Abstract
A therapeutic strategy for prostate cancer (PCa) involves the use of 9-cis-retinoic acid (9cRA) to induce cancer stem cells (CSCs) differentiation and apoptosis. Polyinosinic:polycytidylic acid (PIC) is a Toll-like receptor 3 (TLR3) agonist that induces tumor cells apoptosis after activation. PIC+9cRA combination activates retinoic acid receptor β (RARβ) re-expression, leading to CSC differentiation and growth arrest. Since inorganic arsenic (iAs) targets prostatic stem cells (SCs), we hypothesized that arsenic-transformed SCs (As-CSCs) show an impaired TLR3-associated anti-tumor pathway and, therefore, are unresponsive to PIC activation. We evaluated TLR3-mediated activation of anti-tumor pathway based in RARβ expression, on As-CSC and iAs-transformed epithelial cells (CAsE-PE). As-CSCs and CAsE-PE showed lower TLR3 and RARβ basal expression compared to their respective isogenic controls WPE-Stem and RWPE-1. Also, iAs transformants showed reduced expression of mediators in TLR3 pathway. Importantly, As-CSCs were irresponsive to PIC+9cRA in terms of increased RARβ and decreased SC-markers expression, while CAsE-PE, a heterogeneous cell line having a small SC population, were partially responsive. These observations indicate that iAs can impair TLR3 expression and anti-tumor pathway activated by PIC+9cRA in SCs and prostatic epithelial cells. These findings suggest that TLR3-activation based therapy may be an ineffective therapeutic alternative for iAs-associated PCa.
Keywords: TLR3, RARβ, Prostate cancer, Retinoic acid, Poly (I:C), PIC, Arsenic
Graphical Abstract
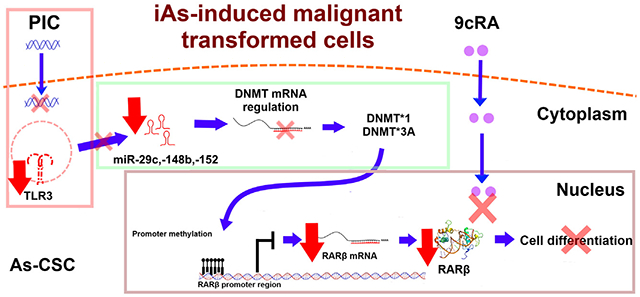
1. Introduction
TLRs represent a family of transmembrane proteins that recognize pathogen associated molecular patterns (PAMPs) and trigger innate immune activation and subsequently the adaptive immunity (Heidarzadeh et al., 2020). For this reason, several TLR agonists are currently being probed as adjuvants for anticancer therapies (Adams, 2009; Barton and Medzhitov, 2003). Specifically, TLR3 antitumor activity is achieved through two independent mechanisms. One of those mechanisms is related to activation of specialized immune cells and the other involves apoptosis induction in cancer cells expressing this receptor (Kawasaki and Kawai, 2014; Premkumar et al., 2010). TLR3 is located in endosomal compartments or at surface of a variety of cells including dendritic and epithelial cells, and responds to activation with double-stranded RNA (dsRNA) (Estornes et al., 2013). TLR3 agonist, Polyinosinic:Polycytidylic acid (PIC) has been proposed as an anti-cancer immunotherapeutic agent due of its ability to induce cancer cell apoptosis (Bernardo et al., 2013a, 2013b). On the other hand, retinoids have been extensively investigated for cancer treatment and prevention (Connolly et al., 2013) since they exert anti-proliferative actions through the regulation of cellular differentiation, proliferation, and apoptosis (Pasquali et al., 2006). Retinoids anti-cancer activity occurs through interaction with their nuclear receptors, retinoic acid receptors, RARα, RARβ, and RARγ (Allenby et al., 1994). RARs form complexes with retinoic X receptors (RXRs) to form RAR/RXR heterodimers that bind to retinoic acid responsive elements (RARE) to activate gene transcription (Bernardo et al., 2013a, 2013b). RARβ expression is well known for its tumor suppressive properties on epithelial cells that results in target genes activation that mediate cancer cell proliferation, apoptosis, and SC differentiation (Bushue and Wan, 2010). This last activity is critical for tumoral cells surveillance since CSCs have been proposed as driving force for cancer development and tumor chemoresistance (Isayev et al., 2019). Therefore retinoids are used as chemotherapeutic agents due to their propriety to induce CSC differentiation towards epithelial cells which are sensitive to anti-cancer drugs (Modarai et al., 2018; Nguyen et al., 2016; Whitworth et al., 2012). Combination of 9-cis retinoic acid (9cRA) and TLR3 activation by PIC currently represents a chemotherapeutic strategy for different kinds of cancers (DeCicco et al., 2001). Although 9cRA antitumor activity related mechanisms are well known, mechanism through which TLR3 exerts its antitumor effects when it is combined with 9cRA, is still not completely defined. However, important insights were described in 2014 by Galli and colleagues (Galli et al., 2013a). Using two prostate cancer (PCa) cell lines (DU145 and TRAMP-C1) they demonstrated that TLR3 activation by PIC induces RARβ gene re-expression through upregulation of miRNAs (miR29b, miR148 and miR152) that target DNA methyltransferases (DNMTs) which normally conduct RARβ gene promoter methylation in aggressive cancer cells. This miRNA-regulated silencing of DNMTs results in an increased expression of RARβ, which causes cell differentiation and sensitization of tumor cells to 9cRA-induced apoptosis (Galli et al., 2013a). Such results demonstrate a crosstalk between TLR3 and 9cRA intracellular signaling pathways and suggest that any impairment in TLR3/RARβ expression will modify the response to PIC/9cRAcombined therapy for PCa. However, in an large percentage of PCa cases, RARβ gene promoter is downregulated or silenced by methylation, making the cancer unresponsive to retinoid treatment (González-Reyes et al., 2011).
Importantly, PCa is associated with chronic exposure to iAs in different populations around the world (Benbrahim-Tallaa and Waalkes, 2008). In vivo and in vitro experiments have shown that iAs drives to over-accumulation of SCs and CSCs, suggesting that these cells have a survival selection advantage to iAs which drives them to malignant transformation (Tokar et al., 2010a, 2010b). Supporting these findings, Tokar et al. demonstrated that prostate SCs (WPE-stem) have innate resistance to iAs-induced lethality and apoptosis, as they show higher expression of anti-apoptotic factors (e.g., Bcl-2, metallothionein (MT)) and decreased expression of apoptotic factors (BAX, caspases 3, 8, 7 and 9). These cells also show higher expression of stress-related genes (e.g. SOD1 and PRODH) compared with parental RWPE-1 cells (Tokar et al., 2010a, 2010b). Given that SCs putatively drive iAs-induced malignant transformation of human prostate cells, and that TLR3 induction by PIC+9cRA leads to activation of an anti-tumor pathway that involves RARβ expression, we hypothesized that TLR3-associated anti-cancer pathway is impaired in As-CSCs, making cells unresponsive to TLR3 activation, thereby inhibiting this protective mechanism. To test this hypothesis, we evaluated TLR3 expression and RARβ anti-tumor associated pathway in normal, non-tumorigenic prostate epithelial and SCs and their isogenic iAs- transformants.
