Abstract
Dopamine (DA) neurons in the ventral tegmental area (VTA) modulate physical activity and feeding behaviors that are disrupted in obesity. Yet, the heterogeneity of VTA DA neurons has hindered determination of which ones might be leveraged to support weight loss. We hypothesized that increased activity in the subset of VTA DA neurons expressing neurotensin receptor-1 (NtsR1) might promote weight loss behaviors. To test this, we used Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) to activate VTA NtsR1 neurons in normal weight and diet-induced obese mice. Acute activation of VTA NtsR1 neurons (24hr) significantly decreased body weight in normal weight and obese mice by reducing food intake and increasing physical activity. Moreover, daily activation of VTA NtsR1 neurons in obese mice sustained weight loss over 7 days. Activating VTA NtsR1 neurons also suppressed how much mice worked to obtain sucrose rewards, even when there was high motivation to consume. However, VTA NtsR1 neural activation was not reinforcing, nor did it invoke liabilities associated with whole-body NtsR1 agonism such as anxiety, vasodepressor response or hypothermia. Activating VTA NtsR1 neurons therefore promotes dual behaviors that support weight loss without causing adverse effects, and is worth further exploration for managing obesity.
Keywords: Ventral Tegmental Area, Dopamine, Neurotensin Receptor-1, DREADDs, Obesity, physical activity, feeding, body weight
1. Introduction
More than one-third of U.S. adults are obese and at increased risk to develop type-2 diabetes (Caspard et al., 2017; Ogden et al., 2013). Complex interactions between genetic factors and obesogenic environment can increase susceptibility for weight gain (Haslam and James, 2005; Thaker, 2017). However, the most recognized cause for obesity is overconsumption of calorie-dense foods combined with a sedentary lifestyle and reduced energy expenditure. Indeed, since ~1970, the energy cost of work has decreased in the United States as the quantity and energy density of foods have increased, and so has the incidence of overweight and obesity (Conway and Rene, 2004; Kruger et al., 2001; Rolls, 2003). Bariatric surgery is increasingly being used to treat severe obesity but there is a debate about its long-term benefit, as the surgery can induce a myriad of complications (Courcoulas et al., 2013; Scheimann et al., 2008). Current non-surgical treatments for obesity, including lifestyle modification and pharmacotherapy, provide limited long-term weight loss (Khera et al., 2016). Individuals who lose weight experience compensatory increases in appetite and diminished metabolic rate, and as a result most regain weight (Sumithran et al., 2011; Wing and Hill, 2001). Understanding how the brain coordinates feeding, physical activity and energy expenditure may be helpful to understand the basic biology of energy balance, and to identify mechanisms to support sustained weight loss.
Dopamine (DA) neurons in the ventral tegmental area (VTA) are essential modulators of feeding and locomotor activity (Narayanan et al., 2010; Zhou and Palmiter, 1995) and might be useful targets to promote behaviors to favor weight loss. However, VTA DA neurons are highly heterogeneous, differing in connectivity, gene expression and how they modulate behavior, ranging from positive and negative reinforcement, decision making and stimulus salience or aversion (Heymann et al., 2020; Lammel et al., 2014, 2011; Perez-Bonilla et al., 2020; Stuber et al., 2015). This heterogeneity has hindered determination of which specific DA neurons might be leveraged to support weight loss. Subsets of VTA DA neurons have begun to be distinguished via their expression of neuropeptide receptors (Heymann et al., 2020; Perez-Bonilla et al., 2020; Woodworth et al., 2018), which hints at their potential contributions to energy balance, given that neuropeptides may be orexigenic or anorectic. One subset of VTA DA neurons co-expresses the Gαq protein-coupled neurotensin receptor-1 (NtsR1), which binds the neuropeptide Neurotensin (Nts) (Tanaka et al., 1990). Nts exerts diverse, site-dependent regulation of pain, sleep, locomotor activity and ingestive behavior, but within the VTA Nts suppresses feeding and promotes locomotor activity - dual behaviors that could support weight loss (Cador et al., 1986; Elliott and Nemeroff, 1986; Schroeder and Leinninger, 2018; Torruella-Suárez and McElligott, 2020). The cellular mediators of Nts effects in the VTA remain unclear, however, because this region contains NtsR1 and DA co-expressing neurons as well as astrocytes expressing neurotensin receptor-2 (NtsR2) (Woodworth et al., 2018). Since previous pharmacological reagents were not truly selective for NtsR1 vs. NtsR2, they have not been sufficient to disentangle their contributions to behavior (Botto et al., 1997; Vita et al., 1998). Likewise, mice constitutively lacking NtsR1 or NtsR2 likely suffer from developmental compensation, and may not reflect the physiology of the normal, adult NtsR1 and NtsR2 systems (Woodworth et al., 2018). Thus, although Nts exerts beneficial weight loss effects via the VTA, the cells and receptors mediating these behaviors are still in question.
There is rationale to home in on NtsR1 in Nts-mediated regulation of body weight because mice genetically lacking NtsR1 overconsume palatable food that promotes weight gain (Kim et al., 2008; Kim and Mizuno, 2010; Opland et al., 2013; Woodworth et al., 2017b). Nts activates VTA DA neurons and promotes DA release (Huang et al., 2020; Leonetti et al., 2004; Sotty et al., 1998). Intriguingly, at least in mice, the subset of all VTA DA neurons that co-express NtsR1 preferentially projects to the nucleus accumbens (NAc), where DA release can modulate ingestive behavior (Woodworth et al., 2018). These data suggest the possibility that activating the VTA NtsR1 subset of DA neurons mediates specific functions amongst all DA neurons, and possibly the Nts-mediated effects in the VTA that evoke weight loss. If true, augmenting NtsR1 signaling could have therapeutic potential to reduce body weight. However, initial interest in clinically modulating NtsR1 waned because systemic or brain-wide treatment with Nts or first-generation NtsR1 agonists suppress feeding but also causes dangerous vasodepression and hypothermia (Carraway and Leeman, 1973; Ciriello and Zhang, 1997; Fantegrossi et al., 2005; Feifel et al., 2010). Recent work shows that activating site-specific Nts or NtsR1-expressing neurons mediates specific physiology without invoking these adverse effects (Kurt et al., 2019; McHenry et al., 2017; Torruella-Suárez et al., 2020; Woodworth et al., 2017b). Thus, here we examined whether selective activation of VTA NtsR1 neurons could promote weight loss behaviors without causing adverse thermoregulatory or cardiorespiratory effects. To test this hypothesis, we expressed Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) in VTA NtsR1 neurons of normal weight and diet-induced obese NtsR1Cre mice, which permitted in vivo activation of VTA NtsR1 neurons. Our data suggest that activating VTA NtsR1 neurons recapitulates Nts-mediated behaviors useful for weight loss without undesirable side effects.
2. Research Design and Methods
2.1. Mice
All mice used herein were bred and housed under a 12hr light/12hr dark cycle, and were cared for by Campus Animal Resources (CAR). We studied male and female heterozygous NtsR1cre mice (NtsR1cre/+) on the C57/Bl6J background, Jackson Stock #033365) and littermate controls (NtsR1+/+, referred to as wild type, Wt). At 4 weeks of age the study mice were individually housed with ad libitum access to water and either chow (Harlan Teklad #7913) or 45% high-fat diet (HFD, Research Diets D12451) for the duration of experiments, unless otherwise specified. Multiple cohorts of NtsR1cre and Wt mice were generated and the mice were tested via multiple tests, as described below. Cohorts were staggered to control for any seasonal effects. Since we did not observe any metabolic or behavioral differences between males and females in our tests, both sexes were pooled. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Michigan State University in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care and National Institutes of Health.
2.2. Surgery
NtsR1Cre mice and Wt littermates (8–15 weeks) were anesthetized (isofluorane/oxygen mixture, 2–4%) and given analgesic (Meloxicam, 5mg/kg), prior to bilateral stereotaxic injection of AAV2-hSyn-DIO-hM3D(Gq)-mCherry (UNC Vector core/Addgene) into the VTA (100 nL per side, A/P: −3.2, M/L: ±0.48, D/V: −4.65) per the mouse brain atlas of Paxinos and Franklin (Franklin and Paxinos, 2008). Mice were allowed to recover for at least 2 weeks prior to metabolic and behavioral testing. Brains were examined via posthoc immunostaining for mCherry and TH (see immunostaining method below) to verify targeting and DREADDq-mCherry expression in the VTA. NtsR1Cre mice were only included in the final data if mCherry-expressing soma were confined to the VTA, and any mice with mCherry soma beyond the VTA were excluded. Mice that were bilaterally targeted in the VTA with DREADDq responded to CNO treatment with robust (>500) wheel rotations, revealing this as a reliable indicator of VTA NtsR1 targeting. Mice with robust unilateral targeting exhibited similar CNO-induced wheel rotations and were also included in the final study data (10% of cases).
2.3. Treatment
Vehicle (VEH, PBS) or the DREADD-ligand clozapine-N-oxide (CNO, 0.3 mg/kg) were administered via i.p. injection. CNO (C0832, Sigma) was dissolved in 10% β-cyclodextrin (C0926, Sigma) in sterile PBS to make 20X CNO stock aliquots (1.2mg/mL), which were diluted with sterile PBS to make the 1X CNO working stock (60μg/mL). We then administered 5μL of 1X CNO per g of body weight. Unless specified otherwise, mice were treated twice a day, 1–2hr after onset of light cycle and 1–2hr before dark cycle, via a crossover design. Thus, all mice received both VEH and CNO with at least 3 days between treatment switch.
2.4. Metabolic Analysis
Mice were analyzed in PhenoMaster metabolic cages (TSE Systems) 2–3 weeks after AAV2-hSyn-DIO-hM3D(Gq)-mCherry injection. Metabolic cages continuously monitored food and water intake, locomotor activity, wheel running, and metabolic parameters (VO2, respiratory exchange ratio [RER], and energy expenditure). Mice acclimated in cages for 48hr prior to receiving daily treatments during the light and dark cycles. Mice received sham injections (day 3), followed by VEH (day 4), CNO (day 5) and then were left in the metabolic cages for a 24hr washout period. Weight was measured just prior to the light cycle injection each day and at the end of the washout period. Ambient temperature was maintained at 20–23°C and airflow rate was adjusted to maintain an oxygen differential around 0.3% at resting conditions.
2.5. Operant Testing
Training Chow-fed mice: Mice were trained to nose-poke for unflavored 20 mg sucrose pellets (TestDiet 1811555) in operant-responding chambers (Med Associates) as previously described (Sharma et al., 2012; Woodworth et al., 2017a). Chow experiments: mice were food restricted to 85% of their body weight during FR1 training sessions, which occurred over 10–16 consecutive days. Each FR1 training session was terminated after 1hr or when the mouse had earned 50 rewards. Once mice achieved 75% response accuracy with ≥20 rewards earned on 3 consecutive days of FR1 training, they were switched to ad libitum food and trained on an FR5 schedule for 3 consecutive days. Mice that failed to reach FR1 criteria after 16 days were removed from the study. Training HFD-fed mice: We and others have found that 85% HFD caloric restriction does not motivate diet-induced obese mice to acquire FR1 criteria, so we modified the conditions to encourage these mice to learn the operant task. After their first FR1 session the HFD mice were weighed and then switched to a restricted chow diet, so as to maintain them at 80% of their starting body weight throughout FR1 training. HFD mice that did not achieve 75% response accuracy with ≥20 rewards earned on 3 consecutive days were removed from the study. FR1 testing was conducted for a maximum of 14–16 consecutive days, at which point any mice failing to meet the above criteria were removed from the study. Mice achieving FR1 criteria proceeded were then trained on an FR5 schedule for 3 consecutive days. Progressive Ratio (PR) Testing for All Mice: After FR5 testing mice were subject to a progressive ratio (PR) schedule where PR=[5e(R*0.2)]-5 with R=number of food rewards earned+1. The PR breakpoint was recorded as the highest ratio completed for each 1 hr test session. Mice were tested until they achieved stable PR, defined as <10% variation in rewards earned over 3 consecutive sessions. Next, mice were treated with VEH or CNO (0.3 mg/kg, i.p.) 30mins before PR testing. To determine if hunger altered responding, mice were fasted overnight, then were treated with VEH or CNO and tested on the PR schedule. Lastly, to determine if satiety modified responding, mice received 3 g of sucrose pellets in their home cage overnight then were PR-tested the next morning.
2.6. Sucrose Preference
Mice were studied in home cages with ad libitum access to two bottles containing liquid. For the first 48 hr both bottles contained water, so as to acclimatize mice to the two-bottle arrangement. The position of the bottles was switched every 24hr to control for place preference effects. Place preference during pretesting is cause for exclusion, but did not occur in our study so no mice were excluded. Next, one of the bottles was replaced with 1% sucrose (Sigma) so that mice had a choice of water or sucrose. All mice received both VEH and CNO injections (2X day) in a crossover design. Mice were given at least 24hr before repeating the experiment with the alternate treatment (e.g., 2 bottles of water for 48hr followed by water vs 1% sucrose for 48hr while being treated). Body and food were weighted every 12hr during the experiment.
