Abstract
Furin is a subtilisin-related endoprotease which processes a wide range of bioactive proteins. Furin is concentrated in the trans-Golgi network (TGN), where proteolytic activation of many precursor proteins takes place. A significant fraction of furin, however, cycles among the TGN, the plasma membrane, and endosomes, indicating that the accumulation in the TGN reflects a dynamic localization process. The cytosolic domain of furin is necessary and sufficient for TGN localization, and two signals are responsible for retrieval of furin to the TGN. A tyrosine-based (YKGL) motif mediates internalization of furin from the cell surface into endosomes. An acidic cluster that is part of two casein kinase II phosphorylation sites (SDSEEDE) is then responsible for retrieval of furin from endosomes to the TGN. In addition, the acidic EEDE sequence also mediates endocytic activity. Here, we analyzed the sorting of furin in polarized epithelial cells. We show that furin is delivered to the basolateral surface of MDCK cells, from where a significant fraction of the protein can return to the TGN. A phenylalanine-isoleucine motif together with the acidic EEDE cluster is required for basolateral sorting and constitutes a novel signal regulating intracellular traffic of furin.
Furin, a member of a family of mammalian enzymes related to the yeast Kex2p and the bacterial subtilisins, is a calcium-dependent serine endoprotease that cleaves proproteins at the C terminus of multibasic sites (reviewed in references 28 and 35).
Although furin is concentrated in the trans-Golgi network (TGN) in the steady state, a significant fraction of the protease cycles among the plasma membrane, endosomes, and the TGN (2, 26). Rat furin is a type I integral membrane glycoprotein composed of a 714-residue luminal domain, a 21-residue transmembrane region, and a 58-amino-acid cytosolic tail (8, 23). The cytosolic tail of furin is necessary and sufficient for TGN localization (2, 5, 26, 33, 40). Several signals that control trafficking of furin have been identified in the cytosolic domain (see Fig. 2). Internalization from the cell surface involves a classical tyrosine-based signal (YKGL) and an acidic amino acid cluster (SDSEEDE) (33, 39, 40). The serine residues in the acidic cluster are subject to phosphorylation by casein kinase II (CKII) (12), and phosphorylation regulates the retrieval of the endoprotease from endosomes to the TGN (12, 25, 39). In PC12 cells, inactivation of the CKII site results in the transfer of furin into mature secretory granules from which the protease is normally excluded (6). The furin tail interacts with the TGN-enriched clathrin adapter AP-1, probably via the connector protein PACS-1 (41). Since the interaction with AP-1 is dependent on the phosphorylation state of the serines in the CKII site (6), removal of furin from mature secretory granules may be mediated by AP-1 and clathrin.
FIG. 2.
Amino acid sequences of the cytosolic domains of wild-type furin tail and mutant Tac-furin tail chimeras and their polarized distribution. Amino acid sequences are shown in the single-letter code, and known sorting signals in the furin tail are underlined. Residues are numbered from left to right, with position 1 corresponding to the presumed initiation methionine. The nomenclature of the mutants is according to that of Voorhees et al. (40); TFT is a chimera combining the luminal and cytosolic domains of Tac and the transmembrane domain of furin; TTF and Tac F are chimeras combining the cytosolic tail of furin and the ecto- and transmembrane domains or complete Tac, respectively. Alanine substitutions in the wild-type sequence are shown in boldface. The polarized distribution of the different constructs is summarized to the right, and the number(s) of the figure(s) showing the data for the corresponding construct is given. Where no data is shown in the text, it is indicated whether the distribution was determined by immunofluorescence (IF) and/or radioactive antibody binding (bdg).
Little is known about trafficking of furin in epithelial cells, where the protease may be delivered from the TGN to the apical or the basolateral plasma membrane domain, or to both domains. In the present study we analyzed the routing of furin in polarized MDCK cell monolayers. Furin was found to be preferentially delivered to the basolateral domain of transfected MDCK cells. Using chimeras combining the ecto- and transmembrane domains of human Tac (interleukin 2 receptor α-chain, or CD25) (18) and the cytosolic domain of furin, we show that the tail of furin is necessary and sufficient for basolateral sorting. Interestingly, basolateral sorting of furin does not rely on the tyrosine signal but requires a novel determinant consisting of an FI motif in conjunction with the nearby acidic amino acid cluster EEDE.
MATERIALS AND METHODS
Materials.
Anti-human furin and anti-rat TGN38 tail antibodies were kindly provided by J.-W. van der Loo (Inter-University of Leuven, Leuven, Belgium) and G. Banting (University of Bristol, Bristol, United Kingdom), respectively. The monoclonal antibodies 7G7 (32) or H93 (31) (the latter was kindly provided by D. Rimoldi, Ludwig Institute for Cancer Research, Epalinges, Switzerland) were used to detect the Tac ectodomain in the chimera. H93 was radioiodinated to specific activities of 2 × 106 to 7 × 106 cpm/μg by using Iodogen (Pierce, Rockford, Ill.), and unincorporated 125I was removed by ion-exchange chromatography on Dowex-1 (Sigma Chemical Co., Buchs, Switzerland) as described previously (22). Protease inhibitor cocktail contained 10 mg each of chymostatin, antipain, leupeptin, and pepstatin A (all from Sigma Chemical Co.) per ml in dimethyl sulfoxide and was used at a 1:1,000 dilution. 125I-labeled NaI was obtained from Amersham Corp., Little Chalfont, Buckinghamshire, United Kingdom. Protein G-Sepharose was from Sigma Chemical Co. and was washed with phosphate-buffered saline (PBS) containing 0.5% Triton X-100 before use. Mowiol 4-88 was from Calbiochem-Novabiochem Corp., La Jolla, Calif., and was used at a 0.1-g/ml concentration supplemented with 0.2% (wt/vol) diazabicyclo(2.2.2)octane (Sigma Chemical Co.).
Cell culture and transfection of MDCK cells.
MDCK cells of strain II were cultured on plastic or, to obtain polarized cell monolayers, on Transwell polycarbonate filter units (Costar Corp., Cambridge, Mass.) as described (11). Units of 6-, 12-, or 24-mm diameter and 0.4-μm pore size were used. To increase expression levels, cells were treated overnight with 10 mM butyrate (20), but similar results were obtained with untreated and treated cells. Cells were transfected by the calcium-phosphate method and selected in medium supplemented with G418 (Calbiochem-Novabiochem Corp.) as detailed elsewhere (11). Resistant clones were analyzed for expression by immunofluorescence, and at least three clones expressing different levels of the particular protein were selected for further analysis. Cells expressing similar levels of the different constructs were compared, and no differences due to expression level were observed. Intactness of cell monolayers grown on Transwell units was verified by measuring the transepithelial electrical resistance.