2. Material and methods
2.1. Cell cultures and treatments
Four isogenic human prostate cell lines were used for this study: 1) immortalized RWPE-1 consisting of differentiated epithelial cells, intermediate cells, and a small population of stem/progenitor cells; 2) RWPE-1 cells transformed by chronic iAs exposure named CAsE-PE, 3) stem/progenitor cells isolated from RWPE-1, named WPE-Stem, and 4) WPE-Stem cells transformed by chronic exposure to iAs and named As-CSC. Cell lines were grown in keratinocyte serum-free medium (KSFM, GIBCO) containing 50 μg/mL of bovine pituitary extract (BPE) and 50 μg/mL of epidermal growth factor (EGF) without antibiotics, incubated at 37 °C in a humidified atmosphere containing 5% carbon dioxide. WPE-Stem and As-CSC were grown in flasks coated with a matrix composed of collagen IV (25 g/mL) (Trevigen, Gaithersburg, MD) and fibronectin (25 μg/mL) (BD Biociences, San Jose, CA). All cell lines were cultivated in triplicate. Cells were exposed to 25 μg/mL of high molecular weight Polyinosinic:Polycytidylic acid (HMW-PIC) (Invivogen, San Diego, CA); 10μM of 9-cys-retinoic acid (9cRA) (Sigma-Aldrich, St. Louis, MO), or a combination of both. Triplicates of untreated cells (NT) were included as controls.
2.2. Western Blot analysis
Total protein was isolated from 80 to 90 % confluent cell cultures using RIPA buffer, total concentration of extracted proteins was determined by Bradford assay. Total protein concentration of each sample was adjusted to 1 μg/μL. Proteins were separated based on their weight and electrophoresis charge in 10 % acrylamide gels. Samples were placed in wells and electrophoresed at 180 V and 300 mAmps for one hour. Proteins were then transferred to nitrocellulose membranes (0.45 μm) at 100 V and 300 mAmps for 1 h. Membranes were then blocked with 5% skim milk in TBS 1x for 1 h in agitation and then incubated overnight at 4 °C with corresponding primary antibody Anti-TLR3 (1:2000), Anti-RARβ (1:2000) or Anti-β-actin (1:2000) (Abcam, Cambridge, CB2 0AX, UK). HRP-labeled secondary antibody (Abcam) was added to membranes and incubated for 2 h at room temperature. Bands corresponding to proteins of interest were visualized by incubating membranes with chemiluminescent HRP substrate (Luminol®) followed by exposure to a photographic film. β-Actin expression was used as loading control.
2.3. Real-time PCR analysis
Cells were grown to 80–90 % confluence, collected by trypsinization (0.025 % trypsin), and pelleted by centrifugation. Pellet was treated with TRIzol® reagent (300 μL per 1 × 106 cells) for total RNA extraction. From each cell line and tested condition, a retro-transcription reaction (RT) was made to prepare cDNA from total RNA. PCR mixture contained 1 μg of RNA, 0.2 μL of random primers (2.5 μg/μL), 0.8 μL of DEPC water, 1 μL of dNTPs (10 mM), 4 μL of 5X RT buffer, 2 μL of DTT, 1 μL of RNAse OUT and 1 μL of SuperScript II, final volume 20 μL. RT reactions were performed using following parameters: 25 °C for 10 min, 50 min for 42 °C and 15 min for 72 °C. Real-time PCR was performed on ABI 7500 (Applied Biosystems) thermocycler using 100 ng of cDNA to analyze CD44, CD133, SOX2, DNMT1 and DNMT3A expression. Each PCR reaction was performed in a total of 10 μL. Reaction consisted of 3 μL of water, 5 μL of Sybr Green Master Mix solution, 0.5 μL of each primer (Forward and reverse) and 1 μL of cDNA. Primer sequences are shown in Table 1S. Reference gene used was 18 s rRNA. Standard thermocycling protocol consisted of 95 °C for 10 min, 95 °C for 2 min, 40 °C for 15 s, and 72 °C for 1 min, for 40 cycles. Expression analysis was performed in High-Resolution Melt (HRM) Software v2.0 program (Applied Biosystems). Changes in relative expression (at 18 s rRNA) were calculated using the 2 ^ −ΔΔCt method.
2.4. miR-29c, miR-148b, miR-152 expression analysis
miRNA purification and cDNA preparation were carried out using Qiagen’s miRNeasy affinity column and miScript® II system. miRNA amplification protocol was: 60 min at 37 °C and 5 min at 95 °C; homogenized gently and kept on ice. Subsequently, 5 μL of final cDNA solution of each sample was taken and diluted 1:10 for later use in real-time PCR. Sequences of miRNA primers are shown in Table 2S. Each reaction was prepared by adding 5 μL of QuantiTech Sybr Green, 1 μL of miScript Universal Primer, 1 μL of miRNA primer, 2 μL of water and 1 μL of cDNA. Thermocycler program consisted of 95 °C for 15 min, subsequently 40 cycles of 94 °C for 5 s, 55 °C by 30 s, 70 °C by 30 s. As internal expression control, UNR6 gene was included. An ABI 7500 was used with HRM Software v2.0 program.
2.5. Statistical analysis
All data are presented as the mean ± SD of three biological replicates. A Student’s t-test was performed, when two groups were compared. To compare all cell lines and the different conditions, two-way ANOVA was conducted with a Tukey test as post hoc analysis. A value of p ≤ 0.05 was considered statistically significant.
3. Results
3.1. TLR3 expression is decreased in prostate cells malignantly transformed with sodium arsenite
To determine TLR3 relative expression, a WB analysis was performed on RWPE-1, CAsE-PE, WPE-Stem, AS-CSC. These cell lines were exposed for 48 h to PIC, 9cRA or the combination (PIC +9cRA). This analysis allowed us to first evaluate whether there was a TLR3 basal expression difference in iAs-transformed compared to untransformed parental cells, as well as to evaluate the ability of different cell lines to respond to treatment with PIC, 9cRA or PIC+9cRA, through their interaction with TLR3 and RARβ, respectively. The results showed that CAsE-PE express less TLR3 when compared to RWPE-1 cells (Fig. 1). The same was observed in As-CSC cells, in which TLR3 basal relative expression was significantly lower than WPE-stem Another important observation is that WPE-stem showed significantly lower TLR3 expression with respect to differentiated cells RWPE-1 (Fig. 1). On the other hand, after treated with PIC only RWPE-1 and WPE-Stem cells, but not CAsE-PE and As-CSC cells, showed a significative increase in TLR3 expression. Similarly, exposure to 9cRA did not exhibited an increase in TLR3 relative expression in CAsE-PE and As-CSC cells with respect to untreated cells, as well as with respect to their RWPE-1 and WPE-Stem controls, which showed a tendency to increase TLR3 expression (Fig. 1B). In CasE-PE and As-CSC exposed to PIC+9cRA, TLR3 relative expression was reduced compared to RWPE-1 and WPE-Stem respectively (Fig. 1A and B)
Fig. 1.
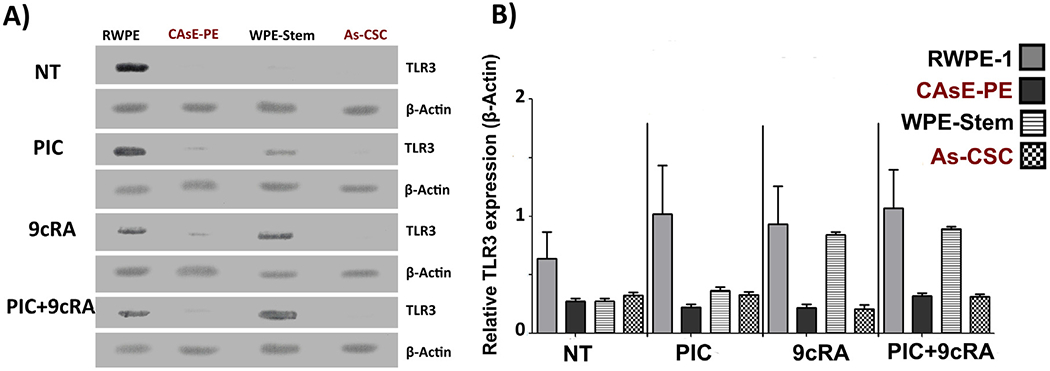
Relative expression of TLR3 in RWPE-1, CAsE-PE, WPE-Stem and As-CSC and post-exposure to treatments. RWPE-1, CAsE-PE, WPE-Stem and As-CSC cells were treated with 25 μg/mL of PIC for 24 h, with 10μM of 9cRA or their PIC+9cRA combination. The expression was determined by WB analysis. Representative tables of RWPE and CAsE-PE were plotted in their untreated condition (NT), condition with PIC, condition with 9cRA and its combination. A) TLR3 protein bands analyzed by WB. B) Densitometric analysis of the relative expression of TLR3, with respect to β- Actin. n-3, *−p ≤ 0.05 compared to its control.