2.7. Conditioned Place Preference (CPP)
CPP (San Diego Instruments) boxes had two distinguishable chambers with different visual and tactile cues (gray wall and smooth floor or striped wall and rough floor) separated by a small center chamber (Woodworth et al., 2017b). Mice had a 15-minute pretest on day 1, during which they were allowed to roam freely between chambers. After pretest data were collected, we assigned which chamber was paired with either VEH or CNO injection with an unbiased, counterbalanced strategy so that approximately half the mice received CNO pairing in the preferred side and half received CNO in the non-preferred side. Mice then underwent conditioning on days 2–5, where each morning they received an injection of VEH and were placed in the VEH-paired side for 30 minutes. After the morning session, mice were returned to their housing environment and 4 hours later they were injected with CNO and were placed in the CNO-paired side for 30 minutes. VEH and CNO injections occurred 5 minutes before mouse placement in the corresponding chamber. Posttest was conducted on day 6, where mice were untreated and were allowed to roam freely amongst both chambers for 15 minutes. The time and locomotor activity in each side of the box was detected as laser beam breaks using manufacturer’s software.
2.8. Chronic Activation
In home cages, HFD-fed mice received twice-daily treatments with either VEH or CNO for 7 days. Body weight and food intake were measured during the light cycle, and on days 1, 5 and 7 mice were placed in open field boxes 2–4 hours after receiving VEH or CNO. Locomotor activity was measured for 30 min using a digital CCD camera and video-tracking software (Clever Sys) (Eagle et al., 2015). At least 7 days passed before repeating the experiment with the opposite treatment.
2.9. Nestlet Shredding
Mice were treated with VEH or CNO in their home cages during the light cycle. After 30 minutes, bedding, food and water were removed, and a pre-weighed, new cotton nestlet was added to the cage. After 90 minutes, intact remnants of the nestlet were removed from the cage, air dried overnight and weighed the following day.
2.10. Marble Burying
Mice received VEH or CNO in home cages during the light cycle. 30 minutes later they were placed in the middle of a cage with 10 evenly dispersed marbles on top of the bedding. After 30 minutes mice were returned to their home cage. Photos taken before and after the test were used to determine the number and percentage of marbles buried (e.g., if 2/3 of the marble was covered).
2.12. Temperature
Core-body temperature was measured with a rodent rectal thermometer (BIO TK9882) immediately after VEH or CNO treatment, then every 30 minutes up to 120 minutes and for a final measure at 180 minutes. A minimum of 3 days of rest were given before repeating the experiment with opposite treatment.
2.13. Telemetry
Under isofluorane/oxygen mixture (2–4%), mice were implanted with subcutaneous PA-C10 radiotelemetry transmitters (DSI, Harvard Bioscience), attached to a catheter inserted into the abdominal aorta via the femoral artery (Diaz-Otero et al., 2016). Transmitters measured mean arterial pressure, activity, heart rate, and systolic and diastolic blood pressure every 10 minutes throughout the experiment. After 3 days of surgical recovery, mice received twice-daily sham injections (day 4) followed by VEH or CNO via crossover design (days 5 and 6).
2.14. Perfusion and Immunofluorescence
Mice that did not have telemeters implanted received a lethal i.p. dose of pentobarbital (Fatal Plus, Vortech) 90 minutes after VEH or CNO treatment, followed by transcardial perfusion with 0.2M PBS (pH 7.4) and then 10% neutral-buffered formalin (Fisher Scientific). Brains were removed, postfixed in 10% formalin overnight at 4°C and dehydrated with 30% sucrose in PBS for 2–3 days. Mice that did have a telemeter implanted also received a lethal i.p. dose of pentobarbital 90 minutes after VEH or CNO treatment, but their brain was removed and postfixed, as formalin perfusion would damage telemeters. Brains were then coronally sectioned (30μm) for immunofluorescence, as per (Woodworth et al., 2018). Sections were exposed to primary antibodies including rabbit-dsRed/mCherry (1:1000, Clontech, AB_10013483), chicken-GFP (1:2000, Abcam, ab13970), mouse anti-TH (1:1000, Millipore, AB_2201528) and goat-cFOS (1:500, Santa Cruz Biotechnology, sc-52-G), followed by species-specific secondary antibodies conjugated to Alexa-568 (1:200, LifeTech, AB_2534017) or Alexa-488 (1:200, Jackson ImmunoResearch, 703–545-155). Sections mounted onto slides were analyzed via an Olympus BX53 fluorescence microscope with FITC and Texas Red filters. Images were collected using Cell Sens software and a Qi-Click 12 Bit cooled camera and analyzed using Photoshop (Adobe). Masks applied in Photoshop to enhance brightness and/or contrast were applied uniformly to all samples.
2.15. Cell Counting
Two representative levels of the VTA (approximately Bregma −3.38 and −2.98) were chosen for counting, and a 20x image of the right and left VTA hemisphere was collected from each. Images were then coded with non-descriptive names and a blinded investigator used Photoshop to count the number of magenta and green labeled cells in each image. For mCherry and TH immunostaining, a cell was considered co-labeled if both labels overlapped in the whole soma. For mCherry and cFos immunostaining, a cell was counted as co-labeled if the nucleus contained cFos and was surrounded by mCherry in the soma. The number of co-localized and singly-labeled cells were summed from the two sections of each brain and used to calculate the percentage of labeled cells. Graphs depict the average percentage of cells ± SEM.
2.16. Statistics
Student’s t-tests and 2-way ANOVA were calculated using Prism 7 (GraphPad). Repeated measures 2-way ANOVA with Sidak post-test was used when each mouse received both VEH and CNO, and when data from the same mice were collected at different time points. Ordinary 2-way ANOVA with Tukey post-test was used if mice did not receive both treatments. A p-value of <0.05 was considered statistically significant. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3. Results
3.1. VTA NtsR1 Neurons are a Subset of all DA Neurons that Can be Activated with DREADDs
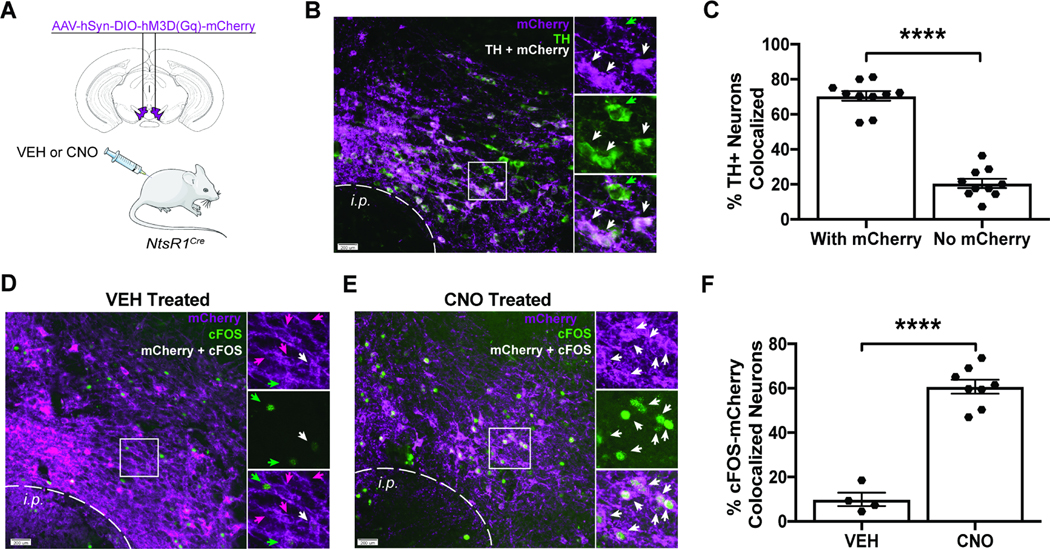
NtsR1cre mice were injected in the VTA with cre-dependent AAV2-hSyn-DIO-hM3D(Gq)-mCherry to express hM3Dq-mCherry (excitatory DREADD) in NtsR1 neurons (Figure 1A). After VEH or CNO treatment (90 min), brains were analyzed for mCherry (hM3Dq-mCherry expressing neurons, purple) and tyrosine hydroxylase (TH, a marker of DA neurons, green) via immunofluorescence (Figure 1B). ~70% of VTA TH neurons co-expressed NtsR1 (Figure 1A - white arrows and Figure 1C), consistent with previous reports (Woodworth et al., 2018, 2017a). Moreover, CNO significantly increased the proportion of mCherry neurons containing cFOS (a marker of depolarization) over VEH treatment (Figure 1D-F, p<0.0001). These data confirm that DREADDs can be used to activate VTA NtsR1 neurons.
Figure 1. VTA NtsR1 Neurons are a Subset of all DA Neurons that Can be Activated Using DREADDs.
A) NtsR1Cre mice were injected in the VTA with AAV2-hSyn-DIO-hM3D(Gq)-mCherry to express hM3Dq-mCherry (excitatory DREADD) in VTA NtsR1 neurons. B) Representative immunofluorescent-labeled images from n = 10 mice showing that tyrosine hydroxylase (TH, marker of DA neurons, green) and mCherry (NtsR1 cells that express hM3Dq-mCherry, purple) are co-expressed in a subset of VTA neurons (white). Panels on right are digital enlargements of boxed region, where white arrows indicate colocalized hM3DqmCherry:TH, and green arrows identify TH-only neurons. C) Percentage of TH neurons co-labeled with hM3Dq-mCherry. D-F) Mice were treated with D) VEH (n = 4) or E) CNO (n = 8) 90 minutes before brain collection, followed by immunofluorescent labeling for mCherry (purple) and cFOS (a marker of neuronal depolarization, green). Panels on right are digital enlargements of boxed region, in which magenta arrows indicate neurons that only express NtsR1-hM3Dq-mCherry, green arrows identify cFOS-only neurons and white arrows indicate co-localized mCherry:cFOS neurons. mCherry-expressing NtsR1 soma are apparent in VEH and CNO-treated brains, but F) CNO significantly increased the percentage of cFOS:mCherry colocalized neurons over VEH treatment. Scale bars = 200 μm. Graphs depict the average percentage of cells ± SEM. ****p < 0.0001 via Student’s t-test.
3.2. Activation of VTA NtsR1 Neurons Promotes Weight Loss Behaviors in Normal Weight Mice
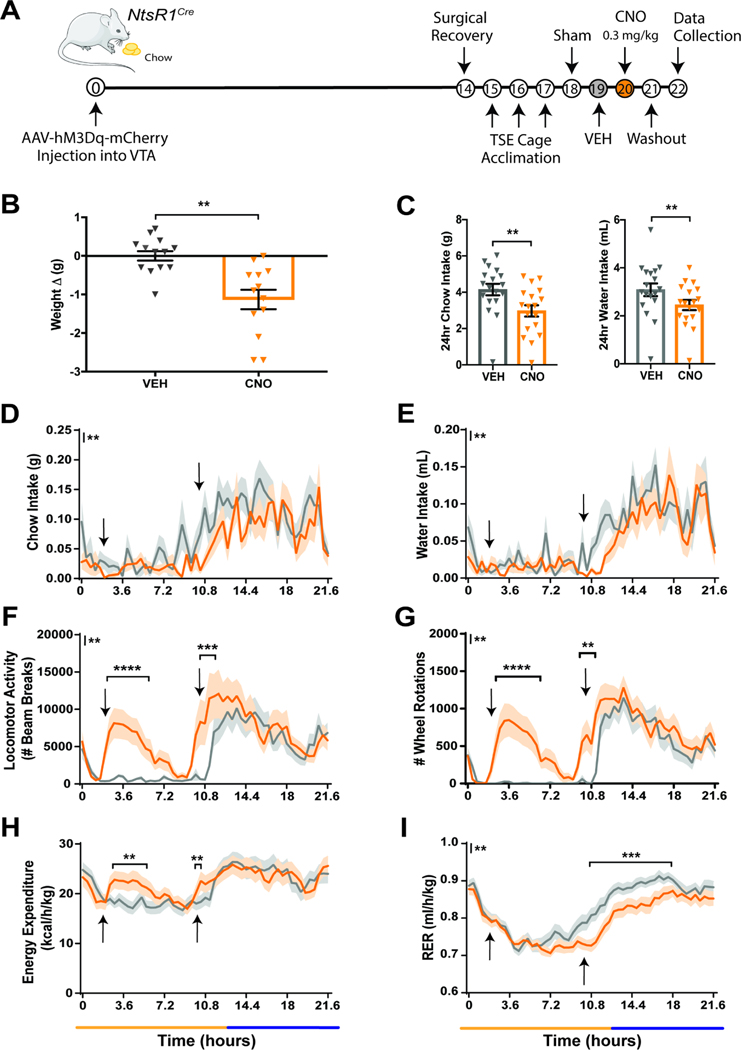
To determine how activating VTA NtsR1 neurons impacts energy balance, we treated normal weight, chow-fed NtsR1Cre mice expressing VTA NtsR1-hM3Dq-mCherry with VEH or CNO while in metabolic chambers (Figure 2A). CNO-mediated activation of VTA NtsR1 neurons decreased body weight in these mice over 24 hrs (Figure 2B). Activating VTA NtsR1 neurons modestly decreased food and water intake over 24 hrs (Figure 2C), primarily during the dark cycle (Figure 2D,E; y axis asterisks indicate overall treatment effect between VEH and CNO). VTA NtsR1 neuronal activation also increased overall ambulatory locomotor activity and wheel rotations during the light and dark cycles (Figure 2F,G; y axis asterisks indicate overall treatment effect between VEH and CNO, while asterisks within the graphs indicate significant differences at specific time points). In addition, activating VTA NtsR1 neurons in chow-fed NtsR1Cre mice increased energy expenditure (Figure 2H) and decreased RER (Figure 2I), which respectively indicate increased calories/hour burned and increased use of fat as an energy substrate (although not outside normal range, 0.7–1). The change in RER is not surprising here, as decreased RER can result from decreased food intake. After CNO treatment, NtsR1Cre mice were kept in TSE cages for 24 hrs (washout day, no injection given), but there were no differences between VEH and washout days (Supplemental Figure 1A-F). However, the 24 hrs feeding and locomotor effects were specific to CNO-mediated activation of VTA NtsR1 neurons in NtsR1Cre mice, as we did not observe any CNO-mediated changes in chow-fed Wt littermates analyzed for 24 hrs food intake and locomotor activity (Supplemental Figure 2) or other metabolic parameters (Supplemental Figure 3). These data support that activation of VTA NtsR1 neurons promotes weight loss in normal weight NtsR1Cre mice.
