Abstract
Objective:
To identify latent subgroups among patients with Achilles tendinopathy, describe patient characteristics and clinical attributes that defined each subgroup, and develop a clinical classification model for subgroup membership.
Study Design:
Cross-sectional study.
Methods:
145 (73 men) age (mean±SD) 51±14 with clinically diagnosed Achilles tendinopathy completed a baseline evaluation including demographics and medical history, patient-reported outcome measures, clinical exam, tendon structure measures using ultrasound imaging and continuous shear wave elastography, and a functional test battery. Subgroups were identified using Mixture Modeling. We compared the subgroups using one-way ANOVA or Chi-Square tests and Tukey’s post-hoc to identify their defining attributes. We developed a clinical classification model using Logistic Regression and ROC curves.
Results:
Three latent subgroups were identified and named by their distinctive patient characteristics and clinical attributes. Activity-dominant (n=67) had the highest physical activity level, function, and quality of life, reported mild symptoms, and were youngest. Psychosocial-dominant (n=56) had the worst symptoms, impaired function, heightened psychological factors, poorest quality of life, minimal tendon structural alterations, were obese and predominately female. Structure-dominant (n=22) had the most tendon structural alterations, severe functional deficits, moderate symptoms and psychological factors, reduced quality of life, were the oldest, obese, and predominately male. The clinical classification model correctly classified 85% (123/145) of participants.
Conclusion:
Three Achilles tendinopathy subgroups were identified that differed in patient characteristics and clinical attributes.
Keywords: tendon, mixture modeling, clinical classification, Achilles tendon pain, tendinitis
INTRODUCTION
Treatment for Achilles tendinopathy has evolved over the past two decades reflected by a growing understanding of pathophysiology. 38,63,68 Exercise therapy is the current gold standard for treating Achilles tendinopathy, however not all patients achieve full recovery. 4,19,36,39 Up to 40% of patients continue to report poor outcomes following 12 weeks of treatment. 5,19,28,45,48,53,57 Recovery remains poorly defined for tendinopathy which impedes the ability to measure success in rehabilitation.57 Symptom resolution, return to participation, and normalization of tendon structure are all important, but individually may not ensure complete recovery for all patients. 26,62
Interindividual differences among patients with Achilles tendinopathy are poorly understood due to insufficient reporting of patient characteristics.49 Because of this paucity, clinicians have limited evidence to inform their treatment plan or determine a patient’s propensity and time needed to achieve recovery. All patients with Achilles Tendinopathy will continue to be treated the same until we as clinicians understand what makes patients different and which factors influence treatment outcomes. If patients could be classified into distinct subgroups by their specific deficits and other related factors, treatment could shift from a one size fits all approach57 to an individualized treatment strategy. In order to understand if there are ways to improve treatment strategies for patient-specific recovery, it is important to evaluate if all patients diagnosed with Achilles tendinopathy are affected the same or are there subgroups that might need additional or modified treatment strategies.
Mixture Modeling is a method for classifying individuals into heterogeneous subgroups within a population when the groups are not known a priori.43 This model-based approach focuses on relationships among individuals, and identifies patterns among individuals based on who are more similar and separates those who are less similar. Mixture modeling has helped derive targeted treatment approaches for disorders that are multifaceted in nature (e.g. low back pain).64 No previous study has applied mixture modelling to Achilles tendinopathy. The purpose of this study was three-fold; first to identify the number of patient subgroups with Achilles tendinopathy, second to describe which patient characteristics and clinical attributes define each subgroup, and third to develop a clinical classification model for identifying subgroup membership.
METHODS AND MATERIALS
A cross-sectional study was conducted within two larger longitudinal studies for patients with Achilles tendinopathy. Selection criteria were consistent with the parent studies; we analyzed baseline data from the parent studies.
Participants
Participants were asked to provide informed consent if they were at least 18 years old and had a clinical diagnosis of Achilles tendinopathy (insertional or midportion). The clinical diagnosis was established by 1) pain on palpation at either the calcaneal insertion or the midportion of the Achilles tendon 2) reported pain with loading 3) reported impaired function (e.g. reduced ability to participate in ADL/work/sport). 34,55 Exclusion criteria included previous Achilles tendon rupture, diagnosis of bursitis only, or another injury that limited their ability to complete the tests. All participants were recruited from local physicians, physical therapy clinics, and advertisements. Data were collected between November 2014 and December 2019. Data extracted from both studies were approved by the Institutional Review Board at the University of Delaware.
Variables
To be as inclusive as possible, 14 variables were selected on the basis of 1) outcome measure in previous tendinopathy studies, 2) clinically meaningful, 3) established as associated with Achilles tendinopathy and 4) collected in the parent studies (FIGURE 1). These selected variables represent five domains of tendon health59 and promotes a biopsychosocial appraisal of the patient suffering from tendinopathy.
FIGURE 1. Domains and outcome measures of tendon health.
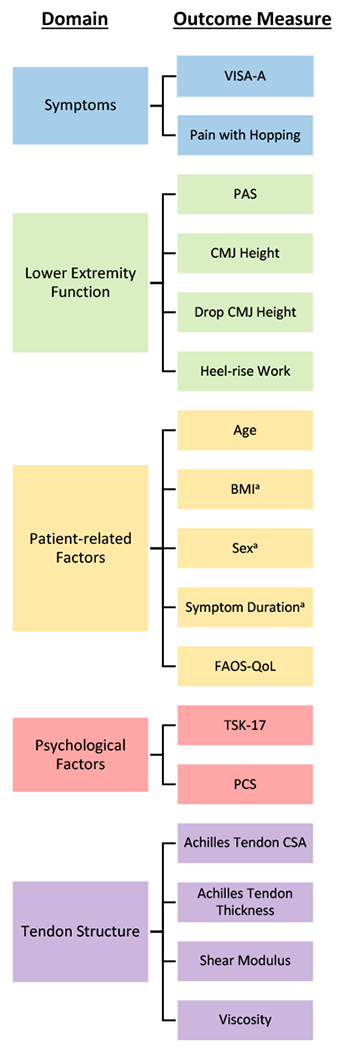
Abbreviations: VISA-A, Victorian Institute of Sport Assessment-Achilles; PAS, Physical Activity Scale; CMJ, counter movement jump; BMI, body mass index; FAOS-QoL, Foot and Ankle Outcome Score-Quality of Life; TSK, Tampa Scale of Kinesiophobia; PCS, Pain Catastrophizing Scale; CSA, cross-sectional area.
a Sex, BMI, and symptom duration were not included in the Mixture Model.
