Abstract
Objectives
This study aimed to describe the longitudinal evolution of neutralizing antibody titres (NtAb) in three different cohorts of healthcare workers (HCWs), including vaccinated HCWs with and without a previous SARS-CoV-2 infection and previously infected unvaccinated HCWs. COVID-19 was mild or asymptomatic in those experiencing infection.
Methods
NtAb was tested before BNT162b2 mRNA COVID-19 vaccine (V0), 20±2 days after the first dose (V1_20), 20±3 days (V2_20) and 90±2 days (V2_90) after the second dose in vaccinated HCWs and after about 2 months (N_60), 10 months (N_300) and 13 months (N_390) from natural infection in unvaccinated HCWs. NtAb were measured by authentic virus neutralization with a SARS-CoV-2 B.1 isolate circulating in Italy at HCW enrolment.
Results
Sixty-two HCWs were enrolled. NtAb were comparable in infected HCWs with no or mild disease at all the study points. NtAb of uninfected HCWs were significantly lower with respect to those of previously infected HCWs at V1_20, V2_20 and V2_90. The median NtAb fold decrease from V2_20 to V2_90 was higher in the uninfected HCWs with respect to those with mild infection (6.26 vs 2.58, p=0.03) and to asymptomatic HCWs (6.26 vs 3.67, p=0.022). The median Nabt at N_390 was significantly lower than at N_60 (p=0.007).
Conclusions
In uninfected HCWs completing the two-dose vaccine schedule, a third mRNA vaccine dose is a reasonable option to counteract the substantial NtAb decline occurring at a significantly higher rate compared with previously infected, vaccinated HCWs. Although low, Nabt were still at a detectable level after 13 months in two-thirds of previously infected and unvaccinated HCWs.
Keywords: Healthcare workers, BNT162b2 mRNA COVID-19 vaccine, Neutralizing antibodies, COVID-19, Mild disease, Asymptomatic
Introduction
The BNT162b2 COVID-19 vaccine is known to induce a rapid production of neutralizing antibodies (Lustig et al., 2021; Vicenti et al., 2021b); however, there are very limited data on their long-term kinetics. Favresse et al. (2021b) described a robust humoral response 90 days after the first dose of vaccine both in previously seropositive and seronegative subjects, but a significant antibody decrease in respect to the higher level reached occurred within this period. Interestingly, the administration of a third dose of the BNT162b2 vaccine, about two months from the second dose, to solid-organ transplant recipients significantly improved the immunogenicity of the vaccine (Kamar et al., 2021). Taken together, this data suggest that a three-month period following the first dose of vaccine may be crucial to identify subjects requiring dedicated vaccine schedules.
The main aim of this study was to analyse early (about 3 weeks) and late (about 12 weeks) changes in neutralizing antibody titres (NtAb) after the second BNT162b2 SARS-CoV-2 vaccination dose in healthcare workers (HCWs) with a previous mild or asymptomatic COVID-19 infection with respect to uninfected HCW. Moreover, it describes long-term humoral responses of previously infected HCWs who were unvaccinated.
Materials and Methods
The study population included HCWs who were vaccinated following asymptomatic or mild infection according to WHO classification (World Health Organization, 2020), a control group of vaccinated HCWs who had never been positive in all of the tests performed for hospital surveillance, and a small cohort of previously infected, unvaccinated HCWs. SARS-CoV-2 infection was diagnosed in the Veneto area in the period 01 March–30 May 2020, and had a negative molecular test since the end of the prescribed quarantine for the surveillance hospital program. All of the vaccinated participants received the BNT162b2 mRNA vaccine and the second dose was administered three weeks after the first dose.
In vaccinated participants, NtAb were tested at four time points: V0 (before receiving the first dose), V1_20 (20±2 days after the first dose), V2_20 (20±3 days after the second dose) and V2_90 (90±2 days after the second dose). In the unvaccinated participants who acquired natural SARS-CoV-2 infection, the study points that were analysed included N_60 (baseline, after about 2 months from diagnosis), N_300 after a median of 291 days and N_390 after a median of 394 days. The study was approved by the local Ethics Committee and all participants gave written informed consent.
