline), and six 420 mg phosphatidylcholine capsules (410 mg total choline, no free choline). The egg whites from four large eggs contained only nominal total choline (2.80 ± 0.23 mg).
Of the 89 participants consented, 82 (92%) completed the study (Figure 1). Baseline characteristics of these participants are shown in Table 1. The median concentrations of plasma and 24-hour urine TMAO did not vary significantly at baseline among the five groups. All participants showed normal renal function.
Figure 1: Study Design.
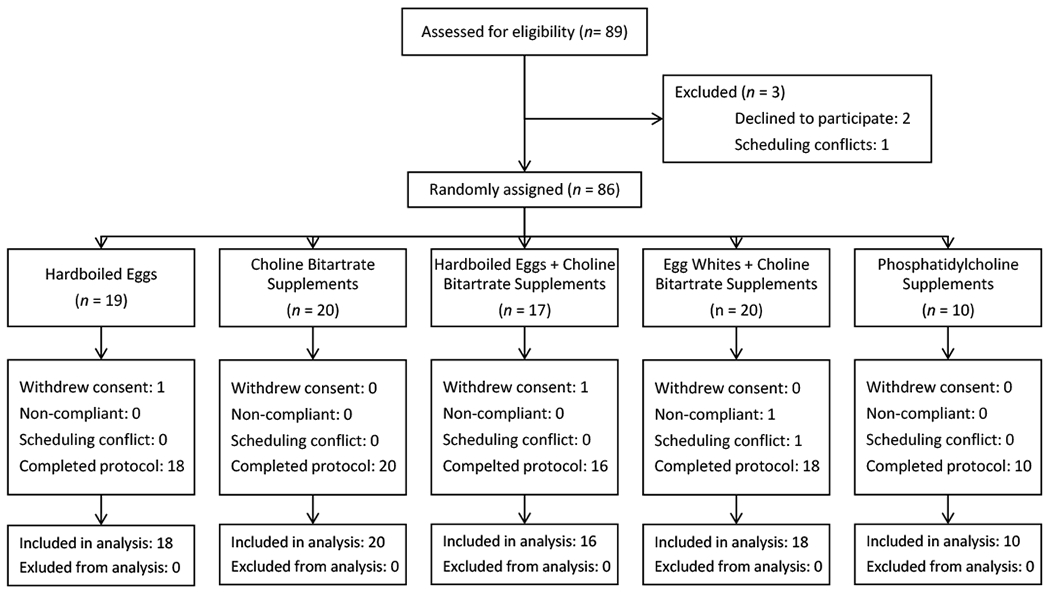
Participants were randomly assigned to one of five different intervention arms. All interventions provided a roughly equivalent dose of choline with the exception of Arm 3, which, as a combination of Arms 1 and 2, provided approximately twice as much choline as the other four arms.
Table 1:
Baseline Subject Characteristics
| All Participants | Arm 1 Whole Eggs | Arm 2 Choline Tablets | Arm 3 Whole Eggs + Choline Tablets | Arm 4 Egg Whites + Choline Tablets | Arm 5 PC Capsules | |
|---|---|---|---|---|---|---|
| Number of Participants | 82 | 18 | 20 | 16 | 18 | 10 |
| Age | 28.0 (24.0-38.8) | 25.5 (23.3-28.8) | 27.0 (23.0-37.0) | 37.0 (24.0-50.3) | 30.0 (25.0-35.8) | 31.5 (25.8-47.3) |
| Gender (% male) | 41.5% | 55.6% | 55% | 6.3% | 44.4% | 40% |
| Race (% white) | 81.7% | 72.2% | 75% | 100% | 88.9% | 70% |
| BMI | 26.9 (22.8-31.9) | 26.3 (22.8-31.1) | 25.7 (22.5-27.8) | 25.9 (22.3-30.0) | 31.0 (26.9-33.5) | 29.4 (24.5-31.6) |
| eGFR | 99.8 (82.6-115.6) | 109.4 (99.0-125.0) | 101.1 (89.9-117.4) | 88.3 (82.4-110.8) | 86.1 (80.9-102.8) | 100.8 (76.6-104.5) |
| Microalbumin:Creatinine | 7.2 (4.9-11.5) | 6.2 (4.7-7.8) | 6.1 (4.5-11.4) | 8.2 (6.6-12.8) | 7.8 (4.8-11.1) | 7.1 (5.2-9.9) |