3.2. As-CSCs and CAsE-PE showed an impaired expression of miR-29c, −148b, −152, DNMT1 and DNMT3a
The observation that TLR3 expression is decreased in CAsE-PE and As-CSC, as well the inability of PIC alone or in combination with 9cRA to increase its expression, suggests a downstream alteration in anti-tumor pathway described by Galli et al. (2014) induced by TLR3 activation in these cells. To assess this possibility, basal expression (without treatment) of miR-29c, 148b, −152 miRNAs that target DNMTs mRNAs was analyzed on RWPE-1, WPE-Stem, CAsE-PE, and As-CSC. They were also evaluated after being exposed to PIC, 9cRA alone or in combination. Results showed that miR-29c, −148b and −152 basal relative expression was significantly lower in CAsE-PE and As-CSC cells compared to their control cells RWPE-1 and WPE-stem (Fig. 2A). Unlike RWPE-1 cells, CAsE-PE cells exposed to PIC did not change miR-29c relative expression, which targets DNMT3A. Similar results were found in AS-CSCs cells exposed to PIC (Fig. 2B). miR-148b and miR-152 (which target DNMT1 and DNMT3A messenger respectively) expression, neither of them changed after CAsE-PE or As-CSC were exposed to PIC. However, in this case, PIC alone did not exhibit effect on WPE-Stem cells regarding miR148b expression, both compared with its basal expression and compared to RWPE-1, untransformed epithelial cells (Fig. 2A and B). Exposure to 9cRA alone induced a decrease in miR-29c and miR-148b expression in WPE-Stem compared to basal conditions, and this treatment did not change expression of any of assayed miRNAs in RWPE-1, CAsE-PE, or As-CSC compared to basal conditions (Fig. 2C). When CAsE-PE and As-CSC cells were exposed to PIC+9cRA, contrary to what was reported, miR-29c, -148b and -152 expression did not increase when compared to their respective control cells. In contrast, miR-29c and -152 expression were both decreased with respect to their control cells. And miR-148b, it showed no modification in all cell lines with respect to its basal condition after exposure to PIC or 9cRA (Fig. 2D). As it had been assumed, decreased the TLR3 expression observed in CAsE-PE and As-CSC cells has a direct impact on PIC’s ability to activate its anti-tumor pathway. This was corroborated by an observed decreased miR-29c, -148b and -152 expression in these iAs-transformed cells, compared to RWPE-1 and WPE-Stem, respectively, even when exposed to PIC+9cRA.
Fig. 2.
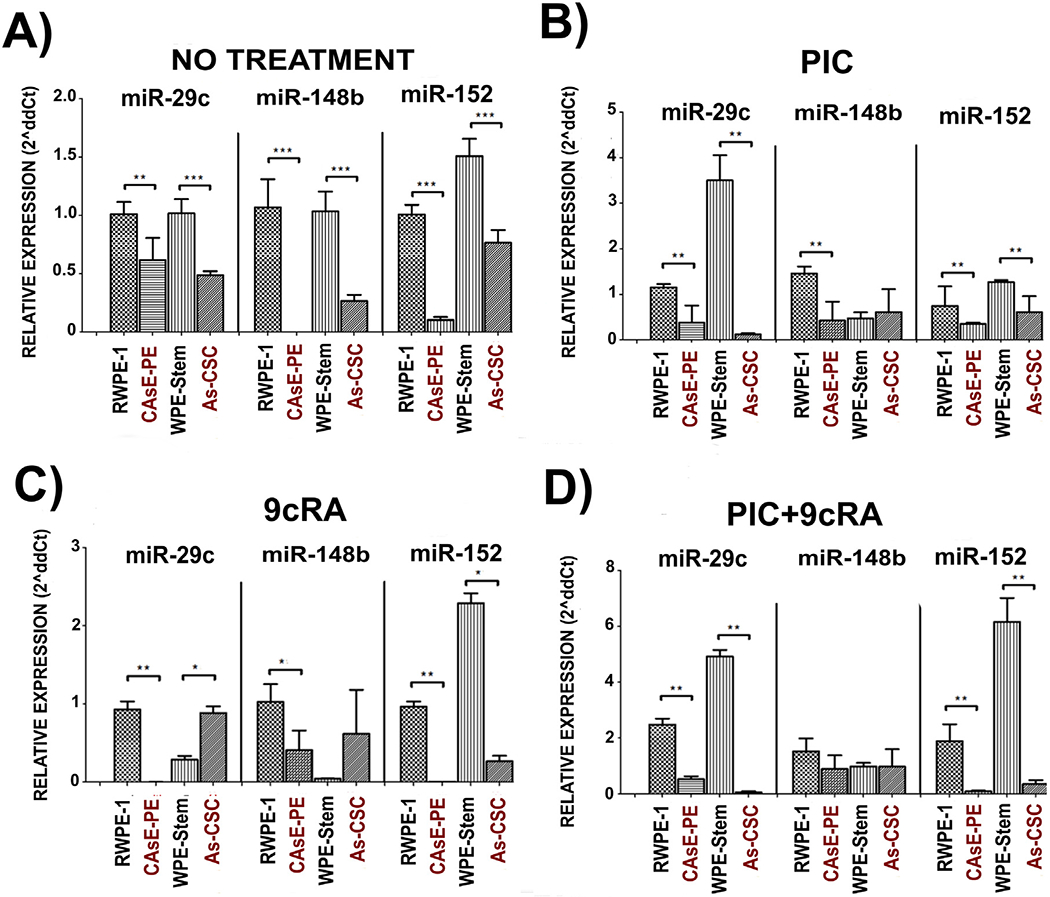
Relative expression of miR-29c, -148b, -152 in RWPE-1, CAsE-PE, WPE-Stem and As-CSC cells. A) DNMT1 y DNMT3A expression with no treatment, in B) exposed to PIC (25 μg/mL),in C) exposed to 9cRA (10μM), and in D) with PIC+9cRA. The results were determined by expression analysis with 2^(−ΔΔCt) in triplicates and with respect to β- Actin gene. Data with n-3, *-p ≤ 0.05, **p ≤ 0.025 compared to its control.