For SARS-CoV-2 virus neutralization, two-fold serial dilutions (starting at 1:10 dilution) of heat-inactivated sera were incubated with 100 TCID50 of SARS-CoV-2 virus (lineage B.1) at 37°C, 5% CO2 for 1 h in 96-well plates. At the end of incubation, 10,000 pre-seeded Vero E6 cells per well (ATCC catalog no. CRL-1586) were treated with serum-virus mixtures and incubated at 37°C, 5% CO2. Each run included an uninfected control, a virus back titration to confirm the virus inoculum, and a known SARS-CoV-2 neutralizing serum yielding a median (interquartile range [IQR]) titre of 69 (59.3–69.9) in five independent runs. After 72 hours, cell viability was determined through the commercial kit Cell-titre Glo2.0 (Promega, Wisconsin, USA) following the manufacturer's instructions. The serum neutralization titre (ID50) was defined as the reciprocal value of the sample dilution that showed a 50% protection of virus cytopathic effect. Sera with ID50 titres ≥10 were defined as SARS-CoV-2 positive and neutralizing; sera with ID50 <10 were defined as negative and scored as 5 for statistical analysis (Vicenti et al., 2021a).
Continuous variables were expressed as the median (IQR), whereas categorical variables were indicated as absolute number and frequency. The Mann-Whitney U test, Wilcoxon signed rank sum test, Chi-squared test and Fisher's exact test were applied as appropriate. Statistical analyses were performed using MedCalc® Statistical Software version 20.009 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021) and the limit of significance for all analyses was established at p<0.05.
Results
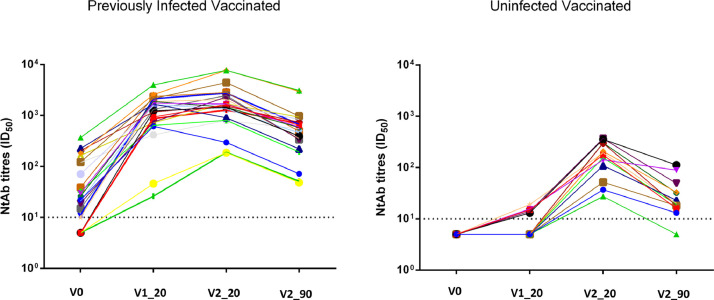
Sixty-two HCWs were enrolled. A complete set of data was available for 23 previously infected and vaccinated HCWs (14 with asymptomatic infection and nine with mild symptoms), 13 uninfected, vaccinated HCWs, and nine previously infected unvaccinated HCWs. A description of the study population, including vaccinated HCWs, is reported in Table 1 and Figure 1, Figure 2 . NtAb values were comparable in HCWs with asymptomatic infection and with mild disease at all four study points. NtAb values of the uninfected HCWs were significantly lower with respect to those of the previously infected HCWs at V1_20, V2_20 and V2_90. At the last control (V2_90), median NtAb was lower than at V2_20 in HCWs with mild disease (p=0.007), asymptomatic (p=0.001) and uninfected (p=0.001). Likewise, V2_90 NtAb were lower than V1_20 NtAb in HCWs with mild disease and in asymptomatic (p=0.028 and p=0.003, respectively): conversely, V2_90 was higher than V1_20 in uninfected HCws (p=0.002). However, median NtAb at V2_90 was still significantly higher than median NtAb at V_0 both in HCWs with past mild disease (p=0.01) and in those experiencing asymptomatic infection (p=0.001).
Table 1.
Description of the main characteristics and neutralizing antibody titres at V0, V1_20, V2_20 and V2_90 in the overall study population and asymptomatic HCWs or with mild disease.