Construction of Tac-furin tail chimeras.
Recombinant DNA techniques were carried out according to standard procedures. The human furin cDNA in the pCDNA3 expression vector was kindly provided by N. Seidah (University of Montreal, Montreal, Quebec, Canada) via G. van der Goot (University of Geneva, Geneva, Switzerland) and directly used for transfections. The construction of Tac-furin tail chimeras encoding the membrane proximal or distal part of the furin tail as well as truncation mutants has been described elsewhere (40); these constructs were subcloned into the expression vector pCB6. Additional constructs carrying point mutations or deletions were generated by PCR. Native tail sequences were thereby replaced with the mutated tails by using a unique StuI site in the furin tail and a BamHI site present in the polylinker of the vectors. Alternatively, mutations were generated by a single-step PCR-based method employing two complementary mutagenic primers (42). All fragments synthesized by PCR were verified by sequencing. The sequences of the different primers are available upon request.
Steady-state distribution and surface appearance of furin.
To determine the steady-state distribution of expressed proteins, cells grown on coverslips were washed with PBS containing 0.9 mM CaCl2 and 0.5 mM MgCl2 (PBS+) and fixed for 20 min in 2% paraformaldehyde. After quenching with 50 mM NH4Cl in PBS+ (10 min) and permeabilization of cells with saponin (0.1% in PBS+), nonspecific binding sites were blocked for 30 min with 10% goat serum in PBS+. Cells were then incubated with the antifurin or anti-Tac monoclonal antibodies (dilution, 1:100 in 10% goat serum in PBS+) and then with a labeled goat anti-mouse antibody (dilution, 1:250 in 10% goat serum in PBS). For colocalization experiments, TGN38 was labeled with a rabbit anti-TGN38 antibody followed by a labeled goat anti-rabbit antibody. To analyze the surface appearance of transfected proteins, cells were incubated at 37°C for 30 min in the presence of the monoclonal antibodies. For cells grown on Transwell filters, the antibodies were added either to the apical chamber or the basolateral chamber. Cells were then washed, fixed, permeabilized, and stained with a labeled second antibody. After being washed with PBS+, cells were mounted in Mowiol and viewed in a Zeiss Axiophot fluorescence microscope with a 63× Apachromat oil immersion lens. Pictures were acquired with a Color Cool View camera (Photonic Science) and Image Pro Plus version 3.0 software (Media Cybernetics, Silver Spring, Md.) and processed with Photoshop version 5.0 software (Adobe Systems, Inc.). Identical parameters for image acquisition and processing were used after addition of apical and basolateral antibodies.
To quantitate surface distribution of the proteins generated by the chimeras, 125I-labeled H89 (1 μg/ml) were allowed to bind to cell monolayers from the apical or basolateral compartment for 60 min on ice. Unbound antibody was removed by washing, filters were cut out of the holder, and bound radioactivity was measured. Specific binding (2,000 to 30,000 cpm, depending on whether the construct produced impaired TGN retention and/or endocytosis) was calculated by subtracting background counts (50 to 300 cpm) obtained from filters with untransfected cells.
RESULTS
Furin is delivered to the basolateral surface of MDCK cells.
Since it is not known if furin is sorted to the apical or basolateral surface in epithelial cells, we first analyzed the intracellular routing of the protein in MDCK cells transfected with a human furin cDNA. Cells stably expressing furin were identified by immunofluorescence by using a monoclonal antibody specific for a luminal epitope in the human protein.
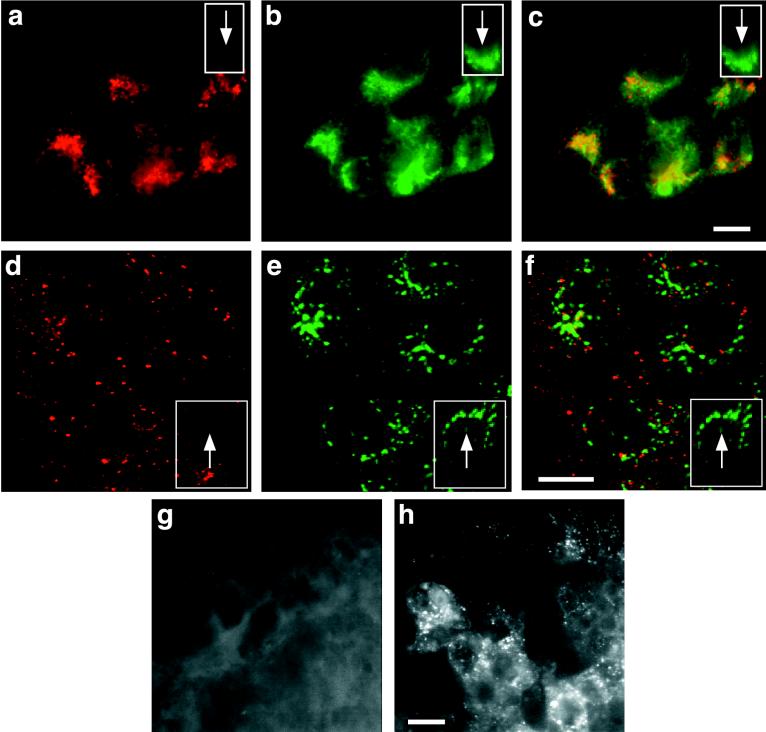
To characterize the steady-state distribution of furin, MDCK cells grown on coverslips were fixed, permeabilized, and stained with the antifurin antibody. As expected, furin localized to a perinuclear compartment, which was morphologically similar to the TGN (Fig. 1a). Labeling for furin was specific since the antibody did not stain untransfected cells (Fig. 1a to c, insets). Even though furin and TGN38 (19) utilize different signals for retrieval to the TGN (1, 16), the two proteins showed extensive colocalization (Fig. 1b and c). Little if any specific labeling for furin was observed on nonpermeabilized cells (data not shown), indicating that few furin molecules were present on the cell surface at any given time. However, if transfected cells were incubated for 60 min at 37°C in medium containing antifurin antibodies (1 μg/ml) prior to fixation and permeabilization, the antibodies were internalized and delivered to a perinuclear compartment (Fig. 1), showing that a detectable fraction of furin is delivered to the cell surface, where it can bind and internalize the antibody. No uptake of antibody was observed in untransfected control cells (Fig. 1d to f, insets), indicating that antibody uptake requires binding to furin molecules that reach the cell surface and is not due to internalization of antibodies in the fluid phase. Interestingly, if cells that had internalized antifurin antibodies were stained for TGN38 (Fig. 1), only a small amount of the internalized antifurin antibodies colocalized with the TGN marker (Fig. 1f, yellow color). Since the luminal domain of furin plays a role in lysosomal transport of aggregated molecules (43), antibody-induced cross-linking may divert internalized furin to lysosomes; this interpretation is consistent with the observation that the Tac-furin tail chimera, which lacks the luminal domain of furin implicated in lysosomal transport, was efficiently retrieved to the TGN (see below, Fig. 3).
