Abstract
Background:
Many preclinical cancer studies use mice with varied phenotypes to monitor tumor treatment. We compared survival and optical imaging characteristics of strains with varied coat colors harboring luciferase-expressing disseminated lymphoma.
Results:
Luciferase-expressing lymphoma cells (Raji-luc) were injected via tail vein into severe combined immunodeficient (SCID) and Rag2-IL2rg (R2G2) mice and survival was tracked. Tumor signals were obtained by imaging ventral and dorsal aspects of mice. Signal attenuation by isolated mouse pelts was measured in vitro. R2G2 mice had decreased survival compared to SCID mice (17 vs. 32 days, p<0.001) despite similar bioluminescence signal when mice were imaged dorsally (p=0.37). However, signal was 17.3-fold higher in R2G2 mice compared to SCID (p<0.001) when imaged ventrally. Isolated dark R2G2 dorsal pelts attenuated signal more than ventral pelts when placed over cells in vitro.
Conclusions:
Mouse pelt color and imaging aspect are critical considerations for quantifying bioluminescent tumor signal and the R2G2 mouse useful for strain may prove preclinical targeted therapy studies.
One Sentence Summary:
Mouse coat color variation strongly impact bioluminescent signal from luciferase-expressing tumor cells, affecting tumor-cell quantification.
Keywords: Bioluminescent imaging, preclinical cancer therapy, mice, lymphoma, targeted radionuclide therapy
BACKGROUND
Optical imaging of luciferase-expressing tumor cells is a powerful technique for preclinical cancer studies [1]. Small tumor-cell numbers can be accurately quantified in vivo with high repeatability [1] and repeated imaging of the same animal allows quantification of cancer-cell growth and treatment response [2]. Patients with non-Hodgkin lymphoma (NHL) frequently present with multi-organ tumor involvement and small foci of disease. Disseminated-disease animal models are thus attractive for modeling such cancers and for testing therapies [3]. However, preclinical studies using disseminated luciferase-expressing tumors cells, including for NHL, often use mouse strains that vary greatly in genotype and phenotype, which may confound comparison of studies [4]. Here, we compared bioluminescent signal profiles of two mouse strains with strikingly different immunodeficiency and coat-color phenotypes, using a disseminated NHL model with Burkitt lymphoma (Raji) cells expressing luciferase. We hypothesized that the highly immunodeficient Rag2-IL2rg (R2G2) mice would exhibit more rapid tumor-cell growth and decreased survival compared to severe combined immunodeficient (SCID) mice. We also evaluated the effects of coat color and imaging aspect (dorsal vs. ventral) on bioluminescent tumor signal attenuation, comparing R2G2 mice with dark dorsal and light tan ventral coats with SCID mice with white (albino) dorsal and ventral coats.
METHODS
Cell lines and cell culture
The aim of this study was to compare features of bioluminescent in vivo lymphoma imaging between the traditional SCID mouse strain and the more recently developed R2G2 strain. A luciferase-expressing Raji Burkitt Lymphoma (Raji-luc) cell line stably transfected to express firefly luciferase joined to eGFP [5]. was generously provided by Dr. Irving Weissman (Stanford University). Raji-luc cells were maintained in RPMI 1640 with L-glutamine,10% fetal bovine serum, and 1% penicillin-streptomycin at 37°C with 5% CO2.
Animals and the disseminated lymphoma model
Animal studies were approved by our Institution’s Animal Care and Use Committee. Raji-luc cells were centrifuged at 4°C for 5 minutes at 34 x g, supernatants were removed, and cells were suspended in PBS to 107 cells/mL. 8–10 week old female severe combined immunodeficient mice (SCID, C.B-17/lcrHsd-Prkdscid, strain #182, Envigo, Indianapolis, IN) and Rag2-IL2rg double knockout-mice (R2G2, B6:129-Rag2tm1Fwa/IL2rgtm1Rsky/DwlHsd, strain #021, Envigo) were anesthetized and injected via the tail vein with 106 Raji-luc lymphoma cells. Mice were monitored and euthanized if meeting defined criteria, including signs of distress, >20% weight loss, or, most typically, hind limb paralysis.