| Overall (23 HCW) |
Asymptomatic HCWs (n=14) |
HCWs with mild disease (n=9) |
p | Control group (n=13) |
p vs asymptomatic | p vs mild | |
| Male, n (%) | 9 (39.1) | 4 (28.6) | 5 (55.6) | 0.382 | 4 (30.8) | 1 | 0.384 |
| Age (years)a | 42 (33-47) | 33 (33-44) | 46 (43-50) | 0.026 | 59 (36-60) | 0.0460 | 0.569 |
| Days from SARS-Cov-2 infection to V0a | 292 (267-300) | 291 (247-300) | 294 (282-300) |
na | na | na | |
| NtAb titre (ID50) at V0a | 26 (10.3-121.4) |
21.5 (5-38.7) |
164.2 (13.7-210) |
0.076 | 5 | na | na |
| Subjects with undetectable NtAb at V0, n (%) | 5 (21.7) | 4 (28.6) |
1 (11.1) | 0.61 | na | na | na |
| NtAb titre (ID50) at V1_20a | 1314.2 (726-1884.3) |
1378.2 (615-1888.1) |
1314.2 (878.5-2047) |
0.5161 | 5 (5-14.5) |
<0.0001 | 0.0001 |
| Subjects with undetectable NtAb at V1_20, n (%) | 0 | 0 | 0 | 1 | 7 (58.8) | 0.001 | 0.016 |
| NtAb titre (ID50) at V2_20a | 1637.8 (997.3-2426.7) |
1450.3 (797.1-2310) |
1707.5 (1371.5-3769.2) |
0.2076 | 176 (94.7-299.7) |
0.0003 | 0.0001 |
| Subjects with undetectable NtAb at V2_20 | 0 | 0 | 0 | na | 0 | na | na |
| NtAb titre (ID 50) at V2_90a | 546 (338.4-724.2) |
520.5 (342-669.9) |
647 (308.4-1439.7) |
0.438 | 20 (17.5-37) | <0.0001 | 0.0001 |
| Subjects with undetectable NtAb at V2_90, n (%) | 0 | 0 | 0 | na | 1 (7.7) | 0.481 | 1 |
Date shown as median and range
p refers to differences between HCWs with asymptomatic or mild disease and to the differences between control group and HCWs with asymptomatic or mild disease
Bold: significant p values
na: not applicable
HCWs: healthcare workers
ID50: reciprocal value of the sample dilution that showed a 50% protection of virus cytopathic effect
V0: before receiving the first dose
V1_20: 20±2 days after the first dose
V2_20: 20±3 days after the second dose
V2_90: 90±2 days after the second dose
Figure 1.
Neutralizing antibody titres in previously infected (n=23) and uninfected healthcare workers (n=13) tested at V0, V1_20, V2_20 and V2_90.
Data are reported as longitudinal course and as individual ID50 values at each study time
The same symbols indicate the same HCWs at different time points
ID50: reciprocal value of the sample dilution that showed a 50% protection of virus cytopathic effect
V0: before receiving the first dose
V1_20: 20±2 days after the first dose
V2_20: 20±3 days after the second dose
V2_90: 90±2 days after the second dose
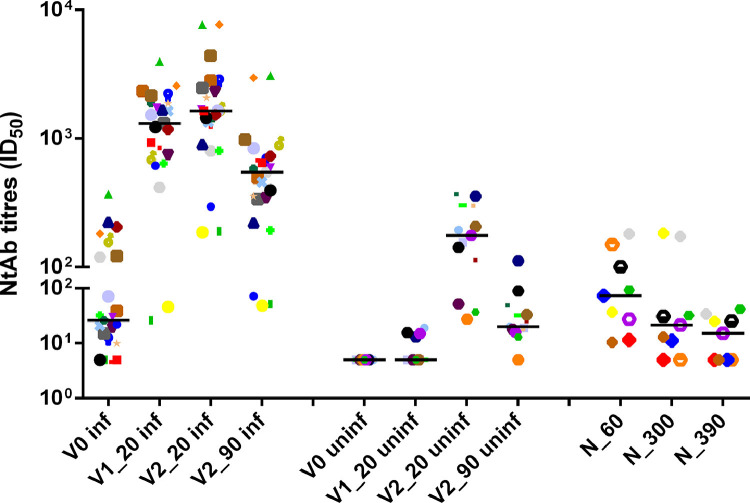
Figure 2.
Neutralizing antibody titres in previously infected (n=23) and uninfected healthcare workers (n=13) tested at V0, V1_20, V2_20 and V2_90, and in unvaccinated HCWs (n=9) tested at N_60, N_300 and N_390.
Data are reported as individual ID50 values and as median value at each study time
The same symbols indicate the same HCWs at different time points
ID50: reciprocal value of the sample dilution that showed a 50% protection of virus cytopathic effect
V0: before receiving the first dose
V1_20: 20±2 days after the first dose
V2_20: 20±3 days after the second dose
V2_90: 90±2 days after the second dose
N_60: baseline, after about 2 months from diagnosis
N_300: after a median of 291 days from diagnosis
N_390: after a median of 394 days from diagnosis
Among HCWs with previous infection, five had no detectable NtAb at V_0 after a median interval from infection of 263 (IQR 199-284) days, which was shorter than those of the 18 HCWs with detectable NtAb al V_0 (296, IQR 288-302 days, p=0.048). Nevertheless, median NtAbs at V2_90 were comparable in the group with undetectable and detectable NtAb at V_0 (respectively, 395, IQR 50.7-660.2 and 564, IQR 342-839, p=0.233), while NtAb were significantly higher in HCWs with detectable NtAb at V_0, both at V1_20 (1656.6, IQR 744-2131.8 vs 874, IQR 41-1005.3; p=0.03) and V2_20 (1695.5, IQR 1347.6-2751.3 vs 1276.9, IQR 188.9-1498.8; p=0.036).
