Abstract
COVID-19 pandemic continues to be a global health crisis. The gut microbiome critically affects the immune system, and some respiratory infections are associated with changes in the gut microbiome; here, we evaluated the role of nutritional and lifestyle habits that modulate gut microbiota on COVID-19 outcomes in a longitudinal cohort study that included 200 patients infected with COVID-19. Of these, 122 cases were mild and seventy-eight were moderate, according to WHO classification. After detailed explanation by a consultant in clinical nutrition, participants responded to a written questionnaire on daily sugar, prebiotic intake in food, sleeping hours, exercise duration and antibiotic prescription, during the past 1 year before infection. Daily consumption of prebiotic-containing foods, less sugar, regular exercise, adequate sleep and fewer antibiotic prescriptions led to a milder disease and rapid virus clearance. Additionally, data on these factors were compiled into a single score, the ESSAP score (Exercise, Sugar consumption, Sleeping hours, Antibiotics taken, and Prebiotics consumption; 0–11 points), median ESSAP score was 5 for both mild and moderate cases; however, the range was 4–8 in mild cases, but 1–6 in moderate (P = 0·001, OR: 4·2, 95 % CI 1·9, 9·1); our results showed a negative correlation between regular consumption of yogurt containing probiotics and disease severity (P = 0·007, OR: 1·6, 95 % CI 1·1, 2·1). Mild COVID-19 disease was associated with 10–20 min of daily exercise (P = 0·016), sleeping at least 8 h daily, prescribed antibiotics less than 5 times per year (P = 0·077) and ate plenty of prebiotic-containing food.
Key words: Probiotics, Gut microbiota, COVID-19, Exercise
Introduction
SARS-COV-2 infection, a new strain of coronavirus, has resulted in a pandemic (COVID-19) of acute respiratory distress syndrome in humans. Egypt is one of the countries that has been affected by this worldwide crisis, and in early 2021, nearly 170 000 cases were confirmed with more than 9500 deaths(1). A fear of future waves of the infection has disabled the world and nations are working hard to defeat this virus. Manifestations of COVID-19 show a wide spectrum and can also mimic the clinical presentation of various gastrointestinal (GI) diseases as anorexia, nausea and diarrhoea have been commonly described in patients with confirmed COVID-19 infection(2).
Some respiratory infections are associated with a change in the gut microbiome, referred to as the gut–lung axis, which is involved in lung immune homoeostasis(3,4). This axis is expected to be mutual, that is, any alteration in the gut microenvironment will have an effect on the pulmonary system through the action of gut endotoxins and disturbances in innate and adaptive immunity(5). The gut microbiome masterminds the gut immune system by fine tuning the equilibrium between pro-inflammatory TH17 and anti-inflammatory responses via Tregs(6) to be protective against respiratory infections, as its depletion or absence in mice leads to impairment of the immune responses and worsens the outcomes following viral infections(7). Our diet plays a critical role in defining the bacterial population in the gut microenvironment(8), and probiotic ‘live microorganisms’ have been shown to have anti-inflammatory properties as they regulate multiple signalling pathways and toll-like receptor activity(9). Prebiotics are a non-digestible food ingredient that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, thereby improving host health. They are associated not only with a healthy gut but also with improving lung immunity(10). Microbiota composition in humans is also affected by sugar intake, exercise, sleeping hours, antibiotic use and other environmental factors, all of which can have long-term effects on the immune system(11). We hypothesised that diet and lifestyle factors that modulate gut microbiota can affect COVID-19 disease outcomes, and therefore, the aim of this study was to evaluate the role of nutritional habits and lifestyle on the degree of severity COVID-19 infection in Egyptian patients. Additionally, we propose a prognostic nutrition and lifestyle scoring system (Exercise, Sugar consumption, Sleeping hours, Antibiotics taken, and Prebiotics consumption (ESSAP) score) that can predict isease outcome and link potential benefits of gut microbiota in boosting our immune system to fight against COVID-19.
Patients and methods
Study design and participants
This longitudinal cohort single centre study was conducted in patients whose nasopharyngeal swab samples tested positive for COVID-19 by the PCR between May and mid-July 2020. Patients were recruited from the COVID-19 out-patient clinic, Kasr Al-Aini School of Medicine, Cairo University Hospitals. Inclusion criteria were any adult patient (aged 18–75 years) suffering from mild or moderate COVID-19 infection, according to WHO COVID-19 criteria(12) and treated according to the Egyptian ministry of health and population management recommendations that had been approved by the scientific COVID-19 committee of the Faculty of Medicine, Cairo University. After diagnosis by PCR, mild and moderate cases were administered paracetamol (1 g every 8 h), along with hydroxychloroquine 400 mg twice daily on the first day and maintained on 200 mg twice daily for 6 d. They were also provided vitamin C (1 gm), Zn (50 mg), acetylcysteine (600 mg), lactoferrin two sachets daily, prophylactic anticoagulant or a similar therapeutic based on D-dimer levels(13).
The following exclusion criteria were applied: patients with chronic GI or immune diseases, those on immunosuppressive medications and those with severe COVID-19 infection. Severe infection was defined according to WHO guidelines as respiratory rate ≥ 30/min, oxygen saturation < 93 % at rest, partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) < 300 mm Hg and more than 50 % lesion progression in lung imaging within 24–48 h.
Data collection
To correlate disease severity with nutritional and lifestyle habits, we followed up 200 cases of mild or moderate COVID-19 that had been confirmed by PCR till they tested negative twice by PCR and their symptoms had resolved.
Data on clinical and demographic parameters, such as age, sex, BMI, underlying co-morbidities (diabetes mellitus, hypertension, chronic pulmonary, kidney, liver or heart disease), clinical symptoms and vital signs, were collected. Clinical symptoms of every patient were recorded using a ‘yes/no’ checklist that asked about constitutional symptoms (fever, fatigue and bony aches), respiratory symptoms (cough, dyspnoea and sore throat), neurological symptoms (headache, loss of taste and loss of smell) and GI symptoms (nausea, vomiting, abdominal pain and diarrhoea).
Phone calls and scheduled video chat on commercial platforms were used to follow up patients throughout their illness, from ‘home quarantine’ when symptomatic or upon testing positive to full recovery, that is, resolution of their symptoms and testing negative twice (48 h apart) by PCR.
Samples for laboratory assessment were collected within 24 h of symptoms onset and analyses consisted of complete blood count (serum creatinine and urea) and liver function tests (alanine aminotransferase, aspartate aminotransferase and total bilirubin), serum C-reactive protein (CRP), D-dimer, ferritin, total lipid profile and lactate dehydrogenase. Initial chest computed tomography was performed and its result was recorded. A single deep nasopharyngeal swab was obtained within 5–6 d after symptom onset, and swabs were placed in viral transport medium for rapid transportation to the laboratory. Molecular diagnosis of COVID-19 used real-time RT-qPCR assay that employed Taqman-based probes to conduct in vitro transcription of SARS-COV-2 RNA, followed by DNA amplification and fluorescence detection. Materials and reagents were supplied by VIASURE for use on a Rotor-Gene instrument (QIAGEN GmbH).