3.2.1. As-CSCs and CAsE-PE also showed increased DNMT1, DNMT3A and DNMT3B gene expression
Results described in sections above indicated that CAsE-PE and As-CSC, show a decreased TLR3 basal expression, as well as decreased miR-29c, -148b and -152 expression which appears not to be modified after exposure to PIC, 9cRA or their combination. Based on anti-tumor pathway described by Galli et al., the next step to be analyzed was DNMTs expression, both basal and after exposure to PIC, 9cRA and the combination. Analysis of CAsE-PE and As-CSC exhibited an increased DNMT1 and DNMT3A basal expression with respect to not transformed RWPE-1 and WPE-Stem cells (Fig. 3A), which could imply that, even before exposing to treatments, anti-tumor pathway described by Galli et al. already shows an important impairment in related intermediary molecules. When all cell lines were exposed to PIC, DNMT1 and DNMT3A expression significantly decreased in CAsE-PE cells compared to basal levels. However, such decreased expression was not sufficient to reach low basal expression levels of DNMT1 and DNMT3A observed in RWPE-1. For As-CSC cells, treatment with PIC showed no change in any of DNMTs assayed, this when compared to the already increased basal level and to WPE-Stem (Fig. 3B). These PIC-induced modifications in DNMTs expression observed in CAsE-PE, (which is mainly composed of differentiated cells and a proportion of stem cells), but not in As-CSCs, with respect to basal conditions, suggest that SC status plays a key role in the tumor ability to respond to this treatment. Besides 9cRA exposure also led to a decreased DNMT1 and DNMT3A expression with respect to its basal condition in all cell lines, in CAsE-PE cells these DNMTs transcript levels are not as low as in RWPE-1 (Fig. 3C). And for As-CSCs, DNMT1 and DNMT3A expression did not change after exposure to 9cRA compared to its basal condition and to WPE-Stem (Fig. 3C). When exposed to PIC+9cRA, CAsE-PE and As-CSC cells, showed an increased DNMT1 and DNMT3A expression with respect to RWPE-1 and WPE-Stem correspondingly, however, it was also observed a trend to decrease when compared to their not treated condition, but still, without reaching basal levels shown by their corresponding controls (Fig. 3D).
Fig. 3.
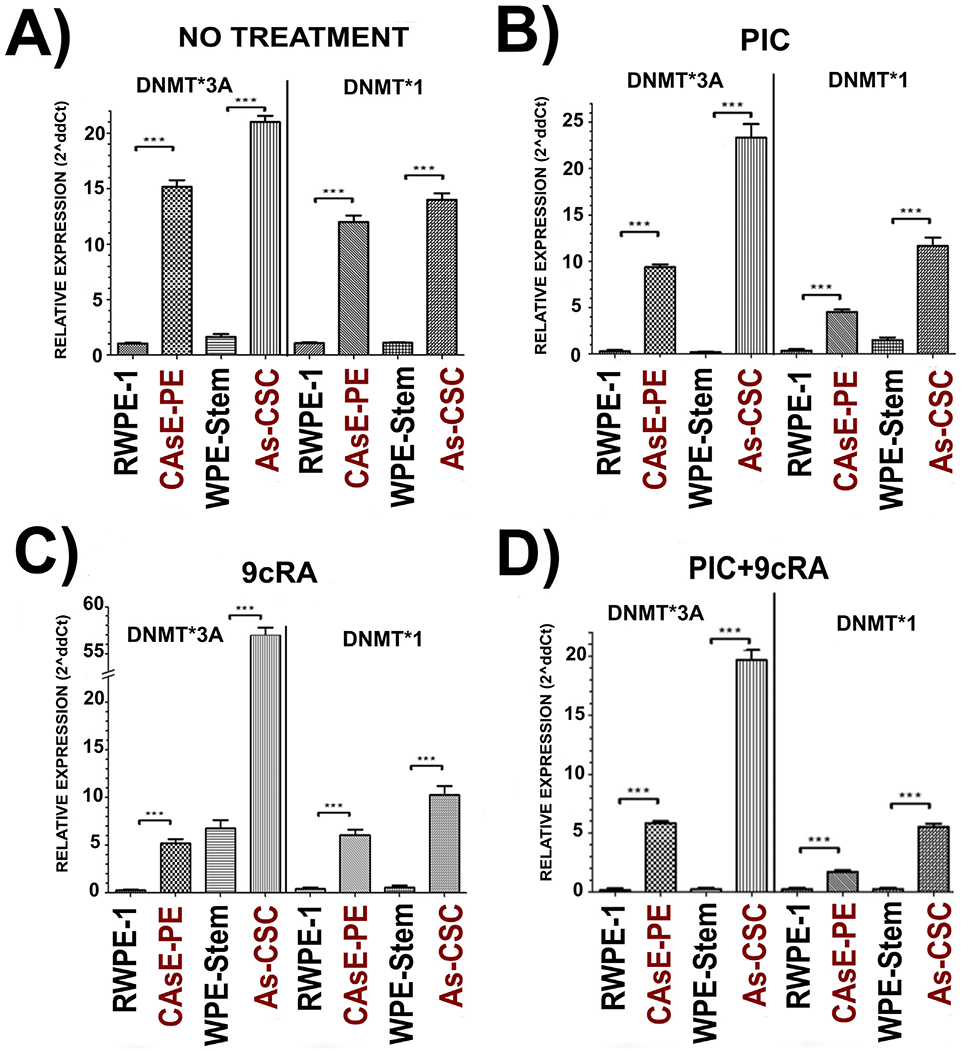
Relative expression of DNMT1 and DNMT3A in RWPE-1, CAsE-PE, WPE-Stem and As-CSC cells. A) DNMT1 y DNMT3A expression with no treatment, in B) exposed to PIC (25 μg/mL),in C) exposed to 9cRA (10μM), and in D) with PIC+9cRA. The results were determined by expression analysis with 2^(−ΔΔCt) in triplicates and with respect to β- Actin gene. Data with n-3, *-p ≤ 0.05, **p ≤ 0.025 compared to its control.
3.3. RARβ expression is downregulated in CAsE-PE and As-CSC
RARβ gene promoter is commonly methylated in cancer through the action of DNMTs (Yaqinuddin et al., 2009) leading to a decrease in RARβ protein synthesis. Consequently, an increased DNMTs expression can in turn have a negative impact on this retinoic acid receptor (RARβ) expression and on response to treatment with 9cRA, especially on the expression of RARβ-regulated genes. RT-PCR, WB and densitometry analysis for total proteins were performed to evaluate RARβ expression in all cell lines, basal and after exposure to PIC, 9cRA and the combination. In basal conditions, CAsE-PE cells exhibited a significant lower RARβ expression when compared to RWPE-1 under the same conditions. Correspondingly, when these cells were exposed to PIC, 9cRA, and their combination, not significant increase in RARβ expression with respect to RWPE-1 and basal condition was observed in CasE-PE (Fig. 4). As for As-CSC, RARβ basal relative expression did not show any difference from that observed in WPE-Stem cells. In contrast, when both lines were exposed to treatments (PIC, 9cRA and their combination), As-CSC did not modify RARβ relative expression, compared with WPE-Stem cells which showed a slight but significant increment in RARβ expression (Fig. 4). These observations are consistent with reported increased DNMTs expression in DU-145 cells, suggesting a critical role of methylation in RARβ gene silencing (Galli et al., 2013b).
Fig. 4.
