Abstract
Purpose:
Examine National Cancer Database (NCDB) data to comparatively evaluate overall survival (OS) between transarterial radioembolization (TARE) and systemic therapy in hepatocellular carcinoma (HCC) with major vascular invasion (HCC-MVI).
Materials and Methods:
1514 HCC-MVI patients receiving first-line TARE or systemic therapy were identified from the NCDB. OS was compared by propensity-score matched Cox regression and landmark analysis. Efficacy was also compared within a target trial framework.
Results:
TARE usage doubled between 2010 and 2015. Pre-treatment intervals were longer for TARE than for systemic therapy (mean (median) 66.5 (60) days versus 46.8 (35) days, respectively, p < 0.0001). Propensity-score matched and landmark-time adjusted analysis associated TARE with HR 0.74 (95% CI 0.60 to 0.91, p = 0.005) and median OS 7.1 months (95% CI 5.0 to 10.5) versus 4.9 months (95% CI 3.9 to 6.5) for systemically-treated patients. Target trial emulation involving 236 patients with unilobular HCC-MVI, low comorbidities, creatinine < 2.0 mg/dL, bilirubin < 2.0 mg/dL, and INR < 1.7, associated TARE with HR 0.57 (95%CI 0.39 to 0.83, p = 0.004) and median OS 12.9 months (95% CI 7.6 to 19.2) versus 6.5 months (95% CI 3.6 to 11.1) for the systemic therapy arm.
Conclusion:
Propensity-score matched analyses involving pragmatic and target trial HCC-MVI cohorts associated TARE with significant survival benefits over systemic therapy. While not a substitute for prospective trials, these findings suggest rising use of TARE for HCC-MVI is accompanied by improved OS. Further trials of TARE in HCC-MVI are needed.
Keywords: hepatocellular carcinoma, selective internal radiation therapy, radioembolization, propensity score, landmark analysis, comparative effectiveness, survival
INTRODUCTION
Major vascular invasion (MVI), usually involving the portal vein or its branches, places HCC at an advanced-stage and confers a poor prognosis1. The Barcelona Clinic Liver Cancer staging system and guidelines from the American Association for the Study of Liver Disease and European Association for the Study of the Liver presently recommend systemic therapy as first-line treatment for HCC involving MVI in patients with preserved liver function2–4. Notwithstanding, some specialized centers report using transarterial radioembolization (TARE) to treat patients with advanced HCC and MVI5,6.
TARE has been found safe and effective across various stages of HCC in prospective trials and retrospective studies 7–11. However, its superiority over the standard of care in terms of overall survival (OS) has not been shown in patients with HCC and MVI. Two randomized controlled trials (RCTs) (SARAH12 and SURveNIB13) have compared TARE to sorafenib in advanced HCC. No survival advantage to either treatment was identified by these trials, however both involved heterogeneous cohorts not limited to those with MVI. Furthermore, neither were adequately powered to support sub-group analyses. A trial focused specifically on HCC with MVI (YES-P trial, NCT01887717) had been initiated but was closed prematurely due to poor accrual14. As questions regarding optimal treatment of HCC with MVI remain unanswered, this study analyzed the National Cancer Database (NCDB) to examine the efficacy of TARE in this context, taking advantage of several statistical methods to address forms of bias that may affect observational studies.
MATERIALS AND METHODS
Study Endpoint, Cohort, and Objective
The main objective was to compare the observational endpoint of OS between TARE and systemically treated patients with HCC-MVI balanced on other clinically relevant covariates.
Data Collection
Institutional review board approval under 45 CFR 46.110 and 21 CFR 56.110 was granted to access the NCDB participant user file (PUF). Only cases diagnosed from 2010 to 2015 were included since clinical and follow-up data were complete only for those years at time of analysis. To avoid confounding, cases involving multiple cancer diagnoses were excluded. HCC was identified by International Classification of Disease – Oncology 3rd edition histology code 8170 with major vascular involvement (HCC-MVI) further identified based on cancer stage and extent in accordance with American Joint Commission on Cancer (AJCC) 7th edition staging that defines MVI as invasion of branches of the main portal vein or one or more hepatic veins. The resulting dataset included 6211 HCC-MVI cases.
Of these, 421 patients were identified as initially treated with TARE and 1939 patients as initially treated with single-agent systemic therapy. All other patients including those initially treated by locoregional therapy or combinations of TARE and systemic therapy were excluded (Figure 1). Immunotherapy, which did not receive US approval until 2017 and comprised initial treatment in 0.46% of screened cases, was not considered systemic therapy for the study purpose. Among those remaining, 117 and 729 respectively were excluded because of missing laboratory data with no significant differences noted between cases excluded and not excluded except for higher proportions of Charlson-Deyo Comorbidity Score (CDCS) > 0 among cases remaining in both treatment groups (p = 0.043 and 0.002, respectively). The resulting pre-match dataset comprised 304 TARE-treated and 1210 systemically treated patients with no missing data (Figure 1).
