Abstract
Heightened sensation seeking is associated with an increased risk of substance use disorder in clinical populations. In rats, sensation seeking is often examined by measuring locomotor reactivity to a novel environment. So-called high responders (HR) acquire self-administration of psychostimulants more quickly and consume higher amounts of drug compared to low responder (LR) rats, indicating that the HR trait might confer a stronger addiction propensity. However, studies of addiction-like behaviors in HR vs LR rats have typically utilized self-administration paradigms that do not dissociate individual differences in the hedonic/reinforcing and motivational properties of a drug. Moreover, little attention has been given to whether HR rats are more susceptible to drug-access conditions that promote a state-dependent addiction phenotype. We report that on a behavioral economics task, HR rats have higher preferred brain-cocaine levels compared to LR rats but do not differ with respect to their demand elasticity for cocaine. In contrast, when tested on an intermittent access schedule of cocaine self-administration, which has been shown to promote several addiction-related endophenotypes, HR rats exhibit greater escalation of intake and more drastic reductions in cocaine demand elasticity. Together, these data indicate that the HR trait does not confer higher extant addiction behavior, but rather that this phenotype is associated with a propensity for addiction that remains dormant until it is actuated by intermittent drug intake. These findings reveal a ‘trait’ (HR) by ‘state’ (intermittent drug intake) interaction that produces a strong addiction-like phenotype.
Keywords: behavioral economics, demand elasticity, cocaine prime, state vs trait, self-administration, novelty seeking
1.0. Introduction
A major goal of addiction research is to identify behavioral biomarkers that predict addiction vulnerability. Epidemiological studies indicate that heightened sensation seeking, defined as an enhanced willingness to take risks for the sake of increases in stimulation and arousal, is associated with a greater propensity to experiment with addictive drugs and thus may predispose to addiction (Mahoney III et al., 2015; Zuckerman and Neeb, 1979). Significant effort has been dedicated to studying this phenomenon in laboratory animals, whereby rodents (typically rats) are screened for their propensity to exhibit high locomotor reactivity to a novel inescapable environment (high responder, or HR, phenotype) (Belin et al., 2011; Belin and Deroche-Gamonet, 2012; Piazza et al., 1989). Compared to low responder (LR) rats, HR rats exhibit a propensity to acquire self-administration of psychostimulants more quickly, to self-administer higher amounts of drug, and to exhibit greater locomotor reactivity to a non-contingent injections of psychostimulants (Hooks et al., 1991a, b; Klebaur et al., 2001; Marinelli and White, 2000; Piazza et al., 1989). One study reported that following extended cocaine self-administration (~60d), rats selectively bred to exhibit a HR phenotype exhibit stronger behaviors reminiscent of human addiction, including cue-controlled cocaine seeking, persistent drug seeking in the absence of drug availability and cued reinstatement of extinguished responding (Flagel et al., 2016). In contrast however, another study reported no relationship between a spontaneous HR phenotype and propensity to develop a multiphenotypic addiction-like state following a similar duration of cocaine self-administration (Deroche-Gamonet et al., 2004). Thus, although there appears to be a clear relationship between the HR phenotype and cocaine reactivity, its relationship to addiction per se remains to be fully understood.
How to measure ‘addiction’ represents an ongoing challenge (Ahmed, 2012; Deroche-Gamonet et al., 2004). A major limitation of many self-administration schedules is that they are dependent on drug dose, pharmacokinetics and baseline shifts in consumption (tolerance), making it difficult to identify the relative contribution of each of these factors to drug-seeking behavior (Bentzley et al., 2013). Moreover, the link between self-administration behavior and ‘addiction-like’ endophenotypes, such as relapse propensity and compulsive responding is tenuous (Quinn et al., 2018). The behavioral economics (BE) approach has emerged as a powerful paradigm for concurrently measuring multiple aspects of drug behavior, including demand elasticity (α), which reflects how quickly drug consumption falls with increases in price and is thus a measure of drug motivation (Bentzley et al., 2013). In the case of psychostimulants, α is orthogonal to preferred drug intake under null cost conditions (Q0), meaning that drug motivation can be assessed without the confound of individual differences in baseline cocaine intake (Bentzley et al., 2013). Demand elasticity (α), but not Q0, is a strong predictor of several addiction-relevant behaviors for stimulants (Bentzley et al., 2014; James et al., 2019a), and thus may offer a preferable index of ‘trait’ addiction propensity. To date, however, the link between sensation seeking and economic demand for cocaine is untested.
In addition to individual risk factors, drug exposure itself plays a major role in determining addiction outcomes (Ahmed and Koob, 1998). Thus, it is interesting to consider whether HR animals are also at greater risk of transitioning to addiction under drug consumption conditions that promote compulsive drug seeking. Studies to this end have generally focused on the amount of cocaine consumed as a key factor, where animals are given continuous access to cocaine over extended periods of time (Ahmed and Koob, 1998). For example, when rats are provided daily extended (10h) access to cocaine, HR rats exhibit greater escalation of cocaine intake compared to their LR counterparts (Mantsch et al., 2001), which is argued to reflect a transition from controlled drug use to ‘addiction’ (Ahmed and Koob, 1998). Similarly, as noted above, selectively bred HR rats exhibit stronger addiction-like behaviors across several behavioral indices following prolonged (~60d) daily access to cocaine (Flagel et al., 2016). Emerging evidence, however, indicates that the pattern of cocaine intake may be more important than the overall amount of drug consumed in determining addiction outcomes (Allain et al., 2015). Indeed, the intermittent access (IntA) self-administration procedure, whereby brief periods of drug availability are separated by longer periods of non-drug-availability within a single session, has recently been shown to promote more profound addiction-like behaviors compared to extended access paradigms (Algallal et al., 2019; Allain and Samaha, 2019; James et al., 2019b; Kawa et al., 2019b; Zimmer et al., 2012). There is significant variability in the extent to which IntA promotes a shift in motivation for cocaine (Garcia et al., 2020; James et al., 2019a), however the potential factors contributing to this variability, including individual differences in sensation seeking, have not been determined.
Here, we show that HR rats exhibit higher cocaine consumption at null cost than LR animals, but do not differ in their demand elasticity. In contrast, HR rats exhibit greater escalation of cocaine intake across the IntA procedure, as well as greater IntA-induced reductions in demand elasticity (increases in drug motivation). Together these data indicate that the HR trait reflects a dormant vulnerability that is actuated by intermittent drug intake, pointing to a trait (HR) x state (IntA) interaction in the development of addiction-related behaviors.
2.0. Methods
2.1. Animals
Adult male Sprague-Dawley rats (n=120, ~300g, 6–8w upon arrival; Charles River Laboratories, Kingston, NY) were pair housed on a reverse 12:12-hour light/dark cycle in a temperature and humidity-controlled animal facility. Animals had ad libitum access to food and water throughout the experiments. Animals were acclimated to the animal facility for at least 1w prior to any surgical or behavioral procedures. All procedures were approved by Rutgers University New Brunswick Institutional Animal Care and Use Committee and conducted in accordance with their guidelines.
2.2. Drugs
Cocaine HCl powder was obtained through the National Institute of Drug Abuse Drug Supply Program and was dissolved in 0.9% sterile saline.
2.3. Surgery
Rats were anesthetized with isoflurane gas and administered an analgesic (rimadyl; 5mg/kg). An intravenous catheter was implanted into the right jugular vein, as described previously (McGlinchey et al., 2016), with the port exiting between the scapulae. During recovery and throughout the entirety of the study, cefazolin (0.1ml; 10mg) and heparin (0.1mL; 100U) were flushed daily through the i.v. catheter.