FIG. 1.
Furin is sorted to the basolateral cell surface in MDCK cells. MDCK cells expressing human furin were grown on coverslips, fixed, and stained with an antifurin antibody (a) or with an anti-TGN38 antibody (b). Furin is enriched in a perinuclear compartment that resembles the compartment carrying TGN38, and superposition of the two panels indicates extensive colocalization (c, yellow color). Cells not expressing furin do not stain with antifurin (panels a to c, insets). For panels d, e, and f, cells were incubated with antifurin antibody present in the medium for 60 min at 37°C prior to fixation, permeabilization, and staining with a labeled secondary antibody (d) or with anti-TGN38 (e). Merging of the two images reveals little colocalization of internalized antifurin antibodies and endogenous TGN38 (f), probably reflecting lysosomal delivery of cross-linked furin. Untransfected cells do not internalize the antifurin antibodies (panels d, e, and f, insets), indicating that antibody uptake did not occur by fluid-phase endocytosis. For panels g and h, polarized MDCK cell monolayers were incubated at 37°C in the presence of antifurin antibodies added to the apical (g) or basolateral (h) compartment. Antibodies are selectively internalized from the basolateral surface. Bars: 10 μm.
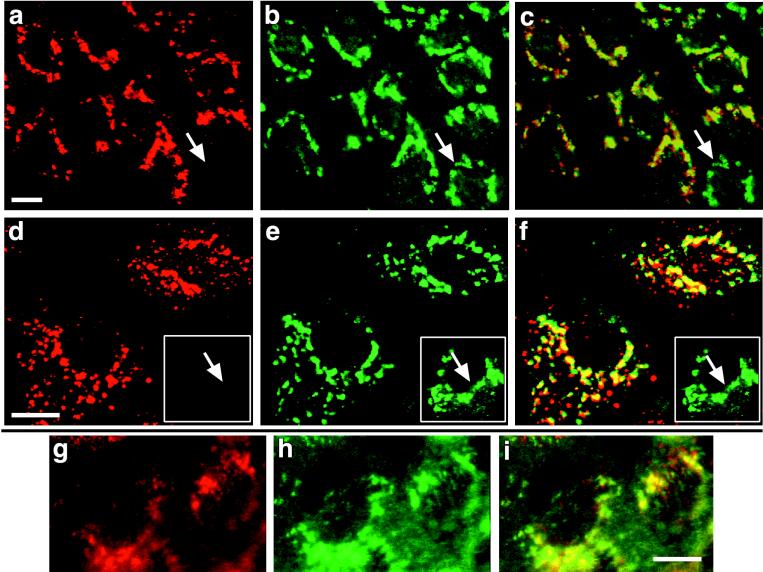
FIG. 3.
Localization and endocytosis of the Tac-furin tail chimera TTF. MDCK cells expressing TTF grown on coverslips were fixed and stained with an anti-Tac antibody (a) or an anti-TGN38 antibody (b). TTF is enriched in a perinuclear compartment that resembles the compartment carrying TGN38, and superposition of the two panels indicates extensive colocalization (c, yellow color). Cells not expressing TTF do not stain with anti-Tac (panels a, b, and c, arrows). For panels d and e, cells were incubated for 60 min at 37°C in the presence of anti-Tac antibodies to allow for the internalization of antibody and then fixed, permeabilized, and stained with a labeled secondary antibody (d) or with anti-TGN38 (e). Merging the two images reveals significant colocalization of internalized anti-Tac antibodies and endogenous TGN38 (f). Untransfected cells do not internalize anti-Tac (panels d, e, and f, arrows in insets), indicating that anti-Tac uptake did not occur by fluid-phase endocytosis. For panels g, h, and i, polarized MDCK cell monolayers were incubated at 37°C in the presence of anti-Tac antibodies added to the basolateral compartment. Fixed cells were then incubated with a labeled secondary antibody to detect the anti-Tac antibody (g) or stained with an anti-TGN38 antibody (h). Superposition of the two panels indicates extensive colocalization (i, yellow color), indicating that a significant fraction of anti-Tac antibodies internalized from the basolateral surface was retrieved to the TGN. Bars, 10 μm.
To determine if furin molecules appear on the apical or basolateral surface of polarized MDCK cells, monolayers grown on Transwell units were incubated in the presence of antifurin antibodies added to the apical or basolateral compartment at 37°C. As shown in Fig. 1g, little uptake of antibodies from the apical compartment was detected. In contrast, antibodies present in the basolateral chamber were readily internalized (Fig. 1h). As already shown for cells grown on glass coverslips (Fig. 1d to f), untransfected MDCK cell monolayers grown on Transwell filters did not internalize detectable amounts of antibody from either surface (not shown).
These experiments show that in polarized MDCK cells furin is sorted to the basolateral plasma membrane.
Basolateral sorting is mediated by determinants in the membrane distal half of the cytosolic domain of furin.
We next analyzed whether the cytosolic domain of furin is necessary and sufficient for basolateral transport. For this purpose, we took advantage of chimeras combining the ecto- and transmembrane domains of human Tac (interleukin 2 receptor α-chain, or CD25) and the wild-type or mutant cytosolic domains of rat furin (TTF or Tac F; Fig. 2). At least three MDCK cell clones stably expressing each construct were selected for further analysis. TGN retention and endocytosis of the different constructs expressed in MDCK cells were very similar to those of the same constructs expressed in NRK cells (data not shown [40]).