| Plasma TMAO (uM) | 2.3 (1.5-3.5) | 2.0 (1.4-3.5) | 1.9 (1.4-3.4) | 2.3 (1.5-2.8) | 2.6 (1.8-5.3) | 2.8 (2.0-5.1) |
Unless otherwise noted, values are reported as median (Q1-Q3). BMI = body mass index; eGFR = estimated glomerular filtration rate; PC = phosphatidylcholine; TMAO = trimethylamine-N-oxide
After four weeks of intervention, plasma TMAO concentrations increased significantly from baseline to end-of-study in participants given choline tablets alone (1.9 [1.4 - 3.4] vs. 11.1 [7.1 - 25.4] μM; P<0.0001), choline tablets plus whole eggs (2.3 [1.5 - 2.8] vs. 12.3 [5.1 - 26.5] μM ; P<0.0001), and choline tablets plus egg whites (2.6 [1.8 - 5.3] vs. 28.1 [9.2 - 44.1] μM ; P<0.0001, Figure 2). However, no significant difference was observed in plasma TMAO concentration in participants given eggs alone (2.0 [1.4 - 3.5] vs. 2.3 [1.9 - 3.7] μM; P=0.20) or phosphatidylcholine capsules (2.8 [2.0 - 5.1] vs. 3.4 [2.6 - 7.1] μM; P=0.27). The results from the 24-hour urine collections (Figure 3A) mirrored those observed in plasma, with significant increases noted only in participants taking choline tablets (P<0.001 each arm) and not in those ingesting eggs alone (P=0.28) or phosphatidylcholine capsules (P=0.11). These changes occurred quickly, appearing after one week of intervention, and were maintained throughout the study period (eFigure 1). TMAO levels in spot urine samples (Figure 3B) followed a similar trend. Additionally, while no difference was observed in the baseline TMAO between groups, a highly significant inter-group difference was observed after 28 days of intervention (eFigure 2). The participants consuming choline tablets, with or without eggs, had significantly elevated TMAO levels when compared to those consuming either eggs only or phosphatidylcholine capsules.