In vivo tumor-cell imaging
Bioluminescence optical imaging was performed on live mice using the IVIS Lumina XR system with Living Image 4.2 software (Perkin Elmer, Waltham, MA) on the day of cell injection (day 0) and bi-weekly thereafter until sacrifice or termination of the study. Mice (18–21 g) were injected intraperitoneally with 200 μL of 15 μg/μL luciferin (D-luciferin (luciferin, LUCK-1G, Gold Biotechnology, Olivette, MO) in PBS. Imaging occurred 15–25 minutes after luciferin injection [1]. Mice were placed on black paper and imaged from the dorsal aspect, immediately followed by imaging from the ventral aspect. Automated settings were used to avoid overexposure. Bioluminescence was quantified as radiance (photons/second/cm2/steradian) using oval regions of interest that spanned the head to the base of the tail and that completely covered the animal’s transverse axis.
In vitro tumor imaging with mouse pelt overlay
In vitro bioluminescence imaging was performed with Raji-luc cells. 100 μL of media with 0 to 106 cells were plated in Costar opaque flat-bottom 96-well plates (Corning, Corning, NY, USA, Product Number 3916). Cells were allowed to equilibrate in the plates at 37˚C for 1–4 hr prior to imaging. 100 μL of RPMI or RPMI with 300 μg/mL D-luciferin was then added to each well, plates were incubated for 10–20 minutes at room temperature, and images were acquired with the IVIS system using automated exposure settings of Living Image 4.2 software. To verify linearity of signal over the cell range used, a 96-well plate was prepared with 3 replicates of 0 cells, or 100 to 106 cells in 10-fold increments, and an image was acquired. After overlay of a 12 × 8 matrix of regions of interest, flux was determined. As expected, the signal was linear over this range (R2 0.997) with no detectable saturation. Next, five separate plates were made, each containing 12 replicates per plate of 100, 103, 104, 105 or 106 Raji-luc cells. To evaluate attenuation of Raji-luc bioluminescence signal by pelts (skin and fur) from euthanized R2G2 and SCID mice, dorsal and ventral pelts were overlaid flat on a 96-well plate cover and serially transferred over these four plates, with images acquired using automated exposure settings. Flux was quantified as described above.
Statistical analysis
All statistical analyses were performed with GraphPad Prism version 8 (GraphPad Software, LaJolla, CA, USA). Tests used included ANOVA with repeated measures, unpaired student’s t-test, and the Log-rank Mantel-Cox test, as indicated in the text and figure legends.
RESULTS
SCID and R2G2 mice exhibit different coat colors
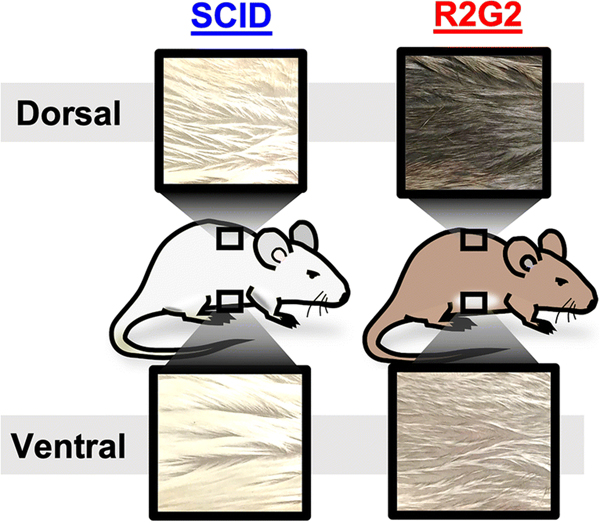
SCID and R2G2 mice strains are both immunodeficient; however, they differ in genotype and phenotype (TABLE 1). R2G2 mice are much more immunodeficient than are SCID mice. Another readily identifiable phenotypic difference between SCID and R2G2 mice is coat color (FIG. 1). SCID mice have a white (albino) coat that is similar in color on the ventral and dorsal aspects. Conversely, R2G2 mice have a light chinchilla (light tan) ventral coat with darker fur on the dorsal coat.