Tac chimeras containing the intact cytosolic tail of furin (TTF) displayed a TGN-like steady-state distribution (Fig. 3a) and were able to internalize anti-Tac antibodies added to the medium at 37°C (Fig. 3d). Colocalization experiments with TGN38 (Fig. 3b and e) confirmed the predominant localization of the chimeras to the TGN under steady-state conditions (Fig. 3c). Furthermore, a significant fraction of the internalized chimera was retrieved from the cell surface to the TGN (Fig. 3f), similar to the behavior of TTF expressed in NRK cells (40). Vesicles that stain for internalized anti-Tac antibody but lack TGN38 may constitute endosomal compartments involved in the uptake and transfer of TTF to the TGN. Untransfected control cells were not stained (Fig. 3a to c) and also failed to take up antibody (Fig. 3d to f, insets), showing that antibody uptake was specific and required binding to the chimera. Thus, while furin and TTF localized to the TGN at equilibrium, antibodies internalized by furin accumulated in an endosomal compartment whereas those internalized by TTF were retrieved to the TGN.
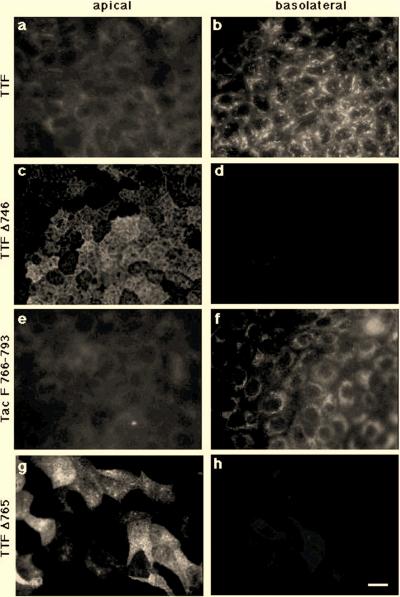
Like wild-type furin, TTF was delivered to the basolateral surface of polarized MDCK cell monolayers since antibody uptake occurred preferentially from the basolateral as compared to the apical compartment (Fig. 4a and b). Detection of antibodies taken up from the basolateral compartment via TTF (Fig. 3g) and TGN38 (Fig. 3h) revealed extensive colocalization (Fig. 3i), indicating that a significant fraction of basolaterally internalized TTF was retrieved to the TGN. In contrast, an internalization-defective tailless TTF chimera containing only the 10 amino acids of the membrane proximal portion of the furin tail (TTF Δ746) (Fig. 2) (40) was delivered to the apical cell surface, as evidenced by the punctate staining pattern characteristic of the apical plasma membrane, which has numerous microvilli (Fig. 4c and d). Since Tac itself and the chimera combining Tac and the transmembrane domain of furin were delivered to the apical surface (see below, Fig. 8), the results show that the cytosolic tail of furin is both necessary and sufficient for basolateral sorting.
FIG. 4.
The cytosolic domain of furin is necessary and sufficient for basolateral sorting and requires a signal in the membrane distal half of the tail. MDCK cells expressing TTF (a and b), TTFΔ746 (c and d), Tac F766-793 (e and f), or TTFΔ765 (g and h) were grown as polarized cell monolayers and incubated at 37°C for 30 min with anti-Tac antibody added to the upper (panels a, c, e, and g) or lower (panels b, d, f, and h) compartment. Cells were then washed on ice, fixed, and permeabilized, and anti-Tac antibodies were visualized by using a labeled second antibody. The chimera containing the wild-type tail (TTF) is transported to the basolateral surface (panels a and b), and the deletion of the tail in TTFΔ746 abolishes basolateral delivery (panels c and d). A chimera containing the membrane distal part of the furin tail (Tac F766-793) is expressed on the basolateral surface (panels e and f), whereas TTF Δ765, encoding the membrane proximal half of the tail, is transported apically (panels g and h). Bar, 10 μm.
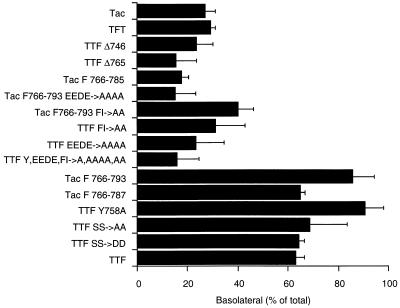
FIG. 8.
Quantitation of the steady-state distribution of different Tac-furin tail constructs. MDCK cells expressing the Tac-furin tail chimeras indicated were grown on Transwell filters. To quantitate the surface expression of the different chimeras, radiolabeled anti-Tac antibody was allowed to bind from either the apical or the basolateral compartment on ice. Similar distributions were obtained for different clones expressing different levels of chimeric proteins. Quantitation of the binding for each clone was obtained from three to five independent experiments, each carried out in duplicate or triplicate. Statistical analysis showed that the distribution of constructs containing the FI and EEDE motifs was significantly different from that of Tac, while the distribution of constructs lacking the FI and EEDE significantly differed (P < 0.005, Student’s t test) from that of TTF, confirming the critical role of the FI and EEDE in basolateral sorting.
To further define the region in the cytosolic domain of furin required for basolateral sorting, we analyzed chimeras containing either the membrane proximal (TTF Δ765) or membrane distal (Tac F766-793) half of the furin tail (Fig. 2). While Tac F766-793 preferentially internalized anti-Tac antibodies from the basolateral compartment (Fig. 4e and f), antibodies were internalized from the apical chamber by cells expressing TTF Δ765 (Fig. 4g and h). These results thus indicate that the membrane distal part of the furin tail carries information required for basolateral sorting.
In conclusion, the above data show that the cytosolic domain of furin is necessary and sufficient for basolateral transport and that basolateral sorting information is carried by the membrane distal part of the furin tail.
Basolateral sorting requires a phenylalanine-isoleucine motif.
To identify amino acids required for basolateral sorting, we next analyzed several deletion and point mutants in the context of the membrane distal part of the furin tail (Fig. 2). C-terminal truncations in the context of Tac F766-793 revealed an important role for the FI at positions 785 and 786 in basolateral sorting. While Tac F766-787 was still delivered to the basolateral cell surface (Fig. 5a and b), deletion of the FI in Tac F766-785 interfered with basolateral sorting (Fig. 5c and d). As expected, both constructs still internalized anti-Tac antibodies (40). Also, C-terminal deletions in the context of the complete furin tail (i.e., TTF Δ787) did not affect basolateral sorting but removal of the FI in TTF Δ785 resulted in apical delivery (Fig. 2; data not shown).