Figure 2: TMAO Concentration across Study Arms.
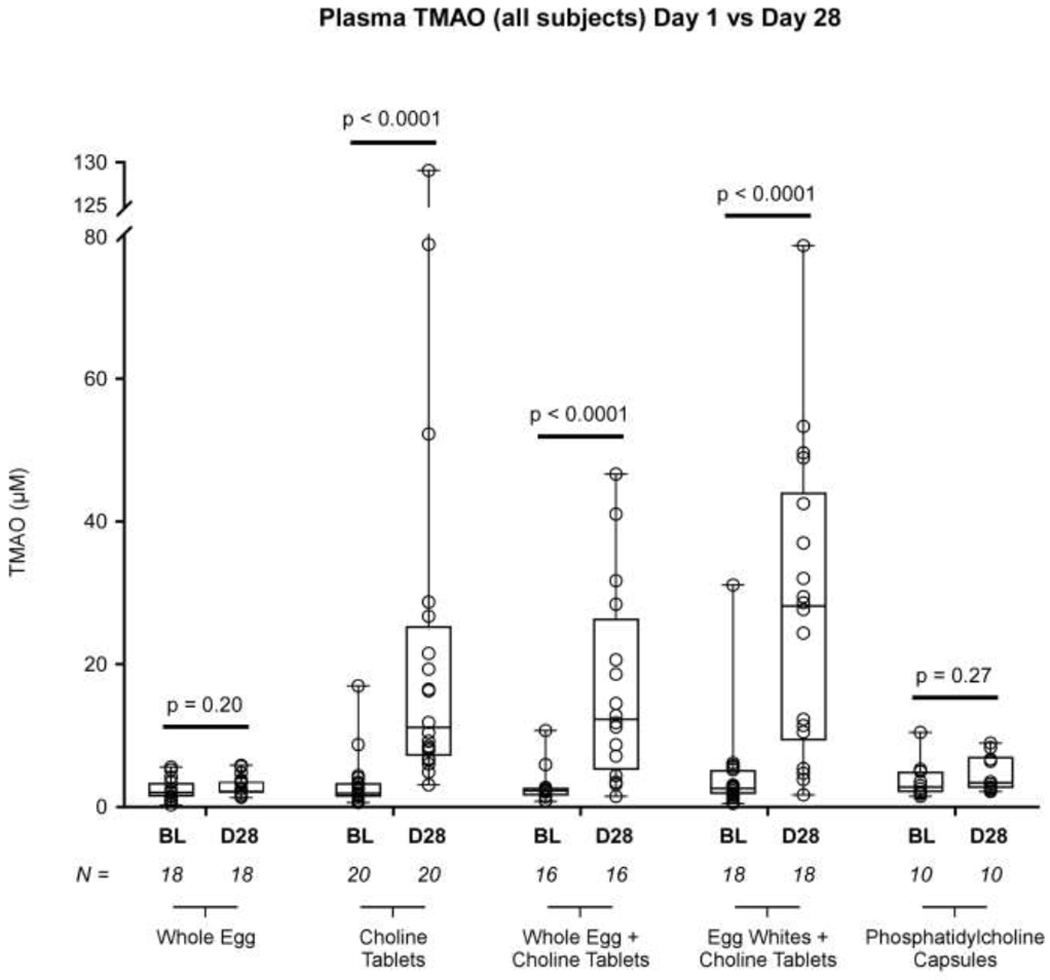
Comparing plasma TMAO concentration (uM) across all arms between the baseline (no intervention) visit and the final (intervention) visit, TMAO increased in the three arms involving the ingestion of a choline bitartrate tablet, but failed to show a significant increase when participants consumed only eggs or only phosphatidylcholine capsules (the two groups of participants that did not consume the choline bitartrate tablets). In this graph, the box indicates the 25th – 75th percentiles, with the solid line inside the box at the median. Each open circle represents an individual TMAO value. BL = Baseline, D28 = Day 28 (final study visit). P values were calculated with the Wilcoxon signed rank test, with values less than 0.05 deemed significant.
Figure 3: TMAO Production across Study Arms.
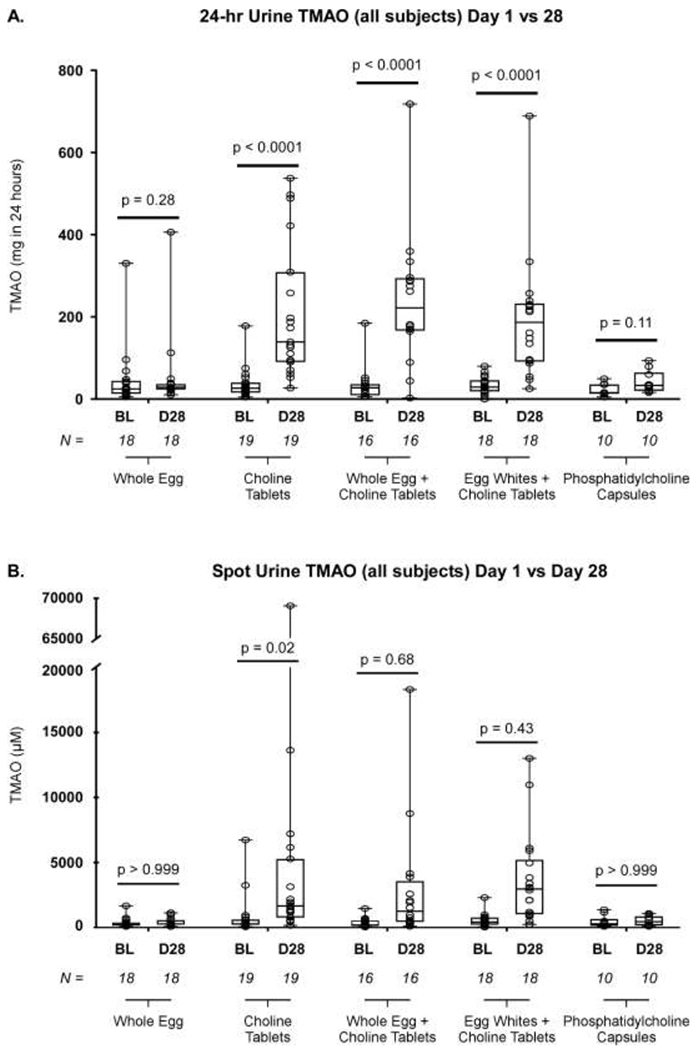
A. Comparing the baseline visit and the final (Day 28) visit, 24-hour urine TMAO increased when participants consumed oral choline bitartrate tablets (either alone or in combination with eggs), but did not increase in the two arms where participants did not consume oral choline bitartrate tablets. B. A similar pattern was observed in spot urine. In this graph, the box indicates the 25th – 75th percentiles, with the solid line inside the box at the median. Each open circle represents an individual TMAO value. BL = Baseline, D28 = Day 28 (final study visit). P values were calculated with the Wilcoxon signed rank test, with values less than 0.05 deemed significant.