Figure 1:
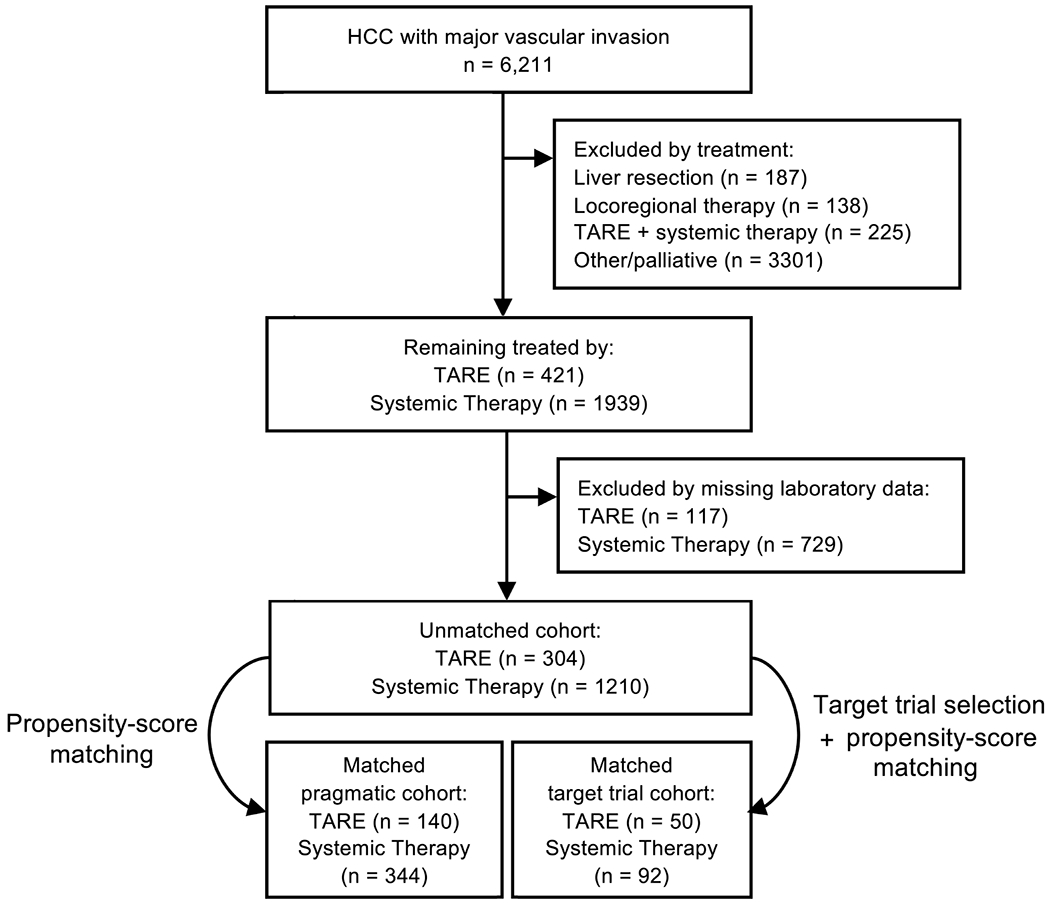
CONSORT Diagram
Statistical methods
Statistical methods are described in the Supplementary Methods. Since OS is measured by NCDB from the diagnosis date, times to treatment are tantamount to guaranteed periods of survival. This potential source of guarantee-time bias was addressed by placing a conditional landmark at 60 days with landmark sensitivity assessed at 30 and 90 days15.
Covariate selection and treatment propensity modeling
Applying a causal framework16, the following covariates were prospectively chosen to model treatment propensity: age, sex, diagnosis year, facility type, Charlson-Deyo Comorbidity Score (CDCS), creatinine level (mg/dL), bilirubin level (mg/dL), international normalized ratio (INR), alpha feto-protein (AFP) range, tumor focality, lobar extent, and maximum unidimensional size. The propensity model included diagnosis year to account for changing treatment availability and overall improved cancer care over time. The included laboratory values are relevant to both treatment and prognosis in liver disease. CDCS, ranging 0 (no comorbidities) to 3 (high comorbidity), substituted for oncologic performance status17. Propensity scores were calculated by logistic regression and matching was completed by nearest-neighbor, 3:1 maximum ratio, no replacement, and 0.1 caliper18. The covariate balance criterion was standardized mean difference < 0.1.
Target trial emulation (TTE)
TTE is described in recent publications16,19. YES-P (NCT01887717), an RCT initiated in 2014 and prematurely terminated in 2017, provided a reasonable target trial as it was designed to compare TARE to sorafenib in patients with portal vein invasion (clinicaltrials.gov protocol Prot_000.pdf accessed November 27, 2020). TTE study design, including intention to treat (ITT), eligibility criteria, treatment arms, and causal estimands is described in the Supplementary Materials. In brief, TTE involved patients age > 18 years with AJCC stage 3B treatment naïve HCC-MVI, total bilirubin < 2.0 mg/dL, INR < 1.7, creatinine < 2.0 mg/dL, CDCS 0 or 1, unilobular disease, and no extrahepatic extension. Because randomization dates are non-existent for TTE, diagnosis dates provided start times for survival analysis.
RESULTS
Descriptive and multivariable analysis
Patient characteristics are summarized in Table 1. Sex distribution confirmed HCC male preponderance20. Significantly more TARE was used at academic/research facilities. TARE was part of initial HCC treatment in 7.14% of advanced cases in 2010 and in 15.9% in 2015. TARE was the only treatment that increased in usage year over year (Figure 2). Mean (standard deviation) times between diagnosis and treatment for TARE and systemic therapy were 66.5 (47.5) days versus 46.8 (45.7) days respectively (p < 0.0001). Among censored patients, mean (standard deviation) follow-up intervals were 34.3 (21.8) months for TARE and 36.6 (28.3) months (p = 0.54) for systemic therapy. On multivariable Cox regression analysis (Table 2), higher mortality was significantly associated with bilirubin level, INR, alpha feto-protein (AFP) level 400ng/mL or higher, and tumor size 50 mm or greater. Significantly lower mortality was associated with unifocal tumors, treatment with TARE, and treatment at non-community cancer programs. The overall fit of a multivariable model that included 2-way treatment interactions did not differ significantly from that of the main effects model.
Table 1:
Characteristics of the study cohort
| Systemic Therapy | TARE | p | |
|---|---|---|---|
| n | 1210 | 304 | |
| Age (mean (SD)) | 62.10 (8.66) | 63.98 (8.83) | 0.001 |
| Sex = Female (%) | 233 (19.3) | 59 (19.4) | 1.000 |
| Charlson-Deyo Comorbidity Score (%) | 0.030 | ||
| 0 | 620 (51.2) | 177 (58.2) | |
| 1 | 287 (23.7) | 66 (21.7) | |
| 2 | 88 (7.3) | 26 (8.6) | |
| 3 | 215 (17.8) | 35 (11.5) | |
| Facility_Type (%) | <0.001 | ||
| Community | 53 (4.4) | 5 (1.6) | |
| Comprehensive | 268 (22.1) | 39 (12.8) | |
| Academic/research | 742 (61.3) | 227 (74.7) | |
| Network | 147 (12.1) | 33 (10.9) | |
| Year (%) | <0.001 | ||
| 2015 | 226 (18.7) | 93 (30.6) | |
| 2014 | 246 (20.3) | 86 (28.3) | |
| 2013 | 231 (19.1) | 43 (14.1) | |
| 2012 | 195 (16.1) | 36 (11.8) | |
| 2011 | 192 (15.9) | 24 (7.9) | |
| 2010 | 120 (9.9) | 22 (7.2) | |
| Bilirubin | 1.89 (1.60) | 1.31 (1.09) | <0.001 |
| mean (SD) | |||
| Creatinine | 1.40 (1.15) | 1.25 (1.03) | 0.041 |
| mean (SD) | |||
| International Normalized Ratio | 1.37 (0.67) | 1.31 (0.66) | 0.118 |
| mean (SD) | |||
| Alpha-Fetoprotein (%) | 0.066 | ||
| <40 ng/mL | 307 (25.4) | 75 (24.7) | |
| 40 to 399 ng/mL | 242 (20.0) | 76 (25.0) | |
| 400 ng/mL or higher | 600 (49.6) | 131 (43.1) | |
| Unknown | 61 (5.0) | 22 (7.2) | |
| Tumor Extent (%) | 0.004 | ||
| Single lobe | 661 (54.6) | 194 (63.8) | |
| Multiple lobes | 470 (38.8) | 101 (33.2) | |
| Unspecified | 79 (6.5) | 9 (3.0) | |
| Maximum tumor size (%) | 0.098 | ||
| <50 mm | 281 (23.2) | 67 (22.0) | |
| 50 mm or greater | 731 (60.4) | 201 (66.1) | |
| Not determined | 198 (16.4) | 36 (11.8) |
Figure 2.