Symptoms
The Victorian Institute of Sport Assessment-Achilles50 (VISA-A) and self-rated pain with hopping evaluated pain and symptoms. The VISA-A is valid and reliable measure of symptom severity in patients with Achilles tendinopathy and is scored 0-100, where a lower score indicates more pain and symptoms. Participants performed 2 trials of 25 single leg hopping. Self-rated pain with hopping was recorded using a numerical pain rating scale17 from 0 (no pain)-10 (worst pain imaginable).
Lower Extremity Function
Jump performance and calf muscle endurance were measured via a single leg countermovement jump (CMJ), drop countermovement jump (Drop CMJ), and a heel-rise endurance test using MuscleLab® measurement system (Ergotest Innovation, Porsgrunn, Norway).58 Participants needed to jump at least 1cm for MuscleLab® to register a trial. Participants received a zero for height if they were unable to jump ≥1cm for a trial. Participants who declined to attempt a jump for any reason were assigned no value for that trial. Average jump height for the CMJ and Drop CMJ were calculated from up to three attempted trials per test. Total heel-rise work was measured in joules (heel-rise height x repetitions x body mass). Physical activity level during the past week was assessed using the Physical Activity Scale25 (PAS). The PAS is measured on a scale from 1-6, where 1 indicates hardly any physical activity and >5 indicates vigorous physical activity for several days per week.
Patient-related Factors
Body Mass Index (BMI) was calculated from measured height and weight. Participant age and sex were collected and considered clinically relevant as tendon mechanical properties and morphology are different between sexes and change throughout the lifespan. 30,47 Quality of life was measured with the Foot and Ankle Outcome Score – Quality of life51 (FAOS-QoL) and considered to be a patient-related factor as it is “an individual’s subjective evaluation of their overall well-being in the context of their own experiences.”9 A higher score (0-100) indicates a higher quality of life. Self-reported duration of symptoms (number of months) was collected since injury duration is proposed to affect nociception and affects quality of life.18 Injury side (unilateral, bilateral) and location (insertional, midportion, both) were also recorded.
Psychological Factors
The Tampa Scale of Kinesiophobia (TSK-17) measured fear of movement.21,32,33 A higher TSK-17 score (17-68) indicates greater fear of movement and a score of ≥37 indicates high kinesiophobia.21 The Pain Catastrophizing Scale65 (PCS) measured pain catastrophizing. Participants reflect on past painful experiences and indicate the degree to which they experienced catastrophizing thoughts or feelings. A higher PCS score (0-52) indicates higher degree of pain catastrophizing.
Tendon Structure & Mechanical Properties
Achilles tendon structure was assessed using B-mode ultrasound imaging (frequency of 10MHz and depth of 3.5 cm) using a GE Logiq e ultrasound scanner (GE LOGIQ e, GE Healthcare, Chicago, IL). All images were taken with the participant lying prone with the feet hanging off the edge of the table. Measurements included tendon thickness, and cross-sectional area (CSA) at the thickest portion using previously described reliable procedures.60,70
Achilles tendon mechanical properties were measured using continuous shear wave elastography (cSWE), which has excellent reliability and validity. 13,14,66 This method is similar to commercial SWE16, however cSWE uses an external actuator to generate shear waves and allows for extrapolation of two separate viscoelastic properties: shear modulus (i.e. stiffness) and viscosity (rate-dependent stiffness) of the tendon.13
STATISTICAL ANALYSIS
Mixture Modeling was used to identify the number of subgroups (best-fitting model) using the 14 variables described above (FIGURE 1). Measures for all analyses were taken from the most symptomatic limb (self-reported). The limb with the lower VISA-A50 score was identified as “most symptomatic” for participants with bilateral symptoms. Mixture Modeling was performed in Mplus (Muthén and Muthén, version 8.3). Missing data were handled using Mplus with a robust maximum likelihood (ML) estimator. A summary of missing data is presented in SUPPLEMENTAL FILE 1.
Determining the number of subgroups depends on a number of factors in addition to fit statistics.23,27,42 Fit statistics included Akaike’s Information Criterion3 (AIC), Bayesian Information Criterion54 (BIC), and sample-adjusted BIC54 (ABIC); all of which have been considered among the strongest indicators among the fit statistics of subgroup enumeration.42 The best fitting model should have the lowest AIC, BIC, and ABIC values.27 Entropy criterion represents the ability of the model to provide well-separated subgroups; a higher value (0-1) indicates the model has both strong separation between subgroups and strong cohesion within subgroups.8 The Vuong-Lo-Mendell-Rubin (VLMR), sample-adjusted VLMR, and Bootstrap Likelihood Ratio (BLR) tests were used to compare statistical significance between the current model to one with one less subgroup (e.g. 3 vs. 2).27 Finally, we ensured that each subgroup included > 5% of the sample69 and used clinical expertise to interpret meaningful differences among subgroups.
Following subgroup enumeration, all variables were compared across subgroups using ANOVAs for continuous variables and chi-square tests for categorical variables and Tukey’s post-hoc test using SPSS (Version 26). Sex, BMI, symptom duration, injury location, and injury side were used for post-hoc comparison across subgroups. All analyses were 2-sided, where p < .05 was considered statistically significant. All results are reported as mean ±SD, unless otherwise stated. To help illustrate the group differences visually across domains, variables were rescaled to z-scores and adjusted so better performance is indicated by higher positive values in FIGURES 2 AND 3.
FIGURE 2. Comparison of subgroup performance on outcome measures separated by tendon health domain. (dotted line represents total sample).
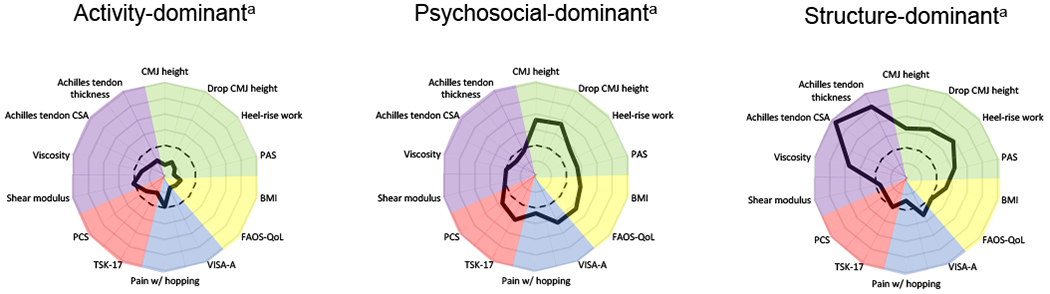
Abbreviations: CMJ, countermovement jump; PAS, Physical Activity Scale; BMI, body mass index; FAOS-QoL, Foot and Ankle Outcome Score-Quality of Life; VISA-A, Victorian Institute of Sport Assessment-Achilles; TSK, Tampa Scale of Kinesiophobia; PCS, Pain Catastrophizing Scale; CSA, cross-sectional area.
a Variables were rescaled by standardizing and adjusted so less distance from center represents less deficit or better performance.