Eight millilitres of blood was drawn from all subjects after fasting for 12 h and divided into three tubes as follows – 2 ml blood was evacuated in an ethylenediamine tetraacetic acid tube for complete blood count which was done on CELL-DYN 3200 automated hematology analyser (Abbott); 5 ml of blood was drawn into a plain vacutainer tube to isolate serum for analysing total bilirubin, aspartate aminotransferase, alanine aminotransferase, urea, creatinine, cholesterol, TAG, HDL, LDL, CRP, lactate dehydrogenase and ferritin on COBAS 6000 Auto analyser (Roche Diagnostics GmbH). The final one ml of blood was evacuated into a fluoride vacutainer tube for fasting blood sugar assay. All laboratory evaluations were performed at the Clinical Chemistry Department, Faculty of Medicine, Cairo University, Cairo, Egypt.
The study protocol conformed to ethical guidelines of the Declaration of Helsinki, 1975(14), was approved by the Research Ethics Committee, Faculty of Medicine, Cairo University (REC n-86-2020) and was registered on clinical trial.gov of the US National library of Medicine (cinicalTrial.gov identifier: NCT04447144) under the title ‘Nutritional Habits, and coronavirus disease (COVID-19) outcome’. Policy of data confidentiality was strictly followed. The aim was explained clearly, and informed consent was obtained from all participants before enrolment. All authors had access to the study data and reviewed and approved the final manuscript.
A questionnaire on nutrition and lifestyle habits that obtained data on patterns during the 12 months before infection was filled by all patients in written forms after a consultant in clinical nutrition explained the contents of the questionnaire (original in Arabic, the English translated form is attached). Two authors, who are experts in clinical nutrition, designed and evaluated whether the proposed questions could effectively capture the role of nutrition and lifestyle habits in modulating gut microbiota. A biostatistician verified that the questionnaire was free of errors, such as double-barreled or confusing questions.
We recruited thirty patients with COVID-19 for a pilot study to assess the validity of the questionnaire. Based on clinical data, disease outcomes and statistical data analysis, we also proposed scores for each item that affected outcome, that is, the ESSAP score (0–11 points), which assessed exercise, sugar consumption, sleeping hours, antibiotic use, and prebiotic consumption in the past 12 months.
After establishing the face validity of our questionnaire, we completed the study with the rest of the 170 patients with COVID-19.
Nutritional habits
Probiotics
Dosage of probiotic foods is based solely on the number of live organisms present; thus, therapeutic yogurts contain >106 CFU per ml and a 100 g serve size will provide sufficient probiotic bacteria(15). Among all the commercial yogurt brands in the Egyptian market, only one product provided information on type of bacteria (Bifidobacterium). Further, CFU values were not provided on the packaging of full cream or skimmed yogurts. However, Bassuoni et al have worked out that a single 135 g daily portion of probiotic yogurt (PY) provided 1·4 × 109 CFU of Bifidum bacteria(16) Thus, a score of 0–3 was designed to assess the frequency of consumption of single serve PY as 0 = never ate; 1 = ≤1 per week; 2 = 2–6 times weekly; and 3 = every day.
Prebiotics
The recommended daily intake of dietary fibre is 25 g for women and 38 g for men, and this component can provide prebiotics in the diet. The Dietary Guidelines for Americans 2010 Committee suggests that foods high in prebiotics (beans, wheat, onions, garlic, artichoke, honey, banana, barley, tomato, rye, soyabean, peas) should be consumed to cover the recommended daily fibre amount(17). Fava bean is a traditional breakfast in Egypt which is consumed by all socio-economic classes, and 100 g contains 9·03 g fibre(18). Traditional Egyptian bread weighs about 90–100 g and is made from whole wheat. As 100 g whole-wheat grain contains 13·5 g fibre(19), bread provides 12–13·5 g fibre, which is the main type of carbohydrate consumed in large amounts. Therefore, scores ranging from 0 to 4 were designed based on the diversity of food commonly consumed by the Egyptian population that are rich in prebiotics. Consuming one type, irrespective of the amount, cannot provide 25 g of fibre; however, consuming two–six different types may provide close to 25 g of fibre, while eating seven or more types will easily provide the required amount. Thus, score 0 indicated once daily consumption; score 1 = 2 items consumed daily; score 2 = 3–4 types daily; score 3 = 5–6 types daily; and score 4 = ≥ 7 types consumed daily.
Sugar intake
The American Heart Association recommends an added-sugar limit of no more than 100 energy intake per day (about 6 teaspoons or 24 g of sugar) for most women and no more than 150 energy intake per day (about 9 teaspoons or 36 g of sugar) for most men(20) Accordingly, we designed our score for a maximum of 9 teaspoons as follows: score 0 = 10 or more teaspoons; score 1 = 3–9 teaspoons; and score 2 = very small amounts, for example, 8 g or ‘2 teaspoons’.
Lifestyle and exercise
According to the US Department of Health and Human Services 2008 Physical Activity Guidelines for American(21), for substantial health benefits, adults should do at least 150 min/week (average of 21 or more minutes per day) of moderate-intensity activity (e.g., aerobic activity that increases a person’s heart rate and breathing to some extent, brisk walking, dancing, swimming or bicycling) in installments of at least 10 min. Further, they should be preferably spread throughout the week, and additional health benefits are gained by engaging in physical activity beyond this amount.
Based on this, we designed scores from 0 to 2, where 0 = less than 10 min, 1 = 10–20 min and 2 = more than 20 min. Household chores were considered as less than 10 min because we could not establish exact duration or whether it was continuous or interrupted, and also because it is not stated in the above guidelines.
Sleeping hours
Sleep recommendations provided by the National Sleep Foundation (USA), the American Academy of Sleep Medicine, the Sleep Research Society (USA) and 24-hour movement guidelines (Canada) suggest 7–9, 7–8 and ≥ 7 h, respectively. Therefore, we decided to score responses based on a total of 8 sleeping hours (night and day naps), on average, where 0 = < 8 h and 1 = ≥ 8 h.
Antibiotic use
It is scored as either 0 or 1 based on the frequency of use in the last 12 months, where score 0 = ≥ 6 and 1 = ≤ 5 times.
In this scoring system, values of 0 or lower are the worst possible while the highest scores imply following or even exceeding international recommendations.