2.4. Experiment 1: Examining the relationship between novelty-induced locomotor activity, non-contingent cocaine exposure and cocaine self-administration
2.4.1. Selection of HRs and LRs to novelty-induced locomotor activity
All subjects were tested for their locomotor response to a novel environment 1w after catheter implantation. Locomotor tests were performed in clear acrylic open field boxes (42cm x 42cm x 30cm), which were housed inside sound attenuating chambers. SuperFlex monitors and software (Omintech Electronics Inc, Columbus, OH) tracked locomotor activity via infrared light beam breaks (16×16 array), as in previous publications from our group (James et al., 2018; 2019b; McGlinchey et al., 2016). Locomotor activity was recorded as beam breaks in 5min epochs for a period of 2h, and total distance traveled during this time was used to determine an animal’s activity score. Each animal was designated as HR or LR depending on whether their activity fell above or below the upper or lower tertile of the overall cohort, respectively. Tertiles were used to capture more extreme ends of the overall population; this was aided by the size of our overall cohort. One day after the novel locomotor reactivity test, a subpopulation of rats was tested for novelty preference using procedures described elsewhere (Belin et al., 2011); data from this test will be presented in a separate manuscript.
2.4.2. Locomotor Reactivity Induced by Cocaine
Following classification of rats into HR/LR groups, we first sought to confirm in a subpopulation of these rats (n=49) the previously reported observation that HR rats respond differentially to an acute, experimenter-administered injection of cocaine. This was evaluated in the same open field apparatus as described in the locomotor assessment. On the day immediately following locomotor or novelty preference testing, rats were placed into the open field and baseline locomotor activity was recorded for 30min. Rats were then removed from the open field, given an injection of cocaine (10mg/kg, i.p.) and then immediately placed back in the open field. Locomotor activity was recorded for 120min.
2.4.3. Cocaine Self-Administration Training
Two days after the phenotyping test, rats began training to self-administer cocaine. Self-administration sessions were conducted in sound attenuating operant chambers, which were connected to MED-PC IV software (Med-Associates, St Albans, VT, USA). Rats were trained on a fixed ratio 1 (FR1) schedule to press an active lever for cocaine infusions (0.2mg/50μl infusion; 3.6s), which were paired with a light and tone cue (white light above the active lever; 78‐dB, 2900‐Hz tone). Infusions were followed by a 20s time-out period where active lever presses did not elicit an infusion or cues. Inactive lever presses at any time did not result in drug infusions or cues. Rats trained in 2h sessions for at least 6d, until they reached criteria (>20 infusions, <25% variability) across 3d, before being tested on the BE paradigm. This schedule of cocaine self-administration is not considered to promote the development of addiction-like behaviors (Ahmed and Koob, 1998; James et al., 2019b; Zimmer et al., 2012).
2.4.4. BE procedure and demand curve fitting
Following self-administration training, rats were trained on a within-session behavioral economics procedure to determine demand for cocaine (Bentzley et al., 2013). Using an FR1 schedule, rats received access to decreasing doses of cocaine in successive 10min intervals on a quarter logarithmic scale (383.5, 215.6, 121.3, 68.2, 38.3, 21.6, 12.1, 6.8, 3.8, 2.2 and 1.2 μg/infusion) over a 110min session, achieved by decreasing pump infusion duration (Bentzley et al., 2013). Each infusion was paired with a light and tone cue and animals could not receive additional infusions by responding on the active lever during this period. Rats were tested on the BE paradigm for a minimum of 6d and until the last 3d generated alpha (α) and consumption (Q0) values (described below) that were within ±25% of the mean of those days (Bentzley et al., 2014). These mean values derived from the final 3d of BE testing were used as our measure of baseline demand, which was tested for correlations with novelty reactivity.
An exponential demand equation was fit to each animals’ data from every BE session (Bentzley et al., 2013; Hursh and Silberberg, 2008) and two parameters were derived: Q0 and α, where Q0 refers to cocaine consumption (mg) at null cost and α represents demand elasticity, or rate of consumption decline with increasing cost (inversely scaled with drug motivation and other addiction behaviors (Bentzley et al., 2014; James et al., 2019a)).
2.5. Experiment 2: Examining the relationship between novelty-induced locomotor activity and the addiction-promoting effects of intermittent access to cocaine
We next sought to explore the extent to which the HR/LR model can predict animals’ propensity to respond to the intermittent access (IntA) self-administration paradigm, which has been shown to robustly promote enhanced addiction-like behaviors for cocaine (Algallal et al., 2019; James et al., 2019b; Kawa et al., 2019b); (Zimmer et al., 2012) and other drugs of abuse (Fragale et al., 2020). A subset of rats (n=21) from Experiment 1 was trained to self-administer cocaine on an intermittent access schedule for 14d (James et al., 2019b; Zimmer et al., 2012). This subgroup was representative of all rats tested in Experiment 1 across all behavioral measures (see Results). In this paradigm, rats were given access to cocaine in twelve 5min drug access periods separated by 25min-duration timeouts with no drug access, resulting in a 6h session. The beginning of drug access periods was signaled by a 5s light and tone cue and a single priming injection of cocaine (1s, 0.055mg) to prime the catheter line, before the levers were extended and the house light was turned on. During this period, cocaine infusions (1s, 0.055mg) were elicited by responses on the active lever (FR1 schedule) and were paired with a light and tone cue. There was no post-infusion timeout period. Immediately following the 14d of IntA training, rats were re-tested on the behavioral economics paradigm, as above, for a minimum of 6d and until the α and Q0 values were stable (<25% variability) over the last 3 days.
2.6. Data analysis
All analyses were carried out using Prism Graphpad versions 6, 8 and 9. Experiment 1: For novel locomotor reactivity and cocaine prime tests, data were analyzed using a 2 ‘phenotype’ (HR, LR) x 24 ‘time’ (5 min bins) mixed effects ANOVA. Baseline activity prior to cocaine prime injection was analyzed using a separate 2 ‘phenotype’ (HR, LR) x 6 ‘time’ (5 min bins) mixed effects ANOVA. Data from the first 5d of self-administration training were compared using a 2 ‘phenotype’ (HR v LR) x 2 ‘lever’ (active, inactive) x 5 ‘time’ (days 1–5) mixed effects ANOVA (active and inactive lever data are presented on different graphs for clarity). Infusion data were analyzed using a similar ‘phenotype’ x ‘time’ ANOVA. For all correlational analyses, Q0 and α were normalized using log transformation, and correlations were analyzed using Pearson’s R regression. A computer error in a subset of rats n=7/group for both HRs and LRs meant that total locomotor activity across the 2h session was calculated, but 5 min epoch data was not collected (as in Figures 1a+1b). One animal was excluded due to its Q0 value being identified as statistical outlier (>3 SDs beyond the group mean) and one animal was excluded due to unexpected death. One HR animals’ inactive lever data was excluded from day 4 of self-administration training data analyses due to an abnormally high number of responses (>1400), which was attributed to a mechanical error with the lever. Experiment 2: As with Experiment 1, HR and LR groups were identified by tertile split. Changes in cocaine intake were calculated for each animal as the difference between intake on d14 and d1. Post-IntA α and Q0 values were converted to percentages of pre-IntA values (post/pre x100); these values were tested for correlations with novelty-induced locomotor scores. Changes in α and Q0 values from pre- to post-IntA were compared across groups using a 3 ‘phenotype’ (HR, middle, LR) x 2 ‘time’ (pre-IntA, post-IntA) ANOVA. For all repeated measures ANOVAs, sphericity was tested using a Geisser-Greenhouse test and fractional degrees of freedom were used to compute a p value when necessary. For other comparisons, normality of data was assessed using a Shapiro-Wilk test; in one case where normality was violated (comparison of pre- vs. post-IntA α values in Experiment 2), a non-parametric Mann-Whitney-U test was used. An α=0.05 was adopted for all statistical tests.