TABLE 1.
Genotypic and phenotypic differences in SCID and R2G2 mice. Strain-dependent underlying genotype defects, immunodeficiency, and radiosensitivity profiles differ between mouse strains.
| MOUSE STRAIN | GENOTYPE DEFECT | IMMUNODEFICIENCY | RADIOSENSITIVITY |
|---|---|---|---|
|
| |||
| SCID | Disrupted Protein Kinase, DNA-Activated, Catalytic Subunit gene (prkdc, DNA damage sensor) | ++ Impaired T & B cell development “Leaky” immunity | +++ Disrupted double-stranded DNA repair |
| R2G2 | Double-knockout Recombination activating gene 2 (Rag2, essential for making mature T & B cells) Common gamma chain gene (IL2rg, a component of many interleukin receptors) | ++++ Defective lymphocyte development and disrupted interleukin receptors. “Non-Leaky” immunity | −/+ Maintained DNA repair |
FIGURE 1.
SCID and R2G2 mice exhibit different coat characteristics. Photographic images of mouse coats from the dorsal and ventral aspects. R2G2 mice exhibit dark dorsal coats and light tan ventral coats, while SCID mice are relatively uniformly albino colored.
Disseminated lymphoma tumor bioluminescent signal differs between mouse strains and between imaging aspect in R2G2, but not SCID, mice
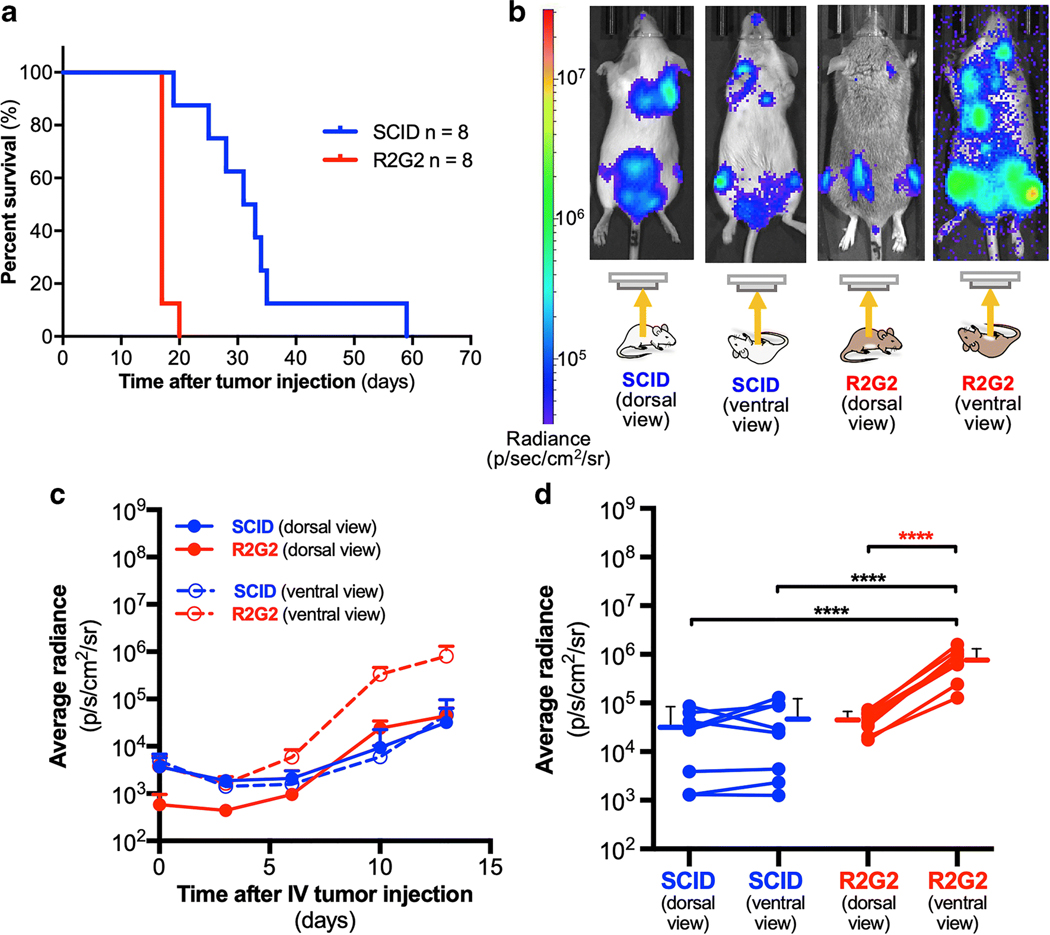
We evaluated viability and tumor-cell detection by bioluminescence in SCID and R2G2 mice. Disseminated lymphoma was established by intravenous injection with Raji-luc cells, and mice were followed over time post-injection. R2G2 mice had decreased survival compared to SCID mice (FIG. 2A, median survival, 17 days vs. 32 days, p<0.001, Log-Rank Mantel Cox), suggesting tumor cells grew more rapidly in R2G2 mice than in SCID mice, as expected by the greater immunodeficiency of the R2G2 mice.