Importantly, examination of plasma choline levels showed significant and roughly comparable increases in all arms across the study period (eggs only [P=0.005], choline tablets only [P<0.001], choline tablets with whole eggs [P<0.001], choline tablets with egg whites [P=0.009], and phosphatidylcholine capsules [P=0.04]), indicating compliance with the study protocol (eTable 1, eFigure 3).
Because TMAO elevation has been mechanistically linked to platelet hyperresponsiveness and thrombosis potential, we compared platelet reactivity across the study period and between the study arms. Subjects in the three arms that involved taking choline tablets showed increased platelet responsiveness, as measured by sub-maximal agonist (ADP) induced aggregation (Figure 4A). The enhanced platelet responsiveness was observed across the study period and paralleled the increase of plasma TMAO in these groups. In contrast, no significant changes in platelet responsiveness were seen when the intervention was either eggs only or phosphatidylcholine capsules, again mirroring the results seen in plasma TMAO levels. Consistent with prior studies showing a direct effect of TMAO on platelet calcium signaling and function,7, 11 platelet responsiveness was strongly correlated with TMAO levels in both plasma (r=0.51, P<0.0001, N=49) and 24-hour urine (r=0.53, P<0.0001, N=49) across all study groups (Figure 4B,C).
Figure 4: Platelet Aggregation across Study Arms.
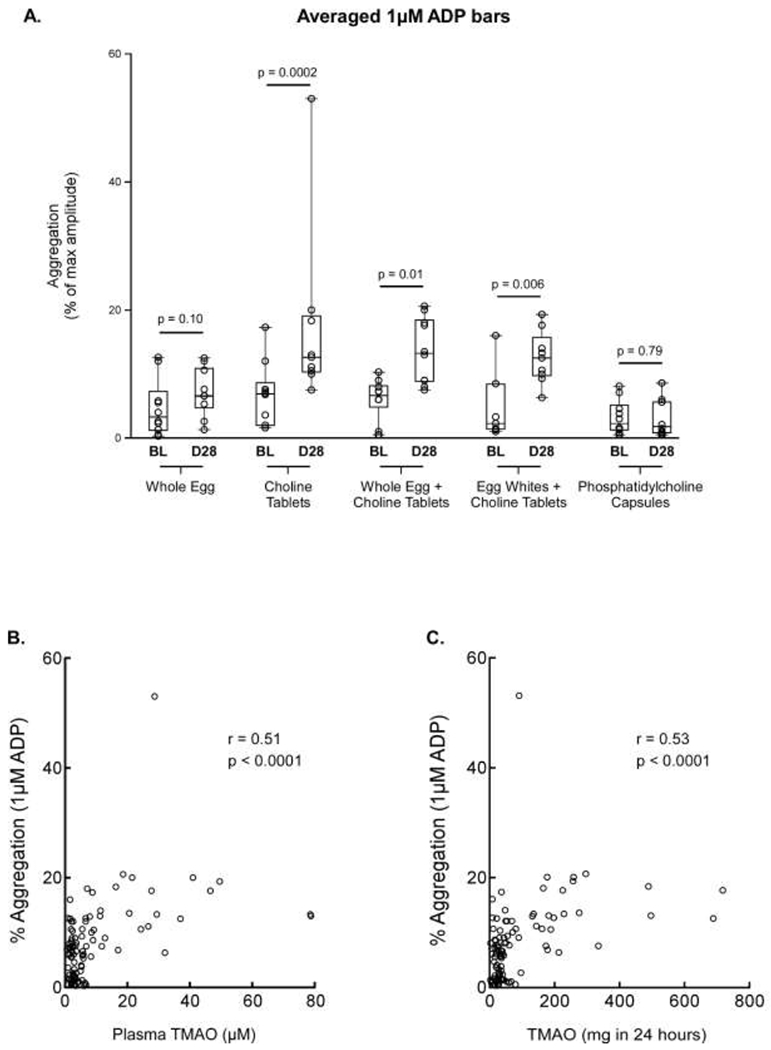
A. Comparison of platelet aggregation in 10 subjects between the baseline visit and the final (Day 28) visit, shown as measured aggregation in response to submaximal (1uM) ADP across all study arms in plasma. The box indicates the 25th – 75th percentiles, with the solid line inside the box at the median. B. Correlation between platelet responsiveness (as monitored by platelet aggregometry elicited by 1 μM ADP as agonist) and plasma TMAO concentration. C. Correlation between platelet responsiveness (as monitored by platelet aggregometry elicited by 1 μM ADP as agonist) and 24-hour urine TMAO levels. Spearman rank correlation and p values are also shown. BL = Baseline, D28 = Day 28 (final study visit).
Lipid panels, including total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, non-HDL cholesterol, and triglycerides, were run at baseline and end-of-study visits and compared (eTable 2). No significant differences were noted in any component of the lipid panel in any arm.
DISCUSSION
In this study of healthy volunteers, one month of daily egg consumption at a considerable level (four large eggs daily) failed to significantly increase TMAO in fasting plasma, spot urine, or 24-hour urine collections. However, significant increases in plasma and urinary TMAO were observed when an equivalent mass of total supplemental choline was consumed as free choline. We also examined the effects of a comparable total choline content delivered in phosphatidylcholine capsules, since the majority of choline found in eggs is glycerol bound as phosphatidylcholine within the egg yolk. The results of this intervention mirrored the egg-only intervention: no observed increase in plasma or urinary TMAO or in platelet responsiveness. Our findings therefore emphasize that the form of choline intake (free choline vs choline moiety in phosphatidylcholine) may differentially influence systemic TMAO levels and adverse physiologic consequences.