FIG. 5.
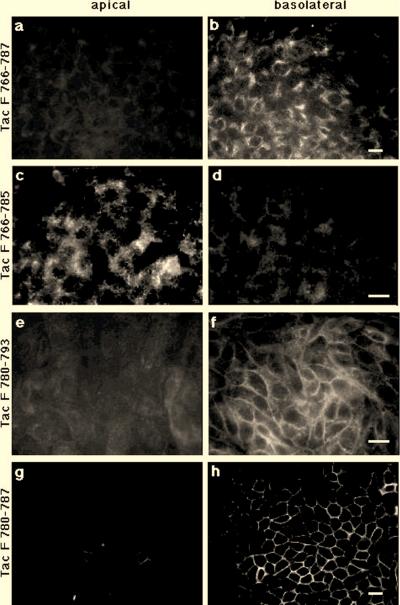
Basolateral sorting requires a membrane-distal phenylalanine-isoleucine motif. MDCK cells expressing Tac F766-787 (a and b), Tac F766-785 (c and d), Tac F780-793 (e and f), or Tac F780-787 (g and h) were grown as polarized cell monolayers and incubated at 37°C for 30 min with anti-Tac antibody added to the upper (panels a, c, e, and g) or lower (panels b, d, f, and h) compartment. Cells were then washed on ice, fixed, and permeabilized, and anti-Tac antibodies were visualized by using a labeled second antibody. Tac F766-787, a truncation mutant that carries the FI, is sorted basolaterally (panels a and b), but removal of the FI in Tac F766-785 abolishes basolateral transport (panels c and d). N-terminal truncation mutants containing the FI, Tac F780-793 or Tac F780-787, are delivered basolaterally (panels e through h). Bars, 10 μm.
Similarly, mutants bearing N-terminal deletions in the context of the membrane distal half of the furin tail containing the FI motif (i.e., Tac F780-793) were delivered to the basolateral cell surface (Fig. 5e and f). Even Tac F780-787, which encodes only an eight-amino-acid short tail containing the FI, was sorted basolaterally (Fig. 5g and h). As expected, the deletions of the acidic amino acid cluster in Tac F780-793 and Tac F 780-787 affected the ability of the chimeras to internalize anti-Tac antibodies (40).
Taken together, these experiments identify the FI motif as a critical element for basolateral sorting of furin.
The basolateral sorting activity of the FI requires the acidic amino acid cluster.
To confirm the role of the FI for basolateral sorting, we generated point mutations affecting the different signals in the context of chimera containing the membrane distal half of the furin tail (Tac F766-793) (Fig. 2). As expected, replacement of the FI by alanines in the context of the C-terminal half of the tail (Tac F766-793 FI→AA) led to apical delivery of the protein (Fig. 6a and b). Surprisingly, however, while deletion of the acidic amino acid cluster in Tac F780-793 or Tac F780-787 did not affect basolateral sorting (see above, Fig. 5e–h), the alanine substitutions for the EEDE motif in Tac F766-793 EEDE→AAAA interfered with basolateral sorting (Fig. 6c and d). This observation indicated that although the FI was able to independently mediate basolateral sorting in short truncations, the acidic amino acid cluster was also required in the context of a larger furin tail. We therefore extended our analysis to point mutants generated in the context of the complete furin tail (see Fig. 2).
FIG. 6.
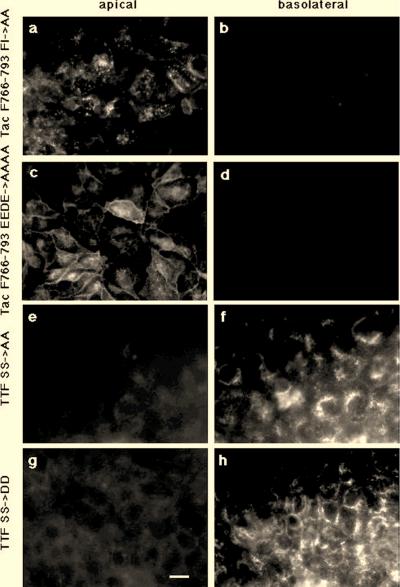
The acidic amino acid cluster EEDE and the FI motif but not serine phosphorylation are required for basolateral sorting. MDCK cells expressing Tac F766-793 FI→AA (a and b), Tac F766-793 EEDE→AAAA (c and d), TTF SS→AA (e and f) or TTF SS→DD (g and h) were grown as polarized cell monolayers and incubated at 37°C for 30 min with anti-Tac antibody added to the upper (panels a, c, e, and g) or lower (panels b, d, f, and h) compartment. Cells were then washed on ice, fixed, and permeabilized, and anti-Tac antibodies were visualized by using a labeled second antibody. Mutation of the FI and EEDE but not that of the serines subject to phosphorylation by CKII affects basolateral transport. Bar, 10 μm.
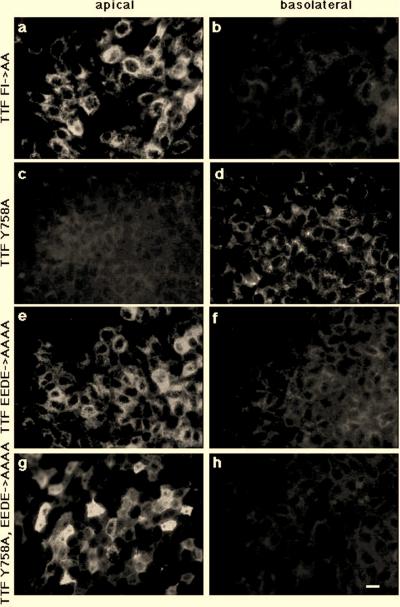
As shown in Fig. 7a and b, the FI was critical for basolateral sorting in the context of the complete furin tail, since the replacement of the FI by alanines in TTF FI→AA (Fig. 2) affected basolateral sorting. Analysis of mutations affecting either the F or the I individually indicated that each of the two hydrophobic residues was independently important for basolateral sorting (Fig. 2; data not shown). TTF Y758A, carrying the inactivated tyrosine-based endocytosis signal (Fig. 2), was still transported basolaterally (Fig. 7c and d). However, the replacement of the acidic amino acid cluster in TTF EEDE→AAAA (Fig. 2) resulted in apical transport (Fig. 7e and f), confirming the importance of the EEDE motif already observed for the Tac F766-793 EEDE→AAAA construct. Likewise, the replacement of both the tyrosine and the acidic amino acid cluster in TTF Y785→A, EEDE→AAAA led to the expression of the protein on the apical surface (Fig. 7g and h).