FIGURE 2.
R2G2 mice show decreased survival and increased bioluminescent signal when imaged in the supine position compared SCID mice in a disseminated lymphoma model. (A) Median survival of R2G2 mice (17 days) is significantly shorter than that of SCID mice (32 days), p<0.001, Log-rank Mantel-Cox test). (B) Images of R2G2 and SCID mice obtained 13 days after intravenous injection of 1 million Raji-luc cells, with overlaid radiance values. (C) Bioluminescent signal quantification after averaging individual regions of interest capturing an entire mouse (SCID, n = 8 and R2G2, n = 8). The average signal for each strain is plotted at different time points for the first 13 days. (D) Bioluminescent signal is compared within the same strain with animal imaged prone and supine at day 13 after intravenous injection of 1 million Raji-luc cells. Data points with connecting lines represent values from a single animal. Lines and bars adjacent to individual points represent mean ± standard deviation. **** p<0.0001 One-way ANOVA with repeated measures. Asterix in red indicates same p-value but with paired t-test.
At various time points after tumor-cell injection, mice were injected intraperitoneally with luciferin, the substrate of luciferase, and bioluminescence was measured from the dorsal and ventral aspects. As expected, both strains exhibited disseminated lymphoma, as indicated by multiple, dispersed tumor foci (FIG. 2B). Qualitative observations (FIG. 2B) suggested bioluminescent signal was equal when SCID mice were imaged either dorsally or ventrally, which was confirmed by quantitative analysis (FIG. 2C). For R2G2 mice, bioluminescent signal from the ventral aspect was elevated over the time course, compared to SCID mice imaged from either aspect (FIG. 2C), indicating faster tumor-cell growth in R2G2 mice, consistent with the viability studies. Notably, when an R2G2 mice was imaged from the dorsal aspect, the detected bioluminescent signal was clearly diminished compared to imaging the same mouse from the ventral aspect (FIG. 2D). Comparison of individual mice at day 13 post-tumor cell injection confirmed an 18.4-fold decreased bioluminescence in R2G2 mice imaged from the dorsal aspect compared to the ventral aspect (0.44 ± 0.19 ×105 dorsal aspect vs. 8.1 ± 4.9 ×105 ventral aspect, radiance [p/s/cm2/sr], p<0.001, unpaired t-test) with no difference observed with the SCID mice (FIG. 2D).