The strong association between TMAO levels and platelet reactivity observed across all study groups corroborates the previously reported strong association between TMAO levels and incident thrombotic event risks.10 It is also informative to the finding in meta-analysis that each 10μmol/L increase in TMAO levels corresponded to a 7.6% increase in mortality over the average study period (4.3 ± 1.5 yrs).6
The essential role of the intestinal microbiome in the metabolism of choline and subsequent TMAO production has been previously demonstrated in extensive animal and human studies.1, 3 Our current findings suggest that the form of choline consumed may affect its bioavailability for gut TMA generation, with phosphatidylcholine, either as a supplement or within eggs, being less bioavailable than free choline. Therefore, an important distinction must be made between the amount of dietary choline consumed and the form of that dietary choline, with the latter playing a larger role in determining downstream TMAO generation. So while choline is considered important for early neurologic development and may help protect against neural and liver damage,15, 16, 33, 34 our observations caution against presumptive generalized associations between dietary nutrients and clinical outcomes in the absence of reliable objective assessments.35 We caution any generalization of dietary nutritional intake as beneficial or harmful when dietary supplements may have differential or unanticipated interactions with our gut microbiota.
To our knowledge, this is the first published prospective randomized controlled trial comparing the effects of various formats of dietary choline on circulating TMAO levels and the potential physiological effects of platelet responsiveness. Our present results corroborate the findings of DiMarco et al. that, in healthy participants, increased choline consumption from a naturally occurring source like eggs may not substantially raise plasma TMAO concentration. After four weeks of consuming four hardboiled eggs per day, no significant differences in TMAO levels between baseline and end-of-study were observed. However, the equivalent total choline dose delivered through supplement tablets greatly increased the concentration of TMAO in both plasma and urine. This increase was independent of whether or not the choline tablets were ingested with whole eggs, egg whites, or without eggs. And consuming eggs (whole or egg white alone) with the choline tablets did not increase the degree to which plasma TMAO levels increased, implying that the choline contained within the egg did not play a large role in determining the final blood concentration of TMAO. Further, any potential impact of egg white anti-microbial peptides on ingested choline or phosphatidylcholine dependent TMAO levels was not significant.