FIG. 7.
The EEDE cluster and the FI motif are required for basolateral sorting in the context of the complete furin tail. MDCK cells expressing TTF FI→AA (a and b), TTF Y758A (c and d), TTF EEDE→AAAA (e and f), or TTF Y758A, EEDE→AAAA (g and h) were grown as polarized cell monolayers and incubated at 37°C for 30 min with anti-Tac antibody added to the upper (panels a, c, e, and g) or lower (panels b, d, f, and h) compartment. Cells were then washed on ice, fixed, and permeabilized, and anti-Tac antibodies were visualized by using a labeled second antibody. Mutations affecting either the FI motif or the EEDE motif in TTF FI→AA, TTF EEDE→AAAA, or TTF Y758A, EEDE→AAAA lead to impaired basolateral sorting (panels a, b, and e through h); inactivating the tyrosine-based endocytosis signal in TTF Y758A has no effect on basolateral transport (panels c and d). Bar, 10 μm.
These results thus identify the FI and the acidic amino acid cluster as important determinants for basolateral sorting, in the context of both the membrane distal half and the complete tail of furin.
Serine phosphorylation is not required for basolateral sorting of furin.
The acidic amino acid cluster required for basolateral sorting of furin is part of a CKII phosphorylation site including serines S772 and S774. Thus, the effect of mutating the EEDE motif could be indirect and reflect an inactivation of the CKII phosphorylation consensus sequence. We therefore directly analyzed whether serine phosphorylation plays a role in basolateral targeting of furin by substituting for the two serines either alanines (TTF SS→AA) or, to mimic the negative charge of phosphoserine, aspartic acids (TTF SS→DD) (Fig. 2). As shown in Fig. 6e through h, TTF SS→AA and TTF SS→DD were delivered to the basolateral surface, indicating that serine phosphorylation does not play a role in basolateral sorting.
Quantitation of the polarized surface distribution of different constructs.
To confirm the polarized surface transport of the different chimeras observed in the immunofluorescence assay, we quantitated the steady-state distribution of key constructs to the apical and basolateral surfaces by using radioiodinated anti-Tac antibodies. For this purpose, cells grown as polarized monolayers were cooled on ice and radioiodinated antibodies were added to the apical or basolateral chamber. After unbound antibodies were washed off, the radioactivity bound to the filters was determined.
Tac alone, a tailless Tac construct (TTFΔ746), and a Tac construct carrying the furin transmembrane domain (TFT) were predominantly found on the apical domain. In the case of the furin tail chimera containing intact FI and EEDE determinants, 60 to 80% of the surface molecules were localized to the basolateral domain (Fig. 8). Mutation of either the FI or the EEDE signals resulted in a relocalization of the constructs from the basolateral to the apical surface, confirming the critical role of the FI and EEDE in basolateral sorting. With the exception of Tac F766-793 FI→AA, the apical or basolateral distribution of the clones was significantly different from a nonpolarized (i.e., 50% apical and 50% basolateral) distribution. Furthermore, the analysis of the insertion of a cohort of newly synthesized molecules labeled with [35S]methionine and [35S]cysteine into the apical and basolateral surfaces was consistent with a role for the FI and EEDE motifs in basolateral sorting, but the low fraction of labeled protein reaching the cell surface led to relatively large standard deviations (not shown). The polarized distribution of several additional constructs was analyzed (data not shown) and the results, summarized in Fig. 2, were consistent with the established importance of the FI and EEDE motifs in basolateral sorting.
In support of the antibody uptake experiments, the biochemical binding data thus confirm the critical role for the FI and EEDE motifs in basolateral sorting of the Tac-furin tail chimera.
DISCUSSION
Furin is selectively concentrated in the TGN, but a significant fraction of the enzyme cycles among the plasma membrane, endosomes, and the TGN, indicating that the accumulation in the TGN reflects a kinetically favored location in a dynamic process. The intracellular routing of furin and the signals involved have been characterized in detail (12, 33, 39, 40). In contrast to endocytosis and TGN retrieval, little is known concerning the pathway by which furin exits the TGN. Here, we show that furin is delivered to the basolateral surface of epithelial MDCK cells and that basolateral sorting involves an FI motif together with the acidic amino acid cluster.
Concentration of furin in the TGN does not require the ecto- or transmembrane domains, but the cytosolic tail is both necessary and sufficient for TGN localization (2, 5, 26, 33, 40). Interestingly, while both furin and the Tac-furin tail chimera were concentrated in the TGN at equilibrium, antibodies internalized via furin were less efficiently transferred to the TGN than those internalized via TTF. Since the luminal domain of furin plays a role in lysosomal transport of aggregated furin molecules (43), antibody-induced cross-linking may also be responsible for lysosomal delivery of cross-linked furin molecules in the endocytic pathway.
Two signals in the cytosolic domain of furin control the intracellular routing of the enzyme (12, 33, 39, 40). A tyrosine-based signal of the YXXØ (where Ø stands for a bulky hydrophobic amino acid) type (YKGL) and an acidic amino acid cluster contribute to the internalization of the protein (12, 33, 39, 40). TGN localization, in contrast, requires only the acidic amino acid cluster (SDSEEDE) (12, 33, 39, 40) and phosphorylation of the serines in the two overlapping CKII phosphorylation sites modulates retrieval to the TGN (6, 41). Since epithelial and nonepithelial cells transport “apical” and “basolateral” membrane proteins from the TGN to the cell surface via two distinct routes (27, 44), our results showing that furin is sorted to the basolateral cell surface in MDCK cells may be relevant for the exocytic transport of furin in general. It is unclear whether furin reaches the cell surface directly from the TGN or indirectly via endosomes and whether particular signals direct furin into one pathway or the other. Newly synthesized transferrin receptor has been shown to reach endosomes prior to its appearance on the plasma membrane of HEp-2 cells (7), and the asialoglycoprotein receptor also reaches endosomes prior to its appearance on the basolateral cell surface of MDCK cells (17). However, it remains to be shown whether transit through endosomes in polarized cells is a general feature of basolateral transport.