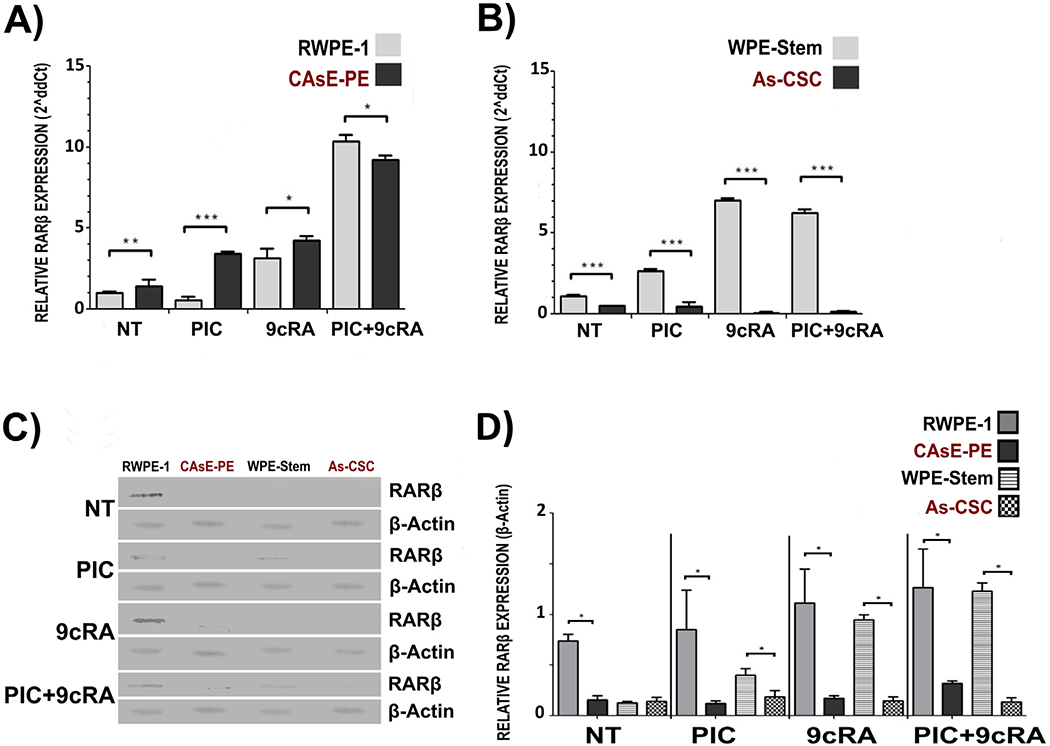
Relative basal expression of RARβ in RWPE-1, CAsE-PE, WPE-Stem and As-CSC and post-exposure to treatments. RWPE-1, CAsE-PE, WPE-Stem and As-CSC cells were treated with 25 g/mL of PIC for 24 h, with 10 μM of 9cRA or their PIC+9cRA combination. The expression was determined by WB analysis. Representative tables of RWPE and CAsE-PE were plotted in their untreated condition (NT), condition with PIC, condition with 9cRA and its combination. A) RARβ relative mRNA expression in RWPE-1 and CAsE-PE B) RARβ Relative mRNA expression in WPE-Stem and As-CSC C) RARβ protein bands analyzed by WB D) Densitometric analysis of the relative expression of TLR3, with respect to β- Actin. n-3, *-p ≤ 0.05 compared to its control.
3.4. Analysis of differentiation markers
Due to impairment in TLR3 antitumor pathway observed in previous experiments, an analysis of SC markers expression was performed to determine the ability of PIC, 9cRA or the combination to induce a change in SC markers expression in iAs-transformed cells. We analyzed the relative expression of SC-associated genes such as CD44, CD133 and SOX2 under basal conditions and after exposure to PIC, 9cRA and PIC+9cRA. At basal level, these genes expression in CAsE-PE was significantly increased with respect to RWPE-1; As-CSC cells also showed a significant increase in CD44, CD133 and SOX2 expression with respect to WPE-Stem (Fig. 5A). When CAsE-PE cells were exposed to PIC+9cRA, relative expression of CD44 was decreased to levels similar to those observed in RWPE-1. As-CSC exposed to PIC+9cRA showed a decreased CD44 expression compared to its basal expression, but its expression was still higher than WPE-stem (Fig. 5B). These results were consistent with previous analyses, where iAs-transformed cells, showed a lower TLR3 basal expression than non-cancerous cells, which was associated with impaired expression of miRNAs, DNMT1 and DNMT3A. Status of latter proteins also correlated with RARβ protein expression and with SC-markers expression.
Fig. 5.
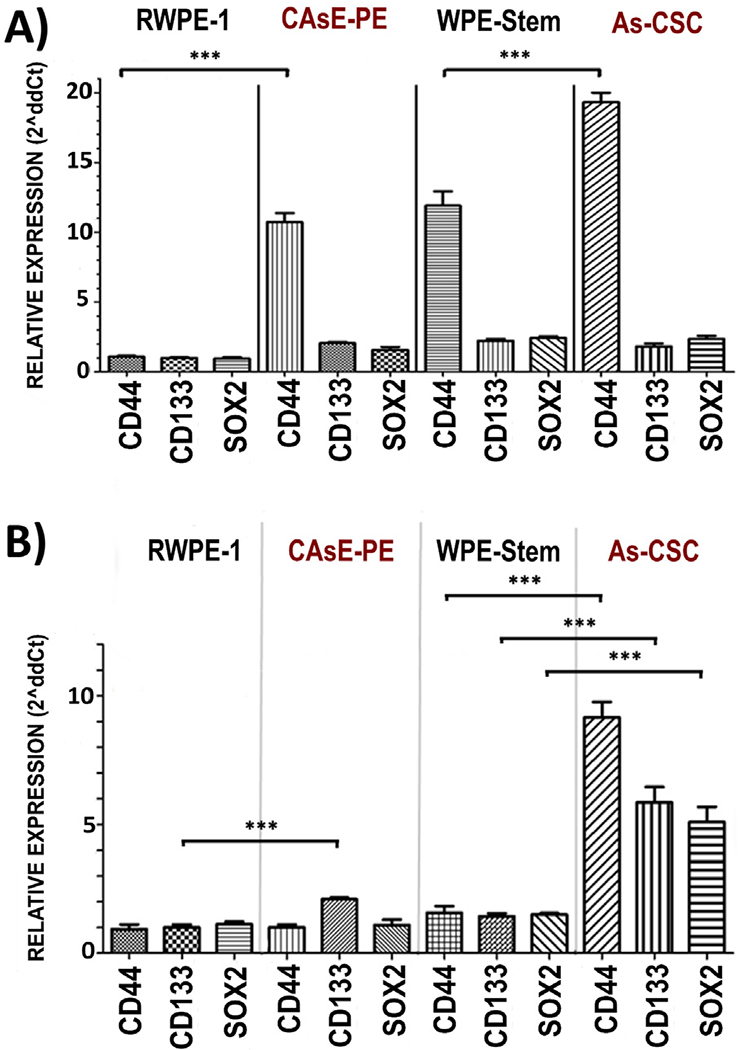
Relative expression of CD44, CD133 and SOX2 in RWPE-1, CAsE-PE, WPE-Stem and As-CSC with respect to β- Actin. RWPE-1, CAsE-PE, WPE-Stem and As-CSC cells were treated with 25 g/mL of PIC for 6 h, with 10 μM of 9cRA or its PIC+9cRA combination. The expression was determined by expression analysis with 2^ (−ΔΔCt). Representative tables of RWPE-1, CAsE-PE, WPE-Stem and As-CSC were plotted in their untreated condition (NT), condition with PIC, condition with 9cRA and its combination. n-3, *−p ≤ 0.05, **p ≤ 0.025 compared to its control.