Since TMAO is almost exclusively cleared from the blood through renal excretion,36–38 the TMAO concentration in spot urine collections and the total urinary TMAO excreted over a 24-hour period provide complementary insights into systemic TMAO beyond what can be captured in a single blood draw. The similar patterns observed in plasma and 24-hour urine samples across measured metabolites offers strong evidence that the observed increases are real and not dependent on possible confounding variables like the length of time since last ingesting choline.
Despite prospective intervention assignment and blinded, objective endpoint assessments, our studies are not without limitations. Even with prospective randomization, the relatively small sample sizes within each group created uneven distributions of some baseline characteristics (e.g. Arm 3 was 94% female). Additionally, participants’ dietary intake beyond the assigned interventions was not controlled during the study period. We also do not know if the significant differences in TMAO levels seen after four weeks would continue with longer-term dietary exposure, but other studies suggest that the pattern would have continued.32, 39 Perhaps the most important limitation of these studies is that all participants showed no evidence of renal disease. Estimated glomerular function and urine albumin-to-creatinine ratio were shown to be within the normal range in all participants. Thus the translatability of the results to other patient populations, like those with impaired renal function, is unclear. We therefore advise caution in recommending choline supplementation for patients with impaired renal function or cardiovascular disease, and even for healthy subjects with normal renal function. Choline is a common and widely available supplement, frequently included in multi-vitamins.14 Further, many prepared foods and protein supplements contain phosphatidylcholine as an additive, providing another source of dietary choline and further obviating the need for choline supplementation beyond dietary sources. If choline deficiency is suspected, it may be more prudent to recommend natural sources of choline, like eggs, over supplements.
CONCLUSION
Despite their high choline content, daily consumption of four eggs over four weeks did not substantially raise fasting concentrations of TMAO or impact platelet reactivity as monitored by aggregometry amongst healthy participants with normal renal function. Similar results were observed when an equivalent amount of total choline was consumed as phosphatidylcholine capsules. In contrast, an equivalent amount of total choline delivered in choline bitartrate tablets substantially raised plasma and urine TMAO levels and increased platelet aggregation responsiveness. These findings imply that the form of choline ingested may differentially influence gut-microbiota dependent TMAO generation. More importantly, these observations strongly caution the overall generalization of health benefits or risks of dietary supplements based solely on their chemical compositions without directly measuring plasma levels of metabolites or demonstrating their biological consequences.
Supplementary Material
Clinical Significance.
Daily dietary free choline supplementation for 28 days significantly raised fasting TMAO levels in plasma and urine and increased platelet aggregation responsiveness to submaximal agonist.
The daily consumption of eggs or phosphatidylcholine, containing an equivalent total choline content to the free choline, failed to increase TMAO or platelet aggregation.
The form of choline in dietary nutrients differentially contributes to gut microbiota-dependent TMAO generation and systemic TMAO levels.
ACKNOWLEDGEMENTS
Sources of Funding:
This work was supported by grants from the National Institutes of Health (NIH) and the Office of Dietary Supplements (R01HL103866, R01HL126827, P01 HL147823). Mass spectrometry studies were performed on instrumentation housed in a facility supported in part through a Shimadzu Center of Excellence award. SLH also notes partial support from the Leonard Krieger Fund and the Foundation Leducq.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures:
SLH reports being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics, being a paid consultant for Procter & Gamble, and having received research funds from Procter & Gamble, and Roche Diagnostics. SLH also reports being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab and Procter & Gamble. WT served as a paid consultant for Sequana Medical AG and received honoraria from Springer Nature and American Board of Internal Medicine, all unrelated to the present topic. The other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors had access to the data and participated in the writing of this manuscript.
Clinical Trial Registration: https://clinicaltrials.gov/ Identifier: NCT03039023.