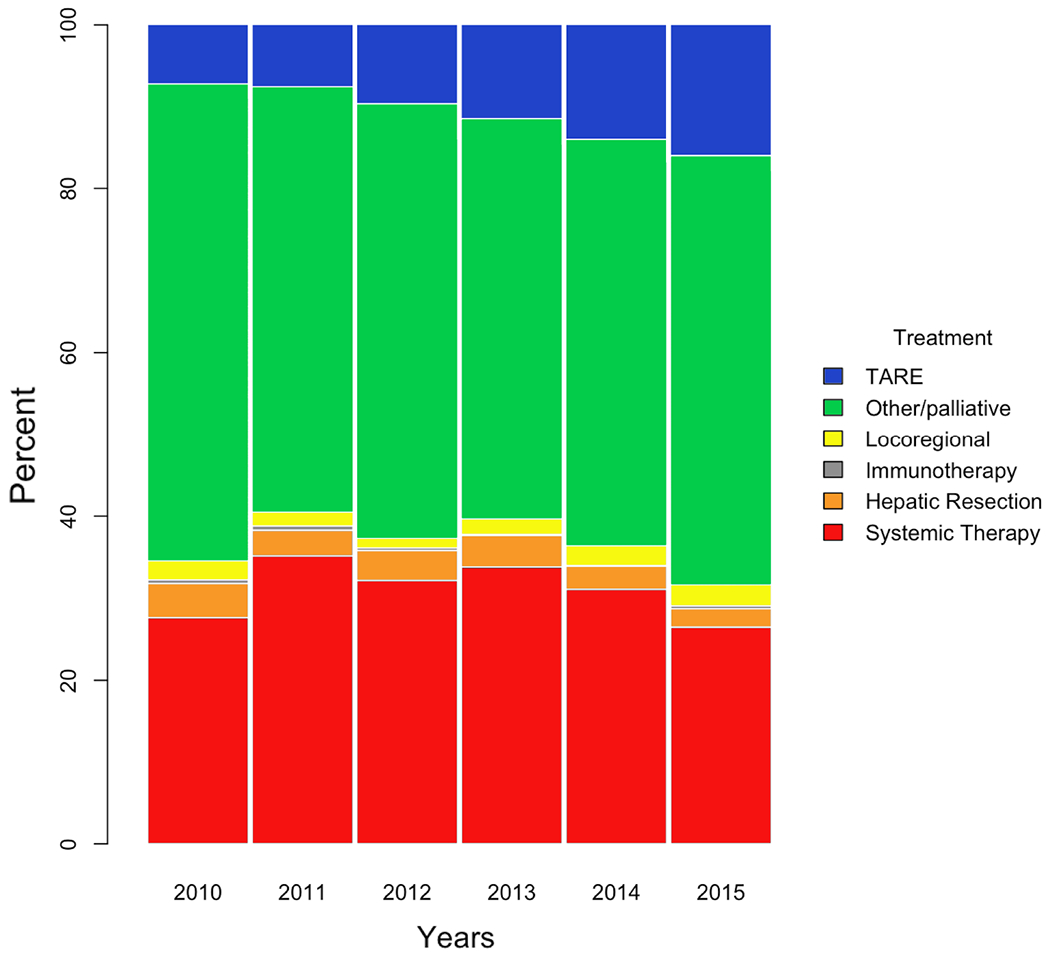
Percentage bar plot showing treatment distribution from 2010 to 2015 (n = 6211). TARE was the only treatment for advanced HCC that increased in utilization every year.
Table 2:
Multivariable Cox proportional hazards regression results
| Variable | HR (95%CI) | P value |
|---|---|---|
| Treatment (TARE vs. Systemic) | 0.78 (0.67-0.90) | <0.001 |
| Age | 1 (0.99-1.01) | 0.924 |
| Sex (Female vs. Male) | 1.07 (0.93-1.23) | 0.369 |
| Year of Diagnosis (vs. 2015) | ||
| 2014 | 0.99 (0.84-1.17) | 0.915 |
| 2013 | 0.91 (0.76-1.10) | 0.338 |
| 2012 | 1.04 (0.86-1.25) | 0.686 |
| 2011 | 1.15 (0.95-1.39) | 0.142 |
| 2010 | 1.13 (0.91-1.40) | 0.270 |
| Cancer Program Type (vs. Community) | ||
| Comprehensive | 0.74 (0.55-0.99) | 0.048 |
| Academic/Research | 0.68 (0.51-0.89) | 0.009 |
| Network | 0.71 (0.52-0.96) | 0.036 |
| Charlson-Deyo Comorbidity Score (vs. 0) | ||
| 1 | 1.00 (0.87-1.15) | 0.999 |
| 2 | 1.05 (0.85-1.30) | 0.647 |
| 3 | 1.09 (0.93-1.27) | 0.293 |
| Creatinine (unit mg/dL) | 1.05 (0.99-1.10) | 0.071 |
| Bilirubin (unit mg/dL) | 1.07 (1.04-1.11) | <0.001 |
| International normalized ratio | 1.11 (1.03-1.20) | 0.008 |
| Alpha feto-protein range (vs. < 40 ng/mL) | ||
| 40 to 399 ng/mL | 1.09 (0.92-1.28) | 0.312 |
| 400 ng/mL or higher | 1.29 (1.13-1.48) | <0.001 |
| Multiple lobe involvement | 1.19 (0.85-1.65) | 0.317 |
| Unifocal (solitary) tumor | 0.52 (0.33-0.81) | 0.003 |
| Tumor size 50 mm or greater | 1.23 (1.07-1.41) | <0.001 |
Propensity-score matching
Matching produced a cohort of 144 TARE-treated and 344 systemically-treated patients with all covariate standardized mean differences below 0.1 (Figure 3). Emulating effects of randomization, post-match propensity score distribution was nearly identical (Figure 4).