Statistical analyses
Data management and analyses were performed using the Statistical Package for Social Sciences (ver. 25). Numerical data were summarised using means and standard deviation or medians and ranges, as appropriate. Categorical data were summarised as numbers and percentages. Normality of numerical data was established using the Kolmogorov–Smirnov test or the Shapiro–Wilk test.
Chi-squared or Fisher’s tests were used for between-group comparisons of categorical data, as appropriate. Between-group comparisons of normally distributed numeric variables used the Student’s t test, while the Mann–Whitney test was used for non-normally distributed numeric variables. Logistic regression was used to arrive at adjusted OR and to calculate the magnitude of the effect of the various risk factors. OR and 95 % CI were also computed (95 % CI that was not 1·0 was considered significant). All tests were two-sided and P values < 0·05 were considered significant(22).
Results
Of the 200 patients with COVID-19 included, 122 were categorised as mild while seventy-eight were moderate. All patients were managed according to Egyptian MOH recommendations. Females accounted for 59 % of the mild cases and 43·6 % of the moderate cases (P = 0·033), mean ages were 37 and 45 years, respectively, for mild and moderate cases, and while 68·6 % of mild cases were younger than 50 years of age, 65·9 % of moderate cases were older than 50 years (P < 0·001). Means and standard deviations of BMI values were 29·0 (sd 5·8) and 31·1 (sd 6·1) kg/m2 for mild and moderate cases, respectively (P = 0·016).
A significant positive correlation between COVID-19 severity and co-morbidities was observed (Table 1). Clinical presentation in 147 patients involved constitutional and respiratory symptoms, while 53 had GI symptoms such as diarrhoea; of the latter, 31 (58·5 %) were classified as having mild COVID-19 while 41·5 % had moderate disease.
Table 1.
Correlation between medical co-morbidities and their correlation to COVID-19 severity
(Numbers and percentages)
| COVID-19 | ||||||
|---|---|---|---|---|---|---|
| All | Mild | Moderate | ||||
| Factors | n 200 | n 122 | % | n 78 | % | P |
| DM | ||||||
| No | 170 | 111 | 65·3 | 59 | 34·7 | 0·003* |
| Yes | 30 | 11 | 36·7 | 19 | 63·3 | |
| HTN | ||||||
| No | 166 | 107 | 64·5 | 59 | 35·5 | 0·027* |
| Yes | 34 | 15 | 44·1 | 19 | 55·9 | |
| Chronic lung disease | ||||||
| No | 185 | 117 | 63·2 | 68 | 36·8 | 0·022* |
| Yes | 15 | 5 | 33·3 | 10 | 66·7 | |
| Chronic liver disease | ||||||
| No | 198 | 121 | 61·1 | 77 | 38·9 | NA |
| Yes | 2 | 1 | 50·0 | 1 | 50·0 | |
| Cardiac | ||||||
| No | 191 | 120 | 62·8 | 71 | 37·2 | 0·015* |
| Yes | 9 | 2 | 22·2 | 7 | 77·8 | |
NA, not applicable.
Significant.
Patients categorised as moderate COVID-19 displayed significant lymphopenia (1784 v. 2240/µl; moderate v. mild, P = < 0·001), higher CRP and serum ferritin levels (CRP, 12 v. 6 mg/l; ferritin, 160 ng/ml v. 100 ng/ml, respectively, P = 0·012), liver enzymes (aspartate aminotransferase and alanine aminotransferase) and total bilirubin (P = 0·011).
There was a significant negative correlation between PY intake and SARS-COV-2 infection severity as 71·2 % of mild cases had never consumed PY, while those who ate PY on a weekly basis (105 ml) suffered a more severe COVID-19 course (P = 0·027) (Fig. 1).
Fig. 1.
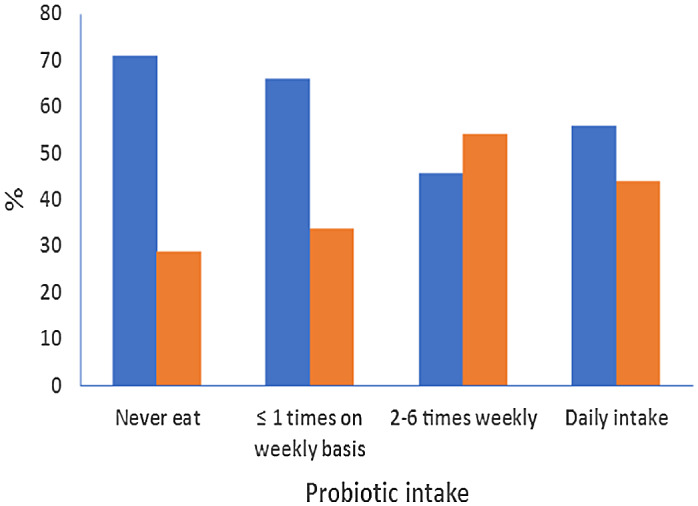
Correlation between probiotic (yogurt) intake and COVID-19 severity.  , mild COVID;
, mild COVID;  , moderate COVID.
, moderate COVID.
The incidence of diarrhoea was higher in the patients who had never consumed PY (32·7 %) but lower in those who consumed variable amounts of PY (19·1, 30·4 and 26 %), as shown in Fig. 2.
Fig. 2.
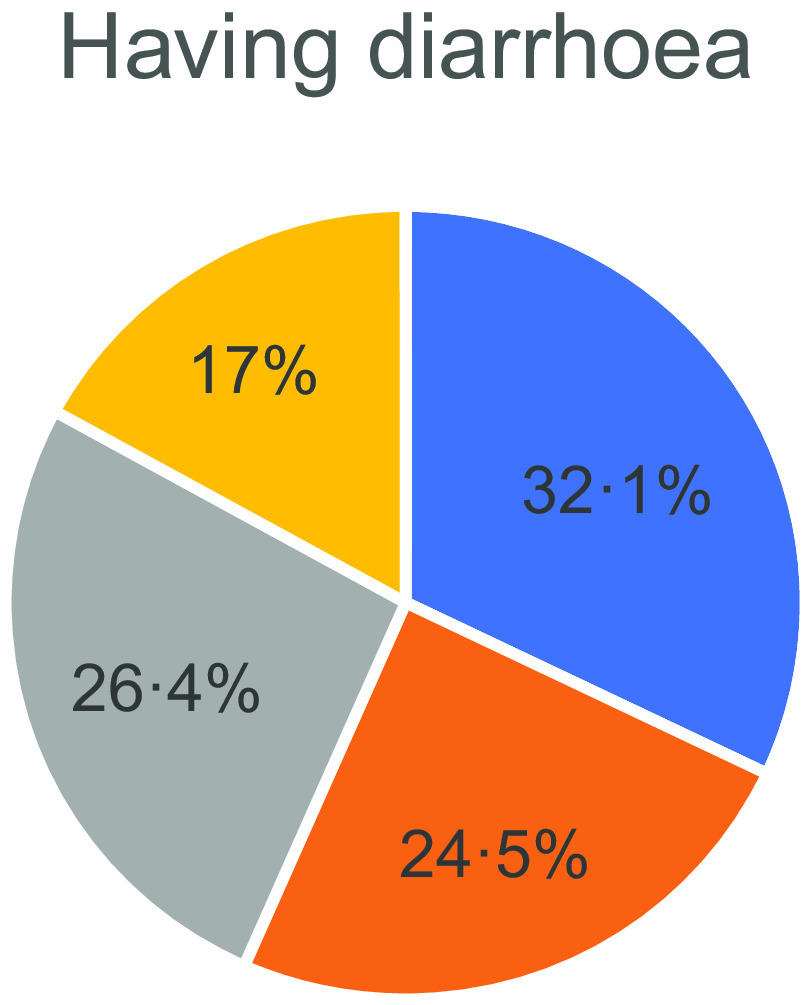
Relation between diarrhoea and probiotic intake.  , never eat probiotics (0);
, never eat probiotics (0);  , ≤ 1 times on weekly basis (1);
, ≤ 1 times on weekly basis (1);  , 2–6 times weekly (2);
, 2–6 times weekly (2);  , daily intake of probiotics (3).