REFERENCES
- 1.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao ME, Liao PD, Zhao XJ, Wang L. Trimethylamine-n-oxide has prognostic value in coronary heart disease: A meta-analysis and dose-response analysis. BMC Cardiovasc Disord. 2020;20:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine n-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med. 2018;22:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe-generated metabolite trimethylamine-n-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur Heart J. 2017;38:2948–2956 [DOI] [PubMed] [Google Scholar]
- 7.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. Trimethylamine-n-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu W, Buffa JA, Wang Z, Warrier M, Schugar R, Shih DM, Gupta N, Gregory JC, Org E, Fu X, Li L, DiDonato JA, Lusis AJ, Brown JM, Hazen SL. Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine n-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. J Thromb Haemost. 2018;16:1857–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih DM, Zhu W, Schugar RC, Meng Y, Jia X, Miikeda A, Wang Z, Zieger M, Lee R, Graham M, Allayee H, Cantor RM, Mueller C, Brown JM, Hazen SL, Lusis AJ. Genetic deficiency of flavin-containing monooxygenase 3 ( fmo3) protects against thrombosis but has only a minor effect on plasma lipid levels-brief report. Arterioscler Thromb Vasc Biol. 2019;39:1045–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WHW, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite tmao enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skye SM, Zhu W, Romano KA, Guo C-J, Wang Z, Jia X, Kirsop J, Haag B, Lang JM, DiDonato JA, Tang WW, Lusis AJ, Rey FE, Fischbach M, Hazen SL. Microbial transplantation with human gut commensals containing cutc is sufficient to transmit enhanced platelet reactivity and thrombosis potential. Circulation Research. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AB, Gu X, Buffa JA, Hurd AG, Wang Z, Zhu W, Gupta N, Skye SM, Cody DB, Levison BS, Barrington WT, Russell MW, Reed JM, Duzan A, Lang JM, Fu X, Li L, Myers AJ, Rachakonda S, DiDonato JA, Brown JM, Gogonea V, Lusis AJ, Garcia-Garcia JC, Hazen SL. Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Nat Med. 2018;24:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Li Y, Rimm EB, Hu FB, Albert CM, Rexrode KM, Manson JE, Qi L. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among us women and men. Am J Clin Nutr. 2016;104:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIH. National institutes of health’s (nih) dietary supplement label database (dsld) version 7.0.7. [Google Scholar]
- 15.Korsmo HW, Jiang X, Caudill MA. Choline: Exploring the growing science on its benefits for moms and babies. Nutrients. 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeisel SH. Choline: Critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agriculture USDo, Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM, Zeisel SH, Dacosta KA, Mar M. Usda database for the choline content of common foods, release two. 2008 [Google Scholar]
- 18.Steiber A, Kerner J, Hoppel CL. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol Aspects Med. 2004;25:455–473 [DOI] [PubMed] [Google Scholar]
- 19.Mazidi M, Katsiki N, Mikhailidis DP, Pencina MJ, Banach M. Egg consumption and risk of total and cause-specific mortality: An individual-based cohort study and pooling prospective studies on behalf of the lipid and blood pressure meta-analysis collaboration (Ibpmc) group. J Am Coll Nutr. 2019;38:552–563 [DOI] [PubMed] [Google Scholar]
- 20.Zhong VW, Van Horn L, Cornelis MC, Wilkins JT, Ning H, Carnethon MR, Greenland P, Mentz RJ, Tucker KL, Zhao L, Norwood AF, Lloyd-Jones DM, Allen NB. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. 2019;321:1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Lam TH, Jiang CQ, Zhang WS, Zhu F, Jin YL, Woo J, Cheng KK, Thomas GN. Egg consumption and the risk of cardiovascular disease and all-cause mortality:Guangzhou biobank cohort study and meta-analyses. Eur J Nutr. 2019;58:785–796 [DOI] [PubMed] [Google Scholar]