FIGURE 3. Comparison of similar and differing performance on outcome measures and respective tendon health domains.
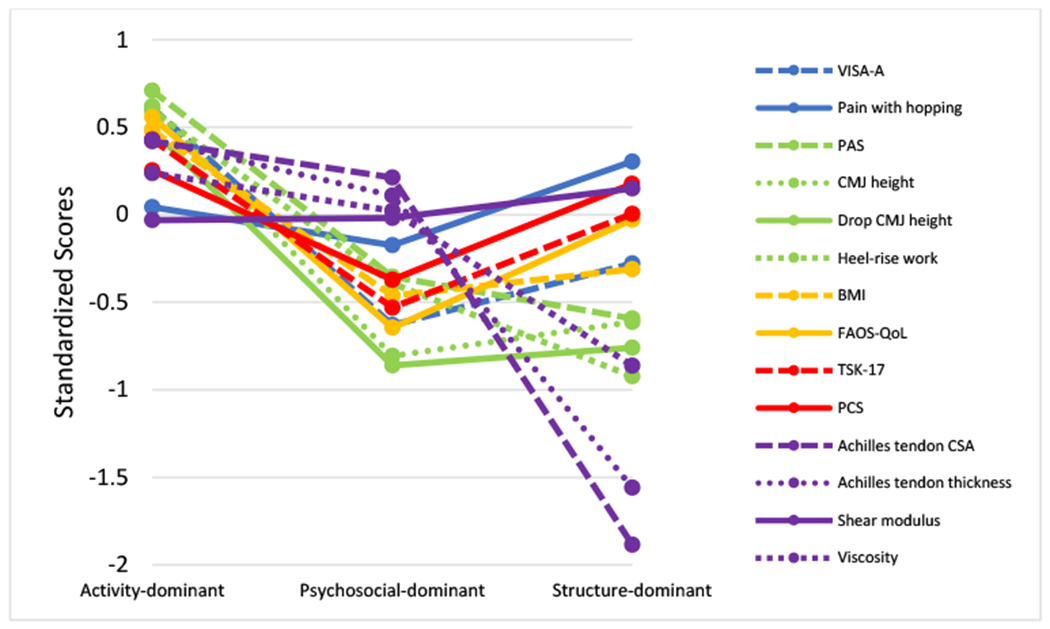
Abbreviations: VISA-A, Victorian Institute of Sport Assessment-Achilles; PAS, Physical Activity Scale; CMJ, countermovement jump; BMI, body mass index; FAOS-QoL, Foot and Ankle Outcome Score-Quality of Life; TSK, Tampa Scale of Kinesiophobia; PCS, Pain Catastrophizing Scale; CSA, cross-sectional area.
a Variables were rescaled by standardizing and adjusted so a higher standardized score indicates less deficit or better performance for each variable.
The clinical classification model (FIGURE 4) was developed post-hoc using the results to provide clinicians with a tool to classify patients using outcome measures that are accessible in clinical practice. Initially a Regression Tree approach was attempted using the variables included in the mixture model, but results were unstable. Instead, a two-step ROC process was employed iteratively to differentiate between subgroups by using cut-scores for each variable that jointly maximized sensitivity and specificity using Youden’s Index.20 For each variable, individuals were scored as having or not having met the criteria.
Figure 4. Clinical classification flowchart.
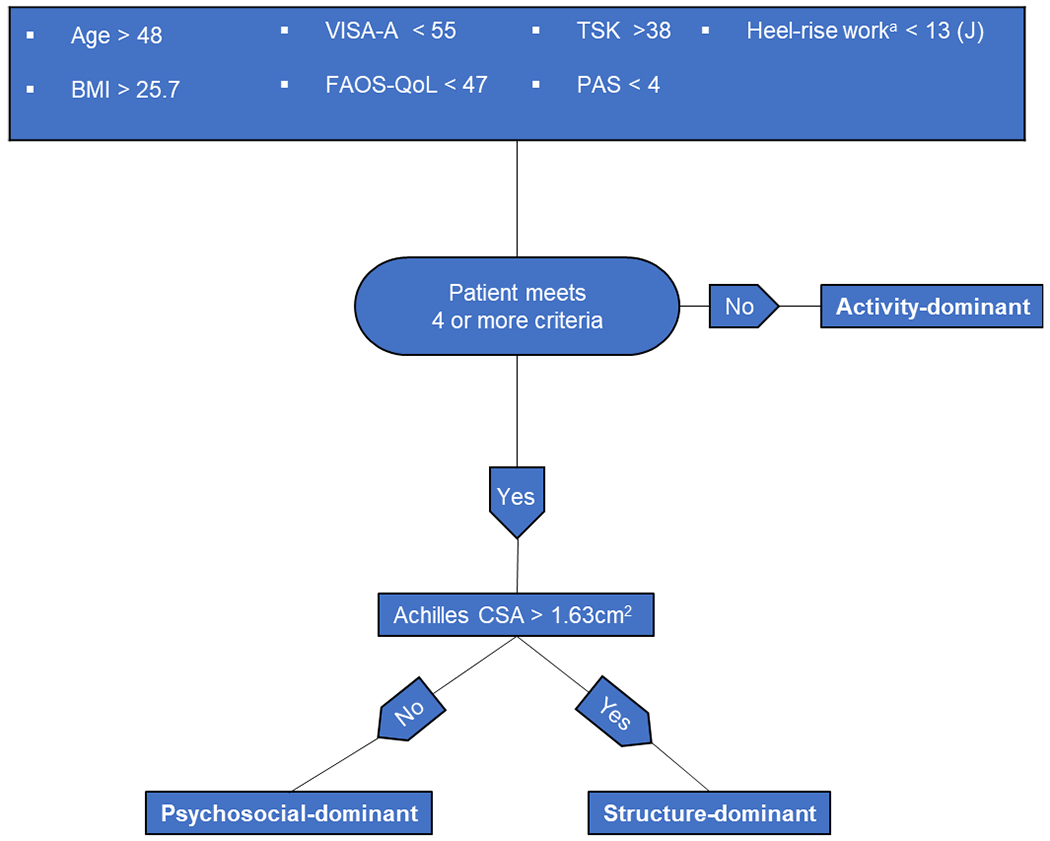
Abbreviations: BMI, body mass index; VISA-A, Victorian Institute of Sport Assessment-Achilles; FAOS-QoL, Foot and Ankle Outcome Score-Quality of Life; TSK, Tampa Scale of Kinesiophobia; PAS, Physical Activity Scale; CSA, cross-sectional area.
a Formula for estimating heel-rise work (J): heel-rise work (J) = 59.44 x repetitions + BMI(5.87) − 7.3.