In the uninfected vaccinated group, seven of 13 HCWs had undetectable NtAb at V1_20 and all but one had detectable NtAb at V2_90. The median NtAb fold change decrease from V2_20 to V2_90 was higher in uninfected HCWs with respect to those with mild infection (6.26 vs 2.58, p=0.03) and to asymptomatic HCWs (6.26 vs 3.67, p=0.022).
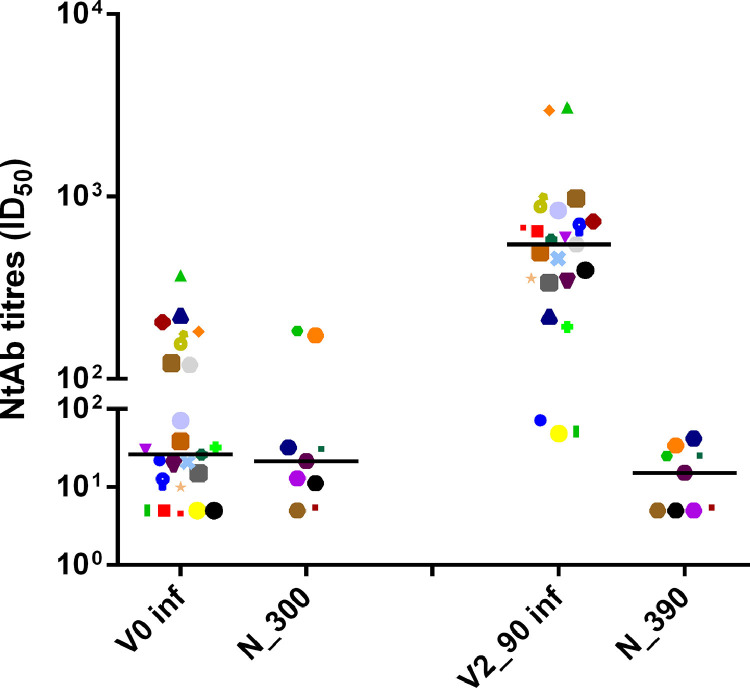
A similar figure in longitudinal evolution of NtAb was observed when a whole cohort of 40 previously infected participants (27 asymptomatic and 13 with mild disease), including 17 without an intermediate time point, was considered: seven (17.5) had no NtAb detectable at V0 and the fold change between V2_20 and V2_90 was 3.670. A detailed description is given in the Supplementary Table and Supplementary Figure 1. The nine previously infected unvaccinated HCWs had a median age of 49 years (IQR 45-56 years). Median NtAb at N_390 was significantly lower in respect to N_60 (15.2, IQR 5-27.6 vs 73.5, IQR 23.3-113, p=0.007). The median value at N_300 was intermediate (21.5, IQR 9.6-67.2). No HCWs had undetectable NtAb at N_60, two had undetectable NtAb both at N_300 and at N_390, and one only at N_390. The data are reported in Figure 3 and Supplementary Figure 2. NtAb titres in previously infected and uninfected vaccinated HCWs and in previously infected unvaccinated HCWs are summarized in Table 2 .
Figure 3.
Neutralizing antibody titres at N_300 (after a median of 291 days from diagnosis in unvaccinated HCWs), corresponding to V0 in vaccinated HCWs and at N_390 (after a median of 394 days from diagnosis), corresponding to the last time point of vaccinated HCWs (V2_90: 90±2 days after the second dose).
Data are reported as individual ID50 values and as median value at each study time.
The same symbols indicate the same HCWs at different time points.
ID50: reciprocal value of the sample dilution that showed a 50% protection of virus cytopathic effect
V0: before receiving the first dose
V2_90: 90±2 days after the second dose
N_300: after a median of 291 days from diagnosis
N_390: after a median of 394 days from diagnosis
Table 2.