Figure 1. Classification of rats by novelty-induced locomotor activity.
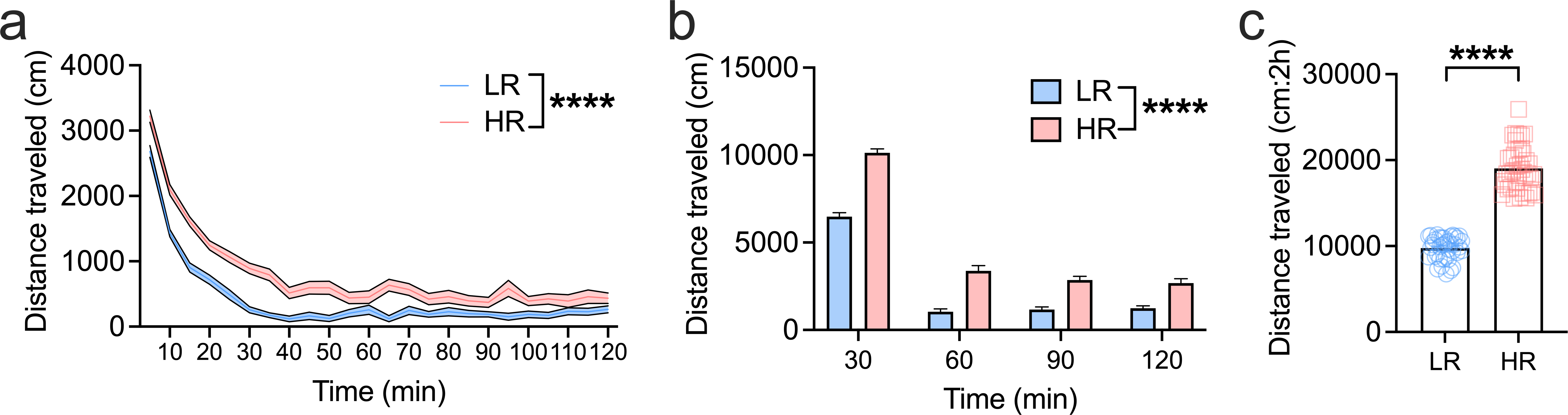
a) Two-hour time course of locomotor activity in high (HR) and low responder rats (LR). Classification of rats as HRs or LRs was based on the group tertile split of total locomotor activity across the 2h test. In addition to a main effect of ‘phenotype’, indicating higher overall activity in HR rats, there was a ‘phenotype’ x ‘time’ interaction with HR rats differing significantly from LR rats in the initial 50 mins of the test (see Results for details). b) Differences in locomotor activity between HRs and LRs were sustained for the duration of the novelty-induced locomotor test session when data were binned into 30 min periods (main effect of ‘phenotype’). c) When locomotor activity was summed across the 2h test, HRs exhibited higher overall activity compared to LRs. (n=40/group). ****P<0.0001. Bar charts depict mean ± SEM.
3.0. Results
3.1. Experiment 1:
3.1.1. Locomotor reactivity to novelty
Rats were first screened for sensation seeking by assessing locomotor reactivity to a novel environment over 2h and were then divided into HRs and LRs based on a tertile split. Across the 2h test, we observed a main effect of ‘phenotype’, such that locomotor activity was significantly greater in HR rats compared to LR rats (F1,1536=307.0, p<0.0001) (Fig. 1a). There was also a ‘phenotype’ x ‘time’ interaction (F23,1536=2.931, p<0.0001) and post-hoc analyses revealed that HR rats exhibited significantly higher activity at every time point in the first 50min of the test, as well as at 65min (Sidak’s multiple comparisons test, p’s<0.05). This group difference was also evident when locomotor activity was summed into 30min bins (F1,256 =234.8, p<0.0001) (Fig. 1b) and when locomotor activity was summed across the entire 2h (t78 = 20.19, p<0.0001) (Fig. 1c).
3.1.2. HR rats exhibit a greater locomotor response to non-contingent cocaine injections
To determine the relationship between sensation seeking and cocaine-related behaviors, we first compared the extent to which the HR/LR phenotype predicted locomotor reactivity to a non-contingent cocaine injection in a 2h test. Analysis of the initial 30min baseline period prior to receiving cocaine revealed a significant ‘phenotype’ x ‘time’ interaction (F5,150=3.219, p=0.0086; Figure 2a), although subsequent post-hoc comparisons failed to identify significant differences between HR and LR rats at any time point (p’s>0.05). When comparing total activity during this baseline period, HRs exhibited significantly greater activity compared to LRs (t30=3.105, p=0.0041; data not shown) and this activity was positively correlated with total activity during the novel locomotor reactivity task (R2=0.1196; p=0.01492; data not shown), indicating that the HR trait was stable across days. Analysis of activity following the cocaine priming injection revealed a significant ‘phenotype’ x ‘time’ interaction (F23,690=1.782, p=0.0138), however subsequent post-hoc analyses again failed to find significant differences between groups at any time point (p’s>0.05). Notably however, there was a main effect of ‘phenotype’ (F1,30=7.723, p=0.0093), indicating that HR rats exhibited higher overall cocaine-induced locomotor activity across the 2h following the injection. Similarly, when data were summed into 30min bins, we observed a main effect of ‘phenotype’, such that HR rats exhibited higher activity across the entire 2h duration of the test (F1, 30=7.748; p=0.0092; Fig. 2b). Across all animals, there was a significant positive correlation between locomotor reactivity to the novel environment and total activity during the 2h cocaine prime test (R2=0.1540; p=0.0053; Fig. 2c).
Figure 2. HR rats exhibit greater locomotor reactivity to acute cocaine.
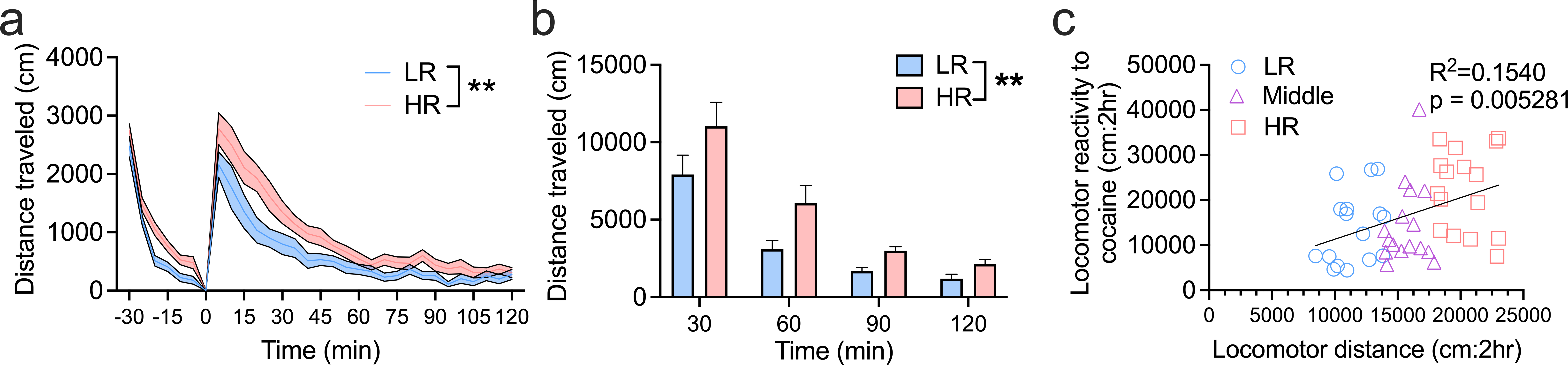
a) Time course of locomotor activity in HRs and LRs before and after a single injection of cocaine (10mg/kg, i.p.) at time 0 min. b) When locomotor reactivity to cocaine was collapsed into 30 min bins, HRs showed a greater activity across all time points compared to LRs (main effect of ‘phenotype’). c) Locomotor reactivity to novelty predicts total locomotor reactivity in the 2h following cocaine priming injection. **p<0.01. Bar chart depicts mean ± SEM. HR: n=16; LR: n=16.