RESULTS
The best-fitting model by information criteria (AIC, BIC, aBIC) identified three latent subgroups (SUPPLEMENTAL FILE 2). The VLMR and aVLMR suggested two subgroups, although three was close to significantly better. The bootstrap version (BLR) was uninformative, and entropy was good for all models. Ultimately, three subgroups were deemed most appropriate. The three patient subgroups were labeled Activity-dominant (n=67), Psychosocial-dominant (n=56), and Structure-dominant (n=22) (TABLE 1) based on their respective distinguishing clinical features (FIGURE 2).
TABLE 1.
Comparison of Patient Characteristics.
| Total Sample n=145a | Activity-dominant n=67 (46%)a | Psychosocial-dominant n=56 (39%)a | Structure-dominant n=22 (15%)a | ANOVA P-value |
P-value (Activity-dominant vs. Psychosocial-dominant)b | P-value (Activity-dominant vs. Structure-dominant)b | P-value (Psychosocial-dominant vs. Structure-dominant)b | |
|---|---|---|---|---|---|---|---|---|
| Symptoms | ||||||||
| VISA-A Score | 53±21 | 66±16 | 40±18 | 47±19 | < .001 | < .001 | < .001 | .254 |
| Pain with Hopping, NPRS | 2±4 | 3±2 | 3±2 | 0±3 | .485 | .663 | .799 | .510 |
| Lower Extremity Function | ||||||||
| Physical Activity Scale | 5±2 | 5±1 | 3±2 | 3±2 | < .001 | < .001 | < .001 | .999 |
| CMJ Height, cm | 6.4±3.6 | 8.6±3.0 | 3.5±1.9 | 4.2±2.1 | < .001 | < .001 | < .001 | .701 |
| Drop CMJ Height, cm | 6.6±3.5 | 8.5±3.3 | 3.2±2.3 | 3.6±2.9 | < .001 | < .001 | < .001 | .949 |
| Heel-rise Work, J | 1470±1209 | 1832±838 | 1062±1415 | 336±937 | < .001 | < .001 | < .001 | .037 |
| Heel-rise endurance test, repetitions | 21±13 | 28±9 | 16±14 | 10±10 | < .001 | < .001 | < .001 | .061 |
| Patient-related Factors | ||||||||
| Age, years | 51±14 | 44±13 | 55±12 | 62±8.7 | < .001 | < .001 | < .001 | .048 |
| BMI, kg/m2 | 27.6±6.74 | 24.3±3.8 | 30.7±7.1 | 30.7±5.9 | < .001 | < .001 | < .001 | .999 |
| Sex, M:F | 73:72 c | 37:30 c | 19:37c | 17:5c | .001 | .087 | .042 | .001 |
| Duration of Symptoms, months | 10.2±25.7 | 12±25.1 | 10.3±20.4 | 8±20.6 | .409 | .949 | .380 | .526 |
| FAOS-QoL Score | 44±19 | 54±16 | 32±15 | 43±14 | < .001 | < .001 | 0.014 | .011 |
| Injury Location | MP:100; I:36; Both:9 | MP:48; I:16; Both: 3 | MP:36; I:16; Both: 4 | MP:16; I:4; Both: 2 | .643 | .976 | .708 | .624 |
| Bilateral Symptoms Incidence Rate | 67 (46%) c | 34 (51%) c | 22 (39%) c | 11 (50%) c | .420 | .418 | .998 | .672 |
| Psychological Factors | ||||||||
| Tampa Scale of Kinesiophobia | 38±5 | 36±5 | 41±4 | 38±5 | < .001 | < .001 | .181 | .081 |
| Pain Catastrophizing Scale | 5±8 | 6±8 | 9±13 | 5±8 | .002 | .002 | .942 | .065 |
| Tendon Structure | ||||||||
| Achilles Tendon CSA, cm2 | 1.0±0.56 | 0.77±0.3 | 0.88±0.31 | 2.06±0.14 | < .001 | .158 | < .001 | < .001 |
| Achilles Tendon Thickness, cm | 0.78±0.28 | 0.65±0.2 | 0.74±0.24 | 1.22±0.14 | <.001 | .052 | <.001 | < .001 |
| Shear Modulus, kPA | 97.76±16.55 | 97.25±16.26 | 97.47±15.32 | 100.24±20.9 | .791 | .998 | .781 | .821 |
| Viscosity, kPa*s | 52.59±12.6 | 55.6±11.44 | 52.86±12.26 | 41.69±12.11 | < .001 | .465 | < .001 | .003 |
Abbreviations: VISA-A, Victorian Institute of Sport Assessment-Achilles; NPRS, Numerical Pain Rating Scale; CMJ, Countermovement jump; M:F, Male:Female; NT, not tested; BMI, body mass index; FAOS-QoL, Foot and Ankle Outcome Score-Quality of Life; MP, Midportion; I: Insertional; CSA, cross-sectional area.
Values are presented as mean±SD. Pain with hopping, Physical Activity Scale, heel-rise work, duration of symptoms, and Pain Catastrophizing Scale are presented as median±IQR.
Bold values indicate significant post-hoc comparison (p<0.05).
Chi Square test was used to compare distribution among subgroups
Activity-dominant
Compared to the other subgroups, Activity-dominant reported the highest PAS, VISA-A and FAOS-QOL scores, lowest TSK-17, lowest BMI, and were youngest (TABLE 1). CMJ and Drop CMJ heights were significantly higher and this subgroup produced nearly twice and five times the heel-rise work compared to Psychosocial-dominant and Structure-dominant, respectively (TABLE 1 AND FIGURE 3). Achilles tendon thickness was significantly less than the other subgroups, and CSA and viscosity were significantly better than Structure-dominant.