, daily intake of probiotics (3).
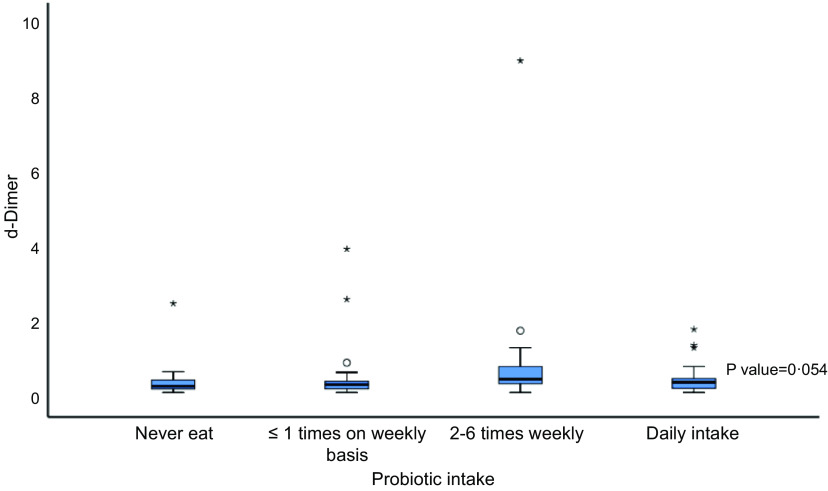
Prevalence of diabetes was similar among groups consuming variable amounts of PY. Serum ferritin levels were higher in patients who ate PY daily, while lowest median CRP (5·6 mg/l) was seen in patients who never ate PY. Similarly, D-dimer levels were lower in patients who never consumed PY or did so only infrequently (Fig. 3).
Fig. 3.
D-dimer in relation to probiotic intake.
The results of the analysis and the correlation between nutrition and lifestyle questionnaire scores and COVID-19 disease severity are shown in Table 2. None of the patients ate ≥ 5 types of food that contain prebiotics; thus, all responses were scored between 0 and 2 with mild cases distributed almost equally among patients who ate between 1 and 4 types daily.
Table 2.
Correlation between nutritional and lifestyle habits and severity of SARS-COV-2
(Numbers and percentages)
| SARS-COV-2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| All | Mild | Moderate | ||||||
| Score | n 200 | n 122 | % | n 78 | % | P | ||
| Probiotic N | Never eat | 0 | 52 | 37 | 71·2 | 15 | 28·8 | 0·027 |
| ≤1 time on weekly basis | 1 | 68 | 45 | 66·2 | 23 | 33·8 | ||
| 2–6 times weekly | 2 | 46 | 21 | 45·7 | 25 | 54·3 | ||
| Daily intake | 3 | 34 | 19 | 55·9 | 15 | 44·1 | ||
| Prebiotic N | Only one type of prebiotics daily | 0 | 36 | 25 | 69·4 | 11 | 30·6 | 0·735 |
| 2 types of prebiotics daily | 1 | 124 | 71 | 57·3 | 53 | 42·7 | ||
| 3–4 types of prebiotics daily | 2 | 40 | 26 | 65·0 | 14 | 35·0 | ||
| 5–6 types of prebiotics daily | 3 | 0 | 0 | 0·0 | 0 | 0·0 | ||
| ≥7 types of prebiotics daily | 4 | 0 | 0 | 0·0 | 0 | 0·0 | ||
| Sugar intake N | ≥10 teaspoon daily | 0 | 38 | 21 | 55·3 | 17 | 44·7 | 0·469 |
| 3–9 teaspoon daily | 1 | 97 | 60 | 61·9 | 37 | 38·1 | ||
| ≤2 teaspoon daily | 2 | 65 | 41 | 63·1 | 24 | 36·9 | ||
| Antibiotic N | ≥6 times per year | 0 | 19 | 8 | 42·1 | 11 | 57·9 | 0·077 |
| ≤5 times per year | 1 | 181 | 114 | 63·0 | 67 | 37 | ||
| Sleep N | <8 h daily | 0 | 100 | 59 | 59·0 | 41 | 41·0 | 0·563 |
| ≥8 h | 1 | 100 | 63 | 63 | 37 | 37 | ||
| Exercise N | Non | 0 | 13 | 6 | 46·2 | 7 | 53·8 | 0·016 |
| Less than 10 min daily | 1 | 105 | 58 | 55·2 | 47 | 44·8 | ||
| 10–20 min daily | 2 | 82 | 58 | 70·0 | 24 | 29·3 | ||
| ≥20 min daily | 3 | 0 | 0 | 0·0 | 0 | 0·0 | ||
Daily sugar intake and frequency of antibiotic use were positively correlated with disease severity. Specifically, 44·7 % of patients who reported regular intake of ≥ 10 teaspoons (0 score) had moderate disease which reduced to 36·9 % in patients with a regular intake of ≤ 2 teaspoons daily (score 2). Next, 57·9 % of the patients who had been prescribed antibiotics ≥ 6 times/year suffered from moderate COVID-19 disease, while 63 % of the mild cases reported fewer prescriptions.
Exercise was the factor that was positively and most significantly correlated with disease severity. Among patients who did not exercise at all (0 score), 53·8 % underwent a moderate COVID-19 course, while 70 % (P = 0·016) of those who exercised 10–20 min every daily (score 1) experienced mild disease. None of our patients accomplished 20 min of daily exercise.
Among patients reporting total sleeping time ≥ 8 h/d on a regular basis, 37 % were categorised as having moderate disease, while this was 41 % among those who slept less than 8 h/d.