Description of neutralizing antibody titres in previously infected and uninfected vaccinated HCWs (tested at V0, V1_20, V2_20 and V2_90) and in unvaccinated HCWs (tested at N_60, N_300 and N_390).
| V0 (ID50) |
V1_20 (ID50) |
V2_20 (ID50) |
V2_90 (ID50) |
||
| Previously infected vaccinated HCWs (n=23) |
26 (10.3-121.4) |
1314.2 (726-1884.3) |
1637.8 (997.3-2426.7) |
546 (338.4-724.2) |
|
| Uninfected vaccinated HCWs (n=13) |
. | 5 | 5 (5-14.5) |
176 (94.7-299.7) |
20 (17.5-37) |
| N_60 (ID50) |
N_300 (ID50) |
N_390 (ID50) |
|||
| Unvaccinated HCWs (n=9) |
73.5 (23.3-113) |
21.5 (9.6-67.2) |
na | na | 15.2 (5-27.6) |
Data are expressed as median and interquartile range
N_300 was defined as corresponding to V0 in vaccinated HCWs and N_390 was defined as corresponding to V2_90
na: not applicable
HCWs: healthcare workers
ID50: reciprocal value of the sample dilution that showed a 50% protection of virus cytopathic effect
V0: before receiving the first dose
V1_20: 20±2 days after the first dose
V2_20: 20±3 days after the second dose
V2_90: 90±2 days after the second dose
N_60: baseline, after about 2 months from diagnosis
N_300: after a median of 291 days from diagnosis
N_390: after a median of 394 days from diagnosis
Discussion
Real world data have demonstrated that SARS-CoV-2 vaccines prevent infection in more than 90% of cases (Butt et al., 2021; Dagan et al., 2021). Accordingly, a 74% reduction in the proportion of cases and 81% reduction in the proportion of symptomatic cases have been reported in HCWs, based on the Italian government's open data directory (Mateo-Urdiales et al., 2021).
Neutralizing antibody responses are a correlate of protection (Khoury et al., 2021) and their measurement through live virus assays is the reference method to investigate the magnitude and duration of immunity following vaccination or natural infection. This study evaluated the long-term neutralizing response to SARS-CoV-2 in different HCW groups representative of three possible scenarios: (i) mild or asymptomatic infection followed by two-dose vaccination about 10 months after diagnosis; (ii) absence of infection, as confirmed by regular monitoring, followed by two-dose vaccination; and (iii) past mild or asymptomatic infection not followed by vaccine for a very long time period. This last condition is now no longer allowed in Italy, since previously infected HCWs get vaccinated and therefore constitutes an unicum.
In agreement with published data (Vicenti et al., 2021b), previously infected HCWs vaccinated with BNT162b2 demonstrated a strong humoral response after the first dose, with a modest further increase 20 days after the second dose. The current data provide additional support on the long-term efficacy of the vaccine booster after a very long time from infection, with a median interval of ten months. The kinetics of NtAb decay in these HCWs compared with those following a single vaccine dose need further study: a significant antibody decline 3 months after the first dose in both seronegative and seropositive individuals who received two doses was recently described (Favresse et al., 2021b). However, the current results were obtained 3 months after the second vaccination, in a well characterized population, with a very long-term humoral memory: the interval between infection and first vaccine dose (median of 292 days, with IQR 267-300) was much loner with respect to that reported by Favresse et al (2021a) (mean 99 days with range 34-337 days). Of note, five (21.7%) of 23 previously infected HCWs had no detectable NtAb before vaccination; nevertheless, they experienced a strong response at V2_90, which was comparable to HCWs with detectable NtAb at V0, supporting the persistence of long-lasting B memory after natural infection (Wang et al, 2021).
Further, the current results support the observation that infected vaccinated HCWs experience a significantly slower NtAb decay than uninfected vaccinated HCWs after 3 months from second dose, indicating the ability of the immune system to mount very high NtAb after repeated antigen stimulation. Although natural infection and vaccination may lead to different responses, and residual and recall immune responses may impact different immune protection, the role of humoral immunity in the first phase of viral infection is well established as a major early obstacle to viral spread, counteracting viral replication and evolution as well as immune escape. Thus, measuring circulating antibody levels, their durability, specificity, and recall kinetics is crucial for understanding and predicting the durability of protection (Cromer et al., 2021). As recently reported, among vaccinated HCWs, the breakthrough infections were correlated with NtAb detected within the week immediately before (Bergwerk et al., 2021). The advantage of high NtAbs against the infection by viral variants was also reported (Cohen et al., 2021). Based on data reported here, a third mRNA vaccine dose is reasonable in uninfected HCWs, to further boost immunity and recapitulate the effect observed in previously infected vaccinated HCWs. However, the identification of specific target groups and the definition of the best timing for vaccination need further analysis with a more prolonged follow-up on a larger cohort. The current study included a group of previously infected unvaccinated HCWs who were tested thrice after SARS-CoV-2 infection: about 2 months later, after a median of 291 days (defined as corresponding to V0 in vaccinated HCWs) and after a median of 394 days (defined as corresponding to the last time point of vaccinated HCWs). The lack of a study time corresponding to N_60 for vaccinated HCWs is regrettable; however, the main aim of this study was the long-term humoral response, and N_390 and V2_90 were definitely corresponding. It is believed that the description of a control group with a protracted follow-up was a useful contribution to the understanding of the durability of NtAb in this particular category of subjects: middle age, previously healthy and without comorbidities and with mild or asymptomatic infection. These individuals are now almost all vaccinated.