Figure 2. Acute Activation of VTA NtsR1 Neurons Promotes Activity and Suppresses Feeding in Normal Weight Mice.
Chow fed NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons were analyzed in metabolic cages while treated with VEH and then CNO. A) Timeline of experiment in days. B) Change in body weight measured 24 hr after VEH or CNO treatment, analyzed with 2-tailed paired t-test (n=13). C) 24hr cumulative chow (left) and water (right) intake, analyzed with 2-tailed paired t-test (n=18). For D-I, data is collected every 21–27 minutes and the x axis spans the light (yellow line) and dark cycle (dark blue line) in a ~22-hr period. Data in D-I were analyzed by repeated measures 2-way ANOVA with Sidak post-tests (n=18). Arrows identify VEH or CNO injection times. D) Chow intake, E) water intake, F) ambulatory locomotor activity, G) wheel rotations, H) energy expenditure, and I) RER. Graphed data represent mean ± SEM. Mark next to the y-axis in D-I indicates overall significant differences between treatments, see Table 1 for T and F values. p*<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3.3. Activating VTA NtsR1 Neurons Suppresses DA-Dependent Palatable Food Consumption in Normal Weight Mice
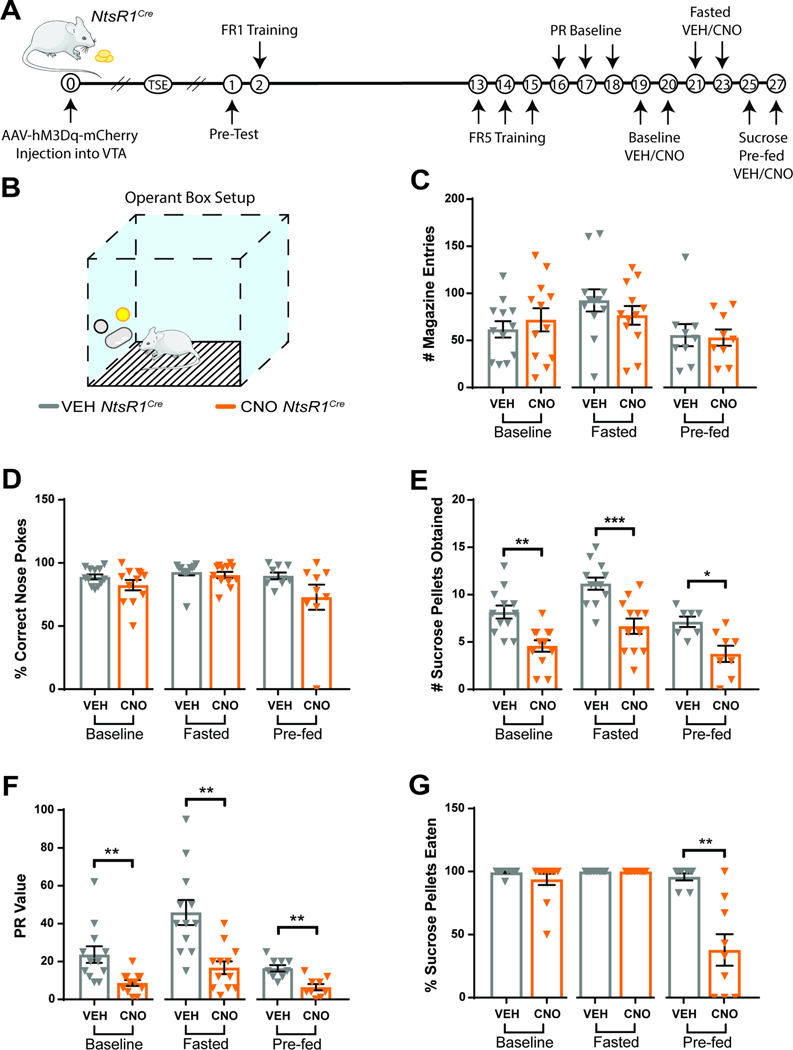
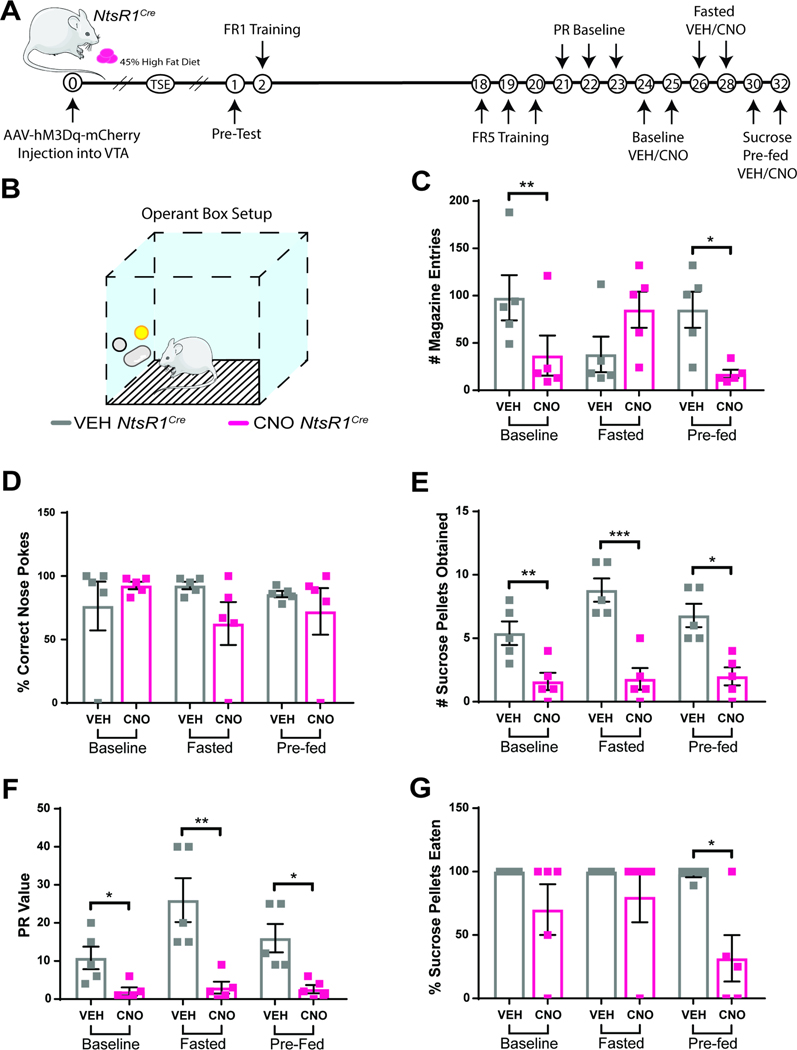
Since DA neurons modify goal directed behaviors to obtain rewards (Salamone and Correa, 2012) we examined how the subset of DA neurons expressing NtsR1 impacts operant responding for sucrose pellets (a test of DA-dependent willingness to work for food rewards). Mice were tested during three motivational states: baseline, fasted, and sucrose pre-fed (sated) (Figure 3A,B). Activation of VTA NtsR1 neurons did not disrupt anticipatory behavior, confirmed by the similar number of magazine entries after VEH and CNO treatment (Figure 1C). CNO-mediated activation of VTA NtsR1 neurons decreased the total number of all nose pokes mice performed (Supplemental Figure 4) but it did not alter the percentage of correct nose pokes (Figure 3D); this indicates that mice performed the task less, but when they did, they were able to perform it correctly. However, CNO-mediated activation of VTA NtsR1 neurons reduced the number of sucrose pellets mice obtained and their PR breakpoint (willingness to work for sucrose) across all motivational states (Figure 3E,F). Interestingly, when VTA NtsR1 neurons were activated, chow-fed NtsR1Cre mice ate 90–100% of the sucrose pellets obtained during baseline and fasted conditions, but pre-fed mice only ate ~40% of the sucrose pellets (Figure 3G). Thus, in a sated state, activating VTA NtsR1 neurons reduced operant responding, thereby reducing the receipt of palatable rewards as well as suppressing their consumption. Conversely, CNO-treatment had no effect on behavior of chow-fed Wt littermates (Supplemental Figure 5). Notably, activation of VTA NtsR1 neurons did not restrain preference for freely-available 1% sucrose solution over water, a test measuring hedonic value (Supplemental Figure 6). Overall, these results suggest that VTA NtsR1 activation suppresses intake primarily by decreasing the performance of effort-requiring behaviors, including those required to obtain food.
Figure 3. VTA NtsR1 Neuron Activation Suppresses Palatable Food Consumption in Normal Weight Mice.