Psychosocial-dominant
Psychosocial-dominant demonstrated the highest psychological factors (TSK-17, PCS), and lowest FAOS-QoL scores compared to the other subgroups (TABLE 1). This subgroup was older than Activity-dominant and significantly worse, although similar to Structure-dominant, for the following variables: VISA-A, PAS, CMJ and Drop CMJ heights (FIGURES 2 AND 3). Psychosocial-dominant produced over 3 times more heel-rise work than Structure-dominant, but significantly less than Activity-dominant. Achilles tendon thickness, CSA, and viscosity measures were similar to Activity-dominant, but were significantly better than Structure-dominant. This subgroup was predominately obese and 66% female.
Structure-dominant
Structure-dominant demonstrated the largest Achilles tendon thickness and CSA, lowest viscosity, and produced the lowest heel rise work (TABLE 1 AND FIGURE 2). This subgroup was the oldest, were predominately obese, and 77% male. Structure-dominant reported similar physical activity levels, but significantly higher quality of life compared to the Psychosocial-dominant.
Clinical Classification
Only variables with a cut-point ROC AUC >.725 (BMI, TSK-17, Age, PAS, FAOS-QoL, Heel-rise work, and VISA-A) were kept in the final model (FIGURE 4). The presence of missing data (SUPPLEMENTAL FILE 1) caused most combinations of potential predictors to have too few individuals in the Structure-dominant subgroup using ROC curves. Alternatively, multinomial Logistic Regression suggested that CSA > 1.63 cm2 was be used to accurately classify 86% (18/21) of Structure-dominant participants while excluding everyone in the other two subgroups (FIGURE 4). Having 4 or more of the 7 criteria accurately classified individuals 85% of the time (105/123). Using both these rules successfully classified 85% (123/145) of participants (FIGURE 4).
DISCUSSION
We identified three subgroups, Activity-dominant, Psychosocial-dominant, and Structure-dominant within the general population of patients with Achilles tendinopathy using statistical modeling. The subgroups were identified by testing model fit using 14 variables commonly associated with tendinopathy including clinical exam findings, patient-reported outcome measures, ultrasound imaging, patient-related factors, and lower extremity functional tests.
Our latent subgroups share parallels with the theoretical continuum model of tendon pathology, which proposed clinical heterogeneity among patients based on imaging, clinical findings, and histological evidence.10 Consistently, our results supports the importance of evaluating all domains of tendinopathy that may impact tendon and patient health.67 While our findings cannot inform treatment recommendations, the distinguishing features of each subgroup reveals three differential patient profiles (FIGURE 2) that may explain the variability observed in both clinical practice and research outcomes.12,35,52,53 Using the clinical classification model (FIGURE 4), clinicians can prospectively identify a patient’s subgroup membership and recognize unique considerations for each subgroup and their potential obstacles to recovery.
Activity-dominant was the majority subgroup. These individuals demonstrated minimal performance impairments, suggesting a higher tendon-load capacity than the other subgroups. This may be because they have less pathological tendons. Athletes with early-onset tendinopathy symptoms have demonstrated slight (25%) increases in tendon CSA with unaltered tendon mechanical properties compared to healthy controls. However, symptom duration was not a distinguishing factor among subgroups. Lower kinesiophobia may explain why Activity-dominant participants reported higher quality of life and high activity levels, or vice versa. If tendon structure dictates physiological capacity, then symptomatic patients with minimal alterations in tendon structure might present with minimal functional impairment.63 Future research is needed to explore how Activity-dominant patients respond to treatment. Due to the apparent minimal impact on tendon health, Activity-dominant individuals may represent the majority of patients who achieve good-excellent results following 12 weeks of treatment.7,46,48,56
Psychosocial-dominant demonstrated minimal tendon structure and mechanical properties alterations, similar to Activity-dominant, yet this subgroup performed significantly worse on the functional test battery and averaged nearly 20 points lower on VISA-A (TABLE 1). The higher degree of psychological impact reported by Psychosocial-dominant may provide some explanation. Kinesiophobic patients may avoid excessive loading due to fear of pain making their condition worse. Fear-avoidance behaviors are associated with pain intensity15,40 and could affect loading test performance (premature test cessation or suppressing maximal jump height).24,31 Future research is needed to determine how psychological factors influence recovery times for patients with Achilles tendinopathy treated with exercise.35,53 Loading the Achilles tendon through tolerable pain is safe and non-detrimental61 to recovery. Future research should evaluate if Psychosocial-dominant patients are more reluctant to load their tendon due to kinesiophobia, and explore potential implications for rehabilitation compliance and progress.
Structure-dominant was the minority subgroup. This subgroup had the greatest degree of tendon alteration demonstrated by measures of tendon thickness, CSA, and decreased tendon viscosity (FIGURE 2). Accordingly, 32% of Structure-dominant were unable to perform one heel-rise repetition, which may have indirect effects on other aspects of tendon health. Consistently, Corrigan et al11 reported greater tendon thickening and lower viscosity was associated with worse calf muscle endurance. Some of the differences observed between Structure-dominant and the other subgroups might also be due to body mass and age. In this subgroup, 86% were obese (BMI >30) which can increase Achilles tensile load to 6-10 times for every 1lb of excess weight.1,22,44 From a general health viewpoint, this subgroup’s patient-related factors raise concerns for comorbidities (e.g. metabolic disease, sarcopenia, menopause) which could negatively affect tendon healing and lengthen the recovery timeline.2,53 The extent to which tendon structural changes are reversible in response to non-surgical and surgical treatment remains debated.6,29 Evidence supports the possibility of recovery of mechanical properties associate with ageing.41 Animal studies suggest that there is no decline in tendon synthesis capacity with aging, therefore it is possible that detectable changes in tendon structure may require greater length of time than previous studies have captured.37 Because symptomatic recovery is achievable without functional recovery62 and we considered Structure-dominant to have the greatest impairments in tendon health. Further research is warranted to determine if this subgroup’s propensity for recurrent injury differs compared to the general population. Further study is needed to determine how Structure-dominant patients respond to exercise therapy and if tendon structural adaptations are achievable for these patients.
Limitations and Future Directions
It is possible for patients to fit into more than one subgroup in clinical practice and clinical expertise should not be superseded by any model. The clinical classification model was developed without cross validation using another sample. Therefore, further validation studies are needed. We acknowledge that additional subgroups might exist in youth and elite athletes or other unrepresented cohorts. Although we tried to be as exhaustive as reasonably possible, we were limited to the variables included in both parent studies. Differences in sex distribution among subgroups was an interesting and unexpected result and merits future research. Future prospective studies are needed to determine how subgroups respond to standardized treatment and to investigate the effectiveness of patient-centered treatment based on tendon health deficits.