While most substrates of furin are processed late during their biosynthesis in the TGN, several precursor proteins are processed outside the exocytic pathway. Cleavage of the protective antigen of anthrax toxin occurs at the plasma membrane, and processing of the Pseudomonas exotoxin requires internalization into endosomes (15, 24). In polarized cells, asymmetric recycling of furin may be of physiological relevance for the polarized secretion of processed bioactive compounds (3). Alternatively, a polarized surface expression of furin may allow the domain-selective processing of extracellular or internalized substrates. In LC-PK1 cells expressing the furin substrate prosomatostatin, for example, the unprocessed form is secreted in an unpolarized fashion whereas processed somatostatin is exclusively released into the basolateral medium (3). This finding suggests that prosomatostatin is selectively processed by furin in the basolateral exocytic route.
The cytosolic domain of furin is necessary and sufficient for basolateral sorting. In several proteins, tyrosine-based basolateral sorting signals which in many cases also mediate endocytic activity have been identified. Despite the overall similarities, however, the sequence requirements for endocytosis and basolateral sorting are in most cases not identical (13). The lack of basolateral sorting activity of the YKGL endocytosis signal of furin may reflect its location in a structural context which allows recognition by the endocytic but not by the basolateral sorting machinery. The importance of dihydrophobic signals in basolateral sorting, on the other hand, is not unprecedented, and related motifs (i.e., LL, LI, ML, and LV) mediate basolateral sorting in other proteins, including FcγRIIb2 (mediated by LL) (9), invariant chain (mediated by LI and ML) (29, 37, 38), and CD44 (mediated by LV) (36). Despite the precedent for dileucine-based signals in basolateral targeting, phenylalanine has not been shown to be active in combination with isoleucine. Although the FI motif was sufficient for basolateral sorting in constructs having short cytosolic tails, a second determinant, EEDE, was as important as the FI in the context of the membrane distal half or the complete furin tail. Mutation of the two serines which, together with the EEDE sequence, form the two overlapping CKII sites did not affect basolateral sorting, indicating that serine phosphorylation does not play a role in basolateral sorting. Acidic amino acid clusters play a role in basolateral sorting of other proteins. In the low-density lipoprotein receptor (LDL-R), acidic amino acid clusters similar to the one in furin are critical for the basolateral sorting activity of the membrane distal and proximal tyrosine-based determinants (10, 20, 21). In contrast to furin, however, the acidic sequences in the LDL-R tail are unimportant for endocytosis. Furthermore, the combination of an acidic amino acid cluster upstream of a putative dileucine signal is a novel arrangement of sorting signals, although membrane proximal acidic amino acids are also important in the LI signal in invariant chain (29, 37). While the FI and EEDE play a critical role in basolateral sorting of furin, we cannot exclude the possibility that additional amino acids contribute to this sorting activity.
Several possibilities may explain the requirement for both of the FI and EEDE motifs for basolateral sorting. The FI motif and the EEDE motif may be independent but weak basolateral signals which by themselves cannot override a putative apical sorting signal in the ecto- and/or transmembrane domain of Tac and, presumably, furin. Alternatively, the two motifs together may be required to interact with the basolateral sorting machinery. The EEDE motif may also be important in conferring a particular secondary structure to the furin tail so that it presents the FI motif. Underscoring their importance in furin function, the acidic amino acids and the downstream FI are conserved in the orthologs from human, cow, rat, mouse, hamster, chick, and Xenopus. In the latter organism, the FI sequence is replaced by a dihydrophobic FL.
Cytosolic sorting signals in receptors and transmembrane proteins interact with adapter complexes which in turn recruit clathrin or other coats, thereby leading to the clustering of cargo molecules in pits and transport vesicles (for a review, see reference 34). While AP-1 and AP-3 are implicated in sorting events at the TGN and endosomes, AP-2 acts during endocytosis from the plasma membrane. Tyrosine- and dileucine-based signals can interact with AP-1, AP-2, and AP-3, and while tyrosine signals bind to the μ subunit of APs, an interaction of dileucine-based signals has been observed with the μ subunit and the β subunit of certain adapters (4, 30) (reviewed in reference 14). For furin, in vitro binding studies have shown that the phosphorylated CKII consensus site is able to bind to AP-1 (6), probably via the connector protein PACS-1 (41). However, it is not clear what role, if any, the EEDE cluster and the FI motif play in PACS-1 or AP-1 binding, nor is it clear if these determinants also interact with other adapters.
A question central to understanding the mechanisms of basolateral sorting is the identification of the molecular machinery mediating this sorting event. The similarities between signals required for clathrin-coated pit localization and basolateral sorting implicate adapter-like complexes (10). A role for any of the known adapters in basolateral sorting has neither been proven nor strictly been ruled out and adapters as yet to be characterized may be candidates for this sorting event. Comparison of the requirements of sequences in the furin tail for the different sorting steps to those for binding to particular adapters might yield additional insights.
ACKNOWLEDGMENTS
We thank N. Seidah for providing the human furin cDNA and J.-W. van der Loo, D. Rimoldi, and G. Banting for the antibodies to furin, Tac, and TGN38, respectively. We are indebted to M. Thali for critically reading the manuscript and I. Xenarios and the members of our laboratories for helpful discussions.
The work was supported by the Canton de Vaud and by a grant and a career development award from the Swiss National Science Foundation (to W.H.).