A) Timeline of experiment in days. Break in x-axis indicates variable time after NtsR1Cre mice received intra-VTA AAV2-hSyn-DIO-hM3D(Gq)-mCherry before beginning the operant test described here. B) Depiction of operant box setup. The position of the correct nose-poke (yellow) was counterbalanced between mice. Chow fed NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons (n=8–12) were trained to nose poke for sucrose rewards. Mice were then tested on a PR schedule for operant responding after VEH or CNO treatment during baseline (control), fasted (hungry) and sucrose pre-fed (sated) conditions. C) Magazine entries (where sucrose pellet is deposited). D) Correct nose pokes. E) Number of sucrose pellets obtained. F) PR value (last ratio obtained). G) Percentage of sucrose pellets eaten. Data were analyzed by Ordinary One-Way ANOVA with Sidak post-tests, see Table 1 for T and F values. Graphs represent mean ± SEM. p*<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3.4. Acute Activation of VTA NtsR1 Neurons Promotes Weight Loss Behaviors in Obese Mice
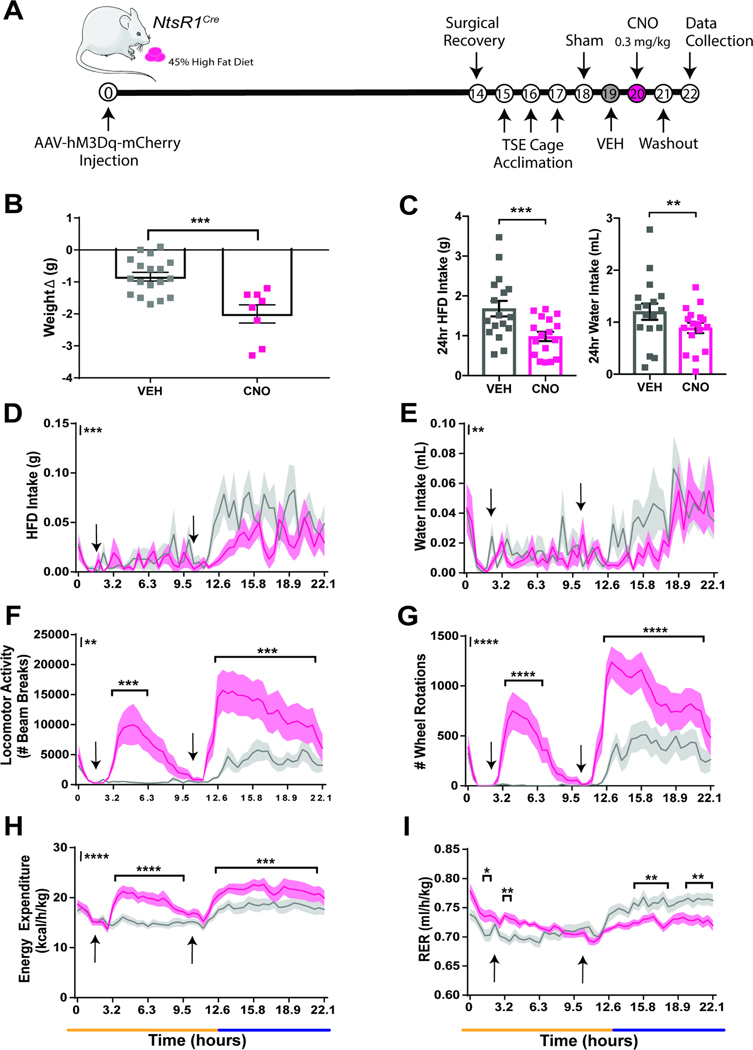
To assess the potential of VTA NtsR1 neurons to treat obesity we DREADD-activated VTA NtsR1 neurons in diet-induced-obese NtsR1Cre mice. Obese NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons were analyzed in metabolic chambers with ad libitum palatable high fat diet (HFD) while receiving twice daily VEH or CNO treatments (Figure 4A). CNO-mediated activation of VTA NtsR1 neurons decreased body weight of obese NtsR1Cre mice over 24 hrs (Figure 4B). Concomitantly, CNO-activation of VTA NtsR1 neurons in these mice decreased HFD and water intake over 24 hrs (Figure 4C), primarily during the dark cycle, when mice consume most food and water (Figure 4D,E). Yet, activation of VTA NtsR1 neurons increased ambulatory locomotor activity and wheel running during both the light and dark cycles (Figure 4F and G). Activation of VTA NtsR1 neurons also increased energy expenditure (Figure 4H) and both increased (light cycle) and decreased (dark cycle) RER (Figure 4I) The y axis asterisks in Figures 4D-I indicate overall treatment effects between VEH and CNO. In addition to significant differences in overall treatment effects, asterisks within the graphs indicate significant differences at specific time points. These effects were absent in obese Wt mice treated with CNO (Supplemental Figures 7 and 8), albeit a modest increase in dark cycle RER that did not mimic the decreased RER in NtsR1Cre mice (Supplemental Figure 8H vs. Figure 4I). After CNO treatment, mice were kept in TSE cages for 24 hours (washout day, no injections given). While the suppression of feeding and drinking was no longer present on washout day, mice still exhibited lower weight, increased activity and wheel rotations during the dark cycle, approximately 24 hrs after the last activation of VTA NtsR1 neurons (Supplemental Figure 9). Overall, these data indicate that activating VTA NtsR1 neurons alters ingestive and locomotor behaviors that promote and maintain weight loss in obese mice.
Figure 4. Acute Activation of VTA NtsR1 Neurons Promotes Activity and Suppresses Feeding in HFD Mice.
VEH or CNO treated HFD NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons were analyzed in metabolic cages. A) Timeline of experiment in days. B) Change in body weight 24 hr after VEH or CNO treatment, analyzed via 2-tailed unpaired t-test (n=8–18). C) 24hr cumulative HFD (left) and water (right) intake, analyzed with 2-tailed paired t-test (n=17). In D-I, data is collected every 24–27 minutes and the x axis spans a light (yellow line) and dark cycle (blue line) over a ~22-hr period. Data in D-I were analyzed by repeated measures 2-way ANOVA with Sidak post-tests (n=17). Arrows identify VEH or CNO injection times. D) HFD intake. E) Water intake. F) Ambulatory locomotor activity. G) Wheel rotations. H) Energy expenditure. I) RER. Graphed data represent mean ± SEM. Body weight average = 41.7 ± 8.2 g. Mark next to the y-axis in D-I indicates overall significant overall differences between treatments, see Table 1 for T and F values. p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3.5. VTA NtsR1 Neuronal Activation in Obese Mice Suppresses DA-Dependent Palatable Food Consumption
We next used operant responding to test whether VTA NtsR1 neurons modulate DA-dependent responding for palatable food in obese mice (Figure 5A,B). Given that activating VTA NtsR1 neurons in obese NtsR1Cre mice notably increased locomotor behavior (Figures 4F), we considered whether this could have impeded their operant responding. As reflected by the number of magazine entries, CNO-mediated activation of VTA NtsR1 neurons in obese NtsR1Cre mice decreased anticipatory behavior in baseline and sucrose pre-fed states, but not in fasted mice (Figure 5C). Together, these data suggest that obese mice with activated VTA NtsR1 neurons can coordinate hunger status with anticipatory locomotor behavior, and that augmented locomotor activity did not prevent them from engaging the magazine. While CNO-mediated activation of VTA NtsR1 neurons did decrease the total number of all nose pokes mice performed in the fasted and pre-fed states (Supplemental Figure 10), it did not diminish the percentage of correct nose pokes (Figure 5D). Thus, mice were able to execute the operant behavior correctly albeit they did so less when VTA NtsR1 neurons were activated. Accordingly, CNO-mediated VTA NtsR1 neuronal activation decreased the number of pellets earned by obese NtsR1Cre mice and their PR breakpoint in all three states (Figure 5E,F). Moreover, when their VTA NtsR1 neurons were activated, obese NtsR1Cre mice ate most of the sucrose pellets they obtained during baseline and fasted states, but pre-fed mice only ate ~30% of their earned sucrose rewards (Figure 5G). As in normal weight mice, activation of VTA NtsR1 neurons in obese mice had no impact on their preference for a freely-available 1% sucrose solution, but did reduce HFD intake and weight (Supplemental Figure 11). Overall, these results suggest that VTA NtsR1 neuronal activation in the obese state does not modify the palatability of food but reduces performance of goal-directed behaviors, including those necessary to obtain food.
Figure 5. Activation of VTA NtsR1 Neurons Suppresses Palatable Food Consumption in HFD Mice.
A) Timeline of experiment in days. Break in x-axis indicates variable time after NtsR1Cre mice received intra-VTA AAV2-hSyn-DIO-hM3D(Gq)-mCherry before beginning the operant testing. B) Depiction of operant box setup. The position of the correct nose-poke (yellow) was counterbalanced between mice. Diet-induced obese NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons (n=5) were trained to respond for sucrose rewards in operant boxes. Mice were then tested via a PR schedule in response to VEH and CNO treatment during baseline (control), fasted and sucrose pre-fed conditions. C) Magazine entries (where sucrose pellet is deposited). D) Percentage of correct nose pokes. E) Number of sucrose pellets obtained. F) PR value (last ratio obtained). G) Percentage of sucrose pellets eaten. Body weight average = 43.4 ± 10.0 g. Data were analyzed by Ordinary One-Way ANOVA with Sidak post-tests, see Table 1 for T and F values. Graphed data represent mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3.6. VTA NtsR1 Neuronal Activation Promotes Sustained Weight Loss in Obese mice
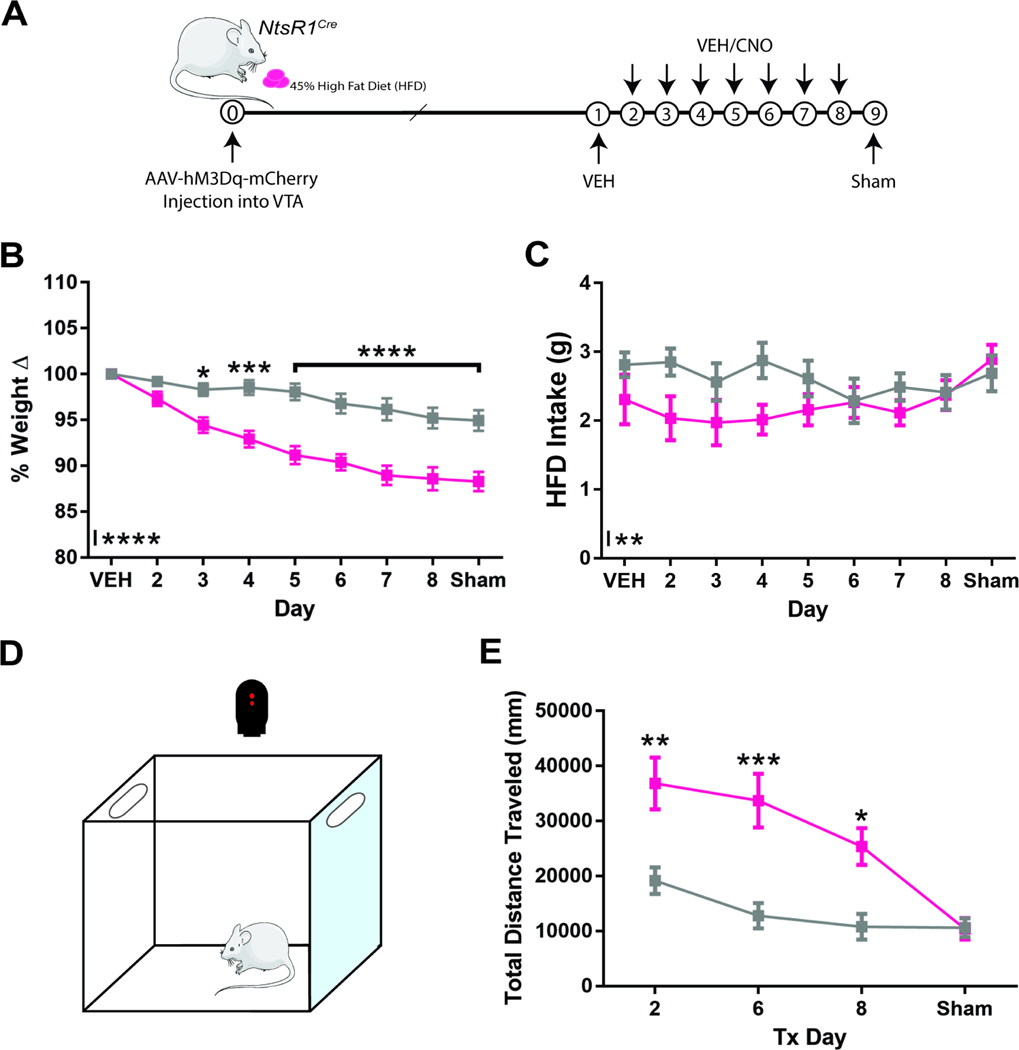
Next, we tested whether the acute weight-reducing effect of activating VTA NtsR1 neurons could be sustained. Diet-induced obese NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons were treated twice daily for 7 days with VEH/CNO in their home cage (Figure 6A). Chronic VTA NtsR1 neuronal activation in obese NtsR1Cre mice decreased body weight, which was sustained for the duration of the treatment period, including after the sham/washout day (Figure 6B). Overall HFD intake was suppressed by CNO treatment (Figure 6C, asterisks at base of y axis indicate overall treatment effect). However, CNO-mediated feeding restraint was more pronounced during the first 4 days of treatment, after which it was comparable to VEH treatment. To assess locomotor activity, mice were placed in open field boxes during the light cycle on treatment days 2, 6, 8 and sham (Figure 6D). CNO-treated obese NtsR1Cre mice exhibited increased physical activity that progressively reduced over the experiment, but was indistinguishable from VEH-treatment on sham/washout day (Figure 6E). Thus, although the feeding suppression and locomotor enhancing effects of activating VTA NtsR1 neurons dissipate over time, repeated activation of VTA NtsR1 neurons can induce and sustain weight loss in obese mice.
Figure 6. VTA NtsR1 Neuronal Activation Promotes Sustained Weight Loss in HFD Mice.