CONCLUSION
The purpose of this study was to identify unobserved heterogeneity among patients with Achilles tendinopathy. We conclude that Achilles tendinopathy subgroups exist among the general population that are distinct in their patient characteristics and clinical attributes.
Supplementary Material
KEY POINTS.
Findings:
Subgroups exist among patients diagnosed with Achilles tendinopathy in the general population. Patients can be classified as Activity-dominant, Psychosocial-dominant, or Structure-dominant.
Implications:
Clinicians should evaluate for subgroup membership and conduct a comprehensive clinical examination that appraises all aspects of tendon and patient health. The presented clinical classification model can inform clinical reasoning to recognize previously unobserved heterogeneity among patients.
Caution:
Patient subgrouping is meant to elucidate the heterogenous clinical presentation of patients and requires further validation. Regardless of subgroup membership, the treatment recommendation remains exercise therapy for patients with Achilles tendinopathy.
Contributors:
All authors planned the study. SLH performed the mixture modeling analysis under the supervision of RTP. RTP performed the statistical analysis for the clinical classification model. All authors contributed to interpretation of the results and writing of the manuscript. All authors have approved the final manuscript.
Patient and public involvement:
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Data Sharing:
Data are available upon reasonable request. Kindly email the corresponding author for the paper to request the relevant data.
Acknowledgments
Funding:
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R21AR067390 and R01AR07203401A1.
Footnotes
Financial disclosure and conflict of interest: None declared.
Ethics Approval: The data used in this study were approved by the University of Delaware Institutional Review Board (ID:1090153-18 and ID:1063764-12) and all subjects gave informed written consent to participate.
REFERENCES
- 1.Abate M, Oliva F, Schiavone C, Salini V. Achilles tendinopathy in amateur runners: Role of adiposity (Tendinopathies and obesity). Muscles Ligaments Tendons J. 2012;2(1):44–48. [PMC free article] [PubMed] [Google Scholar]
- 2.Abate M, Salini V, Schiavone C. Achilles tendinopathy in elderly subjects with type II diabetes: the role of sport activities. Aging Clin Exp Res. 2016;28(2):355–358. doi: 10.1007/s40520-015-0391-7 [DOI] [PubMed] [Google Scholar]
- 3.Akaike H Factor analysis and AIC. Psychometrika. 1987;52:317–332. doi: 10.1007/BF02294359 [DOI] [Google Scholar]
- 4.Alfredson H, Cook J. A treatment algorithm for managing Achilles tendinopathy: New treatment options. Br J Sports Med. 2007;41(4):211–216. doi: 10.1136/bjsm.2007.035543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfredson H, Pietilä T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic achilles tendinosis. Am J Sports Med. 1998;26(3):360–366. doi: 10.1177/03635465980260030301 [DOI] [PubMed] [Google Scholar]
- 6.Alfredson H, Zeisig E, Fahlström M. No normalisation of the tendon structure and thickness after intratendinous surgery for chronic painful midportion Achilles tendinosis. Br J Sports Med. 2009;43(12):948–949. doi: 10.1136/bjsm.2008.050955 [DOI] [PubMed] [Google Scholar]
- 7.Beyer R, Kongsgaard M, Hougs Kjær B, Øhlenschlæger T, Kjær M, Magnusson SP. Heavy slow resistance versus eccentric training as treatment for achilles tendinopathy: A randomized controlled trial. Am J Sports Med. 2015;43(7):1704–1711. doi: 10.1177/0363546515584760 [DOI] [PubMed] [Google Scholar]
- 8.Celeux G, Soromenho G. An Entropy Criterion for Assessing the Number of Clusters in a Mixture Model. J Classif. 1996;13:195–212. doi: 10.1016/S0167-8655(98)00144-5 [DOI] [Google Scholar]
- 9.Ceravolo ML, Gaida JE, Keegan RJ. Quality-of-Life in Achilles Tendinopathy : An Exploratory Study. Clin J Sport Med. 2018;00(00):1–8. doi: 10.1097/JSM.0000000000000636 [DOI] [PubMed] [Google Scholar]
- 10.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409–416. doi: 10.1136/bjsm.2008.051193 [DOI] [PubMed] [Google Scholar]
- 11.Corrigan P, Cortes DH, Pohlig RT, Silbernagel KG. Tendon Morphology and Mechanical Properties Are Associated With the Recovery of Symptoms and Function in Patients With Achilles Tendinopathy: Orthop J Sport Med. 2020;8(4):1–9. doi: 10.1177/2325967120917271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrigan P, Cortes DH, Pontiggia L, Silbernagel KG. the Degree of Tendinosis Is Related To Symptom Severity and Physical Activity Levels in Patients With Midportion Achilles Tendinopathy. Int J Sports Phys Ther. 2018;13(2):196–207. doi: 10.26603/ijspt20180196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrigan P, Zellers JA, Balascio P, Silbernagel KG, Cortes DH. Quantification of Mechanical Properties in Healthy Achilles Tendon Using Continuous Shear Wave Elastography: A Reliability and Validation Study. Ultrasound Med Biol. 2019;45(7):1574–1585. doi: 10.1016/j.ultrasmedbio.2019.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes DH, Suydam SM, Silbernagel KG, Buchanan TS, Elliott DM. Continuous Shear Wave Elastography: A New Method to Measure Viscoelastic Properties of Tendons in Vivo. Ultrasound Med Biol. 2015;41(6):1518–1529. doi: 10.1016/j.ultrasmedbio.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debenham JR, Malliaras P, Stace R, Littlewood C, Mallows AJ. Cognitive and contextual factors to optimise clinical outcomes in tendinopathy. Br J Sports Med. 2017;52(13):822–823. doi: 10.1136/bjsports-2017-098064 [DOI] [PubMed] [Google Scholar]
- 16.Dirrichs T, Quack V, Gatz M, Tingart M, Kuhl CK, Schrading S. Shear Wave Elastography (SWE) for the Evaluation of Patients with Tendinopathies. Acad Radiol. 2016;23(10):1204–1213. doi: 10.1016/j.acra.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 17.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA. Studies with pain rating scales. Ann Rheum Dis. 1978;37(4):378–381. doi: 10.1136/ard.37.4.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): Association with clinical variables. Pain. 2003;106(3):427–437. doi: 10.1016/j.pain.2003.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Fahlström M, Jonsson P, Lorentzon R, Alfredson H. Chronic Achilles tendon pain treated with eccentric calf-muscle training. Knee Surgery, Sport Traumatol Arthrosc. 2003;11(5):327–333. doi: 10.1007/s00167-003-0418-z [DOI] [PubMed] [Google Scholar]