The dark R2G2 dorsal pelt markedly attenuates Raji-luc bioluminescent signal
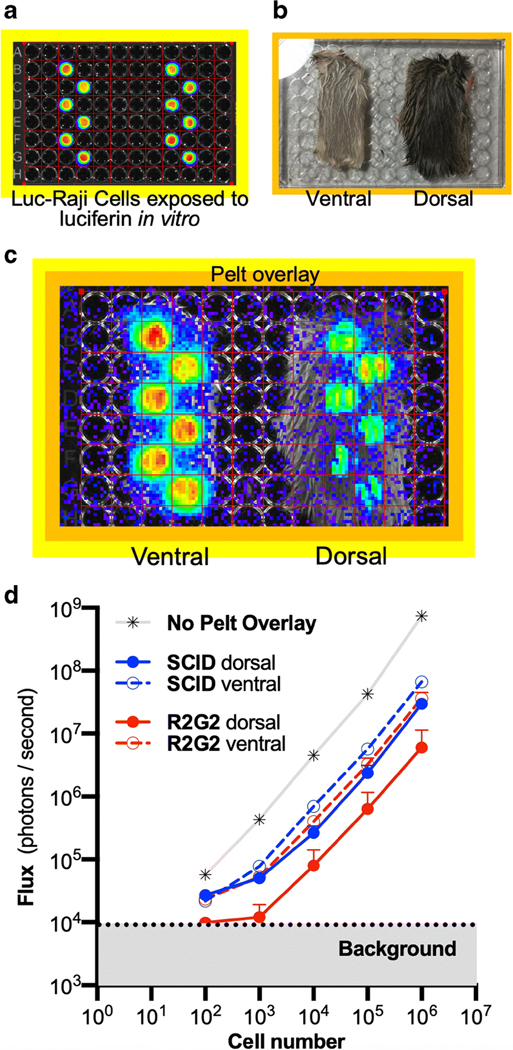
Because of the difference in ventral and dorsal photon transmission in R2G2 mice with disseminated xenograft lymphoma, we investigated the effects of isolated R2G2 and SCID pelts (skin and fur) on photon transmission in vitro. Pelts from the ventral and dorsal surfaces of R2G2 and SCID mice were flat-mounted on the cover of a 96-well plate to make a mobile cartridge that was superimposed on wells containing Raji-luc cells (FIG. 3A, B), and bioluminescence again measured (FIG. 3C). Photon transmission through the ventral and dorsal pelts of SCID, and of the ventral pelt of R2G2 mice was similar, all with an approximately 10-fold attenuation of signal relative to no pelt (FIG. 3D). In contrast, the dark dorsal pelt of R2G2 mice showed much greater photon attenuation, with the transmitted signal reduced to background in wells containing 1,000 or fewer cells. The photon flux through the dorsal R2G2 pelt was approximately 10-fold, or more, lower than the flux transmitted through the SCID dorsal or ventral pelts or the R2G2 ventral pelt. Focusing on the effects at 105 cells, the greatest difference signal attenuation was by the dorsal pelts of R2G2 mice, which demonstrated a 120 ± 80 fold reduction, while SCID dorsal pelts exhibited only a 21.0 ± 5.2 fold signal reduction (p = 0.012, unpaired t-test) comparing fold reduction by dorsal pelts between strains.
FIGURE 3.
Dorsal pelts isolated from R2G2 mice have high bioluminescent signal attenuation. (A) Merged bioluminescence and photographic images of a 96-well plate with individuals wells containing 106 Raji-luc cells exposed to luciferin. (B) Photogram of isolated dorsal and ventral pelts (R2G2 shown) after flat mounting on 96-well plate cover. (C) Merged photograph and bioluminescence image of luciferin treated Raji-luc cells overlaid with R2G2 pelts. (D) Bioluminescent signal of plate in A without or with overlay of the dorsal or ventral portions of the pelts of R2G2 or SCID mice measured as flux vs. cell number. Each data point represents the mean ± standard deviation of six wells with Raji-luc cells. The greatest signal attenuation was by dorsal R2G2 pelts.