From a statistical point of view, after analysing the scores and evaluating the correlation between COVID-19 severity and exercise, sugar intake, sleeping hours, antibiotics received or prebiotic intake, we combined these parameters into a single score (0–11 points), namely the ESSAP score – nutrition and lifestyle gut microbiota modifier health score (score attached).
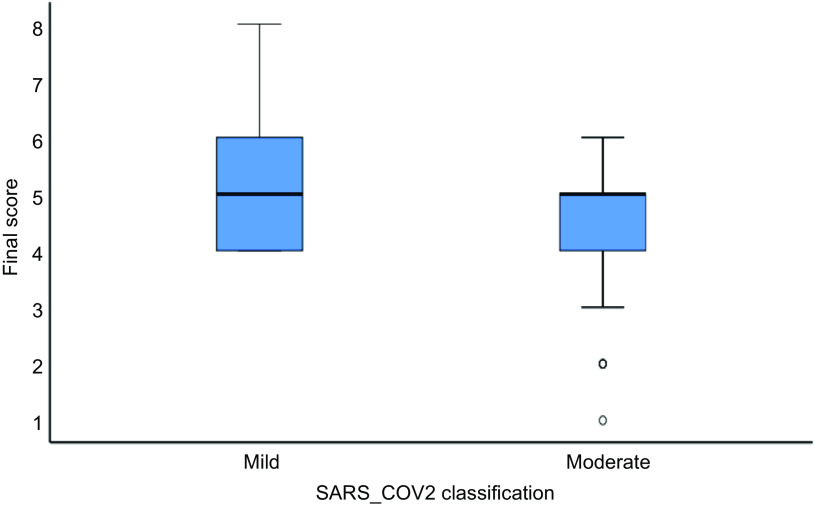
Median ESSAP score was 5 among both mild and moderate cases, but the range was 4–8 among mild cases and 1–6 among moderates (P = 0·001). Furthermore, none of the mild cases recorded a score of less than 4, but twenty patients (16·4 %) had a score of ≥ 7. Among patients with moderate COVID-19, thirty-three (42·3 %) patients had score ≤ 4 and none had scores ≥ 7 (Fig. 4).
Fig. 4.
ESSAP score in mild and moderate COVID-19 cases.
To measure the independent effect of all factors that affect COVID-19 severity, factors which were significant (P < 0·001) during univariate analysis were subjected to stepwise logistic regression analysis (Table 3). The independent factors that could significantly predict COVID-19 severity were, age, DM, probiotic intake and ESSAP score. Specifically, patients aged 50 years or older had a 3·1-fold greater risk of severe infection (P = 0·004, OR: 3·1, 95 % CI 1·4, 6·8), while it was 3-fold for diabetic patients (P = 0·022, OR: 2·9, 95 % CI 1·2, 7·1). Patients who ate PY had 1·6 times higher risk of severity compared with those who did not consume PY (P = 0·007, OR: 1·6, 95 % CI 1·1, 2·1).
Table 3.
Correlation between variables which were significant in the stepwise logistic regression and COVID-19 severity
(Odds ratios and 95 % confidence intervals)
| Factors | B | se | OR | 95 % CI | P |
|---|---|---|---|---|---|
| Age groups | 1·1 | 0·4 | 3·1 | 1·4, 6·8 | 0·004 |
| DM | 1·1 | 0·5 | 2·9 | 1·2, 7·1 | 0·022 |
| Probiotic | 0·4 | 0·2 | 1·6 | 1·1, 2·1 | 0·007 |
| ESSAP score | 1·4 | 0·4 | 4·2 | 1·9, 9·1 | < 0·001 |
| Constant | –2·5 | 0·5 | 0·1 | < 0·001 |
B, regression coefficient. P-value ≤ 0·05 is considered significant.
Discussion
Fighting against SARS-COV-2 infection is essential. The ability to evaluate immune system status and predict COVID-19 disease outcome based on a simple nutrition and lifestyle questionnaire is not only a valuable prognostic tool but also a guide to boost gut microbiota, and ultimately the immune system, to counter infections.
Given the critical role of gut microbiota in modifying the immune system, we designed a questionnaire to obtain information covering a range of nutritional and lifestyle habits that directly supply live bacteria (probiotic), sustain the microbiome (prebiotic) or modify the microbiome (sugar, sleeping hours, exercise and antibiotics). The impact of these factors, namely, consumption of prebiotic-containing foods and sugar, sleeping hours, regular exercise and antibiotics used, was combined into a scoring tool, that is, the ESSAP score – nutrition and lifestyle gut microbiota modifier health score. To identify significant domains in the ESSAP score, we evaluated effect size for all data and found that exercise for at least 20 min daily, sleeping 8 h or more, consumption of ≤ 2 teaspoons of sugar, along with prebiotics-containing food every day and limiting antibiotic use were all associated with mild COVID-19. These results can be explained by the benefits of a diverse gut microbiome on overall immune response. Our results suggest that an ESSAP score of 5 or less is associated with moderate disease severity, while that above 5 was associated with mild disease.
The diet has a crucial role in defining our gut microenvironment(23). Specifically, a healthy microbiota stimulates the development of non-specific and specific immune system components, just after birth and during life, and provides resistance to pathogenic invasion of the mucosa. However, several organisms can overcome these defensive mechanisms by producing pro-inflammatory cytokines(24). Gut microbiota can regulate the production and development of T helper cell types 1, 2, 17 and regulatory T cells, and hence affect overall immune status(25). The relationship between respiratory infections and the gut microbiome is bi-directional, in that respiratory infections also can manipulate the architecture of the gut microbiota(26).
We show that the risk of greater COVID-19 severity was 3·1-fold higher among patients who were 50 years or older. This is related not only to associated co-morbidities but also to age-related decline in the clearance of inhaled particles in the small airways(27). Further, our results clearly show the burden of co-morbidities on disease outcome as diabetes was associated with three times greater risk of severity. This is in agreement with the conclusions of a previous systemic review and meta-analysis which reported that the prevalence of diabetes and cardiovascular and respiratory diseases were significantly higher among critical COVID-19 cases compared with noncritical ones(28). Additionally, excess fat as visceral adipose tissue has been reported to trigger the release of inflammatory mediators that promote a state of chronic inflammation and immune dysregulation(29). Previous studies have also addressed the influence of type 2 diabetes on gut microbiota and suggest the presence of an intricate axis between host immune system and gut microbiota that involves glycolysis, polysaccharide degradation, NEFA modulation and mucin degradation(30,31).