Figure 3:
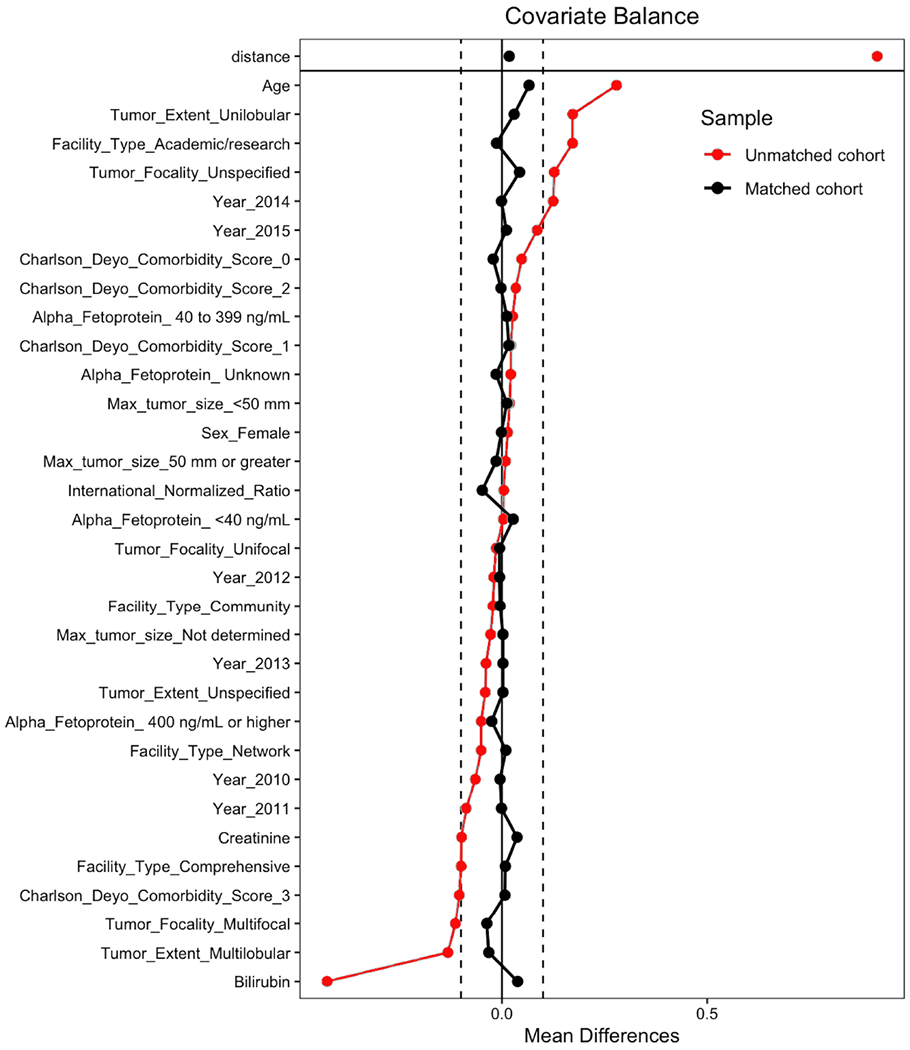
Covariate standardized mean differences before and after propensity-score matching. Vertical hash-lines demarcate 0.1 standardized mean difference as the threshold for covariate balance. Plot also shows a substantial reduction in the overall measure of imbalance (‘distance’).
Figure 4:
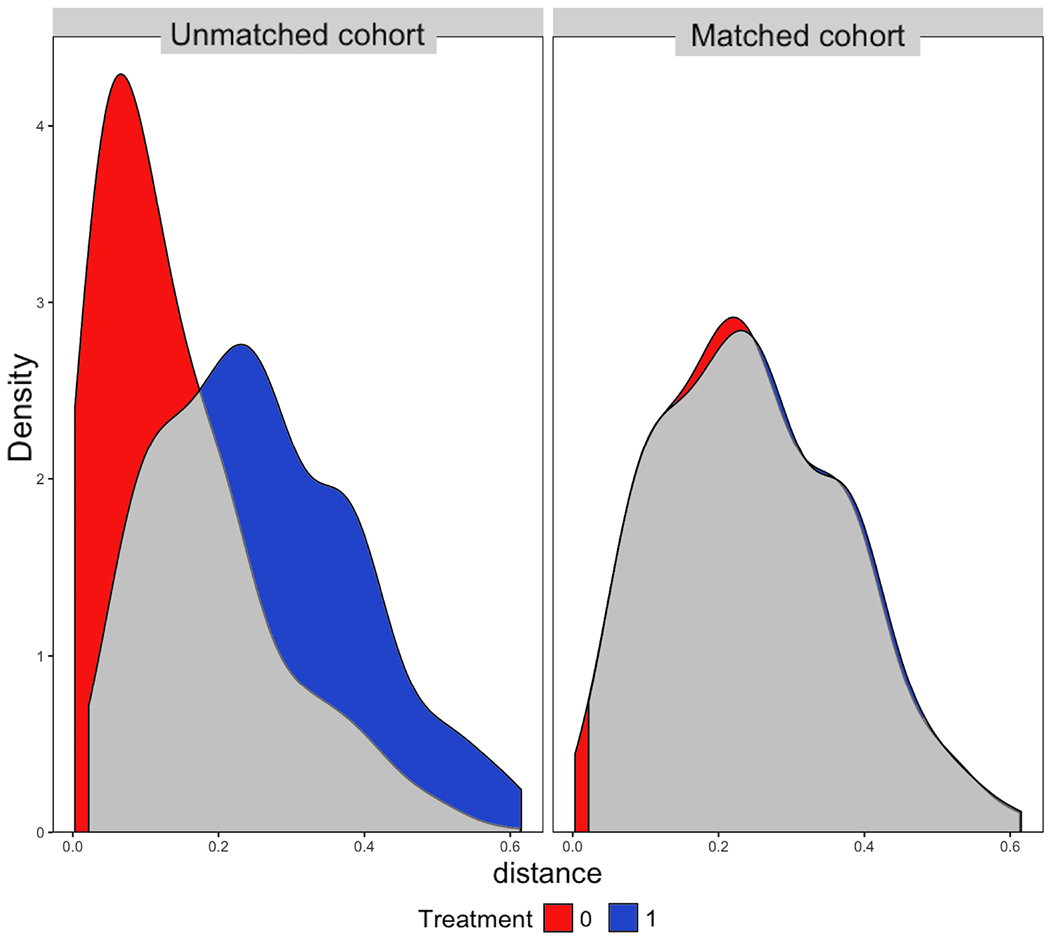
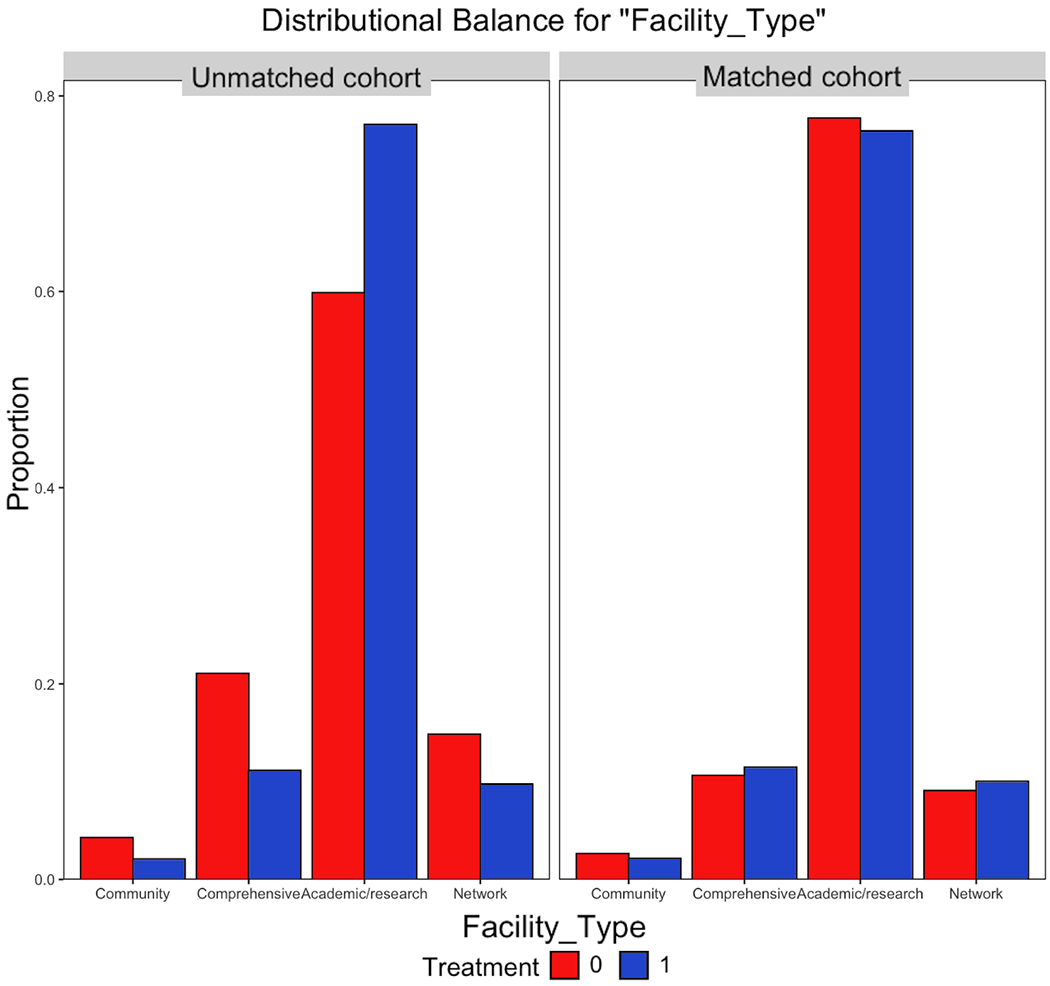
Propensity score distributions before and after matching. A. Consistent with pseudo-randomization, density function plot shows nearly identical post-match propensity-score distributions. B. For example, the distribution of patients among various treatment programs was poorly balanced before matching but well-balanced after matching. 1 = TARE, 0 = Systemic Therapy.
Survival analysis
TARE was associated with hazard ratio (HR) 0.74 (95% CI 0.60 to 0.91, p = 0.005) on Cox regression with 60-day landmark. Median OS was 7.1 months (95% CI 5.0 to 10.5) with TARE and 4.9 months (95% CI 3.9 to 6.5) with systemic therapy (Figure 5A). Significantly improved OS was also found on sensitivity analysis using a 30-day landmark (HR 0.49 (95% CI 0.31 to 0.76, p = 0.002), median OS 11.7 months (95% CI 7.4 to 22.8) vs. 3.9 months (95% CI 2.9 to 6.2)) and a 90-day landmark (HR 0.77 (95% CI 0.65 to 0.92, p = 0.004), median OS 6.7 months (95% CI 5.4 to 9.9) vs. 5.4 months (95% CI 4.5 to 6.7)).