3.1.3. HR rats have a higher preferred level of cocaine intake at low/null cost but do not differ from LR rats in terms of demand elasticity for cocaine
We next examined the relationship between sensation seeking and cocaine self-administration behavior. We trained rats to self-administer cocaine on a simple FR1 schedule before testing them on a BE procedure. Across the first 5d of self-administration training, there was a significant main effect of ‘lever’, indicating that rats made significantly more responses on the active versus inactive lever across all days (F1,77=51.82, p<0.0001; Fig. 3a and 3b). However, there was no significant main effect of ‘phenotype’ (F1,288=1.273, p=0.2601) or ‘day’ (F2.58,198.7=1.967, p=0.1293), nor was there an interaction between any factors (all p’s>0.49). Thus, HR rats did not differ significantly from LR rats with respect to the number of active or inactive lever responses made across the first 5d of self-administration training. Although the number of cocaine infusions earned across the first 5d of self-administration training significantly increased with time (‘day’ main effect: F2.83,254.9=7.059, p=0.0002), there was no ‘day’ x ‘phenotype’ interaction (F4,355=0.9234, p=0.4503) indicating that infusions were similar in HR vs LR rats across the 5d (Fig. 3c). We did however observe a significant positive correlation between locomotor reactivity and the average number of cocaine infusions across the first five self-administration FR1 training sessions (R2=0.03461, p=0.04371; Fig. 3d). Consistent with a general relationship between locomotor scores and cocaine intake, we observed a relationship between locomotor activity and Q0 (preferred cocaine intake) on the BE procedure (R2=0.03357; p=0.04704; Fig. 3e). Correspondingly, HR rats had significantly higher Q0 values compared to LR rats (t78=2.177; p=0.0325; Fig. 3f). In contrast, locomotor reactivity was not predictive of rats’ α values on the BE procedure (R2=0.01533; p=0.1817; Fig. 3g) and HR and LR rats did not differ overall on this index (t78=0.7731; p=0.4418; Fig. 3h).
Figure 3. HR rats exhibit higher levels of low-cost cocaine consumption.
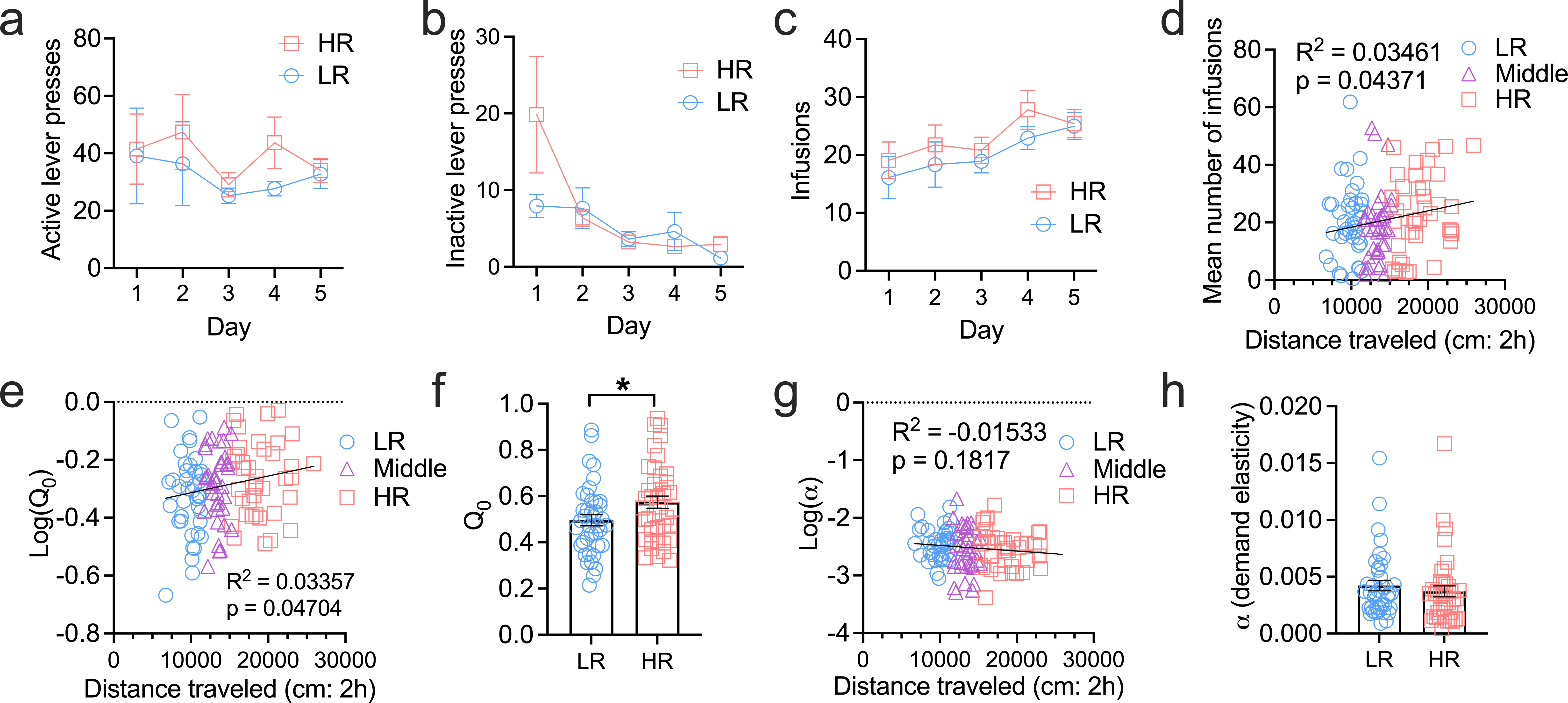
HR and LR rats did not differ on the number of active (a) or inactive lever (b) responses, nor the number of cocaine infusions earned (c) over the first 5 FR1 self-administration training sessions, however locomotor reactivity to a novel environment was positively correlated with the mean number of infusions during the first 5 FR1 self-administration training sessions (d). e) Locomotor reactivity to a novel environment was positively correlated with Q0 values (estimated cocaine intake at null cost) on a behavioral economics procedure. f) HR rats had higher Q0 values compared to LRs. g) There was no relationship between locomotor reactivity and demand elasticity (α) on the behavioral economics procedure. h) HR and LR rats did not differ in terms of baseline α values. *p<0.05. Bar charts depict mean ± SEM. n=40/group, except in panels a-c where one HR rat’s data are not included due to mechanical issues during a self-administration session across the first 5d of training.