- 20.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biometrical J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135 [DOI] [PubMed] [Google Scholar]
- 21.French DJ, France CR, Vigneau F, French JA, Evans RT. Fear of movement/(re)injury in chronic pain: A psychometric assessment of the original English version of the Tampa scale for kinesiophobia (TSK). Pain. 2007;127(1-2):42–51. doi: 10.1016/j.pain.2006.07.016 [DOI] [PubMed] [Google Scholar]
- 22.Gaida JE, Cook JL. Risk Factors for overuse tendinopathy. Australas Musculoskelet Med. 2008;13(2):60–65. [Google Scholar]
- 23.Geiser C Data Analysis with Mplus. Guilford Press; 2013. [Google Scholar]
- 24.Grävare Silbernagel K, Brorsson A, Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: A 5-year follow-up. Am J Sports Med. 2011;39(3):607–613. doi: 10.1177/0363546510384789 [DOI] [PubMed] [Google Scholar]
- 25.GRIMBY G Physical Activity and Muscle Training in the Elderly. Acta Med Scand. 1986;220(S711):233–237. doi: 10.1111/j.0954-6820.1986.tb08956.x [DOI] [PubMed] [Google Scholar]
- 26.Habets B, van den Broek AG, Huisstede BMA, Backx FJG, van Cingel REH. Return to Sport in Athletes with Midportion Achilles Tendinopathy: A Qualitative Systematic Review Regarding Definitions and Criteria. Sport Med. 2018;48(3):705–723. doi: 10.1007/s40279-017-0833-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henson JM, Reise SP, Kim KH. Detecting mixtures from structural model differences using latent variable mixture modeling: A comparison of relative model fit statistics. Struct Equ Model. 2007;14(2):202–226. doi: 10.1080/10705510709336744 [DOI] [Google Scholar]
- 28.Johannsen F, Jensen S, Wetke E. 10-year follow-up after standardised treatment for Achilles tendinopathy. BMJ Open Sport Exerc Med. Published online2018:1–7. doi: 10.1136/bmjsem-2018-000415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Jonge S, Tol JL, Weir A, Waarsing JH, Verhaar JAN, De Vos RJ. The tendon structure returns to asymptomatic values in nonoperatively treated achilles tendinopathy but is not associated with symptoms. Am J Sports Med. 2015;43(12):2950–2958. doi: 10.1177/0363546515605077 [DOI] [PubMed] [Google Scholar]
- 30.Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol. 2003;88(6):520–526. doi: 10.1007/s00421-002-0744-8 [DOI] [PubMed] [Google Scholar]
- 31.Leeuw M, Goossens MEJB, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS. The fear-avoidance model of musculoskeletal pain: Current state of scientific evidence. J Behav Med. 2007;30(1):77–94. doi: 10.1007/s10865-006-9085-0 [DOI] [PubMed] [Google Scholar]
- 32.Lundberg M, Styf J. Kinesiophobia among physiological overusers with musculoskeletal pain. Eur J Pain. 2009;13(6):655–659. doi: 10.1016/j.ejpain.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 33.Lundberg MKE, Styf J, Carlsson SG. A psychometric evaluation of the Tampa Scale for Kinesiophobia - From a physiotherapeutic perspective. Physiother Theory Pract. 2004;20(2):121–133. doi: 10.1080/09593980490453002 [DOI] [Google Scholar]
- 34.Maffulli N, Kenward MG, Testa V, Capasso G, Regine R, King JB. Clinical diagnosis of Achilles tendinopathy with tendinosis. Clin J Sport Med. 2003;13(1):11–15. doi: 10.1097/00042752-200301000-00003 [DOI] [PubMed] [Google Scholar]
- 35.Maffulli N, Walley G, Sayana M, Longo UG, Denaro V. Eccentric calf muscle training in athletic patients with Achilles tendinopathy. Disabil Rehabil. 2008;30(20-22):1677–1684. doi: 10.1080/09638280701786427 [DOI] [PubMed] [Google Scholar]
- 36.Mafi N, Lorentzon R, Alfredson H. Superior short-term results with eccentric calf muscle training compared to concentric training in a randomized prospective multicenter study on patients with chronic Achilles tendinosis. Knee Surgery, Sport Traumatol Arthrosc. 2001;9(1):42–47. doi: 10.1007/s001670000148 [DOI] [PubMed] [Google Scholar]
- 37.Magnusson P, Kjaer M. The impact of loading, unloading, ageing and injury on the human tendon. J Physiol. 2018;0:1–16. doi: 10.1113/JP275450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: Balancing the response to loading. Nat Rev Rheumatol. 2010;6:262–268. doi: 10.1038/nrrheum.2010.43 [DOI] [PubMed] [Google Scholar]
- 39.Martin RL, Chimenti R, Cuddeford T, et al. Achilles Pain, Stiffness, and Muscle Power Deficits: Midportion Achilles Tendinopathy Revision 2018. J Orthop Sport Phys Ther. 2018;48(5):A1–A38. doi: 10.2519/jospt.2018.0302 [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Calderon J, Jensen MP, Morales-Asencio JM, Luque-Suarez A. Pain Catastrophizing and Function in Individuals with Chronic Musculoskeletal Pain. Clin J Pain. 2019;35(3):279–293. doi: 10.1097/AJP.0000000000000676 [DOI] [PubMed] [Google Scholar]
- 41.Narici M V, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disabil Rehabil. 2008;30(20-22):1548–1554. doi: 10.1080/09638280701831058 [DOI] [PubMed] [Google Scholar]
- 42.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Model. 2007;14(4):535–569. doi: 10.1080/10705510701575396 [DOI] [Google Scholar]
- 43.Oberski DL. Mixture Models: Latent Profile and Latent Class Analysis. In: Modern Statistical Methods for HCI.; 2016:275–287. doi: 10.1007/978-3-319-26633-6_12 [DOI] [Google Scholar]
- 44.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Heal Organ - Tech Rep Ser. 2000;894(i-xii):1–253. [PubMed] [Google Scholar]
- 45.Öhberg L, Alfredson H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surgery, Sport Traumatol Arthrosc. 2004;12(5):465–470. doi: 10.1007/s00167-004-0494-8 [DOI] [PubMed] [Google Scholar]