REFERENCES
- 1.Bos K, Wraight C, Stanley K K. TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J. 1993;12:2219–2228. doi: 10.1002/j.1460-2075.1993.tb05870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters P J, Bonifacino J S. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brakch N, Yang X F, Crine P, Cohen P, Boileau G. Predominant basolateral proteolytic processing of prosomatostatin into somatostatin-28 in polarized LLC-PK1 cells. Neuropeptides. 1997;31:393–398. doi: 10.1016/s0143-4179(97)90030-5. [DOI] [PubMed] [Google Scholar]
- 4.Bremnes T, Lauvrak V, Lindqvist B, Bakke O. A region from the medium chain adaptor subunit (mu) recognizes leucine- and tyrosine-based sorting signals. J Biol Chem. 1998;273:8638–8645. doi: 10.1074/jbc.273.15.8638. [DOI] [PubMed] [Google Scholar]
- 5.Chapman R E, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dittie A S, Thomas L, Thomas G, Tooze S A. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Futter C E, Connolly C N, Cutler D F, Hopkins C R. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- 8.Hatsuzawa K, Hosaka M, Nakagawa T, Nagase M, Shoda A, Murakami K, Nakayama K. Structure and expression of mouse furin, a yeast Kex2-related protease. Lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem. 1990;265:22075–22078. [PubMed] [Google Scholar]
- 9.Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13:2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- 11.Hunziker W, Mellman I. Expression of macrophage-lymphocyte Fc receptors in MDCK cells: polarity and transcytosis differ for isoforms with or without coated pit localization domains. J Cell Biol. 1989;109:3291–3302. doi: 10.1083/jcb.109.6.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones B G, Thomas L, Molloy S S, Thulin C D, Fry M D, Walsh K A, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- 14.Kirchhausen T, Bonifacino J S, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 15.Klimpel K R, Molloy S S, Thomas G, Leppla S H. Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proc Natl Acad Sci USA. 1992;89:10277–10281. doi: 10.1073/pnas.89.21.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladinsky M S, Howell K E. An electron microscopic study of TGN38/41 dynamics. J Cell Sci. 1993;17s:41–47. doi: 10.1242/jcs.1993.supplement_17.7. [DOI] [PubMed] [Google Scholar]
- 17.Leitinger B, Hille-Rehfeld A, Spiess M. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc Natl Acad Sci USA. 1995;92:10109–10113. doi: 10.1073/pnas.92.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard W J, Depper J M, Crabtree G R, Rudikoff S, Pumphrey J, Robb R J, Kronke M, Svetlik P B, Peffer N J, Waldmann T A, et al. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984;311:626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- 19.Luzio J P, Brake B, Banting G, Howell K E, Braghetta P, Stanley K K. Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38) Biochem J. 1990;270:97–102. doi: 10.1042/bj2700097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- 21.Matter K, Yamamoto E M, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellman I S, Plutner H, Steinman R M, Unkeless J C, Cohn Z A. Internalization and degradation of macrophage Fc receptors during receptor-mediated phagocytosis. J Cell Biol. 1983;96:887–895. doi: 10.1083/jcb.96.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misumi Y, Oda K, Fujiwara T, Takami N, Tashiro K, Ikehara Y. Functional expression of furin demonstrating its intracellular localization and endoprotease activity for processing of proalbumin and complement pro-C3. J Biol Chem. 1991;266:16954–16959. [PubMed] [Google Scholar]
- 24.Molloy S S, Bresnahan P A, Leppla S H, Klimpel K R, Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992;267:16396–16402. [PubMed] [Google Scholar]
- 25.Molloy S S, Thomas L, Kamibayashi C, Mumby M C, Thomas G. Regulation of endosome sorting by a specific pp2a isoform. J Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molloy S S, Thomas L, VanSlyke J K, Stenberg P E, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musch A, Xu H X, Shields D, Rodriguez-Boulan E. Transport of vesicular stomatitis virus g protein to the cell surface is signal mediated in polarized and nonpolarized cells. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama K. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem J. 1997;327:625–635. doi: 10.1042/bj3270625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odorizzi G, Trowbridge I S. Structural requirements for major histocompatibility complex class II invariant chain trafficking in polarized Madin-Darby canine kidney cells. J Biol Chem. 1997;272:11757–11762. doi: 10.1074/jbc.272.18.11757. [DOI] [PubMed] [Google Scholar]
- 30.Rapoport I, Chen Y C, Cupers P, Shoelson S E, Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rimoldi D, Salvi S, Hartmann F, Schreyer M, Blum S, Zografos L, Plaisance S, Azzarone B, Carrel S. Expression of IL-2 receptors in human melanoma cells. Anticancer Res. 1993;13:555–564. [PubMed] [Google Scholar]
- 32.Rubin L A, Kurman C C, Biddison W E, Goldman N D, Nelson D L. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma. 1985;4:91–102. doi: 10.1089/hyb.1985.4.91. [DOI] [PubMed] [Google Scholar]
- 33.Schäfer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse M L, Kern H F, Klenk H D, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid S L. Clathrin-coated vesicle formation and protein sorting—an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 35.Seidah N G, Chretien M. Eukaryotic protein processing: endoproteolysis of precursor proteins. Curr Opin Biotechnol. 1997;8:602–607. doi: 10.1016/s0958-1669(97)80036-5. [DOI] [PubMed] [Google Scholar]
- 36.Sheikh H, Isacke C M. A di-hydrophobic Leu-Val motif regulates the basolateral localization of CD44 in polarized Madin-Darby canine kidney epithelial cells. J Biol Chem. 1996;271:12185–12190. doi: 10.1074/jbc.271.21.12185. [DOI] [PubMed] [Google Scholar]
- 37.Simonsen A, Bremnes B, Nordeng T W, Bakke O. The leucine-based motif DDQXXLI is recognized both for internalization and basolateral sorting of invariant chain in MDCK cells. Eur J Cell Biol. 1998;76:25–32. doi: 10.1016/S0171-9335(98)80014-9. [DOI] [PubMed] [Google Scholar]
- 38.Simonsen A, Stang E, Bremnes B, Roe M, Prydz K, Bakke O. Sorting of MHC class II molecules and the associated invariant chain (Ii) in polarized MDCK cells. J Cell Sci. 1997;110:597–609. doi: 10.1242/jcs.110.5.597. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi S, Nakagawa T, Banno T, Watanabe T, Murakami K, Nakayama K. Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues within the cytoplasmic domain. J Biol Chem. 1995;270:28397–28401. doi: 10.1074/jbc.270.47.28397. [DOI] [PubMed] [Google Scholar]
- 40.Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan L, Molloy S S, Thomas L, Liu G P, Xiang Y, Rybak S L, Thomas G. Pacs-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 42.Weiner M P, Costa G L, Schoettlin W, Cline J, Mathur E, Bauer J C. Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction. Gene. 1994;151:119–123. doi: 10.1016/0378-1119(94)90641-6. [DOI] [PubMed] [Google Scholar]
- 43.Wolins N, Bosshart H, Küster H, Bonifacino J S. Aggregation as a determinant of protein fate in post-Golgi compartments: role of the luminal domain of furin in lysosomal targeting. J Cell Biol. 1997;139:1735–1745. doi: 10.1083/jcb.139.7.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimori T, Keller P, Roth M G, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]