3.2. Experiment 2:
The HR trait confers susceptibility to state (IntA)-induced addiction
Experiment 1 established that the HR/LR model predicts ‘trait’ cocaine intake but not addiction behaviors per se. Here, we examined whether the HR trait confers a greater susceptibility to the state-induced addiction phenotype that results from IntA. Rats selected to undergo IntA (n=21) were statistically similar to the remaining 97 rats in terms of novelty-induced locomotor scores (mean±SEM: 12697±577cm vs. 14337±445cm, respectively; p=0.1023), cocaine-induced locomotor scores (6093±599cm vs. 6204±280.8cm, respectively; p=0.8637), self-administration behavior across the first 5d (‘phenotype’ main effects: active lever responses, p=0.4143; inactive lever responses, p=0.4980; cocaine infusions, p=0.4288), and baseline demand values (α: 0.00373±0.00043 vs. 0.00405±0.00036, respectively, p=0.6871; Q0: 0.5518±0.0208 vs. 0.5316±0.0172, respectively, p=0.5966). When examining cocaine intake over the 14d of IntA, we observed a significant main effect of ‘phenotype’, such that intake was higher in HR rats compared to LR rats (F1,12=4.764, p=0.0497; Figure 4a). Consistent with this, overall cocaine intake across the 14d was higher in HR rats (t12=2.183; p=0.0497; Figure 4b). Because a key feature of the IntA procedure is an escalation of cocaine intake across the 14d IntA period (James et al., 2019b; Kawa et al., 2016), we next calculated the difference between cocaine intake on d14 vs. d1 for each animal. We found that the extent to which animals escalated their intake was positively and significantly correlated with their novelty-induced locomotor activity score (R2=2.018; p=0.0411; Figure 4c). Consistent with this, escalation of intake was higher in HR rats compared to LR rats (t12=2.345; p=0.0371; Figure 4d).
Figure 4. HR animals are more susceptible to the addiction-promoting effects of the intermittent access procedure.
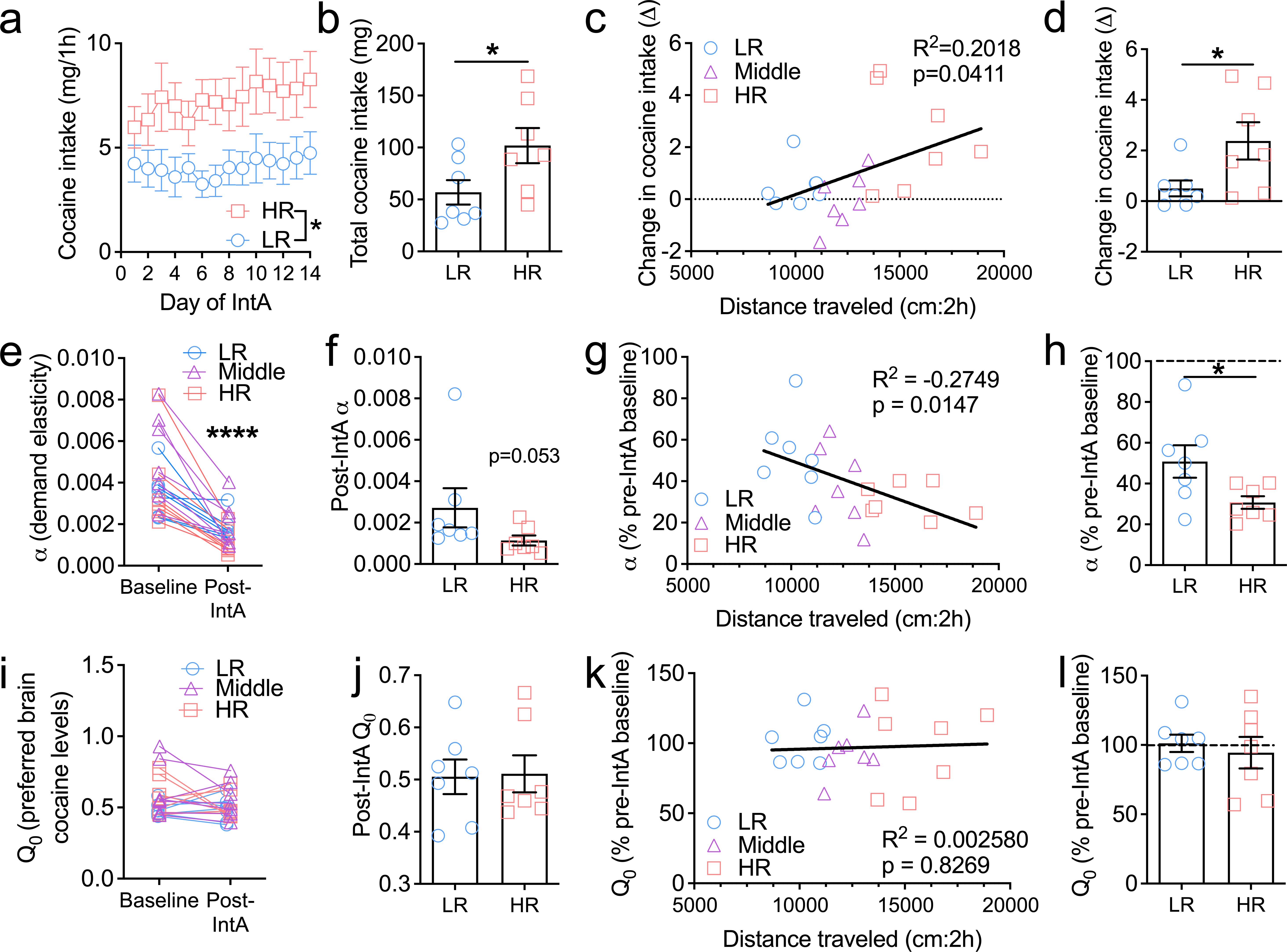
a) HR rats exhibited significantly higher overall cocaine intake across the 14d of IntA to cocaine. b) Cumulative cocaine intake across the 14d of IntA was significantly higher in HR rats compared to LR rats. c) Change in cocaine intake across the 14d of IntA (Δd14-d1) was positively correlated with locomotor reactivity to novelty. d) HR rats exhibited a higher change in cocaine intake (escalation) across the IntA period compared to LR rats (Δd14 intake – Δd1 intake). e) Across all rats, α values were significantly lower (reflecting increased cocaine motivation) following IntA compared to baseline (pre-IntA). f) Post-IntA α values tended to be lower (reflecting higher motivation) in HR rats, compared to LR rats. g) Post-IntA α values, calculated as percentage of baseline α values, were negatively correlated with locomotor reactivity to novelty, indicating that the biggest reductions in α values were in those rats with higher locomotor scores (HR rats). h) Post-IntA α values, as a percentage of baseline values, were significantly lower in HR rats compared to LR rats. i) Across all rats, IntA was not associated with a change in Q0 values (preferred brain-cocaine levels). j) Post-IntA Q0 values were similar between HR and LR rats. k) There was no relationship between IntA-induced changes in Q0 and locomotor reactivity to novelty. l) HR and LR rats did not differ in the extent to which IntA produced changes in Q0 values. *p<0.05, ****p<0.0001. HR, n=7; middle, n=7; LR, n=7. Bar charts depict mean ± S.E.M.