Strengths of the study were the: availability of authentic virus neutralization with a SARS-CoV-2 isolate; homogeneity of the study population with previous asymptomatic or mild disease; long time interval from infection to vaccination; long follow-up after the second dose of vaccination; and different HCW cohorts tested. The main limitation was the number of evaluated participants, but data were immediately described after their availability.
In conclusion, data obtained by analysis of the response to SARS-CoV-2 infection of this multifaceted population may help with clinical use of NtAb to inform health policy, that is: to evaluate the decision to administer a third dose of the vaccine and how to monitor unvaccinated personnel.
Authors’ contributions
IV: performed the laboratory experiments, helped to interpret the findings and to write the paper. MB: helped to interpret the findings. FG: managed the patients and collected the samples. RS: managed the patients and collected the samples. AB: performed the laboratory experiments. DZ: helped to interpret the findings. EM: helped to interpret the findings. FD: performed the laboratory experiments. SGP: designed and coordinated the study, collected and managed the samples and the data, interpreted the findings, and wrote the paper. MZ: supervised the laboratory experiments, helped to interpret the findings, performed the statistical analysis and wrote the paper.
Funding source
This work was supported by University of Padova, grant numbers DOR-2019 and DOR-2020 to SGP
Ethical approval
The study was approved by the local Ethics Committee: all the subjects gave written informed consent to the inclusion.
Conflict of interests
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank Alessia Lai for making the SARS-CoV-2 lineage B strain available for this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.08.052.
Appendix. Supplementary materials
References
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021 doi: 10.1056/NEJMoa2109072. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AA, Omer SB, Yan P, Shaikh OS, Mayr FB. SARS-CoV-2 Vaccine Effectiveness in a High-Risk National Population in a Real-World Setting. Ann Intern Med. 2021 doi: 10.7326/M21-1577. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen KW, Linderman SL, Moodie Z, Czartoski J, Lai L, Mantus G, et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer D, Juno JA, Khoury D, Reynaldi A, Wheatley AK, Kent SJ, et al. Prospects for durable immune control of SARS-CoV-2 and prevention of reinfection. Nat Rev Immunol. 2021;21:395–404. doi: 10.1038/s41577-021-00550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favresse J, Bayart JL, Mullier F, Dogné JM, Closset M, Douxfils J. Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2) Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.004. S1198-743X(21)00224-X. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favresse J, Bayart JL, Mullier F, Elsen M, Eucher C, Eeckhoudt SV, et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg Microbes Infect. 2021:1–8. doi: 10.1080/22221751.2021.1953403. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021 doi: 10.1056/NEJMc2108861. NEJMc2108861Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00220-4. S2213-2600(21)00220-4. https://doi:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo-Urdiales A, Del Manso M, Andrianou X, Spuri M, D'Ancona F, Filia A, et al. Initial impact of SARS-Cov-2 vaccination on healthcare workers in Italy- Update on the 28th of. Vaccine. March 2021;2021 doi: 10.1016/j.vaccine.2021.07.003. S0264-410X(21)00862-8. https://doi:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. World Health Organization. 2020 https://apps.who.int/iris/handle/10665/330893 (accessed 14 July 2021) [Google Scholar]
- Vicenti I, Gatti F, Scaggiante R, Boccuto A, Modolo E, Zago D, et al. Time Course of Neutralizing Antibody in Health Care Workers With Mild or Asymptomatic COVID-19 Infection. Open Forum Infect Dis. 2021;8:ofab312. doi: 10.1093/ofid/ofab312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicenti I, Gatti F, Scaggiante R, Boccuto A, Zago D, Basso M, et al. Single-dose BNT162b2 mRNA COVID-19 vaccine significantly boosts neutralizing antibody response in health care workers recovering from asymptomatic or mild natural SARS-CoV-2 infection. Int J Infect Dis. 2021;108:176–178. doi: 10.1016/j.ijid.2021.05.033. https://doi:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Muecksch F, Schaefer-Babajew D, Finkin S, Viant C, Gaebler C, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.