Figure 5:
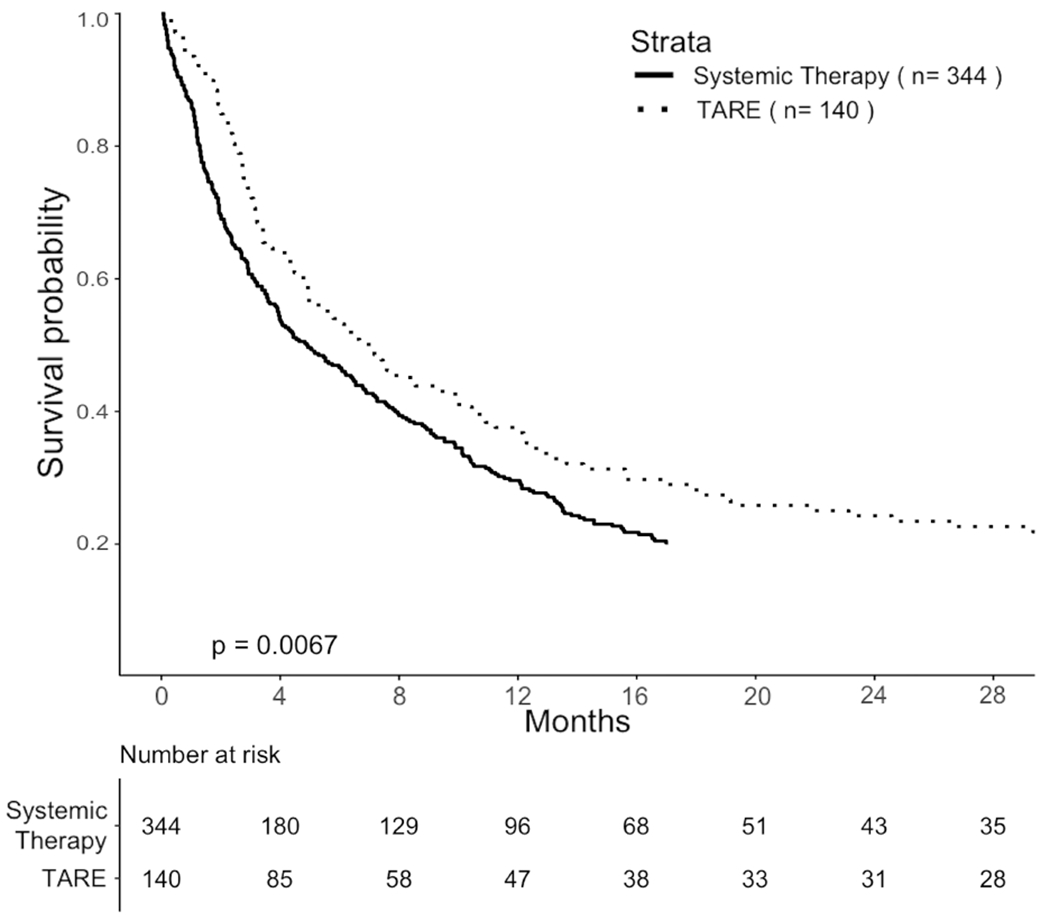
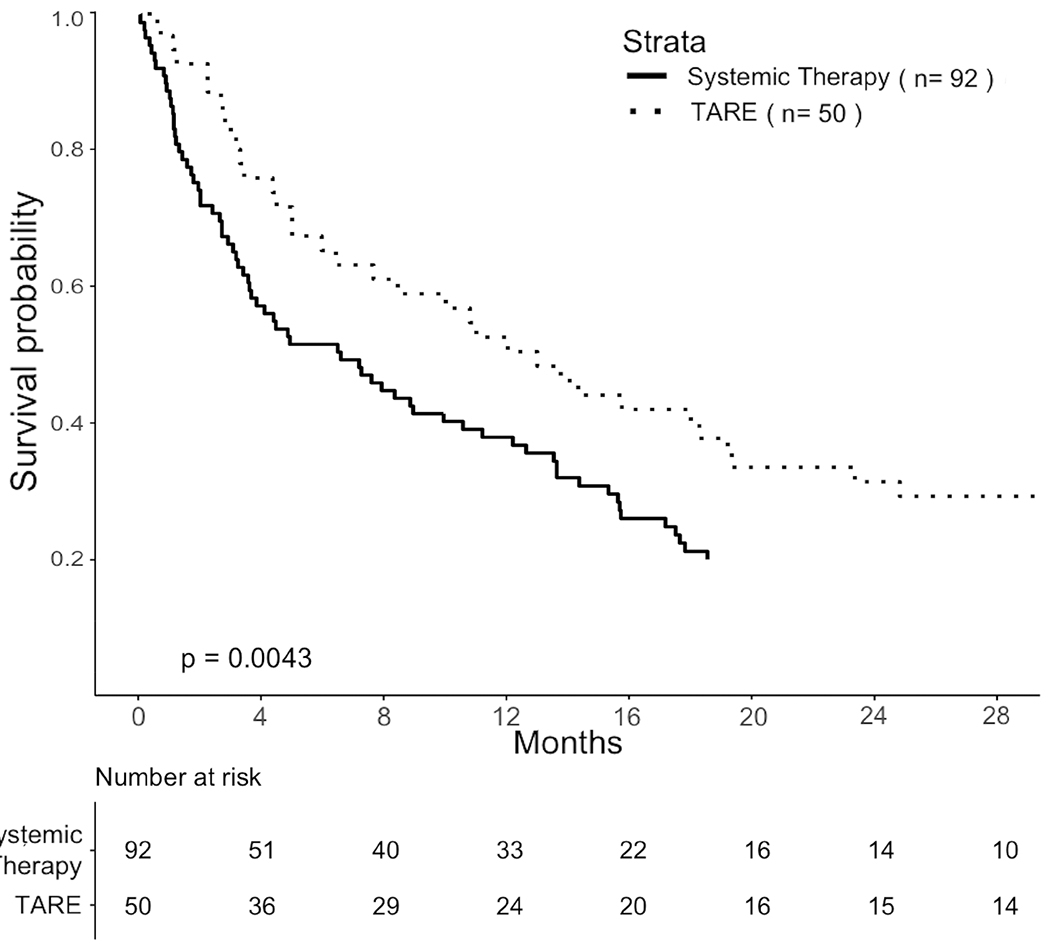
Comparisons of OS in propensity-score matched cohorts. A. Pragmatic cohort with HCC-MVI. B. TTE cohort selected by eligibility criteria based on the YES-P clinical trial protocol.
TTE
The cohort was reduced to 236 patients after applying trial selection criteria. Effects of selection on sample size are summarized in Supplementary Materials. Propensity-score matching produced a matched cohort of 50 TARE-treated and 92 systemically treated patients (Figure 1). On 60-day landmark analysis, TARE was associated with HR 0.57 (95% CI 0.39 to 0.83, p = 0.004). Median OS was 12.9 (95% CI 7.6 to 19.2) months for TARE versus 6.5 (95% CI 3.6 to 11.1) months for systemic treatment (Figure 5B). On landmark sensitivity analysis, HR was 0.65 (95% CI 0.50 to .85, p = 0.002) with median OS 13.4 months (95% CI 8.8 to 18.9) versus 6.0 months (95% CI 5.1 to 9.8) using a 30-day landmark. The HR was 0.72 (95% CI 0.52 to 1.01, p = 0.06) with median OS 13.2 months (95% CI 6.6 to 19.3) vs. 8.9 months (95% CI 4.2 to 14.2) using a 90-day landmark.