When examining changes in demand elasticity (α) as a result of IntA, ANOVA revealed a significant main effect of ‘time’, indicating that demand elasticity values were significantly lower following IntA compared to pre-IntA across all phenotypes (F1,18=56.5, p<0.0001; Figure 4e). There was no main effect of ‘phenotype’ (F2,18=1.20, p=0.3241) or ‘phenotype’ x ‘time’ interaction (F2,18=1.53, p=0.2444), indicating that demand elasticity values and their change as a result of IntA were similar across all groups. When comparing post-IntA α values, HR rats tended to have lower values compared to LR rats, but this failed to reach significance (Mann-Whitney U score=9, p=0.053; Figure 4f). There was a significant correlation between locomotor scores and the extent to which IntA produced a decrease in α (R2=0.2749, p=0.0147; Fig. 4g), such that HR rats exhibited significantly greater reductions in α (increased motivation) compared to LR rats (t12=2.355; p=0.0364; Fig. 4h). IntA had no effect on Q0 values (main effect ‘time’: F1,18=1.85, p=0.1908; main effect ‘phenotype’: F2,18=1.41, p=0.2695; ‘time’ x ‘phenotype’ interaction: F2,18=0.579, p=0.5708; Fig. 4i), nor was there a significant difference in post-Q0 values between HR and LR rats (t12=0.1167, p=0.9090). Similarly, there was no relationship between locomotor-reactivity to novelty and changes in Q0 as a result of IntA (p’s>0.05; Fig. 4k,l)
4.0. Discussion
We report two key findings with respect to the sensation seeking model as a predictor of addiction vulnerability. First, the sensation-seeking trait, as determined by locomotor reactivity to a novel environment, was predictive of rats’ sensitivity to cocaine, but not their motivation for cocaine. HR rats exhibited higher locomotor responses to experimenter-administered cocaine and had a higher preferred level of cocaine intake on a behavioral economics (BE) self-administration procedure, but they did not differ in their willingness to work to defend their preferred level of cocaine intake on the BE task (demand elasticity), a key index of drug motivation and predictor of other addiction-related indices. Second, however, HR rats were more susceptible to the addiction-enhancing effects of IntA compared to LR rats, such that they exhibited greater escalation of cocaine intake and proportionally greater reductions in demand elasticity (increases in cocaine motivation). Thus, our data indicate that addiction susceptibility in HR rats is only revealed by certain drug access conditions, pointing to a ‘trait’ x ‘state’ interaction in the development of addiction-related behaviors.
It is established that locomotor reactivity to a novel environment predicts rats’ behavioral response to experimenter-administered cocaine (Hooks et al., 1991a, b). Here, we confirm that compared to LR rats, HR rats exhibit significantly greater locomotor activity following a 10mg/kg cocaine challenge. These differences have been linked with individual variability in the acquisition of psychostimulant self-administration and consumption (Hooks et al., 1991a, b; Klebaur et al., 2001; Marinelli and White, 2000; Piazza et al., 1989), which is consistent with our observation of a positive correlation between locomotor reactivity and the mean number of cocaine infusions earned across the first 5d of low-effort, FR1 cocaine self-administration training. It is worth nothing however, that in contrast to what has been reported previously (Marinelli and White, 2000), HR and LR rats did not differ in terms of daily cocaine intake across the first 5d of training; the reasons for this are unclear, but may relate to the fact that approximately half of the rats had prior cocaine exposure (in the cocaine prime test) prior to commencing self-administration training (Piazza et al., 1989). Low-effort (FR1) self-administration behavior is a poor predictor of a rat’s ‘addiction’ propensity (Quinn et al., 2018), and thus we next tested rats on a BE schedule that parses an animal’s preferred brain cocaine levels (Q0) from its motivation to maintain that level of intake (α; demand elasticity). Consistent with the relationship observed for FR1 intake, we observed a significant positive correlation between sensation seeking and Q0, such that Q0 values were significantly higher in HR rats. In rats, Q0 may be considered an index of the animal’s ‘hedonic set point’ for cocaine, or their preferred brain-cocaine concentrations (Bentzley et al., 2013). Similarly, when applied to clinical populations, demand analyses indicate that Q0 (also known as demand intensity) is a strong predictor of ‘real world’ drug use and consumption (Bruner and Johnson, 2014; MacKillop and Murphy, 2007). However, the relationship between drug intake and ‘addiction’ propensity per se is less clear. For example, rats classified as ‘addiction-prone’ based on the “three-criteria” cocaine addiction model do not differ from ‘addiction-resilient’ rats in terms of overall drug intake (Belin and Deroche-Gamonet, 2012; Deroche-Gamonet et al., 2004; Quinn et al., 2018). In paradigms where rats self-administer cocaine on a continuous access schedule for 6–12h/d, changes in motivation for drug are often modest and transient (James et al., 2019b; Kippin et al., 2006; Knackstedt and Kalivas, 2007), despite a persistent increase in preferred brain cocaine levels (Q0) (James et al., 2019b). In contrast, several preclinical studies now indicate that an animal’s willingness to defend their preferred brain cocaine levels, as reflected by demand elasticity (α), is more closely related to their propensity to exhibit several addiction-related behaviors (Bentzley et al., 2014; James et al., 2019a). Similarly, clinical studies indicate that demand elasticity, but not demand intensity (Q0), is predictive of treatment outcomes and likelihood of polydrug abuse (MacKillop and Murphy, 2007; Morris et al., 2018). It is interesting, therefore, that in the present experiment individual differences in sensation seeking were unrelated to variability in baseline demand elasticity (α). Our findings align with several recent studies indicating that the HR/LR model is not a strong predictor of ‘trait’ addiction-like behaviors across multiple drugs of abuse (Augier et al., 2018; Swain et al., 2018) and addiction correlates (e.g. incentive salience attribution) (Hughson et al., 2019). Thus, the strength of the HR model in predicting ‘trait’ drug behavior might not lie in its ability to predict addiction propensity per se, but rather preferred drug consumption levels. It is worth noting however, that although statistically significant, the relationship between locomotor reactivity and FR1 intake and Q0 was weak (~R2=0.03 for both indices) and baseline Q0 differences were not observed between HR and LR rats in Experiment 2. Despite this, our data are consistent with many previous reports indicating that novelty seeking is a reliable predictor of low-effort drug intake (Hooks et al., 1991a, b; Klebaur et al., 2001; Marinelli and White, 2000; Piazza et al., 1989).
It is well-recognized that drug exposure itself is a predisposing factor for addiction (Ahmed, 2012). Although there has been a longstanding focus in preclinical studies on the amount of drug consumed (Ahmed and Koob, 1998), recent evidence indicates that the pattern of drug intake might play a more important role in determining the development of an addiction phenotype (Allain et al., 2015). Here, we show that locomotor reactivity to a novel environment was predictive of two key behaviors associated with the IntA schedule of cocaine self-administration. First, HR animals exhibited higher overall intake across the 14d of IntA to cocaine – a finding consistent with higher baseline Q0 values in these rats in Experiment 1 – and also exhibited a significantly greater escalation of intake across the IntA period, compared to LR rats. Second, although a significant decrease in α values following IntA was observed across all rats, proportionally this change was greatest in HR rats. It is unclear if these two observations are causally linked, although it seems likely that the greater escalation of intake in HR rats reflects a motivational shift rather than the development of tolerance (increased preferred brain cocaine levels), as HR and LR rats did not differ in terms of post-IntA Q0 values. It is also possible that increased drug-cue pairings in HR rats during the IntA phase (resulting from a higher number of infusions) might have subsequently affected motivated responding on the BE task, which is highly cue-dependent (Bentzley and Aston-Jones, 2015). One limitation of our study is that we did not test the expression of other addiction-relevant behaviors in HRs vs LRs. Thus, it is unclear whether the sensation-seeking trait is limited to predicting changes in demand following IntA, or if it also has utility for identifying rats at risk of developing other endophenotypes that reflect key diagnostic criteria for substance use disorder. Interesting in this regard is a recent study which reported that rats that exhibit the greatest escalation of cocaine intake over the course of IntA exhibit higher binge-like cocaine intake, more robust locomotor sensitivity to cocaine, and higher levels of cued reinstatement (Garcia et al., 2020). Moreover, we have previously reported that drug seeking during initial abstinence, reinstatement propensity and withdrawal-associated emotional behavior are strongly correlated with post-IntA α values (James et al., 2019a). Thus, it might be anticipated that HR animals would also exhibit bigger shifts in the expression of several addiction endophenotypes compared to LR rats, however this will need to be tested directly by future studies. Indeed, these studies will be critical for evaluating the HR/LR model as an approach for predicting animals that are at-risk of transitioning to a multiphenotypic addiction-like state after IntA. Although previous studies have reported that HR rats exhibit increased locomotor activity only in response to novel environments (and not familiar ones; (Dellu et al., 1996)), future studies should also rule out the possibility that higher spontaneous activity levels contributes to the effects observed here in HR rats; our analyses of inactive lever responding did not indicate significant differences between groups, however homecage monitoring would provide a more robust estimate of basal activity. These studies would thus determine the extent to which the higher activity observed here can be attributed to exposure to a novel environment or a cocaine priming injection versus higher overall basal activity, as well as any relationship between basal activity and addiction propensity following IntA.