4. Discussion
Retinoic acid (RA) and other retinoids are used in advanced PCa therapy (Pasquali et al., 2006). However, RARβ, relevant mediator in PIC-9cRA antitumoral pathway (Bernardo et al., 2013a, 2013b), is silenced in a number of PCa cases as part of the immortalization process of tumor cells (Belinsky, 2005; Swisshelm et al., 1994; Xu, 2007). Target populations in RA-based cancer therapy are SCs and CSCs since they are involved in tumor persistence and recurrence; therefore, strategies are sought that favor RARβ reexpression (Park et al., 2019). A proposed approach in castration-resistant PCa treatment is the use of PIC (TLR3 agonist) and retinoids such as 9cRA, in combination with conventional therapy (Le Naour et al., 2020; Podrazil et al., 2021). This strategy is based on reports showing that TLR3 activation by PIC, synergizes with 9cRA activating different pathways which induce RARβ re-expression in prostate and breast cancer cell lines. (23). Importantly, 9cRA indirectly increase TLR3 expression by controlling expression and activity of transcription factors (Bernardo et al., 2013a, 2013b; Kolla et al., 1996). Critically, iAs induced over-accumulation of prostate SCs and CSCs both in vitro and in vivo suggesting a critical role of SCs in development of iAs-associated cancer (19, 20). Tokar et al. (2010a, 2010b) demonstrated that continuous exposure of WPE-stem cells to 5 μM sodium arsenite for 18 weeks caused a rapid acquisition of CSC phenotype compared with RWPE-1 cells that become transformed after 30 weeks of exposure. As-transformed WPE-stem cells (As-CSCs) are highly invasive and proliferative, secrete high amounts of metalloproteinase-9, and form aggressive tumors when hetero-transplanted into nude mice. These cells also re-express self-renewal-related genes (p63, ABCG2, BMI-1, SHH, OCT-4, NOTCH-1) while expression of tumor suppressor PTEN, was depleted (Tokar et al., 2010a, 2010b). Such observations aroused our interest in evaluating TLR3 expression, as well as the status of anti-tumor pathway intermediaries, leading to RARβ re-expression and SCs differentiation in epithelial and stem cells lines in vitro transformed with iAs. To our knowledge, there is no previous work evaluating this complete TLR3-associtated anti-tumor pathway in PCA associated to iAs exposure. We first measured basal expression levels of several pathway intermediaries (TLR3, miR-29c, miR-148b, miR-152, RARβ, DNMT1, DNMT3A, SOX2, CD44 and CD133) in control cells and in iAs-transformed cells. In general, iAs-transformed CAsE-PE and As-CSC show a lower basal expression of TLR3, miR-29c, miR-148b, miR-152, RARβ, but higher DNMT1, DNMT3A, SOX2, CD44 and CD133 expression compared with their respective controls, RWPE-1 and WPE-stem. Previous studies have shown that PCa cell lines, DU-145, LNCaP and PC3 express similar amounts of TLR3, although a control cell line for comparison was not included (Galli et al., 2010; Paone et al., 2008). Basal expression of miR-29c, miR-148b, and miR-152 were lower in iAs-transformed cell lines compared with their respective controls. These results are consistent with reports showing that these miRNAs are significantly reduced in PC3 and LNCaP cells compared with non-tumor prostate cells from healthy tissues or with HaCaT immortalized human keratinocyte cells (Feng et al., 2019; Li et al., 2018; Sengupta et al., 2018; Zhu et al., 2013, 2014). Also, our observations are consistent with reports indicating that DNMT1 and DNMT3 expression levels in DU145 and PC3 are significantly elevated compared with RWPE-1 (Galli et al., 2013b; Lee et al., 2016). Unlike RWPE-1 and WPE-Stem, CAsE-PE and As-CSC cells did not respond to treatment with PIC, 9cRA or PIC +9cRA in terms of TLR3 expression. This observation suggests that a key target population in treatment of PCa (SCs/CSCs), could be less responsive to activation of antitumor pathway based on TLR3 activation with its synthetic ligand PIC. In a Galli et al. study, DU-145 cells showed increased TLR3 expression after activation when the cells were treated with 9cRA (10μM) and PIC (25 μg/mL) for 48 h. Similar observations were made in transformed murine prostatic cell line, TRAMP, and in breast cancer cell line, MDA-MB-231 (Galli et al., 2013b). The same study showed miR-29c, -148b and -152 expression increased after interaction of TLR3 with PIC (25 μg/mL) for 6 h in DU-145 (Galli et al., 2013b). In our work, CAsE-PE and As-CSC cells exposed to PIC, 9cRA or their combination showed no change in miR-29c, -148b and -152 expression when compared to RWPE-1 and WPE-stem. A similar decrease in expression levels of miRNAs associated with regulation of DNMTs has also been reported in transformed SCs from other tissues. For example, osteosarcoma CSCs (3AB-OS-CSC) exhibit a decreased miR-29 relative basal expression compared to their 3AB-OS source cell line (Di Fiore et al., 2014). In another study, miR-152 expression was significantly lower in hepatic carcinoma cells with CSC-like properties (e.g CD133+) compared with their control counterparts (Huang et al., 2015). Possible targets for miR-29c and miR-148b and -152 include DNMT3A and a DNMT1 correspondingly (Morita et al., 2013; Sengupta et al., 2018). Although CAsE-PE cells show a lower TLR3 expression, exposure of these cells to PIC, 9cRA or their combination (PIC+9cRA) showed a decreased relative expression of DNMT1 and DNMT3A. However, relative expression of both DNMTs still remains significantly higher in CAsE-PE compared with parental RWPE-1. While DNMT1 and DNMT3a levels are higher in As-CSC cells compared with parental WPE-stem, expression of these DNMTs in As-CSC was not altered upon exposure to PIC, 9cRA, or their combination. In our hands, RARβ expression in CAsE-PE did increased cells following exposure to treatments (PIC+9cRA) but not up to the levels observed in RWPE-1 cells, suggesting that, despite showing alterations in antitumor pathway related to TLR3 activation, CAsE-PE cells are still responsive in some extent to treatment with PIC + 9cRA. However, when exposed to treatments, As-CSC cells showed a very poor RARβ expression compared to WPE-stem, in contrast, previous studies show a significantly increased RARβ expression in cell lines like PC3, LNCaP and DU145, treated with 9cRA (10μM) by 48 h with respect to its basal condition (Galli et al., 2013b; Modarai et al., 2018). As indicated Tokar et al., previously demonstrated that exposure of RWPE-1 to iAs induces the enrichment of cells that express CSCs markers (Tokar et al., 2010a, 2010b; Tokar et al., 2017). The relevance of retinoid use in cancer treatment lies in CSCs differentiation induction (Liu et al., 2010). In this sense, the potential cellular response to 9cRA can be determined through associated genes expression with cell differentiation such as CD44, CD133, SOX2, ABCG2, OCT4 and Notch1 (Bushue and Wan, 2010; Kalantari et al., 2017; Tokar et al., 2010a, 2010b). In this study RARβ activation as a transcription factor through the binding to 9cRA, was determined by measuring expression of typical SC markers, SOX2, CD44 and CD133. Exposure of CAsE-PE to PIC+9cRA, resulted in a significant decrease in CD44, CD133 and SOX2 expression, reaching levels similar to RWPE-1 cells. In contrast, As-CSC exposed to PIC+9cRA show no changes in relative expression of these markers compared to no-transformed WPE-stem. These results strongly suggest that iAs-transformed SCs are irresponsive to this treatment. Taken together, results in present study show that epithelial prostate cell lines and SCs malignantly transformed in vitro by iAs have decreased TLR3 basal expression and an impaired antitumor pathway based on induction of RARβ re-expression. These results also showed that treatments with PIC and 9cRA, tend to increase RARβ expression in heterogeneous cell line CAsE-PE, but not so in As-CSC cell line. These observations strongly suggest that iAs transformation has differential effects in CAsE-PE and As-CSCs cells, and further suggest that this transformation makes SCs irresponsive to treatment based on TLR3 activation and cellular differentiation by induction of RARβ expression (Fig. 6). Mechanisms by which this happens remain to be elucidated, however, it is suggested that events involving silencing of genes such as p53, which is a key regulator of TLR3 expression, can play a central role in SC or CSC phenotype development. This study contributes to establishing the basis for routinely identifying cases of PCa associated with iAs exposure, to conduct studies to predict effectiveness of PIC + RA treatments, like evaluation of TLR3 and RARβ status. Additional studies are required to determine mechanisms by which iAs decreases TLR3 expression, which would allow identification of molecular targets to induce TLR3 and RARβ re-expression in human populations exposed to this metalloid.