DISCUSSION
Bioluminsecent imaging has a variety of applications, and is particularly attractive in cancer imaging [6,7]. The present study compared the bioluminescent signal of disseminated lymphoma modeled in SCID and R2G2 mice. The in vivo studies show that in R2G2 mice, bioluminescent tumor signal attenuation was lower when imaged from the lighter-colored ventral aspect compared to imaging through the darker dorsal aspect. Due to more rapid tumor cell growth in R2G2 mice, as suggested by their greater immunodeficiency and faster rate to lethality and by the observation that at day 13 after injection of Raji-luc tumor cells the average radiance passing through the ventral surface was nearly 17-fold higher in the R2G2 mice than in the SCID mice, despite the slightly darker ventral coat of the R2G2 mice. When the ventral pelts were placed over known quantities of Raji-luc cells and assayed in vitro, the signal in R2G2 mice was half of that in SCID mice, in keeping with greater light absorption by the slightly darker coat. Given the increased attenuation by the dorsal pelt of SCID mice, compared to the ventral pelt, there may also be component of fur density and skin thickness, independent of fur color, contributing to attenuation differences.
Variable attenuation of bioluminescent signal from soft-tissues [8], and, as our data demonstrates, from the animal’s fur and skin (pelt), can greatly affect detection of tumor via optical imaging. Pelt coloration in mammals is mainly due to melanin, including eumelanin and pheomelanin [9], which have high light absorption properties, including attenuation of light produced via bioluminescence [10]. One solution that has been used to address fur-related attenuation differences is frequent shaving or chemical removal of fur before optical imaging [11]. However, fur serves as a natural barrier to infection and removal of the fur, and nicking of skin by shaving, can result in microabrasions that increase the chance of infection. Also, time after shaving then becomes a confounding variable [8,11]. Imaging unshaven animals in long-term survival studies can thus improve throughput for large therapeutic studies, reduce intrasubject variability, and reduce infection in long-term survival studies. In addition to intervening soft tissues [8], strain-dependent coat characteristics are significant sources of bioluminescent signal attenuation and should be considered when conducting studies, partially as attenuation from the pelt could lead to a false (incorrectly low) interpretation of cancer-cell levels. Our study suggests that multiple imaging views and coat color are important considerations when using optical imaging to quantify tumor-cell levels in in vivo preclinical mouse models, notably in the promising R2G2 model.
Our study builds our technical understanding of BLI in a relatively new mouse system. While the incremental knowledge and perhaps the novelty could be viewed as limited, this “limited” knowledge is important as BLI therapy monitoring studies form the foundation for addressing new cancer treatments including radiophamarmaceutical therapies. After completing the studies and analyzing the data, one might argue that the findings are relatively intuitive. However the literature directly addressing mouse coat color on bioluminescence properties is very small. Further, to the best of our knowledge, there are no formal manuscripts available on bioluminescence with the relatively new R2G2 strain. These mice have a strong potential as a model for targeted alpha-particle therapy given their maintained machinery for repairing dsDNA breaks. The remarkable positional variability in the BLI signal is of importance, relevance and novelty to those conducting therapy studies in this species and likely carries important lessons for extrapolating BLI studies into other species, especially those with variable skin pigmentation.
CONCLUSIONS
Mouse strain and imaging aspect are critical considerations for conducting studies of bioluminescence in preclinical models. R2G2 mice are a very attractive model for in vivo radiopharmaceutical therapy studies with alpha and other particle emitters due to their severe immunodeficiency. We suggest care must be taken when comparing quantitative results obtained using different mouse strains, and that multiple aspects should be imaged, with the lighter aspect likely allowing the most sensitive detection of bioluminescence. Also, we recommend publications indicate animal coat color and strain (exact genotype, company, and catalog number) and imaging aspect when presenting bioluminescence results.
KEY POINTS.
KEY POINTS:
Mouse pelt color and imaging aspect are critical considerations for quantifying bioluminescent tumor signal.
There is high variability in survival between mouse strains after intravenous tumor cell delivery.
Preclinical studies with an imaging component should carefully consider source and strain of mice.
QUESTION:
Do the highly immunodeficient Rag2-IL2rg (R2G2) exhibit more rapid tumor-cell growth and decreased survival compared to severe combined immunodeficient (SCID) mice and are there differences in bioluminescent tumor signal dependent on strain and imaging aspect?