Our findings are interesting in light of a previous study that failed to show a link between the HR/LR trait and the expression of compulsive drug taking/seeking in rats following prolonged cocaine self-administration (long access) (Belin et al., 2011), and may point to differing contributions of the HR phenotype in the expression of addiction behaviors following continuous vs. intermittent cocaine self-administration. Indeed, Flagel et al. (2016) reported that bHR rats exhibit stronger addiction-like behaviors compared to LR rats following prolonged daily access to cocaine in daily sessions that were divided into cycling periods of drug availability (40min) and non-drug availability (20min); although different to the IntA schedule used here, this schedule of access is likely to have produced spiking patterns of brain-cocaine levels, and thus to some degree may reflect a trait (bHR) x state (IntA) interaction (although note that Deroche-Gamonet et al. (2004) used a similar schedule and failed to find a relationship between the HR trait and several addiction-like behaviors). Given recent interest in the IntA model described here for its ability to induce a more persistent addiction-like endophenotypes in rats compared to conventional extended access models (Kawa et al., 2019a; James et al., 2019b), the HR/LR model might serve as a useful and rapid behavioral screen to identify those rats most likely to exhibit state-dependent (IntA) potentiation of addiction behaviors, allowing for examination of neural systems that contribute to addiction vulnerability without the confound of cocaine exposure.
Indeed, the present findings raise important questions regarding the neurobiological link between individual differences in sensation seeking and the related addiction behaviors identified here. To date, the large majority of studies examining differences in brain reward function in HR vs LR rats have focused on the mesolimbic dopamine system (Marinelli and White, 2000; Norbury and Husain, 2015). Microdialysis studies indicate that under basal conditions, HR rats have lower DA uptake and higher extracellular DA levels in nucleus accumbens (NAc) compared to LR rats (Chefer et al., 2003). Following a priming injection of cocaine, all rats exhibit a significant increase in extracellular DA levels in NAc, however the magnitude of this change is greatest in HR rats (Chefer et al., 2003; Hooks et al., 1991b). Interestingly, increased accumbal DA release and uptake in NAc is observed following IntA to cocaine (Calipari et al., 2013; Kawa et al., 2019b), and pharmacological blockade of DA signaling in NAc reduces cocaine seeking following IntA (Singer et al., 2018). Thus, higher cocaine-evoked DA release in HR rats might promote higher initial intake and increased preferred brain-cocaine levels (Q0), as observed here. Curiously however, increased DA signaling has been shown to increase motivation on BE, progressive ratio and several other effort-related tasks (Mahler et al., 2019; Salamone et al., 2018), and thus higher DA signaling in HR rats might be expected to also result in higher baseline motivation for cocaine – which we did not observe. Alternatively, other neural systems might be involved in driving the enhanced motivational profile in HR rats. The orexin system is a promising candidate, as we have recently shown that orexin neurons are increased in number and reactivity following IntA to both cocaine and opioids, and normalization of orexin system signaling selectively reduces drug motivation (but not intake at low cost) in IntA rats (Fragale et al., 2020; James et al., 2019a; James et al., 2019b). Notably, rats with higher motivation (lower α values) for cocaine following IntA or a history of polysubstance use are most susceptible to the anti-addiction properties of an orexin-1 receptor antagonist (James et al., 2019a; James et al., 2021), indicating that the HR trait might have utility in identifying individuals most likely to benefit from these forms of therapeutics, which are currently being investigated for clinical use (James and Aston-Jones, 2020; James et al., 2020; Suchting et al., 2019). Attention should also be given to exploring whether HR and LR rats differ in terms of cocaine reactivity in corticolimbic circuits which was recently linked with the expression of the IntA phenotype (James, 2020; Minogianis and Samaha, 2020).
In conclusion, behavioral economic analyses of cocaine demand revealed that the HR/LR sensation-seeking trait is predictive of rats’ preferred brain cocaine levels but not their baseline willingness to expend effort to defend those levels, which is more closely related to the expression of addiction-related behaviors. In contrast, the sensation seeking trait was highly predictive of individual differences in propensity to exhibit state-dependent addiction behaviors, such that HR rats exhibit lower post-IntA demand elasticity (higher motivation) for cocaine, a phenotype closely associated with several addiction indices. Together these findings indicate that the utility of the HR/LR model of sensation seeking may be to identify individuals most at risk of developing addiction after certain patterns of drug consumption.
Acknowledgements:
We would like to thank Ms. Nikki Koll, Ms. Ami Ghadia, Ms. Jasmine Lin and Mr. Allen Cheng for their assistance with behavioral testing, as well as Ms. Nupur Jain for her general lab assistance. We thank Dr. Jennifer Fragale for her helpful discussions about these data. We also thank the NIDA Drug Supply Program for their generous supply of cocaine.
Funding sources:
GAJ was supported by a U.S. Public Health Service award from NIDA to GAJ (R01 DA006214). MHJ was supported by a National Health and Medical Research Council of Australia C.J. Martin Fellowship (No. 1072706) and a National Institute on Drug Abuse (NIDA) K99/R00 Award (K99 DA045765). The authors declare that the research was conducted without any commercial or financial relationships that could be considered a potential conflict of interest.
Footnotes
Declarations of interest: None.