Existing reports also document a reduction in lung damage and improved survival among mice fed with diets rich in fermentable fibres during an influenza virus infection; this effect is thought to be associated with metabolism of ‘SCFA.’(32). Prebiotic-rich foods with high content of fermentable fibres, oligosaccharides and resistant starches, such as legumes, vegetables, some fruits and whole-wheat grain, can beneficially affect gut microbiota as prebiotics are selective substrates for specific beneficial colonic bacteria, as they are neither hydrolysed nor absorbed in the proximal gut(33). Dietary fibre has been suggested as immune modulators because they reduce bacterial translocation by maintaining the integrity of the gut barrier(34). All our patients with COVID-19 ate four or fewer types of foods rich in fibres (fava beans, lentil, wheat, garlic) and scored between 0 and 2. The frequency of mild cases was similar between patients who ate only one type and those who consumed up to four types daily, and this reflects a limitation of our study, that is, that the quantity of each type of food could not be accurately assessed. Thus, fibre consumption was approximated, and a significant impact may have been achieved if patients had reported consuming ≥ 5 types of food which corresponds to scores 3 or 4 and consumption of almost 25 g fibre/d.
Our results show that daily sugar intake of ≤ 2 teaspoons was associated with lower COVID-19 severity. These results concur with those reported previously, that is, that excess sugar consumption in Western diets is not only linked to increased incidence of obesity, diabetes and CVD(35) but also to alterations in the gut microbiome resulting in the evolution of new metamorphic strains(36). Lifestyle habits had a positive impact on COVID-19 outcomes. Specifically, exercise was the most significant factor that was positively correlated with disease severity; however, none of our patients reported > 20 min of regular daily exercise. Exercise is thought to shorten GI transit time, thereby reducing pathogen exposure at the mucosal layer(37). Further, increasing evidence suggests that exercise can modulate gut microbiota by enhancing microbiome diversity, thereby improving immune status(38), and this effect is attributed to increased levels of n-butyrate augmenting B-cell activation(39). Moderate-intensity exercise has been reported to affect neutrophil number and function, host immune system modulation and reduction in pulmonary infections(40,41).
Moderate COVID-19 was higher among patients who slept less than 8 h daily, and 7–8 h of nocturnal sleep has been suggested to negatively affect T-cell activation and immunity through greater β 2-integrin activation and down-regulated Gαs-coupled receptor signalling(42). Gut microbiome diversity also improved with increased sleeping hours and greater sleep quality, with IL-6 providing a coherent link between sleep quality and microbiome diversity(43). Another study has shown that sleep deprivation impairs the gut microbiome in less than 48 h(44).
Several studies have highlighted the consequences of antibiotic use on gut microbiome integrity and such effects can be direct or indirect. While broad-spectrum antibiotics can directly constrain commensal gut bacteria growth and result in gut dysbiosis, susceptible bacteria will be eliminated under fastidious pressure by antibiotics, resulting in an increase in antibiotic-resistant strains that impair host immunity. The underlying mechanisms are thought to involve reduced concentration of peptidoglycan(45) and altered gut lymphoid cells development which lead to diminished IL-22 production(46). Antibiotics that alter gut microbiota have been found to blunt respiratory defenses against S. pneumoniae by interfering with toll-like receptors signalling and alveolar macrophage microbial response, apart from decreasing alveolar IgA production(47). These facts help explain the findings from the present study, that is, that mild COVID-19 was more prevalent among patients reporting less frequent antibiotic use.
A negative correlation between PY intake and disease severity was observed; specifically, PY consumption was associated with 1·6 times greater risk of severity compared with those who never ate it. The GI tract is repeatedly exposed to external factors including food particles, and bacterial antigens which alter them cause what is known as ‘gut dysbiosis’. Certain bacterial species like Bifidobacterium and Lactobacillus are known to have favourable effects in restoring gut microbiota balance; hence, the term ‘probiotic’ has been introduced. Many of the normal gut commensals compete for nourishment and mucosal binding sites and enhance gut mucosal barrier by decreasing the production of deleterious lipopolysaccharides and peptidoglycans(48). In humans, Lactobacillus- and Bifidobacterium-based probiotics have shown improvement in incidence of and outcomes after respiratory infections(49,51).
However, our results on COVID-19 severity and probiotic intake are contrary to the known health benefits of probiotics. This led us to question the safety of probiotics as well. Interestingly, a study published in 2010 reported that probiotics have been theoretically suggested to increase the incidence of systemic infections, lead to deleterious metabolic activities and excessive immune stimulation in susceptible individuals, apart from gene transfer and GI side effects(52). Endocarditis caused by either Lactobacillus or Streptococcus in yogurt has also been reported(53,54). Accumulating evidence also suggests that probiotic use was associated with metabolic concerns including D-lactate production and de-conjugation of bile acids(55). Other studies have raised concerns that probiotics can excessively stimulate the immune system and cause immune dysregulation through over-activation of innate and adaptive immunity(56). Lastly, even lactic acid-rich microbiota have been shown to harbour plasmids containing resistance genes against many antibiotics, thereby increasing chances of resistance development(57).
Several studies have emphasised that probiotic-rich yogurt is healthier than non-probiotic ones. However, commercial yogurt is widely available in two forms – heat-treated fermented cow milk, known as pasteurised yogurt, and probiotic ‘active’ yogurt that is rich in Lactobacillus. Heat and acidity characterise the yogurt manufacturing process and are both fundamental to ensure the product safety. Starter organisms are added to avoid longer incubation periods and thus, poor product quality(58). When yogurt is produced from AFM1-contaminated milk, the toxin with potential carcinogenic properties can still be present during the process of fermentation, even though the developing acidity can reduce AFM1 levels through several mechanisms involving peptidoglycans, cell-wall polysaccharides and proteins(59). Nevertheless, a previous study showed that pasteurisation was not effective in reducing the formation of this toxin(60,62).
Our results also showed no relation between diabetes and the proposed beneficial effect of probiotic consumption on microbiota of patients with diabetes. Several factors have been proposed to affect probiotic bioavailability during processing, including reduced levels of hydrogen peroxide, presence of lactic acid and the interactions between the yogurt starter culture and probiotics(63). Probiotics can be consumed in many forms, including as dietary supplements, and such probiotics should stay stable and viable during storage and consumption(64).
Our results regarding the negative correlation between probiotics and disease severity could be explained by many possible contributing factors, starting from differences in the source and type of milk used for preparing commercial yogurt to possible Aflatoxin M1 contamination. Irrespective of whether the product is rich in live bacteria, possible deleterious effects include defects in enzymatic function during denaturation or type of starter added, which can reduce the stability of yogurt during storage before consumption.
GI symptoms in the form of diarrhoea in patients with COVID-19 in the present study were more frequent in patients who never ate PY than in those who consumed variable amounts. A previous systematic review has reported beneficial effects of probiotics on acute diarrhoea, and probiotic use has been associated with shortened duration of acute diarrhoea(49). Contrastingly, our results did not show any significant difference, again raising the question of commercial PY containing either a negligible amount of bacteria or an insufficiency in the type that has rendered it non-beneficial. Thus, methods of verifying the exact type and amount of the beneficial bacteria in the yogurt box must be established.