Diet-induced obese NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons (n=9–12) were studied to assess long term (7 days) effects of activating VTA NtsR1 neurons. Mice were treated twice-daily with either VEH or CNO to activate VTA NtsR1 neurons, with ad libitum access to 45% HFD. A) Timeline of experiment. Break in x-axis indicates variable time after NtsR1Cre mice received intra-VTA AAV2-hSyn-DIO-hM3D(Gq)-mCherry before receiving the chronic treatments. B) Percentage weight change over 7 days measured in home cages. C) HFD intake measured in home cages. D) Open field box representation. E) Total distance traveled on days 2, 6, 8 and sham, measured in open field box. Graphed data represent mean ± SEM. Body weight average = 46.3 ± 9.1 g. Data were analyzed by 2-way ANOVA with Sidak post-tests. Mark next to the y-axis indicates overall significant differences between treatments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3.7. VTA NtsR1 Neuron-Mediated Suppression of Feeding is not Due to Aversion or Anxiety
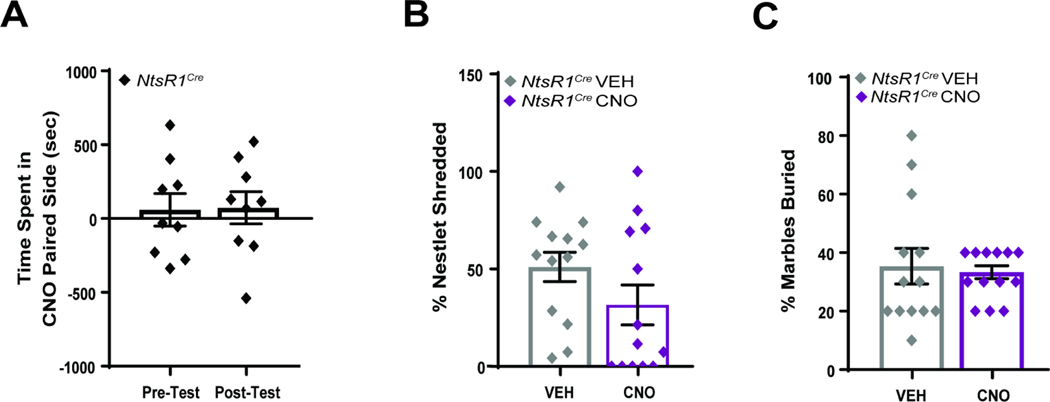
Aversive stimuli or stress can also suppress feeding, so we investigated whether activation of VTA NtsR1 neurons promotes these conditions. Normal weight and obese NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons were pooled for these tests since there were no significant differences between the diet-defined groups. When tested in conditioned place preference chambers paired with either VEH or CNO, NtsR1Cre mice demonstrated neither aversion nor preference for the CNO-activation paired chamber (Figure 7A). Activating VTA NtsR1 neurons did not increase anxiety-like behavior as measured via nestlet shredding or marble burying (Figure 7B,C). Collectively, these data signify that activating VTA NtsR1 neurons does not suppress feeding secondary to aversion or anxiety.
Figure 7. VTA NtsR1 Neuronal Activation Does Not Invoke Aversion or Anxiety.
A) Normal weight and diet-induced obese NtsR1Cre mice expressing hM3Dq-mCherry in VTA NtsR1 neurons (n=9) were assessed via CPP. Mice received CNO in one chamber and VEH treatment in the other, so as to associate the chamber and treatment. Graph depicts time spent in the CNO-paired chamber during the pre- and post-tests, revealing that activation of VTA NtsR1 neurons is neither rewarding nor aversive. B, C) Anxiety and compulsive like behavior was assessed via nestlet shredding and marble burying. B) Percentage of nestlet shredded; no differences were observed between treatments for NtsR1Cre mice (n=13). C) Percentage of marbles buried; no differences were observed between treatments for NtsR1Cre mice (n=13). Data were analyzed with 2-tailed paired t-test. Graphs depict mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
3.8. Activation of VTA NtsR1 Neurons Does Not Induce Hypothermia or Vasodepression
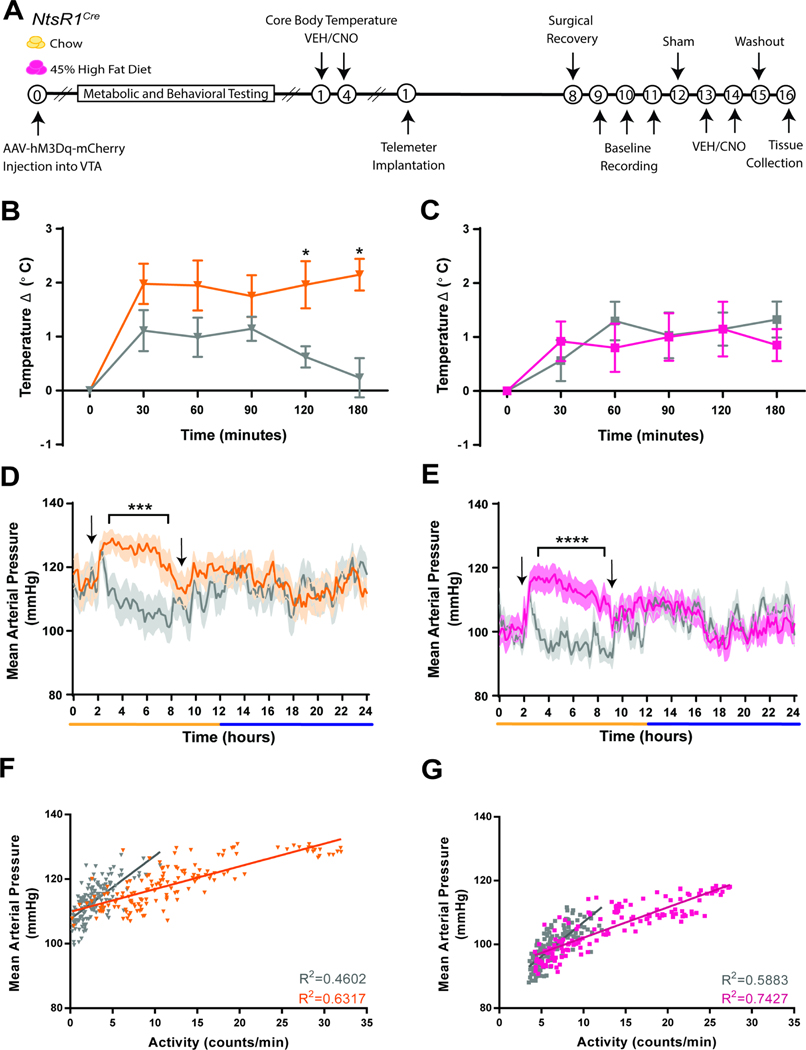
Systemic or brain-wide treatment with Nts or NtsR1 agonists lowers blood pressure and causes hypothermia and cyanosis in rats (Boules et al., 2013; Carraway and Leeman, 1973; Ciriello and Zhang, 1997; Fantegrossi et al., 2005; Feifel et al., 2010; Gully et al., 1996; Zogovic and Pilowsky, 2012), but it is unclear which NtsR1 neurons mediate these adverse effects. We tested whether activation of VTA NtsR1 neurons invokes vasodepression and/or hypothermia in normal weight and obese NtsR1Cre mice (Figure 8A). CNO-mediated activation of VTA NtsR1 neurons significantly increased core body temperature in normal weight but not obese, NtsR1Cre mice (Figure 8B,C), but did not produce the hypothermia observed with systemic NtsR1-agonism. We then implanted telemeters in these mice to measure blood pressure, heart rate and physical activity. CNO-mediated activation of VTA NtsR1 neurons increased mean arterial pressure (Figure 8D,E), and heart rate only during the light cycle (Supplemental Figure 12B, C) though it increased locomotor activity during the light and dark cycles (Supplemental Figure 12D, E). Interestingly, ~60–70% of these cardiovascular effects are attributable to the increased locomotor activity in NtsR1Cre mice (Figure 8F,G and Supplemental Figure 12F, G). Thus, selectively activating VTA NtsR1 neurons does not recapitulate the hypothermia or vasodepression previously described with systemic NtsR1 agonism.
Figure 8. Activation of VTA NtsR1 Neurons does not Induce Hypothermia or Vasodepression.
A) Timeline of experiments. Break in x-axis indicates variable time after NtsR1Cre mice received intra-VTA AAV2-hSyn-DIO-hM3D(Gq)-mCherry before testing. B-C) Core body temperature change every 30 minutes for 120 minutes and at 180 minutes after VEH or CNO treatment in B) normal weight and C) obese mice. D-E) Mean arterial pressure over 24 hr in D) normal weight and E) obese mice. F-G) Activity vs. mean arterial pressure over 24 hr in F) normal weight and G) obese mice. Graphed data represent mean ± SEM. Body weight average for HFD mice = 50.7 ± 11.7 g. Temperature data were analyzed with ordinary 2-way ANOVA and Tukey’s posts-tests (chow n=9–10, HFD n=5–6). Telemetry data were analyzed by repeated measures 2-way ANOVA with Sidak post-tests (chow n=11, HFD n=10). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
4. Discussion
Nts can suppress feeding and increase locomotor activity via the VTA, but prior limitations prevented understanding if these effects are mediated via NtsR1 or NtsR2-expressing cells. Herein we used excitatory DREADDs to show that activating VTA NtsR1 neurons, a subset of all VTA DA neurons, restrains feeding and drinking, promotes locomotor activity, and invokes weight loss in normal weight and obese mice of both sexes. Notably, the weight loss in obese mice persists after washout of the DREADD ligand, suggesting that long-term changes in body weight may be possible via activating VTA NtsR1 neurons. While activating VTA NtsR1 neurons does not reduce preference for palatable food, it dampens how much mice work for it, which might be useful to curb caloric intake. Moreover, experimental activation of VTA NtsR1 neurons does not cause the hypothermia, vasodepression or reinforcement effects invoked by global modulation of DA or NtsR1 signaling. Since activating VTA NtsR1 neurons largely recapitulates the effects of Nts treatment in the VTA, our findings implicate VTA NtsR1 neurons as mediators of Nts-mediated weight loss, and worth exploring as targets to support weight loss without adverse side effects.
Activating all VTA DA neurons produces a range of differential responses, but only some support weight loss (Boekhoudt et al., 2018, 2017; Heymann et al., 2020; Mahler et al., 2019), likely due to the heterogeneity of VTA DA neurons and their projections (Brischoux F, Chakraborty S, Brierley DI et al., 2009; Lammel et al., 2012, 2011; Margolis et al., 2006; Matsumoto and Hikosaka, 2009; Poulin et al., 2014). We reasoned that certain VTA DA neuronal subsets might be useful to invoke weight loss, and used NtsR1 as a genetic marker to identify and modulate one subset. Importantly, since >90% of VTA NtsR1 neurons co-express TH (Woodworth et al., 2017a), they are a subset of the large DA population rather than the comparatively smaller GABA or glutamate containing VTA populations. Thus, VTA NtsR1 neuron-mediated effects are likely due to release of DA rather than GABA or glutamate. While VTA NtsR1 neurons comprise a large portion (~70%) of all VTA DA neurons, they are a subset that selectively projects to the NAc (Woodworth et al., 2018), which plays an important role in modulating goal-directed behaviors (Salamone et al., 2003; Salamone and Correa, 2012). Moreover, given that Nts exerts anorectic actions via the VTA (Cador et al., 1986; Hawkins, 1986), we hypothesized that VTA NtsR1 neurons might contribute to this effect. Indeed, activating the VTA NtsR1 neuronal subset restrained feeding, promoted locomotor activity, and supported weight loss, consistent with the effects of Nts administered into the VTA. These effects were observed in both males and females, which is why we pooled data from both sexes. However, given that DA signaling can differ in males and females (Zachry et al., 2021) it is possible that hormonal or environmental context could influence the role of VTA NtsR1-DA neurons, and this should be explored in the future. Indeed, loss of function NtsR1 variants have been characterized in eating disorders that are more common in females than males (Lutter et al., 2017), suggesting that there may be sex- and context-specific roles for VTA NtsR1 neurons.
A potential caveat of this study is the use of NtsR1Cre mice to genetically modulate VTA NtsR1 neurons, and the use of DREADDs to activate them outside of endogenous regulation. It is recognized that introducing IRES Cre after the stop codon (as is the case in NtsR1Cre mice) can sometimes influence expression of the upstream coding sequence (Song and Palmiter, 2018). Expression of DREADD is thought to be inert, including in DA neurons (Mahler et al., 2019) but some work suggests it could impact neuronal mechanics and dynamics (Smith et al., 2016). While we do not have data to suggest that either of these were factors in our current study, they could conceivably lead to differences in baseline behavior of Wt and NtsR1Cre mice. To control for this possibility we used a within-subjects crossover treatment design, so that NtsR1Cre received both VEH and CNO, and could be used as their own controls (Becher et al., 2018; Smith et al., 2016). We treated Wt mice to assess any potential off-target effects of CNO, which was particularly important for this study since high doses of CNO have been shown to inhibit DA-mediated locomotor activity (MacLaren et al., 2016). Importantly, the low dose of CNO used in these studies does not alter locomotor activity in Wt mice, verifying that it does not exert off-target regulation of the DA system that would confound our studies. Out of an abundance of caution, and in case Wt mice may not be identical to NtsR1Cre mice, we did not directly intercompare their data. Alternatively, we could have compared NtsR1Cre mice injected with cre-inducible DREADDq vs. mCherry, but this study design would prevent the opportunity to discover any potential behavioral differences between Wt and NtsR1Cre mice.