DISCUSSION
Several centers have reported increasing institutional experience in using TARE as the primary treatment for intermediate and advanced stages of HCC5,6,21–23. The present study confirmed this to be a national trend for HCC-MVI even though an RCT comparing TARE against the standard of care has never been completed in this patient population. On propensity-score matched analyses of NCDB data, TARE was associated with significantly longer OS relative to systemic therapy as initial treatment of HCC-MVI in both a pragmatic real-world cohort and a cohort defined by a target trial. However, as these findings are based on observational data, they require careful interpretation alongside any available data from prospective trials that may have included patients with HCC-MVI.
In the SARAH RCT that involved a heterogeneous patient cohort, OS was found not to differ significantly between patients assigned to TARE or sorafenib. However, TARE was associated with longer delays between randomization and treatment (mean 21 versus 3 days). From 237 patients assigned by ITT to TARE, 53 (22%) ultimately did not receive TARE, including 8 that progressed after randomization and 26 that ultimately received sorafenib 12. In contrast, 216 of 222 patients (97%) assigned to sorafenib were treated as planned. Similarly, in the SIRveNIB trial, mean time between randomization and treatment was 29 days for TARE and 7 days for sorafenib 13. Of 182 patients assigned TARE, 52 (29%) were precluded, including 24 with excessive hepatopulmonary shunting and 5 with unfavorable angiographic findings, while 162 of 178 (91%) patients assigned to sorafenib were treated as planned13. Considering these were ITT trials, such imbalances likely exerted a negative impact on the measured survival benefits of TARE. The present study also identified delays to TARE, however for observational survival analysis these time differences were readily addressed through landmark analysis15,24. The study finding that TARE nonetheless was associated with improved OS is encouraging given that treatments for advanced HCC are not expected to prolong survival beyond several months25–27.
Further supporting TARE in this patient population, a recent meta-analysis involving SARAH, SURveNIB, and SORAMIC (comparing TARE plus sorafenib to sorafenib alone) data found TARE non-inferior to sorafenib overall, potentially superior in non-cirrhotic patients and patients with hepatitis B, and associated with significantly fewer treatment-related adverse events in patients with advanced HCC with or without MVI27. However, treatment dosimetry in these trials was based on the empiric, body-surface-area, or the Medical Internal Radiation Dosimetry based models currently approved for clinical practice. As pre-treatment planning becomes more efficient and incorporates more advanced and individualized methods of dosimetry, TARE efficacy and safety should improve further28–30.
This study has several limitations. One limitation is the possibility that treatment propensity modeling did not account for all relevant sources of selection bias. However, the NCDB did adequately supply the propensity model with those factors most strongly influencing survival outcome and treatment selection in HCC, including laboratory parameters such as bilirubin, creatinine, and prothrombin time that impact both liver disease severity and eligibility for TARE. Even though all data sought for the propensity model was available, the NCDB did lack certain details, including TARE-specific information such as the specific embolic agent used, catheter position, and radiation dose distribution. Analyzing such factors might have produced additional insights since complete tumor targeting and absorbed radiation dose to the tumor appear to be independent predictors of progression free survival and OS in TARE-treated intermediate and advanced stage HCC28. Because TARE implementation is heterogeneous across institutions, this lack of detail limits this study to estimating only the average treatment effect without enabling further insights related to TARE delivery. Nonetheless, the study findings may be encouraging for patients faced with making a decision between TARE and systemic therapy. TARE may also further increase in overall efficacy as patient-individualized treatment dosimetry gains traction in clinical practice28–30. In this regard, the present study provides a baseline performance estimate for examining improvements in clinical TARE over time. The NCDB also contains few details about systemic therapy, although sorafenib would be the systemic agent most used for advanced HCC between 2010 and 2015. Another limitation of this study was that the available data comprised only an isolated period of time. Systemic therapy for HCC has evolved substantially in recent years, and now includes combined immune checkpoint and angiogenesis inhibition as a first-line treatment option31. Considering this evolution, this study will mainly serve as a historical account of the potential clinical benefit and increasing use of TARE during a time when all clinical guidelines considered the standard of care for advanced HCC to be systemic therapy with a multi-kinase inhibitor. However, by identifying potential benefits from this previous era, this study helps carry the impetus forward in supporting TARE as an alternative to systemic therapy and provides justification for future studies to compare or combine it with immunotherapy and other contemporary regimens for advanced HCC.
To date, a clinical trial comparing TARE to systemic therapy has not been completed successfully in patients with HCC-MVI. To address this knowledge gap , TTE was performed based on the published protocol of YES-P, an uncompleted RCT for HCC-MVI, adapting its design and analysis plan in an effort to mitigate potential biases that may arise from misalignment between observational data analysis and the ideal analysis engendered by a target trial24,32. In factoring ITT into this analysis, overestimations of survival benefit that could arise from a per-protocol analysis were avoided while recognizing the role of systemic therapy as standard of care for HCC-MVI. The association of TARE with improved OS on a pragmatic analysis comprising the real-world spectrum of HCC-MVI suggests that future clinical trials of TARE in advanced HCC could consider less restrictive eligibility criteria, especially since YES-P was terminated due to poor accrual. Other recent observational data analyses indicate careful broadening of the eligibility criteria could lead to greater patient participation, equity, and external validity in oncology trials33.
With immune checkpoint inhibitor antibodies now approved for first-line treatment of HCC31, a prospective trial comparing TARE with multi-kinase inhibitors is no longer timely. The present challenge will be to design and conduct a study comparing a contemporary first-line systemic regimen (such as immune-checkpoint inhibitor plus anti-angiogenic agent) against first-line TARE (optimized using voxel-based or other personalized dosimetry with streamlined pre-treatment planning), or their combination, in cohorts selected using eligibility criteria that supports brisk trial enrollment and external validity. Furthermore, while OS has traditionally been used as a trial endpoint for supporting regulatory approval, the increasing number of second-line and multidisciplinary treatment options available may justify using objective response and progression free survival as pivotal endpoints. It may become increasingly difficult to estimate the clinical benefits of first-line treatments in terms of OS as second-line treatment strategies evolve and increase in effectiveness. The present study provides observational evidence in pragmatic and target trial cohorts that first-line TARE can impact OS in HCC-MVI and gives estimates on treatment effect size and impact of trial selection criteria to justify and inform future trials. The larger improvement in OS observed with TTE suggests that TARE may have particular benefit in patients with HCC-MVI and unilobar disease.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by U.S. National Institutes of Health grants 5P30CA071789 and R01CA161209-06, Agency for Healthcare Research and Quality grant R25HS023185-01, and Patient-Centered Outcomes Research Institute contract R-IMC-1306-03827.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The corresponding author and all co-authors have no relevant conflicts of interest to disclose. The contents of this work do not necessarily reflect the views of The Queen’s Medical Center. The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC’s NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
The contents of this manuscript has not been presented at SIR or any other professional society meeting.