To the best of our knowledge, we introduce for the first time a lifestyle and nutritional gut microbiota modifier health score and have used it for predicting COVID-19 severity. Nevertheless, there are limitations to our study. First is the relatively small patient number and unequal case distribution between disease severity groups. Next, microbiota gene sequencing or stool analysis for microbiome mapping of each individual was not possible due to isolation and/or home quarantine of the patients with infection. Consequently, this may be regarded as a pilot study and we hope to test this tool on wider scale.
In summary, we designed a nutrition and lifestyle questionnaire that covered factors that can potentially modify our microbiome and show that sustaining the gut microbiome will help reduce the severity of COVID-19. Further, it appears that introduction of bacteria from an external source, specifically PY, may not only be useless but might also have deleterious effects. Future prospective randomised studies are warranted to shed more light on the effects of nutritional habits and lifestyle on COVID-19 disease outcomes and on the role of different types of bacterial species, either from a natural food source or as dietary supplements, on the gut microbiome and on the possible application of the ESSAP score in different infectious and non-infectious diseases.
Acknowledgements
The authors thank Dr. Mona Fathy, Professor of Clinical Pathology, Faculty of Medicine, Cairo University for her expertise and valuable contributions to our study.
All authors have contributed significantly to finish this work; all authors are in agreement with the content of the manuscript. Design of the study: M. A.-E. H., A. A., M. H. E-D. I. Performance of management: M. A.-E. H., A. A., O. A. A., M. T. H., M. W., R. M. L., H. M. A-H., S. A. A. E. Acquisition of data: M. A.-E. H., A. A., O. A. A., M. T. H., M. W., R. M. L., H. M. A-H., S. A. A. E. Analysis of data: M. A.-E. H., D. A. Interpretation of data and drafting the article: M. A.-E. H., A. A., O. A. A., M. T. H, S. A. A. E. Final approval of the version: M. A.-E. H.
All authors declare the absence of any financial or personal relationships with other people or organisations that could inappropriately influence and bias the work.
References
- 1.WHO (2021) Egypt: WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/region/emro/country/eg/ (accessed April 2021).
- 2.Pan L, Mu M, Yang P, et al. (2020) Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, china. A descriptive, cross-sectional, multicenter study. Am J Gastroenterol 115, 766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groves HT, Higham SL, Moffatt MF, et al. (2020) Respiratory viral infection alters the gut microbiota by inducing inappetence. mBio 11, e03236–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maynard CL, Rich RR, Fleisher TA, et al. (2019) The Microbiota in Immunity and Inflammation, Clinical Immunology, 5th ed., 207–219.e1, ISBN 9780702068966. Elsevier. [Google Scholar]
- 5.Negi S, Das DK, Pahari S, et al. (2019) Potential role of gut microbiota in induction and regulation of innate immune memory. Front Immunol 10, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Round JL & Mazmanian SK (2010) Mazmanian Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci 107, 12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominika Ś, Arjan N, Karyn RP, et al. (2011) The study on the impact of glycated pea proteins on human intestinal bacteria. Int J Food Microbiol 145, 267–272. [DOI] [PubMed] [Google Scholar]
- 8.West CE, Dzidic M, Prescott SL, et al. (2017) Bugging allergy; role of pre-, pro- and symbiotic in allergy prevention. Allergol Int 66, 529–538. [DOI] [PubMed] [Google Scholar]
- 9.Trompette A, Gollwitzer ES, Yadava K, et al. (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20, 159–166. [DOI] [PubMed] [Google Scholar]
- 10.Quercia S, Candela M, Giuliani C, et al. (2014) From lifetime to evolution: timescales of human gut microbiota adaptation. Front Microbiol 5, 587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlon MA & Bird AR (2015) The impact of diet and lifestyle on gut microbiota and human health. Nutrients 7, 17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (2020) Clinical Management of COVID-19: Interim Guidance. https://www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed May 2020).
- 13.Egypt Ministry of Health and Population (2020) Diagnosis and Treatment Protocol for COVID 19. Cairo: Egypt Ministry of Health and Population. [Google Scholar]
- 14.World Medical Association (2009) Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Am Coll Dent 81, 14–18. [PubMed] [Google Scholar]
- 15.Lourens-Hattingh A & Viljoen BC (2001) Yogurt as a probiotic carrier food. Int Dairy J 11, 1–17. [Google Scholar]
- 16.Bassuoni R, Soliman M, Hussein L, et al. (2019) Bio-efficiencies of probiotic yoghurt and fermented sour soya supplements on gut microbial health and other associated health biomarkers among Egyptian pre-school to school age children. Int J Clin Nutr Diet 5, 145. [Google Scholar]
- 17.Committee (DGAC) (2010) The Dietary Guidelines for Americans 2010 (USDA and HHS, 2011). https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2010 (accessed April 2021).