- 46.Öhberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: Normalised tendon structure and decreased thickness at follow up. Br J Sports Med. 2004;38(1):8–11. doi: 10.1136/bjsm.2001.000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peter Magnusson S, Hansen M, Langberg H, et al. The adaptability of tendon to loading differs in men and women. In: International Journal of Experimental Pathology. Vol 88.; 2007:237–240. doi: 10.1111/j.1365-2613.2007.00551.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Der Plas A, De Jonge S, De Vos RJ, et al. A 5-year follow-up study of Alfredson’s heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med. 2012;46(3):214–218. doi: 10.1136/bjsports-2011-090035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rio EK, Auliffe SM, Kuipers I, et al. ICON PART-T 2019 – International Scientific Tendinopathy Symposium Consensus : recommended standards for reporting participant characteristics in tendinopathy research (PART-T). 2019;(from 2018):1–4. doi: 10.1136/bjsports-2019-100957 [DOI] [PubMed] [Google Scholar]
- 50.Robinson JM, Cook JL, Purdam C, et al. The VISA-A questionnaire : a valid and reliable index of the clinical severity of Achilles tendinopathy The VISA-A questionnaire : a valid and reliable index of the clinical severity of Achilles tendinopathy. Sport Med. Published online2001:335–341. doi: 10.1136/bjsm.35.5.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roos EM, Brandsson S, Karlsson J. Validation of the Foot and Ankle Outcome Score for Ankle Ligament Reconstruction. Foot Ankle Int. 2001;22(10):788–794. doi: 10.1007/s11420-015-9466-4 [DOI] [PubMed] [Google Scholar]
- 52.Rowe V, Hemmings S, Barton C, Malliaras P, Maffulli N, Morrissey D. Conservative Management of Midportion Achilles Tendinopathy. Sport Med. 2012;42(11):941–967. doi: 10.2165/11635410-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 53.Sayana MK, Maffulli N. Eccentric calf muscle training in non-athletic patients with Achilles tendinopathy. J Sci Med Sport. 2007;10:52–58. doi: 10.1016/j.jsams.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 54.Schwarz G Estimating the Dimension of a Model. Ann Stat. 1978;6:461–464. doi: 10.1214/aos/1176344136 [DOI] [Google Scholar]
- 55.Scott A, Squier K, Alfredson H, et al. ICON 2019: International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br J Sports Med. 2020;54:260–262. doi: 10.1136/bjsports-2019-100885 [DOI] [PubMed] [Google Scholar]
- 56.Shalabi A, Kristoffersen-Wilberg M, Svensson L, Aspelin P, Movin T. Eccentric training of the gastrocnemius-soleus complex ion chronic achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am J Sports Med. 2004;32(5):1286–1296. doi: 10.1177/0363546504263148 [DOI] [PubMed] [Google Scholar]
- 57.Silbernagel KG. Does One Size Fit All When It Comes to Exercise Treatment for Achilles Tendinopathy? J Orthop Sport Phys Ther. 2014;44(2):42–44. doi: 10.2519/jospt.2014.0103 [DOI] [PubMed] [Google Scholar]
- 58.Silbernagel KG, Gustavsson A, Thomeé R, Karlsson J. Evaluation of lower leg function in patients with Achilles tendinopathy. Knee Surgery, Sport Traumatol Arthrosc. 2006;14(11):1207–1217. doi: 10.1007/s00167-006-0150-6 [DOI] [PubMed] [Google Scholar]
- 59.Silbernagel KG, Hanlon S, Sprague A. Current Clinical Concepts: Conservative Management of Achilles Tendinopathy. J Athl Train. 2020;55(5):438–447. doi: 10.4085/1062-6050-356-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silbernagel KG, Shelley K, Powell S, Varrecchia S. Extended field of view ultrasound imaging to evaluate achilles tendon length and thickness: A reliability and validity study. Muscles Ligaments Tendons J. 2016;6(1):104–110. doi: 10.11138/mltj/2016.6.1.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silbernagel KG, Thomeé R, Eriksson BI, Karlsson J. Continued sports activity, using a pain-monitoring model, during rehabilitation in patients with achilles tendinopathy: A randomized controlled study. Am J Sports Med. 2007;35(6):897–906. doi: 10.1177/0363546506298279 [DOI] [PubMed] [Google Scholar]
- 62.Silbernagel KG, Thomeé R, Eriksson BI, Karlsson J. Full symptomatic recovery does not ensure full recovery of muscle-tendon function in patients with Achilles tendinopathy. Br J Sports Med. 2007;41(4):276–280. doi: 10.1136/bjsm.2006.033464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snedeker JG, Foolen J. Tendon injury and repair – A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017;63:18–36. doi: 10.1016/j.actbio.2017.08.032 [DOI] [PubMed] [Google Scholar]
- 64.Stynes S, Konstantinou K, Ogollah R, Hay EM, Dunn KM. Novel approach to characterising individuals with low back-related leg pain: Cluster identification with latent class analysis and 12-month follow-up. Pain. 2018;159(4):728–738. doi: 10.1097/j.pain.0000000000001147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychol Assess. 1995;7(4):524–532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 66.Suydam SM, Soulas EM, Elliott DM, Gravare Silbernagel K, Buchanan TS, Cortes DH. Viscoelastic properties of healthy achilles tendon are independent of isometric plantar flexion strength and cross-sectional area. J Orthop Res. 2015;33(6):926–931. doi: 10.1002/jor.22878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vicenzino B, De Vos RJ, Alfredson H, et al. ICON 2019 - International Scientific Tendinopathy Symposium Consensus: There are nine core health-related domains for tendinopathy (CORE DOMAINS): Delphi study of healthcare professionals and patients. Br J Sports Med. Published online2019:1–8. doi: 10.1136/bjsports-2019-100894 [DOI] [PubMed] [Google Scholar]
- 68.Xu Y, Murrell GAC. The basic science of tendinopathy. Clin Orthop Relat Res. 2008;466(7):1528–1538. doi: 10.1007/s11999-008-0286-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang CC. Evaluating latent class analysis models in qualitative phenotype identification. Comput Stat Data Anal. 2006;50(4):1090–1104. doi: 10.1016/j.csda.2004.11.004 [DOI] [Google Scholar]
- 70.Zellers JA, Bley BC, Pohlig RT, Alghamdi NH. Frequency of pathology on diagnostic ultrasound and relationship to patient demographics in individuals with insertional Achilles tendinopathy. Int J Sports Phys Ther. 2019;14(5):1–9. doi: 10.26603/ijspt201901 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