PERTINENT FINDINGS:
Given similar numbers of lymphoma cells injected intravenously, the highly immunodeficient R2G2 mice had decreased survival compared to SCID mice, despite similar bioluminescent tumor signal when imaged with a dorsal view. Imaging with ventral view revealed increased tumor signal in R2G2 mice compared to SCID mice, and isolated pelts were sufficient to cause significant differences in lymphoma cell bioluminescent signal attenuation in vitro, with the greatest attenuation by the dark R2G2 ventral pelt.
IMPLICATIONS FOR PATIENT CARE:
Reproducible preclinical data serve as the foundation for successful translation of therapies, including radioimmunotherapy for cancer. Preclinical studies use a variety of different mouse strains that vary greatly in genotype and phenotype. This study highlights variation in bioluminescent signal measurement in vivo between strains, which may result in variations between preclinical cancer studies.
ACKNOWLEDGEMENTS
We would like to thank the MIR Preclinical Imaging Facility including Dr. Kooresh Shoghi, Nikki Fettig, Lori Strong, Margaret Morris, and Amanda Roth for their technical expertise in animal handling and imaging.
Funding: These studies were funded in part by the RSNA Resident Research Grant RR1839. MJH received salary support from National Institutes of Health TOP-TIER grant T32-EB021955 during the study design period.
Authors’ information (optional): n/a
FINANCIAL DISCLOSURES
Funding:
These studies were funded in part by the RSNA Resident Research Grant RR1839.
LIST OF ABBREVIATIONS
- Raji-luc
Luciferase-expressing Raji lymphoma cells
- SCID
Severe Combined Immunodeficient
- R2G2
Rag2-IL2rg
- NHL
Non-Hodgkin Lymphoma
Footnotes
DECLARATIONS
Ethics approval and consent to participate:
Consent for publication: All authors provide consent for publication of the current work.
Availability of data and material:
Competing interests: No relevant financial disclosure.
This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Competing interests:
MJH, MSL, KS and RLW have no relevant competing interests.
DISCLAIMERS
No disclaimers are associated with this study.
REFERENCES
- 1.Baba S, Cho SY, Ye Z, Cheng L, Engles JM, Wahl RL. How reproducible is bioluminescent imaging of tumor cell growth? Single time point versus the dynamic measurement approach. Mol Imaging. 2007;6:315–22. [PubMed] [Google Scholar]
- 2.Close DM, Xu T, Sayler GS, Ripp S. In vivo bioluminescent imaging (BLI): noninvasive visualization and interrogation of biological processes in living animals. Sensors (Basel). 2011;11:180–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg DM. Targeted therapy of cancer with radiolabeled antibodies. J Nucl Med. 2002;43:693–713. [PubMed] [Google Scholar]
- 4.Brennan TV, Lin L, Huang X, Yang Y. Generation of luciferase-expressing tumor cell lines. Bio Protoc.2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinde R, Perkins J, Contag CH. Luciferin derivatives for enhanced in vitro and in vivo bioluminescence assays. Biochemistry. 2006;45:11103–12. [DOI] [PubMed] [Google Scholar]
- 7.Puaux A-L, Ong LC, Jin Y, Teh I, Hong M, Chow PKH, et al. A Comparison of Imaging Techniques to Monitor Tumor Growth and Cancer Progression in Living Animals. International Journal of Molecular Imaging. 2011;2011:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virostko J, Chen Z, Fowler M, Poffenberger G, Powers AC, Jansen ED. Factors influencing quantification of in vivo bioluminescence imaging: application to assessment of pancreatic islet transplants. Mol Imaging. 2004;3:333–42. [DOI] [PubMed] [Google Scholar]
- 9.Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 2003;16:523–31. [DOI] [PubMed] [Google Scholar]
- 10.Kollias N, Baqer A. Spectroscopic characteristics of human melanin in vivo. J Invest Dermatol.1985;85:38–42. [DOI] [PubMed] [Google Scholar]
- 11.Curtis A, Calabro K, Galarneau J-R, Bigio IJ, Krucker T. Temporal variations of skin pigmentation inC57BL/6 mice affect optical bioluminescence quantitation. Mol Imaging Biol. 2011;13:1114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]