DREADD-activation of VTA NtsR1 neurons, while artificial, did recapitulate the feeding suppression and locomotor activity induced by Nts or NtsR1 agonists administered into the VTA (Cador et al., 1986; Hawkins, 1986; Woodworth et al., 2017b). Physiologic release of Nts into the VTA also produces some of these behaviors, suggesting a role for Nts-NtsR1 signaling in mediating the effects. For example, some lateral hypothalamic area neurons express Nts (e.g. LHA Nts neurons) and release it to the VTA and other sites (Patterson et al., 2015). Activating LHA Nts neurons promotes locomotor activity, restrains feeding and leads to weight loss in normal weight mice, similar to chemogenetically activating VTA NtsR1 neurons (Woodworth et al., 2017b). However, while activating VTA NtsR1 neurons sustained weight reduction in obese animals, activating LHA Nts neurons did not. This discrepancy may be due to the net activation of LHA Nts projections to the VTA and other brain areas, which initially invokes water intake that increases body weight followed by weight reducing behaviors (Woodworth et al., 2017b). By contrast, activating VTA NtsR1 neurons decreased water intake. One explanation for this effect is that since VTA NtsR1 activation reduces the motivation to obtain food rewards, it could also reduce the motivation to drink, as DA has been associated with the act of drinking and not hydration state (Augustine et al., 2019; Hsu et al., 2020). Alternatively, since drinking is generally coupled with feeding, the feeding suppression observed here may have accordingly reduced fluid intake as well. In any case, since activating VTA NtsR1 neurons did not promote drinking, LHA Nts neurons may mediate drinking via non-VTA projections. Nts-mediated feeding restraint may be mediated via the VTA, as it was observed after activating LHA Nts neurons (Woodworth et al., 2017b) and VTA NtsR1 neurons (herein). Circuit-specific activation methods will be necessary to dissect the physiological role of Nts released to the VTA from the LHA vs. other sites. It has also been suggested that Nts produced within the VTA could engage Nts receptors (Piccart et al., 2015), although in mice there are few Nts-expressing neurons in the VTA compared to the LHA or other brain areas (Schroeder et al., 2019). Whatever the source of endogenous Nts, our data suggest that activating VTA NtsR1 neurons could be a mechanism to reduce feeding and promote weight loss, and may be particularly useful in the context of obesity. Indeed, DREADD-activating VTA NtsR1 neurons elicits prolonged weight loss in obese mice, even 24 hrs after washout of the DREADD ligand. While it remains to be examined, VTA NtsR1 neuronal activation may invoke long-lasting changes in gene and/or protein expression or cell signaling that enable sustained weight reduction. However, the absence of persistent weight loss in normal weight chow-fed mice suggests that activating VTA NtsR1 neurons is unlikely to cause anorexia or adverse effects that might preclude targeting this pathway.
Activation of all VTA DA neurons has been largely characterized as facilitating reward intake. Indeed DREADDq-mediated activation of VTA DA neurons in rats increases cocaine reinstatement but contrarily it decreases free cocaine consumption (Mahler et al., 2019), suggesting that context is important. Likewise, increasing DA signaling has been associated with both increasing and decreasing drug and food intake. Chemogenetic activation of all VTA DA neurons increases responding for sucrose rewards under progressive ratio reinforcement, but psychostimulant drugs that increase DA signaling suppress food intake (Boekhoudt et al., 2018, 2017), indicating the complexities of DA-mediated behavior. Our studies differ from prior bulk activation of all VTA DA neurons because here we only activated the subset expressing NtsR1 that are known to project to the NAc. Moreover, we purposely used a DREADDq approach to activate VTA NtsR1 neurons because NtsR1 is Gq-coupled receptor. While CNO-mediated activation of DREADDq is not the same as Nts or NtsR1 agonist-mediated activation of NtsR1, we reasoned that it was a reasonable proxy, since Nts and NtsR1 agonists administered into the VTA activate DA neurons expressing the Gq-coupled NtsR1and suppress food intake (Cador et al., 1986; Kalivas et al., 1983; Kelley et al., 1989). Likewise, we found that activating VTA NtsR1 neurons reduced feeding and operant responding for palatable food. Because VTA NtsR1 neurons are a subpopulation of all VTA DA neurons that project to the NAc, it is important to consider that their activation could promote DA associated behaviors observed with DA release in the NAc, such as reduction of food intake and hyperlocomotion. It is also worth noting that hyperlocomotion by itself can result in decreased food intake, and we cannot rule out that VTA NtsR1-induced physical activity may contribute to suppressed feeding. However, this elevated locomotor activity did not prevent mice from engaging in anticipatory and operant behaviors, nor from obtaining a freely accessible sucrose solution, suggesting it did not completely impede feeding. VTA NtsR1 activation did reduce overall performance of operant behaviors, which may have secondarily reduced responding for food. Our current findings cannot rule out that activating VTA NtsR1 neurons causes a general reduction in performance of goal-directed behaviors, and thus, a non-specific reduction in operant food intake. Yet, if VTA NtsR1 neuronal activation reduces willingness to engage in the operant food task, it might manifest as a general reduction in operant responding in this paradigm. VTA DA neurons are well established to modulate the incentive salience of food and drug rewards (Berridge et al., 2010; Richard et al., 2013), so it remains possible that VTA NtsR1 neurons could influence how much rewards are “wanted” and will be worked for. Further testing will be required to reveal the physiologic and behavioral meaning of the VTA NtsR1-modulated responding observed herein.
Food intake is also influenced by its hedonic valence (e.g. how much it is liked) and is mediated via a DA-independent mechanism. The divergence in wanting vs. liking (Berridge and Kringelbach, 2015) may explain why activating VTA NtsR1 neurons could not completely prevent operant responding for sucrose; the palatability of the sucrose likely drives some degree of hedonic intake that cannot be mitigated by DA-containing VTA NtsR1 neurons. Indeed, activating VTA NtsR1 neurons did not blunt intake of freely accessible sucrose solution, a test that assesses sucrose’s hedonic value. Mechanistically, these data suggest that DA-containing VTA NtsR1 neurons may not modify the opioid signaling systems that encode food “liking” and so cannot completely blunt intake of freely available, palatable foods that promote weight gain. However, because activating VTA NtsR1 neurons in a sated state restrained goal-directed behavior necessary to obtain palatable food, and to also how much was eaten, these neurons may have promise to prevent overeating and weight gain. Conditioned taste aversion could also account for diminished feeding, and can develop after a single exposure to an aversive stimulus. Although the mice in this study went through multiple trials of VTA NtsR1 activation, their food intake did not significantly differ from VEH after during the washout period, arguing against induction of a conditioned taste aversion. (Supplemental Figures 1 & 9). However, we cannot exclude that taste aversion might contribute to VTA NtsR1-mediated feeding restraint.
We did, however, evaluate whether activating VTA NtsR1 neurons causes other adverse physiology that would preclude leveraging these neurons to treat disease. Some VTA DA signaling has been implicated in promoting anxiety, aversion or reinforcement that can secondarily reduce feeding behavior (Scheggi et al., 2018; Seibenhener and Wooten, 2015). However, our battery of tests shows that activating VTA NtsR1 neurons does not invoke these conditions. We also assessed the cardiorespiratory consequence of activating VTA NtsR1 neurons, since the most widely reported effects of systemic treatment with Nts or NtsR1 agonists are hypotension and decreased core body temperature (Carraway and Leeman, 1973; Ciriello and Zhang, 1997; Fantegrossi et al., 2005; Feifel et al., 2010; Gully et al., 1996). While results can differ by tissue and treatment paradigm, in rodents Nts treatment in the central nervous system treatment is well established to elicit hypothermia and a dose related decrease in mean arterial pressure and heart rate (Boules et al., 2013; Zogovic and Pilowsky, 2012). These clinical liabilities have diminished enthusiasm for pharmacologically modulating the Nts-NtsR1 system to treat disease. However, the advent of tools to site-specifically modulate Nts and NtsR1 neurons revealed that dedicated Nts/NtsR1 circuits modulate specific physiology, which opened the door for the possibility that only certain circuits mediate cardiorespiratory effects (Salamone and Correa, 2012; Tabarean, 2020). We found that activating VTA NtsR1 neurons promoted weight loss behaviors but did not make mice hypothermic or lower their blood pressure. Moreover, VTA NtsR1 neural activation during the light cycle did not reduce, but rather increased mean arterial pressure and heart rate. Physical activity was also increased during VTA NtsR1 activation and part of the mean arterial pressure and heart rate increase effect (during the light cycle) is due to this increase in physical activity. An increase in mean arterial pressure and heart rate is not surprising here, since during exercise, cardiac output increases more than the total peripheral resistance, which results in an increase in mean arterial pressure (Kurtz et al., 2014). Cardiac output, in turn, is dependent on heart rate; an increase in heart rate leads to an increase in cardiac output (Kurtz et al., 2014). Since only 60–70% of the variation in mean arterial pressure and heart rate is explained by increased activity, we cannot rule out that activating VTA NtsR1 neurons may drive other physiology that contributes to elevated body temperature and cardiovascular response. Curiously, activating VTA NtsR1 neurons during the dark cycle, when mice are normally alert and active, had no effect on blood pressure or heart rate. However, dark cycle activation of VTA NtsR1 neurons restrains feeding and promotes locomotor activity. Thus, augmenting VTA NtsR1 activity during wakefulness might promote dual behaviors to support weight loss while avoiding any cardiovascular effects. Taken together, these data suggest that augmenting activity of VTA NtsR1 neurons may be useful to support weight loss behaviors without causing psychiatric or cardiovascular liabilities previously described with systemic NtsR1 agonism.
The experimental activation paradigm we used here reveals biological roles for VTA NtsR1 neurons in feeding and locomotor behaviors relevant to control of body weight. However, the potential of this system for treating obesity will depend on whether pharmacological NtsR1 agonists can selectively and safely promote activity of VTA NtsR1 neurons. For example, site-specific agonists to enhance activation of VTA NtsR1 neurons might thwart the increased appetitive drive that occurs after an initial weight loss, preventing regain while encouraging physical activity without adverse cardiorespiratory effects or disrupting mental state. However, this would require targeting NtsR1 agonists selectively to the VTA, so as to avoid inducing off-target modulation of arousal, hypo-locomotor activity, analgesia, body temperature, blood pressure and heart rate associated with Nts-NtsR1 action in other brain regions (Carraway and Leeman, 1973; Ciriello and Zhang, 1997; Fantegrossi et al., 2005; Feifel et al., 2010). There are reports of NtsR1-Dopamine Receptor-2 heterodimers, and if true, it may be possible to design agonists for these heterodimers that might at least confine the drug to the DA system. Whether such heterodimers exist in vivo, or how they might signal are vital questions that must be resolved to assess the utility of such an approach. Alternatively, better understanding of NtsR1-mediated signaling may identify strategies to promote beneficial behaviors without adverse physiological effects. Thus, while there is much yet to be learned about the biology of NtsR1 in mediating energy balance, our data support further exploration of VTA NtsR1 neurons as potential pharmacological targets to improve the treatment of obesity and its co-morbidities.
Supplementary Material
Table 1.