- 18.Elsheikh EAE, El Tinay AH & Fadul IA (1999) Effect of nutritional status of fava bean on proximate composition, antinutritional factors and in vitro protein digestibility (IVPD). Food Chem 67, 379–383. [Google Scholar]
- 19.Frølich W, Aman P & Tetens I (2013) Whole grain foods and health a Scandinavian perspective. Food Nutr Res 57, 18503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson RK, Appel LJ, Brands M, et al. (2009) Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 120, 1011–1020. [DOI] [PubMed] [Google Scholar]
- 21.US Department of Health and Human Services (2008) Physical Activity Guidelines for Americans. Washington, DC: US Department of Health and Human Services. [Google Scholar]
- 22.Coakes SJ & Steed L (2009) SPSS: Analysis without Anguish using SPSS Ion 14.0 for Windows. Statistics in Medicine 18, 2984–2985. [Google Scholar]
- 23.Dominika Ś, Arjan N, Karyn RP, et al. (2011) The study on the impact of glycated pea proteins on human intestinal bacteria. Int J Food Microbiol 145, 267–272. [DOI] [PubMed] [Google Scholar]
- 24.Mogensen TH (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22, 240–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemente JC, Ursell LK, Parfrey LW, et al. (2012) The impact of the gut microbiota on human health: an integrative view. Cell 148, 1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groves HT, Higham SL, Moffatt MF, et al. , (2020) Respiratory viral infection alters the gut microbiota by inducing inappetence. mBio 11, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrotta F, Corbi G, Mazzeo G, et al. (2020) COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clin Exp Res 32, 1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Z, Peng F, Xu B, et al. (2020) Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 81, e16–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellulu MS, Patimah I, Khaza’ai H, et al. (2017) Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 13, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everard A, Belzer C, Geurts L, et al. (2013) ‘Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity’. Proc Natl Acad Sci 110, 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harsch IA & Konturek PC (2018) The role of gut microbiota in obesity and type 2 and type 1 diabetes mellitus: new insights into ‘Old’ diseases. Med Sci 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trompette A, Gollwitzer ES, Pattaroni C, et al. (2018) Dietary fiber confers protection against flu by shaping Ly6c- patrolling monocyte hematopoiesis and CD8 T cell metabolism. Immunity 48, 992–1005. [DOI] [PubMed] [Google Scholar]
- 33.Arrieta MC, Meddings J & Field CJ (2011) The immunomodulatory effects of dietary fiber and prebiotics in the gastrointestinal tract. In Non digestible Carbohydrates and Digestive Health, pp. 37–77 [Paeschke TM & Aimutis WR, editors]. Ames, IA: Blackwell Publishing Ltd. and Institute of Food Technologists. [Google Scholar]
- 34.Schley PD & Field CJ (2007) The immune-enhancing effects of dietary fibers and prebiotics. Br J Nutr 87, 221–230. [DOI] [PubMed] [Google Scholar]
- 35.Khan TA & Sievenpiper JL (2016) Controversies about sugars: results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Eur J Nutr 55, 25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Rienzi SC & Britton RA (2020) Adaptation of the gut microbiota to modern dietary sugars and sweeteners. Adv Nutr 11, 616–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bermon S, Petriz B, Kajeniene A, et al. (2015) ‘The microbiota: an exercise immunology perspective’. Exerc Immunol Rev 21, 70–79. [PubMed] [Google Scholar]
- 38.Mika A, Van Treuren W, González A, et al. (2015) ‘Exercise is more effective at altering gut microbial composition and producing stable changes in lean mass in juvenile v. adult male F344 rats’. PLoS ONE 10, e0125889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto M, Inoue R, Tsukahara T, et al. , (2008) ‘Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum’. Biosci Biotechnol Biochem 72, 572–576. [DOI] [PubMed] [Google Scholar]
- 40.Peake JM (2002) Exercise-induced alterations in neutrophil degranulation and respiratory burst activity: possible mechanisms of action. Exerc Immunol Rev 8, 49–100. [PubMed] [Google Scholar]
- 41.Jones AW & Davison G (2019) Exercise, Immunity, and Illness. Muscle Exercise Physiology, 317–344. doi: 10.1016/B978-0-12-814593-7.00015-3 [DOI] [Google Scholar]
- 42.Dimitrov S, Lange T, Gouttefangeas C, et al. (2019) Gαs-coupled receptor signaling and sleep regulate integrin activation of human antigen-specific T cells. J Exp Med 216, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith RP, Easson C, Lyle SM, et al. (2019) Gut microbiome diversity is associated with sleep physiology in humans. PLoS One 14, e0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedict C, Vogel H, Jonas W, et al. (2016) Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. J Mol Metab 5, 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willing BP, Russell SL & Finlay BB (2011) Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 9, 233–243. [DOI] [PubMed] [Google Scholar]
- 46.Becattini S, Taur Y & Pamer EG (2016) Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med 22, 458–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robak OH, Heimesaat MM, Kruglov AA, et al. (2018) Antibiotic treatment-induced secondary IgA deficiency enhances susceptibility to Pseudomonas aeruginosa pneumonia. J Clin Invest 128, 3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tlaskova-Hogenova H, Stepankova R, Hudcovic T, et al. (2004) ‘Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases’. Immunol Lett 93, 97–108. [DOI] [PubMed] [Google Scholar]
- 49.Salari P, Nikfar S & Abdollahi M (2012) A meta-analysis and systematic review on the effect of probiotics in acute diarrhea. Inflamm Allergy Drug Targets 11, 3–14. [DOI] [PubMed] [Google Scholar]
- 50.Luoto R, Ruuskanen O, Waris M, et al. (2014) Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: a randomized placebo-controlled trial. J Allergy Clin Immunol 133, 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jespersen L, Tranow I, Eskesen D, et al. (2015) Effect of Lactobacillus. paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. Am J Clin Nutr 101, 1188–1196. [DOI] [PubMed] [Google Scholar]
- 52.Sanders ME, Akkermans LM, Haller D, et al. (2010) Safety assessment of probiotics for human use. Gut Microbes 1, 164–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mackay AD, Taylor MB, Kibbler CC, et al. (1999) Lactobacillus endocarditis caused by a probiotic organism. Clin Microbiol Infect 5, 290–292. [DOI] [PubMed] [Google Scholar]
- 54.Presterl E, Kneifel W, Mayer HK, et al. (2001) Endocarditis by Lactobacillus rhamnosus due to yogurt ingestion? J Infect Dis 33, 710–714. [DOI] [PubMed] [Google Scholar]
- 55.Ku W (2006) Probiotics provoked D-lactic acidosis in short bowel syndrome: case report and literature review. HK J Paediatr 11, 246–254. [Google Scholar]
- 56.Drakes M, Blanchard T & Czinn S (2004) Bacterial probiotic modulation of dendritic cells. Infect Immun 72, 3299–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin CF, Fung ZF, Wu CL, et al. (1996) Molecular characterization of a plasmid- borne (pTC82) chloramphenicol resistance determinant (cat-TC) from Lactobacillus reuteri G4. Plasmid 36, 116–124. [DOI] [PubMed] [Google Scholar]
- 58.Shah NP (2007) Functional cultures and health benefits. Int Dairy J 17, 1262–1277. [Google Scholar]
- 59.Bakirci I (2001) A study on the occurrence of aflatoxin M1 in milk and milk products produced in Van province of Turkey. Food Control 12, 47–51. [Google Scholar]
- 60.Kabak B & Var I (2008) Factors affecting the removal of aflatoxin M1 from food model by Lactobacillus and Bifidobacterium strains. J Environ Sci Health Part B Pesticides 43, 617–624. [DOI] [PubMed] [Google Scholar]
- 61.Montaseri H, Arjmandtalab S, Dehghanzadeh G, et al. (2014) Effect of production and storage of probiotic yogurt on aflatoxin M1 residue. J Food Qual Hazards Control 1, 7–14. [Google Scholar]
- 62.Zakaria AM, Amin YA, Khalil OSF, et al. (2019) Rapid detection of aflatoxin M1 residues in market milk in Aswan Province, Egypt and effect of probiotics on its residues concentration. J Adv Vet Anim Res 6, 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lourens-Hattingh A & Viljoen BC (2001) Yoghurt as probiotic carrier food. Int Dairy J 11, 1–17. [Google Scholar]
- 64.Williams NT (2010) ‘Probiotics’. Am J Health-System Pharm 67, 449–458. [DOI] [PubMed] [Google Scholar]