REFERENCES
- 1.Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015;61(1):184–90. DOI: 10.1002/hep.27443. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69(1):182–236. DOI: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67(1):358–380. DOI: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Fuster J, Bruix J, Barcelona-Clinic Liver Cancer G. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl 2004;10(2 Suppl 1):S115–20. DOI: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 5.Salem R, Gabr A, Riaz A, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018;68(4):1429–1440. DOI: 10.1002/hep.29691. [DOI] [PubMed] [Google Scholar]
- 6.Fidelman N, Kerlan RK Jr. Transarterial Chemoembolization and (90)Y Radioembolization for Hepatocellular Carcinoma: Review of Current Applications Beyond Intermediate-Stage Disease. AJR Am J Roentgenol 2015;205(4):742–52. DOI: 10.2214/AJR.15.14802. [DOI] [PubMed] [Google Scholar]
- 7.Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47(1):71–81. DOI: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 8.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138(1):52–64. DOI: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54(3):868–78. DOI: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57(5):1826–37. DOI: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 11.Salem R, Lewandowski R, Roberts C, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol 2004;15(4):335–45. DOI: 10.1097/01.rvi.0000123319.20705.92. [DOI] [PubMed] [Google Scholar]
- 12.Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18(12):1624–1636. DOI: 10.1016/S1470-2045(17)30683-6. [DOI] [PubMed] [Google Scholar]
- 13.Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol 2018;36(19):1913–1921. DOI: 10.1200/JCO.2017.76.0892. [DOI] [PubMed] [Google Scholar]
- 14.Sposito C, Mazzaferro V. The SIRveNIB and SARAH trials, radioembolization vs. sorafenib in advanced HCC patients: reasons for a failure, and perspectives for the future. Hepatobiliary Surg Nutr 2018;7(6):487–489. DOI: 10.21037/hbsn.2018.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol 2013;31(23):2963–9. DOI: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landsittel D, Srivastava A, Kropf K. A Narrative Review of Methods for Causal Inference and Associated Educational Resources. Qual Manag Health Care 2020;29(4):260–269. DOI: 10.1097/QMH.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 17.Boorjian SA, Kim SP, Tollefson MK, et al. Comparative performance of comorbidity indices for estimating perioperative and 5-year all cause mortality following radical cystectomy for bladder cancer. J Urol 2013;190(1):55–60. DOI: 10.1016/j.juro.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Ho DI, K: King G; Stuart E. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis 2007;15(3):199–236. [Google Scholar]
- 19.Hernan MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. Am J Epidemiol 2016;183(8):758–64. DOI: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology 2020. DOI: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rognoni C, Ciani O, Sommariva S, et al. Trans-arterial radioembolization for intermediate-advanced hepatocellular carcinoma: a budget impact analysis. BMC Cancer 2018;18(1):715. DOI: 10.1186/s12885-018-4636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somma F, Stoia V, Serra N, D’Angelo R, Gatta G, Fiore F. Yttrium-90 trans-arterial radioembolization in advanced-stage HCC: The impact of portal vein thrombosis on survival. PLoS One 2019;14(5):e0216935. DOI: 10.1371/journal.pone.0216935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardarelli-Leite L, Chung J, Klass D, et al. Ablative Transarterial Radioembolization Improves Survival in Patients with HCC and Portal Vein Tumor Thrombus. Cardiovasc Intervent Radiol 2020;43(3):411–422. DOI: 10.1007/s00270-019-02404-5. [DOI] [PubMed] [Google Scholar]
- 24.Hernan MA, Sauer BC, Hernandez-Diaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol 2016;79:70–75. DOI: 10.1016/j.jclinepi.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359(4):378–90. DOI: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 26.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57(4):821–9. DOI: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venerito M, Pech M, Canbay A, et al. NEMESIS: Noninferiority, Individual-Patient Metaanalysis of Selective Internal Radiation Therapy with (90)Y Resin Microspheres Versus Sorafenib in Advanced Hepatocellular Carcinoma. J Nucl Med 2020;61(12):1736–1742. DOI: 10.2967/jnumed.120.242933. [DOI] [PubMed] [Google Scholar]
- 28.Allimant C, Kafrouni M, Delicque J, et al. Tumor Targeting and Three-Dimensional Voxel-Based Dosimetry to Predict Tumor Response, Toxicity, and Survival after Yttrium-90 Resin Microsphere Radioembolization in Hepatocellular Carcinoma. J Vasc Interv Radiol 2018;29(12):1662–1670 e4. DOI: 10.1016/j.jvir.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Kafrouni M, Allimant C, Fourcade M, et al. Retrospective Voxel-Based Dosimetry for Assessing the Ability of the Body-Surface-Area Model to Predict Delivered Dose and Radioembolization Outcome. J Nucl Med 2018;59(8):1289–1295. DOI: 10.2967/jnumed.117.202937. [DOI] [PubMed] [Google Scholar]
- 30.Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021;6(1):17–29. DOI: 10.1016/S2468-1253(20)30290-9. [DOI] [PubMed] [Google Scholar]
- 31.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382(20):1894–1905. DOI: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 32.Lodi S, Phillips A, Lundgren J, et al. Effect Estimates in Randomized Trials and Observational Studies: Comparing Apples With Apples. Am J Epidemiol 2019;188(8):1569–1577. DOI: 10.1093/aje/kwz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harvey RD, Bruinooge SS, Chen L, et al. Impact of Broadening Trial Eligibility Criteria for Patients with Advanced Non-Small Cell Lung Cancer: Real-World Analysis of Select ASCO-Friends Recommendations. Clin Cancer Res 2021;27(9):2430–2434. DOI: 10.1158/1078-0432.CCR-20-3857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