Fig. 6.
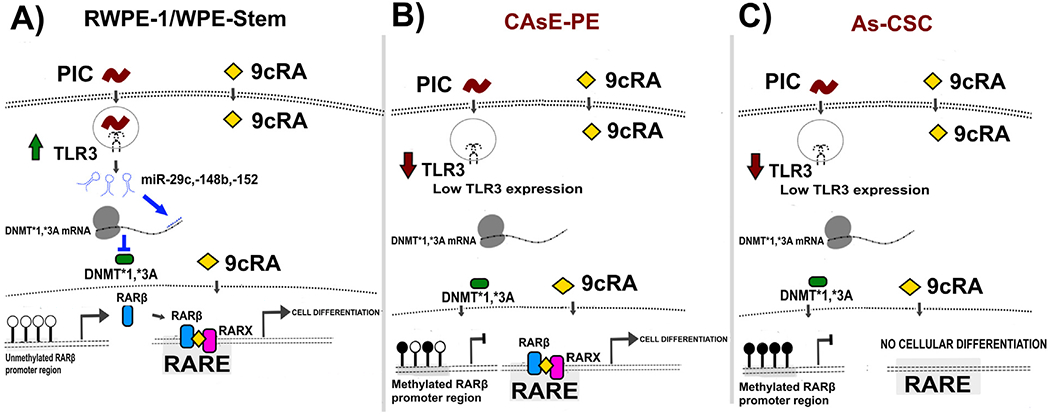
Anti-tumor route related to the activation of TLR3 and its synergy with RAR, proposed model for RWPE-1, CAsE-PE, WPE-Stem and As-CSC.
Supplementary Material
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxlet.2021.07.013.
HIGHLIGHTS.
TLR3 activation through PIC plus the use of 9cRA is a proposed immunotherapy for PCa.
iAs-transformed prostate stem cells (As-CSCs) express significantly less TLR3 than non-exposed parental cells (WPE-stem).
As-CSC cells show an impaired basal TLR3-associated anti-tumor pathway.
As-CSCs are not responsive to TLR3 agonist PIC in combination with 9cRA.
iAs-associated human PCa may be un-responsive to this immunotherapy.
Acknowledgements
The authors thank PhD. Edith Uresti Rivera for providing technical support in the development of the initial phases of this project.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of Competing Interest
The authors declare that they have no knowledge of external financial or personal situations that have an influence on the work reported in this article.
References
- Adams S, 2009. Toll-like receptor agonists in cancer therapy. Immunotherapy 1, 949–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allenby G,Janocha R, Kazmer S, Speck J, Grippo JF, Levin AA, 1994. Binding of 9-cis-retinoic acid and all-trans-retinoic acid to retinoic acid receptors alpha, beta, and gamma. Retinoic acid receptor gamma binds all-trans-retinoic acid preferentially over 9-cis-retinoic acid. J. Biol. Chem 269, 16689–16695. [PubMed] [Google Scholar]
- Barton GM, Medzhitov R, 2003. Toll-like receptor signaling pathways the signal to move : d. discoideum Go Orienteering 300, 1524–1526. [DOI] [PubMed] [Google Scholar]
- Belinsky SA, 2005. Silencing of genes by promoter hypermethylation: key event in rodent and human lung cancer. Carcinogenesis 26, 1481–1487. doi: 10.1093/carcin/bgi020. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waalkes MP, 2008. Inorganic arsenic and human prostate cancer. Environ. Health Perspect 116, 158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo AR, Cosgaya JM, A.A.A.J.-L, 2013a. Synergy between RA and TLR3 promotes type I IFN- dependent apoptosis through upregulation of TRAIL pathway in breast cancer cells. Cell Death Dis. 4, 1–10. doi: 10.1038/cddis.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo AR, Cosgaya JM, Aranda A, Jiménez-Lara AM, 2013b. Synergy between RA and TLR3 promotes type I IFN-dependent apoptosis through upregulation of TRAIL pathway in breast cancer cells. Cell Death Dis. 4, 1–10. doi: 10.1038/cddis.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushue N, Wan YJY, 2010. Retinoid pathway and cancer therapeutics. Adv. Drug Deliv. Rev 62, 1285–1298. doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly RM, Nguyen NK, Sukumar S, 2013. Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin. Cancer Res 19, 1651–1959. doi: 10.1158/1078-0432.CCR-12-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCicco KL, Youngdahl JD, Catharine Ross A, 2001. All-trans-retinoic acid and polyriboinosinic : polyribocytidylic acid in combination potentiate specific antibody production and cell-mediated immunity. Immunology 104, 341–348. doi: 10.1046/j.1365-2567.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore R, Drago-Ferrante R, Pentimali F, Di Marzo D, Forte IM, D’anneo A, Carlisi D, De Blasio A, Giuliano M, Tesoriere G, Giordano A, Vento R, 2014. MicroRNA-29b-1 impairs in vitro cell proliferation, self-renewal and chemoresistance of human osteosarcoma 3AB-OS cancer stem cells. Int. J. Oncol 45, 2013–2023. doi: 10.3892/ijo.2014.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estornes Y, Micheau O, Renno T, Lebecque S, 2013. Dual role of TLR3 in inflammation and cancer cell apoptosis. World’s Larg. Sci. Technol. Med. Open Access B. Publ 247–270. [Google Scholar]
- Feng F, Liu H, Chen A, Xia Q, Zhao Y, Jin X, Huang J, 2019. miR-148-3p and miR-152-3p synergistically regulate prostate cancer progression via repressing KLF4. J. Cell. Biochem 120, 17228–17239. doi: 10.1002/jcb.28984. [DOI] [PubMed] [Google Scholar]
- Galli R, Starace D, Busà R, Angelini DF, Paone A, De Cesaris P, Filippini A, Sette C, Battistini L, Ziparo E, Riccioli A, 2010. TLR stimulation of prostate tumor cells induces chemokine-mediated recruitment of specific immune cell types. J. Immunol 184, 6658–6669. doi: 10.4049/jimmunol.0902401. [DOI] [PubMed] [Google Scholar]
- Galli R, Paone A, Fabbri M, Zanesi N, Calore F, Cascione L, 2013a. microRNA-dependent Reexpression of Functional RAR β and Tumor Regression 3., pp. 1–6. doi: 10.1073/pnas.1304610110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Paone A, Fabbri M, Zanesi N, Calore F, Cascione L, Acunzo M, Stoppacciaro A, Tubaro A, Lovat F, 2013b. Toll-like receptor 3 (TLR3) activation induces microRNA-dependent reexpression of functional RARβ and tumor regression. Proc. Natl. Acad. Sci 110, 9812–9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Reyes S, Fernández JM, González LO, Aguirre A, Suárez A, González JM, Escaff S, Vizoso FJ, 2011. Study of TLR3, TLR4, and TLR9 in prostate carcinomas and their association with biochemical recurrence. Cancer Immunol. Immunother. 60, 217–226. doi: 10.1007/s00262-010-0931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidarzadeh M, Roodbari F, Hassanpour M, Ahmadi M, Saberianpour S, Rahbarghazi R, 2020. Toll-like receptor bioactivity in endothelial progenitor cells. Cell Tissue Res. 1–8. [DOI] [PubMed] [Google Scholar]
- Huang H, Hu M, Li P, Lu C, Li M, 2015. Mir-152 inhibits cell proliferation and colony formation of CD133+ liver cancer stem cells by targeting KIT. Tumor Biol. 36, 921–928. doi: 10.1007/s13277-014-2719-x. [DOI] [PubMed] [Google Scholar]
- Isayev O, Zhu Y, Gasimov E, Werner J, Bazhin AV, 2019. Effect of chemotherapeutic agents on the expression of retinoid receptors and markers of Cancer stem cells and epithelial-mesenchymal transition. Biochem. 84,1424–1432. doi: 10.1134/S0006297919110166. [DOI] [PubMed] [Google Scholar]