References
- Ahmed SH, 2012. The science of making drug-addicted animals. Neuroscience 211, 107–125. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF, 1998. Transition from Moderate to Excessive Drug Intake: Change in Hedonic Set Point. Science 282, 298–300. [DOI] [PubMed] [Google Scholar]
- Algallal H, Allain F, Ndiaye NA, Samaha AN, 2019. Sex differences in cocaine self-administration behaviour under long access versus intermittent access conditions. Addict Biol, e12809. [DOI] [PubMed] [Google Scholar]
- Allain F, Minogianis E-A, Roberts DCS, Samaha A-N, 2015. How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neuroscience & Biobehavioral Reviews 56, 166–179. [DOI] [PubMed] [Google Scholar]
- Allain F, Samaha AN, 2019. Revisiting long-access versus short-access cocaine self-administration in rats: intermittent intake promotes addiction symptoms independent of session length. Addict Biol 24, 641–651. [DOI] [PubMed] [Google Scholar]
- Augier E, Barbier E, Dulman RS, Licheri V, Augier G, Domi E, Barchiesi R, Farris S, Nätt D, Mayfield RD, Adermark L, Heilig M, 2018. A molecular mechanism for choosing alcohol over an alternative reward. Science 360, 1321–1326. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V, 2011. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36, 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V, 2012. Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harbor perspectives in medicine 2, a011940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G, 2015. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci 41, 1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G, 2013. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology (Berl) 226, 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G, 2014. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A 111, 11822–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner NR, Johnson MW, 2014. Demand curves for hypothetical cocaine in cocaine-dependent individuals. Psychopharmacology (Berl) 231, 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR, 2013. Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 38, 2385–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Zakharova I, Shippenberg TS, 2003. Enhanced responsiveness to novelty and cocaine is associated with decreased basal dopamine uptake and release in the nucleus accumbens: quantitative microdialysis in rats under transient conditions. J Neurosci 23, 3076–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV, 2004. Evidence for addiction-like behavior in the rat. Science 305, 1014–1017. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Chaudhury S, Waselus M, Kelly R, Sewani S, Clinton SM, Thompson RC, Watson SJ Jr., Akil H, 2016. Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. Proc Natl Acad Sci U S A 113, E2861–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, James MH, Aston-Jones G, 2020. Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. bioRxiv, 2020.2004.2023.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AF, Webb IG, Yager LM, Seo MB, Ferguson SM, 2020. Intermittent but not continuous access to cocaine produces individual variability in addiction susceptibility in rats. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB Jr., 1991a. Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav 38, 467–470. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB Jr., 1991b. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse 9, 121–128. [DOI] [PubMed] [Google Scholar]
- Hughson AR, Horvath AP, Holl K, Palmer AA, Solberg Woods LC, Robinson TE, Flagel SB, 2019. Incentive salience attribution, “sensation-seeking” and “novelty-seeking” are independent traits in a large sample of male and female heterogeneous stock rats. Sci Rep 9, 2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A, 2008. Economic demand and essential value. Psychol Rev 115, 186–198. [DOI] [PubMed] [Google Scholar]
- James MH, 2020. Mimicking Human Drug Consumption Patterns in Rat Engages Corticostriatal Circuitry. Neuroscience 442, 311–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Aston-Jones G, 2020. Introduction to the Special Issue: “Making orexin-based therapies for addiction a reality: What are the steps from here?”. Brain Res 1731, 146665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Bowrey HE, Stopper CM, Aston-Jones G, 2019a. Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci 50, 2602–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Fragale JE, Aurora RN, Cooperman NA, Langleben DD, Aston-Jones G, 2020. Repurposing the dual orexin receptor antagonist suvorexant for the treatment of opioid use disorder: why sleep on this any longer? Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Fragale JE, O’Connor SL, Zimmer BA, Aston-Jones G, 2021. The orexin (hypocretin) neuropeptide system is a target for novel therapeutics to treat cocaine use disorder with alcohol coabuse. Neuropharmacology 183, 108359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, McGlinchey EM, Vattikonda A, Mahler SV, Aston-Jones G, 2018. Cued Reinstatement of Cocaine but Not Sucrose Seeking Is Dependent on Dopamine Signaling in Prelimbic Cortex and Is Associated with Recruitment of Prelimbic Neurons That Project to Contralateral Nucleus Accumbens Core. International Journal of Neuropsychopharmacology 21, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G, 2019b. Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry 85, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Allain F, Robinson TE, Samaha AN, 2019a. The transition to cocaine addiction: the importance of pharmacokinetics for preclinical models. Psychopharmacology (Berl) 236, 1145–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, Robinson TE, 2016. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology (Berl) 233, 3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Valenta AC, Kennedy RT, Robinson TE, 2019b. Incentive and dopamine sensitization produced by intermittent but not long access cocaine self-administration. Eur J Neurosci 50, 2663–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, See RE, 2006. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 187, 60–67. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT, 2001. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behav Pharmacol 12, 267–275. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Kalivas PW, 2007. Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther 322, 1103–1109. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG, 2007. A behavioral economic measure of demand for alcohol predicts brief intervention outcomes. Drug Alcohol Depend 89, 227–233. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Quintanilla J, Cope ZA, Lin EC, Riedy MD, Scofield MD, Messinger J, Ruiz CM, Riegel AC, España RA, Aston-Jones G, 2019. Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse. J Neurosci 39, 503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney III JJ, Thompson-Lake DG, Cooper K, Verrico CD, Newton TF, De La Garza R, 2015. A comparison of impulsivity, depressive symptoms, lifetime stress and sensation seeking in healthy controls versus participants with cocaine or methamphetamine use disorders. J Psychopharmacol 29, 50–56. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ, 2001. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 157, 31–39. [DOI] [PubMed] [Google Scholar]
- Marinelli M, White FJ, 2000. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci 20, 8876–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G, 2016. Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. The Journal of Neuroscience 36, 8700–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles DR, van den Bree MB, Gupman AE, Newlin DB, Glantz MD, Pickens RW, 2001. A twin study on sensation seeking, risk taking behavior and marijuana use. Drug Alcohol Depend 62, 57–68. [DOI] [PubMed] [Google Scholar]
- Minogianis EA, Samaha AN, 2020. Taking Rapid and Intermittent Cocaine Infusions Enhances Both Incentive Motivation for the Drug and Cocaine-induced Gene Regulation in Corticostriatal Regions. Neuroscience 442, 314–328. [DOI] [PubMed] [Google Scholar]
- Morris V, Patel H, Vedelago L, Reed DD, Metrik J, Aston E, MacKillop J, Amlung M, 2018. Elevated Behavioral Economic Demand for Alcohol in Co-Users of Alcohol and Cannabis. J Stud Alcohol Drugs 79, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury A, Husain M, 2015. Sensation-seeking: Dopaminergic modulation and risk for psychopathology. Behav Brain Res 288, 79–93. [DOI] [PubMed] [Google Scholar]
- Piazza P, Deminiere J, Le Moal M, Simon H, 1989. Factors that predict individual vulnerability to amphetamine self-administration. Science 245, 1511–1513. [DOI] [PubMed] [Google Scholar]
- Quinn RK, James MH, Hawkins GE, Brown AL, Heathcote A, Smith DW, Cairns MJ, Dayas CV, 2018. Temporally specific miRNA expression patterns in the dorsal and ventral striatum of addiction-prone rats. Addict Biol 23, 631–642. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Yang J-H, Rotolo R, Presby R, 2018. Dopamine, Effort-Based Choice, and Behavioral Economics: Basic and Translational Research. Frontiers in Behavioral Neuroscience 12, 52–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BF, Fadanelli M, Kawa AB, Robinson TE, 2018. Are Cocaine-Seeking “Habits” Necessary for the Development of Addiction-Like Behavior in Rats? J Neurosci 38, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting R, Yoon JH, Miguel GGS, Green CE, Weaver MF, Vincent JN, Fries GR, Schmitz JM, Lane SD, 2019. Preliminary examination of the orexin system on relapse-related factors in cocaine use disorder. Brain Res, 146359. [DOI] [PubMed] [Google Scholar]
- Swain Y, Muelken P, LeSage MG, Gewirtz JC, Harris AC, 2018. Locomotor activity does not predict individual differences in morphine self-administration in rats. Pharmacol Biochem Behav 166, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, Roberts DCS, 2012. The Motivation to Self-Administer is Increased After a History of Spiking Brain Levels of Cocaine. Neuropsychopharmacology 37, 1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Neeb M, 1979. Sensation seeking and psychopathology. Psychiatry research 1, 255–264. [DOI] [PubMed] [Google Scholar]