Statistical Analysis Details.
|
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Figure 1. TH, cFOS and mCherry Counts | t | df | p | ||||||||||||
|
|
|||||||||||||||
| C. TH-mCherry/TH-no mCherry | 13.3 | 18 | **** | ||||||||||||
| F. mCherry-cFOS VEH/CNO | 10.2 | 10 | **** | ||||||||||||
|
| |||||||||||||||
| Figure 2. NtsR1Cre Chow TSE | t | df | p | Time F | DFn | DFd | p | Tx F | DFn | DFd | Tx p | Int. F | DFn | DFd | Int. p |
|
| |||||||||||||||
| B. Weight Change | 3.8 | 12 | ** | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| C. Left. 24hr Chow Intake Bars | 3.7 | 17 | ** | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| C. Right. 24hr Water Intake Bars | 3.0 | 17 | ** | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| D. Chow Intake | n/a | n/a | n/a | 6.7 | 54 | 918 | **** | 13.4 | 1 | 17 | ** | 1.1 | 54 | 918 | ns |
| E. Water Intake | n/a | n/a | n/a | 9.8 | 54 | 918 | **** | 9.3 | 1 | 17 | ** | 1 | 54 | 918 | ns |
| F. Ambulatory Locomotor Activity | n/a | n/a | n/a | 9.6 | 54 | 918 | **** | 8.5 | 1 | 17 | ** | 4.4 | 54 | 918 | **** |
| G. Wheel | n/a | n/a | n/a | 14.1 | 54 | 918 | **** | 10.8 | 1 | 17 | ** | 3.5 | 54 | 918 | **** |
| H. Energy Expenditure | n/a | n/a | n/a | 14.9 | 54 | 918 | **** | 2.2 | 1 | 17 | ns | 3.7 | 54 | 918 | **** |
| I. RER | n/a | n/a | n/a | 33.7 | 54 | 864 | **** | 12.9 | 1 | 16 | ** | 3.9 | 54 | 864 | **** |
|
| |||||||||||||||
| Figure 3. NtsR1Cre Chow Operant | F | DFn | DFd | p | Baseline t | df | p | Fasted t | df | p | Pre-fed t | df | p | ||
|
|
|||||||||||||||
| C. Magazine Entries | 1.9 | 5 | 60 | ns | 0.7 | 60 | ns | 1.1 | 60 | ns | 0.2 | 60 | ns | ||
| D. % Correct Nose Pokes | 2.7 | 5 | 60 | * | 1.1 | 60 | ns | 0.4 | 60 | ns | 2.5 | 60 | * | ||
| E. Sucrose Pellets Obtained | 14.1 | 5 | 57 | **** | 3.8 | 57 | ** | 4.7 | 57 | **** | 2.8 | 57 | * | ||
| F. PR Value | 13.22 | 5 | 60 | **** | 2.8 | 60 | * | 5.4 | 60 | **** | 1.6 | 60 | ns | ||
| G. Sucrose Pellets Eaten | 23.3 | 5 | 59 | **** | 0.9 | 59 | ns | 0 | 59 | ns | 7.6 | 59 | **** | ||
|
| |||||||||||||||
| Figure 4. NtsR1Cre HFD TSE | t | df | p | Time F | DFn | DFd | p | Tx F | DFn | DFd | p | Int. F | DFn | DFd | Int. p |
|
| |||||||||||||||
| B. Weight Change | 4.3 | 24 | *** | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| C. Left. 24hr Chow Intake Bars | 4.8 | 16 | *** | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| C. Right. 24hr Water Intake Bars | 3.7 | 16 | ** | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| D. Food Intake | n/a | n/a | n/a | 4.5 | 49 | 784 | **** | 23.4 | 1 | 16 | *** | 1.5 | 49 | 784 | * |
| E. Water Intake | n/a | n/a | n/a | 4.3 | 49 | 784 | **** | 13.9 | 1 | 16 | ** | 1 | 49 | 784 | ns |
| F. Ambulatory Locomotor Activity | n/a | n/a | n/a | 8.1 | 49 | 784 | **** | 15.6 | 1 | 16 | ** | 4.8 | 49 | 784 | **** |
| G. Wheel | n/a | n/a | n/a | 11.1 | 49 | 784 | **** | 37.1 | 1 | 16 | **** | 7.3 | 49 | 784 | **** |
| H. Energy Expenditure | n/a | n/a | n/a | 13.2 | 49 | 784 | 47.35 | 1 | 16 | ** * * | 4.8 | 49 | 784 | ||
| I. RER | n/a | n/a | n/a | 11.9 | 49 | 784 | **** | 1.5 | 1 | 16 | ns | 7.9 | 49 | 784 | **** |
|
| |||||||||||||||
| Figure 5. NtsR1Cre HFD Operant | F | DFn | DFd | p | Baseline t | df | p | Fasted t | df | p | Pre-fed t | df | p | ||
|
|
|||||||||||||||
| C. Magazine Entries | 3.1 | 5 | 24 | * | 2.3 | 24 | ns | 1.8 | 24 | ns | 2.6 | 24 | ns | ||
| D. % Correct Nose Pokes | 0.9 | 5 | 24 | ns | 0.9 | 24 | ns | 1.6 | 24 | ns | 0.7 | 24 | ns | ||
| E. Sucrose Pellets Obtained | 13.2 | 5 | 24 | **** | 3.2 | 24 | * | 5.9 | 24 | **** | 4.0 | 24 | ** | ||
| F. PR Value | 9.1 | 5 | 24 | **** | 1.9 | 24 | ns | 5.1 | 24 | **** | 2.9 | 24 | * | ||
| G. Sucrose Pellets Eaten | 3.7 | 5 | 24 | * | 1.5 | 24 | ns | 1.0 | 24 | ns | 3.4 | 24 | ** | ||
|
|
|||||||||||||||
| Figure 6. HDF Chronic | Time F | DFn | DFd | p | Tx F | DFn | DFd | p | Int. F | DFn | DFd | Int. p | |||
|
|
|||||||||||||||
| B. % Weight Change | 43.6 | 8 | 152 | **** | 23.6 | 1 | 19 | *** | 8.5 | 8 | 152 | **** | |||
| C. HFD Intake | 0.7 | 8 | 180 | ns | 8.5 | 1 | 180 | ** | 0.9 | 8 | 180 | ns | |||
| E. Total Distance | 9.0 | 3.0 | 68.0 | **** | 28.2 | 1 | 68 | **** | 3.5 | 3 | 68 | * | |||
|
|
|||||||||||||||
| Figure 7. Anxiety and CPP | t | df | p | ||||||||||||
|
|
|||||||||||||||
| A. CPP | 0.2 | 8 | ns | ||||||||||||
| B. % Nestlet Shredded | 1.8 | 12 | ns | ||||||||||||
| C. % Marbles Buried | 0.2 | 11 | ns | ||||||||||||
|
|
|||||||||||||||
| Figure 8. Temperature and Telemetry | Time F | DFn | DFd | p | Tx F | DFn | DFd | p | Int. F | DFn | DFd | Int. p | |||
|
|
|||||||||||||||
| B. Chow Temperature | 6.5 | 5 | 96 | **** | 24.4 | 1 | 96 | **** | 1.8 | 5 | 96 | ns | |||
| C. HFD Temperature | 3.1 | 5 | 48 | * | 0.3 | 1 | 48 | ns | 0.4 | 5 | 48 | ns | |||
| D. Chow MAP | 2.5 | 144 | 1440 | **** | 12.1 | 1 | 10 | ** | 4.1 | 144 | 1440 | **** | |||
| E. HFD MAP | 3.3 | 144 | 1296 | **** | 4.9 | 1 | 9 | ns | 5.7 | 144 | 1296 | **** | |||
Highlights.
Activating VTA NtsR1 neurons promotes weight loss in normal weight and obese mice
VTA NtsR1 activation reduces feeding and increases physical activity
Activating VTA NtsR1 neurons reduces the motivation to work for palatable rewards
Activating VTA NtsR1 neurons does not invoke adverse effects
Acknowledgements
Krystal Santiago-Colon was supported by the NIH-NINDS Bridge to the PhD in Neuroscience (BPNP)- ENDURE Program (R25-NS090989). Rabail Khan was supported by the Fulbright Foreign Student Program. Patricia Perez-Bonilla received support from the NIH-funded Integrative Pharmacological Science Training Program (2T32-GM092715-06, PI Anne Dorrance). This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under awards to Patricia Perez-Bonilla (F31-DK121373) and Gina Leinninger (R01-DK103808).
Footnotes
Conflict of Interest: The authors do not have any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Augustine V, Ebisu H, Zhao Y, Lee S, Ho B, Mizuno GO, Tlan L, Oka Y, 2019. Temporally and Spatially Distinct Thirst Satiation Signals. Neuron 103, 249. 10.1016/j.neuron.2019.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher B, Waisman A, Lu LF, 2018. Conditional Gene-Targeting in Mice: Problems and Solutions. Immunity 48, 835–836. 10.1016/j.immuni.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, Difeliceantonio AG, 2010. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 1350, 43–64. 10.1016/j.brainres.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML, 2015. Review Pleasure Systems in the Brain. Neuron 86, 646–664. 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoudt L, Roelofs TJM, De Jong JW, De Leeuw AE, Luijendijk MCM, Wolterink-Donselaar IG, Van Der Plasse G, Adan RAH, 2017. Does activation of midbrain dopamine neurons promote or reduce feeding? Int. J. Obes 41, 1131–1140. 10.1038/ijo.2017.74 [DOI] [PubMed] [Google Scholar]
- Boekhoudt L, Wijbrans EC, Man JHK, Luijendijk MCM, de Jong JW, van der Plasse G, Vanderschuren LJMJ, Adan RAH, 2018. Enhancing excitability of dopamine neurons promotes motivational behaviour through increased action initiation. Eur. Neuropsychopharmacol 28, 171–184. 10.1016/j.euroneuro.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Botto JM, Guillemare E, Vincent JP, Mazella J, 1997. Effects of SR 48692 on neurotensin-induced calcium-activated chloride currents in the Xenopus oocyte expression system: Agonist-like activity on the levocabastine-sensitive neurotensin receptor and absence of antagonist effect on the levocabastine insensi. Neurosci. Lett 223, 193–196. 10.1016/S0304-3940(97)13437-1 [DOI] [PubMed] [Google Scholar]
- Boules M, Li Z, Smith K, Fredrickson P, Richelson E, 2013. Diverse roles of neurotensin agonists in the central nervous system. Front. Endocrinol. (Lausanne). 4, 36. 10.3389/fendo.2013.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, U M, Brischoux F, Chakraborty S, Brierley DI, Ungless MA, 2009. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U. S. A 106, 4894–4899. 10.1073/pnas.0811507106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Kelley AE, Le Moal M, Stinus L, 1986. Ventral tegmental area infusion of substance P, neurotensin and enkephalin: Differential effects on feeding behavior. Neuroscience 18, 659–669. 10.1016/0306-4522(86)90061-8 [DOI] [PubMed] [Google Scholar]
- Carraway R, Leeman SE, 1973. The Isolation of a New Hypotensive Peptide, Neurotensin, from Bovine Hypothalami. J. Biol. Chem 248, 6854–6861. [PubMed] [Google Scholar]
- Caspard H, Jabbour S, Hammar N, Fenici P, Sheehan JJ, Kosiborod M, 2017. Recent Trends in the Prevalence of Type 2 Diabetes and the Association With Abdominal Obesity Lead to Growing Health Disparities in the USA: An analysis of the NHANES surveys from 1999 to 2014. Diabetes. Obes. Metab 20, 667–671. 10.1111/dom.13143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J, Zhang TX, 1997. Cardiovascular effects of neurotensin microinjections into the nucleus of the solitary tract. Brain Res. 749, 35–43. [DOI] [PubMed] [Google Scholar]
- Conway B, Rene A, 2004. Obesity as a disease: no lightweight matter. Obes. Rev 5, 145–151. 10.1111/j.1467-789X.2004.00144.x [DOI] [PubMed] [Google Scholar]
- Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM, 2013. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA - J. Am. Med. Assoc 10.1001/jama.2013.280928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM, 2016. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am. J. Physiol. Heart Circ. Physiol 310, 365–375. 10.1152/ajpheart.00562.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle AL, Gajewski PA, Yang M, Kechner ME, Al Masraf BS, Kennedy PJ, Wang H, Mazei-Robison MS, Robison AJ, 2015. Experience-dependent induction of hippocampal ΔFosB controls learning. J. Neurosci 35, 13773–13783. 10.1523/JNEUROSCI.2083-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott PJ, Nemeroff CB, 1986. Repeated neurotensin administration in the ventral tegmental area: Effects on baseline and d-amphetamine-induced locomotor activity. Neurosci. Lett 68, 239–244. 10.1016/0304-3940(86)90149-7 [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ko MCH, Woods JH, Richelson E, 2005. Antinociceptive, hypothermic, hypotensive, and reinforcing effects of a novel neurotensin receptor agonist, NT69L, in rhesus monkeys. Pharmacol. Biochem. Behav 80, 341–9. 10.1016/j.pbb.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Feifel D, Goldenberg J, Melendez G, Shilling PD, 2010. The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology 58. 10.1016/j.neuropharm.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin K, Paxinos G, 2008. The Mouse Brain in Stereotaxic Coordinates, Compact, 3rd ed.Academic Press. [Google Scholar]
- Gully D, Lespy L, Canton M, Rostène W, Kitabgi P, Le Fur G, Maffrand JP, 1996. Effect of the neurotensin receptor antagonist SR48692 on rat blood pressure modulation by neurotensin. Life Sci. 58, 665–674. 10.1016/S0024-3205(96)80005-1 [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WPT, 2005. Obesity. Lancet 366, 1197–1209. 10.1016/S0140-6736(05)67483-1 [DOI] [PubMed] [Google Scholar]
- Hawkins MF, 1986. Aphagia in the rat following microinjection of neurotensin into the ventral tegmental area. Life Sci. 38, 2383–8. 10.1016/0024-3205(86)90606-5 [DOI] [PubMed] [Google Scholar]
- Heymann G, Jo YS, Reichard KL, McFarland N, Chavkin C, Palmiter RD, Soden ME, Zweifel LS, 2020. Synergy of Distinct Dopamine Projection Populations in Behavioral Reinforcement. Neuron 105, 909–920. 10.1016/j.neuron.2019.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu TM, Bazzino P, Hurh SJ, Konanur VR, Roitman JD, Roitman MF, 2020. Thirst recruits phasic dopamine signaling through subfornical organ neurons. Proc. Natl. Acad. Sci. U. S. A 117, 30744–30154. 10.1073/pnas.2009233117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Masureel M, Qu Q, Janetzko J, Inoue A, Kato HE, Robertson MJ, Nguyen KC, Glenn JS, Skiniotis G, Kobilka BK, 2020. Structure of the neurotensin receptor 1 in complex with β-arrestin 1. Nature 579, 303–308. 10.1038/s41586-020-1953-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Burgess SK, Nemeroff CB, Prange AJJ, 1983. Behavioral and neurochemical effects of neurotensin microinjection into the ventral tegmental area of the rat. Neuroscience 8, 495–505. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Cador M, Stinus L, Le Moal M, 1989. Neurotensin, substance P, neurokinin-α, and enkephalin: injection into ventral tegmental area in the rat produces differential effects on operant responding. Psychopharmacology (Berl). 10.1007/BF00442258 [DOI] [PubMed] [Google Scholar]
- Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S, 2016. Association of pharmacological treatments for obesity withweight loss and adverse events a systematic review and meta-analysis. JAMA - J. Am. Med. Assoc 315, 2424–2434. 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ER, Leckstrom A, Mizuno TM, 2008. Impaired anorectic effect of leptin in neurotensin receptor 1-deficient mice. Behav. Brain Res 194, 66–71. 10.1016/j.bbr.2008.06.024 [DOI] [PubMed] [Google Scholar]
- Kim ER, Mizuno TM, 2010. Role of neurotensin receptor 1 in the regulation of food intake by neuromedins and neuromedin-related peptides. Neurosci. Lett 468, 64–67. 10.1016/j.neulet.2009.10.064 [DOI] [PubMed] [Google Scholar]
- Kruger J, Kohl HW, Miles IJ, 2001. Prevalence of regular physical activity among adults - United States, 2001 and 2005. Morb. Mortal. Wkly. Rep 56, 1209–1212. [PubMed] [Google Scholar]
- Kurt G, Woodworth HL, Fowler S, Bugescu R, Leinninger GM, 2019. Activation of lateral hypothalamic area neurotensin-expressing neurons promotes drinking. Neuropharmacology 154, 13–21. 10.1016/j.neuropharm.2018.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz TW, Lujan HL, DiCarlo SE, 2014. The 24 h pattern of arterial pressure in mice is determined mainly by heart Rate-Driven variation in cardiac output. Physiol. Rep 2. 10.14814/phy2.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC, 2011. Projection-Specific Modulation of Dopamine Neuron Synapses by Aversive and Rewarding Stimuli. Neuron 70, 855–862. 10.1016/j.neuron.2011.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC, 2014. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76, 351–359. 10.1016/j.neuropharm.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC, 2012. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217. 10.1038/nature11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti M, Brun P, Clerget M, Steinberg R, Soubrie P, Renaud B, Suaud-Chagny M-F, 2004. Specific involvement of neurotensin type 1 receptor in the neurotensin-mediated in vivo dopamine efflux using knock-out mice. J. Neurochem 89, 1–6. 10.1046/j.1471-4159.2003.02231.x [DOI] [PubMed] [Google Scholar]
- Lutter M, Bahl E, Hannah C, Hofammann D, Acevedo S, Cui H, McAdams CJ, Michaelson JJ, 2017. Novel and ultra-rare damaging variants in neuropeptide signaling are associated with disordered eating behaviors. PLoS One 12. 10.1371/journal.pone.0181556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DAA, Browne RW, Shaw JK, Radhakrishnan SK, Khare P, España RA, Clark SD, 2016. Clozapine N-oxide administration produces behavioral effects in long-evans rats: Implications for designing DREADD experiments. eNeuro 3, 0219–0216. 10.1523/ENEURO.0219-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM, Buchta WC, Bentzley BS, Quintanilla J, Cope ZA, Lin EC, Riedy MD, Scofield MD, Messinger J, Ruiz CM, Riegel AC, España RA, Aston-Jones G, 2019. Chemogenetic manipulations of ventral tegmental area dopamine neurons reveal multifaceted roles in cocaine abuse. J. Neurosci 39, 503–518. 10.1523/JNEUROSCI.0537-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL, 2006. The ventral tegmental area revisited: Is there an electrophysiological marker for dopaminergic neurons? J. Physiol 557, 907–924. 10.1113/jphysiol.2006.117069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2009. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841. 10.1038/nature08028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR, Stuber GD, 2017. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci 20, 449–458. 10.1038/nn.4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Guarnieri DJ, DiLeone RJ, 2010. Metabolic hormones, dopamine circuits, and feeding. Front. Neuroendocrinol 31, 104–112. 10.1016/j.yfrne.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM, 2013. Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- Opland D, Sutton A, Woodworth H, Brown J, Bugescu R, Garcia A, Christensen L, Rhodes C, Myers M, Leinninger G, 2013. Loss of neurotensin receptor-1 disrupts the control of the mesolimbic dopamine system by leptin and promotes hedonic feeding and obesity. Mol. Metab 2, 423–434. 10.1016/j.molmet.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CM, Wong JMT, Leinninger GM, Allison MB, Mabrouk OS, Kasper CL, Gonzalez IE, Mackenzie A, Jones JC, Kennedy RT, Myers MG, 2015. Ventral tegmental area neurotensin signaling links the lateral hypothalamus to locomotor activity and striatal dopamine efflux in male mice. Endocrinology 156, 1692–1700. 10.1210/en.2014-1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Bonilla P, Santiago-Colon K, Leinninger GM, 2020. Lateral hypothalamic area neuropeptides modulate ventral tegmental area dopamine neurons and feeding. Physiol. Behav 223. 10.1016/j.physbeh.2020.112986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart E, Courtney NA, Branch SY, Ford CP, Beckstead MJ, 2015. Neurotensin induces presynaptic depression of D2 dopamine autoreceptor-mediated neurotransmission in midbrain dopaminergic neurons. J. Neurosci 35, 11144–52. 10.1523/JNEUROSCI.3816-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Zou J, Drouin-Ouellet J, Kim KYA, Cicchetti F, Awatramani RB, 2014. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 9, 930–943. 10.1016/j.celrep.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Castro DC, DiFeliceantonio AG, Robinson MJF, Berridge KC, 2013. Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neurosci. Biobehav. Rev 37, 1919–1931. 10.1016/j.neubiorev.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ, 2003. The supersizing of america: Portion size and the obesity epidemic. Nutr. Today 38, 1723. 10.1097/00017285-200303000-00004 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM, 2003. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: Implications for studies of natural motivation, psychiatry, and drug abuse. J. Pharmacol. Exp. Ther 305, 1–8. 10.1124/jpet.102.035063 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa MM, 2012. The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron 76, 470–485. 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheggi S, De Montis MG, Gambarana C, 2018. Making sense of rodent models of anhedonia. Int. J. Neuropsychopharmacol 21, 1049–1065. 10.1093/ijnp/pyy083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W, 2008. Critical analysis of bariatric procedures in Prader-Willi syndrome. J. Pediatr. Gastroenterol. Nutr 10.1097/01.mpg.0000304458.30294.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder LE, Furdock R, Quiles CR, Kurt G, Perez-Bonilla P, Garcia A, Colon-Ortiz C, Brown J, Bugescu R, Leinninger GM, 2019. Mapping the populations of neurotensin neurons in the male mouse brain. Neuropeptides 76, 101930. 10.1016/j.npep.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder LE, Leinninger GM, 2018. Role of central neurotensin in regulating feeding: Implications for the development and treatment of body weight disorders. Biochim. Biophys. Acta - Mol. Basis Dis 10.1016/j.bbadis.2017.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibenhener ML, Wooten MC, 2015. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp 96. 10.3791/52434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Hryhorczuk C, Fulton S, 2012. Progressive-ratio responding for palatable high-fat and high-sugar food in mice. J. Vis. Exp 63. 10.3791/3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV, 2016. DREADDs: Use and application in behavioral neuroscience. Behav. Neurosci 130, 137–155. 10.1037/bne0000135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song AJ, Palmiter RD, 2018. Detecting and Avoiding Problems When Using the Cre–lox System. Trends Genet. 34, 333–340. 10.1016/j.tig.2017.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Souliere F, Brun P, Chouvet G, Steinberg R, Soubrie P, Renaud B, Suaud-Chagny MF, 1998. Differential effects of neurotensin on dopamine release in the caudal and rostral nucleus accumbens: a combined in vivo electrochemical and electrophysiological study. Neuroscience 85, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Stamatakis AM, Kantak PA, 2015. Considerations when using cre-driver rodent lines for studying ventral tegmental area circuitry. Neuron 85, 439–445. 10.1016/j.neuron.2014.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J, 2011. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med 365, 1597–1604. 10.1056/NEJMoa1105816 [DOI] [PubMed] [Google Scholar]
- Tabarean IV, 2020. Neurotensin induces hypothermia by activating both neuronal neurotensin receptor 1 and astrocytic neurotensin receptor 2 in the median preoptic nucleus. Neuropharmacology 171. 10.1016/j.neuropharm.2020.108069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Masu M, Nakanishi S, 1990. Structure and functional expression of the cloned rat neurotensin receptor. Neuron 4, 847–854. 10.1016/0896-6273(90)90137-5 [DOI] [PubMed] [Google Scholar]
- Thaker VV, 2017. GENETIC AND EPIGENETIC CAUSES OF OBESITY. Adolesc. Med. State Art Rev 28, 379–405. [PMC free article] [PubMed] [Google Scholar]
- Torruella-Suárez ML, McElligott ZA, 2020. Neurotensin in reward processes. Neuropharmacology 167, 1080–05. 10.1016/j.neuropharm.2020.108005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torruella-Suárez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A, Dange K, Patel GK, McHenry JA, Andrew Hardaway J, Kantak PA, Crowley NA, DiBerto JF, Faccidomo SP, Hodge CW, Stuber GD, McElligott ZA, 2020. Manipulations of central amygdala neurotensin neurons alter the consumption of ethanol and sweet fluids in mice. J. Neurosci 40, 632–647. 10.1523/JNEUROSCI.1466-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita N, Oury-Donat F, Chalon P, Guillemot M, Kaghad M, Bachy A, Thurneyssen O, Garcia S, Poinot-Chazel C, Casellas P, Keane P, Le Fur G, Maffrand JP, Soubrie P, Caput D, Ferrara P, 1998. Neurotensin is an antagonist of the human neurotensin NT2 receptor expressed in Chinese hamster ovary cells. Eur. J. Pharmacol 360, 265–272. 10.1016/S0014-2999(98)00678-5 [DOI] [PubMed] [Google Scholar]
- Wing RR, Hill JO, 2001. Successful weight loss maintenance. Annu Rev Nutr. 10.1146/annurev.nutr.21.1.323\r21/1/323 [pii] [DOI] [PubMed] [Google Scholar]
- Woodworth HL, Batchelor HM, Beekly BG, Bugescu R, Brown JA, Kurt G, Fuller PM, Leinninger GM, 2017a. Neurotensin Receptor-1 Identifies a Subset of Ventral Tegmental Dopamine Neurons that Coordinates Energy Balance. Cell Rep. 20, 1881–1892. 10.1016/j.celrep.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth HL, Beekly BG, Batchelor HM, Bugescu R, Perez-Bonilla P, Schroeder LE, Leinninger GM, 2017b. Lateral Hypothalamic Neurotensin Neurons Orchestrate Dual Weight Loss Behaviors via Distinct Mechanisms. Cell Rep. 21, 3116–3128. 10.1016/j.celrep.2017.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth HL, Perez-Bonilla PA, Beekly BG, Lewis TJ, Leinninger GM, 2018. Identification of neurotensin receptor expressing cells in the ventral tegmental area across the lifespan. eNeuro 5. 10.1523/ENEURO.0191-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachry JE, Nolan SO, Brady LJ, Kelly SJ, Siciliano CA, Calipari ES, 2021. Sex differences in dopamine release regulation in the striatum. Neuropsychopharmacology 46, 491–499. 10.1038/s41386-020-00915-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q-Y, Palmiter RD, 1995. Dopamine-Deficient Mice Are Severely Hypoactive, Adipsic, and Aphagic. Cell 83, 1197–1209. [DOI] [PubMed] [Google Scholar]
- Zogovic B, Pilowsky PM, 2012. Intrathecal neurotensin is hypotensive, sympathoinhibitory and enhances the baroreflex in anaesthetized rat. Br. J. Pharmacol 166, 378–389. 10.1111/j.1476-5381.2011.01760.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.