- Kalantari E, Asgari M, Nikpanah S, 2017. Co-expression of putative Cancer stem cell markers CD44 and CD133 in prostate carcinomas. Pathol. Oncol. Res doi: 10.1007/s12253-016-0169-z. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T, 2014. Toll-like receptor signaling pathways. Front. Immunol 5, 1–8. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla V, Lindner DJ, Weihua X, Borden EC, Kalvakolanu DV, 1996. Modulation of interferon (IFN)-inducible gene expression by retinoic acid up-regulation of STAT1 protein in IFN-unresponsive cells. J. Biol. Chem 271, 10508–10514. [DOI] [PubMed] [Google Scholar]
- Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E, 2020. Trial watch: TLR3 agonists in cancer therapy. Oncoimmunology 9 doi: 10.1080/2162402X.2020.1771143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Wang J, Yumoto K, Jung Y, Cackowski FC, Decker AM, Li Y, Franceschi RT, Pienta KJ, Taichman RS, 2016. DNMT1 regulates epithelial-mesenchymal transition and Cancer stem cells, which promotes prostate Cancer metastasis. Neoplasia (United States) 18, 553–566. doi: 10.1016/j.neo.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Fu F, Wan X, Huang S, Wu D, Li Y, 2018. Up-regulated miR-29c inhibits cell proliferation and glycolysis by inhibiting SLC2A3 expression in prostate cancer. Gene 665, 26–34. doi: 10.1016/j.gene.2018.04.086. [DOI] [PubMed] [Google Scholar]
- Liu Te, Xu F, Du X, Lai D, Liu Tianjin, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W, Liu Z, 2010. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol. Cell. Biochem 340,265–273. doi: 10.1007/s11010-010-0426-5. [DOI] [PubMed] [Google Scholar]
- Modarai SR, Gupta A, Opdenaker LM, Kowash R, Masters G, Viswanathan V, Zhang T, Fields JZ, Boman BM, 2018. The anti-cancer effect of retinoic acid signaling in CRC occurs via decreased growth of ALDH+ colon cancer stem cells and increased differentiation of stem cells. Oncotarget 9, 34658–34669. doi: 10.18632/oncotarget.26157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Horii T, Kimura M, Ochiya T, Tajima S, Hatada I, 2013. MiR-29 represses the activities of DNA methyltransferases and DNA demethylases. Int. J. Mol. Sci 14, 14647–14658. doi: 10.3390/ijms140714647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PH, Giraud J, Staedel C, Chambonnier L, Dubus P, Chevret E, Bœuf H, Gauthereau X, Rousseau B, Fevre M, Soubeyran I, Belleannée G, Evrard S, Collet D, Mégraud F, Varon C, 2016. All-trans retinoic acid targets gastric cancer stem cells and inhibits patient-derived gastric carcinoma tumor growth. Oncogene 35, 5619–5628. doi: 10.1038/onc.2016.87. [DOI] [PubMed] [Google Scholar]
- Paone A, Starace D, Galli R, Padula F, De Cesaris P, Filippini A, Ziparo E, Riccioli A, 2008. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-α-dependent mechanism. Carcinogenesis 29,1334–1342. [DOI] [PubMed] [Google Scholar]
- Park J, Park K, Chung W, Park J, Zhang X, Lee SK, Song N, Son SH, Kim KR, Shim JH, 2019. CCL28-induced RAR B Expression Inhibits Oral Squamous Cell Carcinoma Bone Invasion Find the Latest Version : CCL28-induced RAR β Expression Inhibits Oral Squamous Cell Carcinoma Bone Invasion. 129., pp. 5381–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali D, Rossi V, Bellastella G, Bellastella A, Sinisi A, 2006. Natural and synthetic retinoids in prostate Cancer. Curr. Pharm. Des 12, 1923–1929. doi: 10.2174/138161206776873554. [DOI] [PubMed] [Google Scholar]
- Podrazil M, Horvath R, Becht E, Rozkova D, Sochorova K, Hromadkova H, Kayserova J, Lastovicka J, Vrabcova P, Kubackova K, Jarolim L, Babjuk M, Spisek R, Bartunkova J, n.d. Phase I / II clinical trial of dendritic-cell based immunotherapy (DCVAC / PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar V, Dey M, Dorn R, Raskin I, 2010. MyD88-dependent and independent pathways of toll-like receptors are engaged in biological activity of triptolide in ligand-stimulated macrophages. BMC Chem. Biol 10 doi: 10.1186/1472-6769-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D, Deb M, Patra SK, 2018. Antagonistic activities of miR-148a and DNMT1: ectopic expression of miR-148a impairs DNMT1 mRNA and dwindle cell proliferation and survival. Gene 660, 68–79. doi: 10.1016/j.gene.2018.03.075. [DOI] [PubMed] [Google Scholar]
- Swisshelm K, Ryan K, Lee X, Tsou HC, Peacocke M, Sager R, 1994. Down-regulation of retinoic acid receptor β in mammary carcinoma cell lines and its up-regulation in senescing normal mammary epithelial cells. Cell Growth Differ. 5, 133–141. [PubMed] [Google Scholar]
- Tokar Erik J., Diwan BA, Waalkes MP, 2010a. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ. Health Perspect 118, 108–115. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar Erik J., Qu W, Liu J, Liu W, Webber MM, Phang JM, Waalkes MP, 2010b. Arsenic-specific stem cell selection during malignant transformation. J. Natl. Cancer Inst 102, 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Qu W, Waalkes MP, 2017. Arsenic, Stem Cells, and the Developmental Basis of Adult Cancer. 120., pp. 192–203. doi: 10.1093/toxsci/kfq342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth JM, Straughn JM, Atigadda ER, Muccio DD, Buchsbaum DJ, 2012. The use of retinoids in ovarian cancer: a review of the literature. Int. J. Gynecol. Cancer 22, 191–198. doi: 10.1097/IGC.0b013e318236a2ec. [DOI] [PubMed] [Google Scholar]
- Xu XC, 2007. Tumor-suppressive activity of retinoic acid receptor-β in cancer. Cancer Lett. 253, 14–24. doi: 10.1016/j.canlet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqinuddin A, Qureshi SA, Qazi R, Farooq S, Abbas F, 2009. DNMT1 silencing affects locus specific DNA methylation and increases prostate Cancer Derived PC3 cell invasiveness. J. Urol 182, 756–761. doi: 10.1016/j.juro.2009.03.082. [DOI] [PubMed] [Google Scholar]
- Zhu C, Li J, Ding Q, Cheng G, Zhou H, Tao L, Cai H, Li P, Cao Q,Ju X, Meng X, Qin C, Hua L, Shao P, Yin C, 2013. MiR-152 controls migration and invasive potential by targetingTGFα in prostate cancer cell lines. Prostate 73,1082–1089. doi: 10.1002/pros.22656. [DOI] [PubMed] [Google Scholar]
- Zhu W, He J, Chen D, Zhang B, Xu L, Ma H, Liu XG, Zhang YK, Le H, 2014. Expression of miR-29c, miR-93, and miR-429 as potential biomarkers for detection of early stage non-small lung cancer. PLoS One 9,1–7. doi: 10.1371/journal.pone.0087780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxlet.2021.07.013.