- 22.Drouin-Chartier JP, Chen S, Li Y, Schwab AL, Stampfer MJ, Sacks FM, Rosner B, Willett WC, Hu FB, Bhupathiraju SN. Egg consumption and risk of cardiovascular disease: Three large prospective us cohort studies, systematic review, and updated meta-analysis. BMJ. 2020;368:m513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krittanawong C, Narasimhan B, Wang Z, Virk HUH, Farrell AM, Zhang H, Tang WHW. Association between egg consumption and risk of cardiovascular outcomes: A systematic review and meta-analysis. Am J Med. 2020 [DOI] [PubMed] [Google Scholar]
- 24.Spence JD, Jenkins DJ, Davignon J. Egg yolk consumption and carotid plaque. Atherosclerosis. 2012;224:469–473 [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Wang Z, Tang WHW, Hazen SL. Gut microbe-generated trimethylamine n-oxide from dietary choline is prothrombotic in subjects. Circulation. 2017;135:1671–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller CA, Corbin KD, da Costa KA, Zhang S, Zhao X, Galanko JA, Blevins T, Bennett BJ, O’Connor A, Zeisel SH. Effect of egg ingestion on trimethylamine-n-oxide production in humans: A randomized, controlled, dose-response study. Am J Clin Nutr. 2014;100:778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DiMarco DM, Missimer A, Murillo AG, Lemos BS, Malysheva OV, Caudill MA, Blesso CN, Fernandez ML. Intake of up to 3 eggs/day increases hdl cholesterol and plasma choline while plasma trimethylamine-n-oxide is unchanged in a healthy population. Lipids. 2017;52:255–263 [DOI] [PubMed] [Google Scholar]
- 28.Lemos BS, Medina-Vera I, Malysheva OV, Caudill MA, Fernandez ML. Effects of egg consumption and choline supplementation on plasma choline and trimethylamine-n-oxide in a young population. J Am Coll Nutr. 2018:1–8 [DOI] [PubMed] [Google Scholar]
- 29.Whenham N, Lu TC, Maidin MB, Wilson PW, Bain MM, Stevenson ML, Stevens MP, Bedford MR, Dunn IC. Ovodefensins, an oviduct-specific antimicrobial gene family, have evolved in birds and reptiles to protect the egg by both sequence and intra-six-cysteine sequence motif spacing. Biol Reprod. 2015;92:154. [DOI] [PubMed] [Google Scholar]
- 30.Li XS, Wang Z, Cajka T, Buffa JA, Nemet I, Hurd AG, Gu X, Skye SM, Roberts AB, Wu Y, Li L, Shahen CJ, Wagner MA, Hartiala JA, Kerby RL, Romano KA, Han Y, Obeid S, Luscher TF, Allayee H, Rey FE, DiDonato JA, Fiehn O, Tang WHW, Hazen SL. Untargeted metabolomics identifies trimethyllysine, a tmao-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine-n-oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem. 2014;455:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine n-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poly C, Massaro JM, Seshadri S, Wolf PA, Cho E, Krall E, Jacques PF, Au R. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the framingham offspring cohort. Am J Clin Nutr. 2011;94:1584–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchman AL, Dubin MD, Moukarzel AA, Jenden DJ, Roch M, Rice KM, Gornbein J, Ament ME. Choline deficiency: A cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 1995;22:1399–1403 [PubMed] [Google Scholar]
- 35.Ludwig DS, Ebbeling CB, Heymsfield SB. Improving the quality of dietary research. JAMA. 2019 [DOI] [PubMed] [Google Scholar]
- 36.Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine n-oxide (tmao) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hai X, Landeras V, Dobre MA, DeOreo P, Meyer TW, Hostetter TH. Mechanism of prominent trimethylamine oxide (tmao) accumulation in hemodialysis patients. PLoS One. 2015;10:e0143731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bain M, Faull R, Fornasini G, Milne R, Evans A. Accumulation of trimethylamine and trimethylamine-n-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrology Dialysis Transplantation. 2006;21:1300–1304 [DOI] [PubMed] [Google Scholar]
- 39.Koeth RA, Lam-Galvez BR, Kirsop J, Wang Z, Levison BS, Gu X, Copeland MF, Bartlett D, Cody DB, Dai HJ, Culley MK, Li XS, Fu X, Wu Y, Li L, DiDonato JA, Tang WHW, Garcia-Garcia JC, Hazen SL. L-carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J Clin Invest. 2019;129:373–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


