Abstract
The neuropeptide oxytocin (OXT) and its receptor (OXTR) modulate interpersonal relationships, particularly mother–child interactions. DNA methylation (DNAm) changes of the OXTR gene were observed in individuals who experienced Childhood Maltreatment (CM). A modulatory role of single nucleotide polymorphisms (SNP) within OXTR in association with CM on the regulation of OXTR was also postulated. Whether these CM-induced epigenetic alterations are biologically inherited by the offspring remains unknown. We thus investigated possible intergenerational effects of maternal CM exposure on DNAm and OXTR gene expression, additionally accounting for the possible influence of three SNP: rs53576 and rs2254298 (OXTR gene), and rs2740210 (OXT gene). We used the Childhood Trauma Questionnaire to classify mothers into individuals with (CM+) or without CM (CM−). Maternal peripheral immune cells were isolated from venous blood (N = 117) and fetal immune cells from the umbilical cord (N = 113) after parturition. DNA methylation was assessed using MassARRAY. Taqman assays were performed for genotyping and gene expression analyses. Among mothers, CM was not associated with OXTR mean methylation or gene expression. However, four CpG sites showed different methylation levels in CM− compared to CM+. In mothers, the OXTR rs53576 and OXT rs2740210 allelic variations interacted with CM load on the OXTR mean methylation. Maternal and newborns’ mean methylation of OXTR were positively associated within CM− dyads, but not in CM+ dyads. We show gene×environment interactions on the epigenetic regulation of the oxytocinergic signaling and show the intergenerational comparability of the OXTR DNAm might be altered in infants of CM+ mothers.
Subject terms: Clinical genetics, Physiology, Human behaviour
Introduction
Childhood maltreatment (CM) includes experiences of physical, sexual and/or emotional abuse, as well as physical and emotional neglect during childhood and adolescence [1]. CM often occurs repetitively or even chronically and therefore, constitutes a major threat to the physical, mental, and emotional development of a child [2–5]. Consequences of CM can last into adulthood [2–6], presumably via epigenetic mechanisms, e.g. via CM-associated alterations in DNA methylation (DNAm). Higher DNAm can result in less gene transcription activity, especially when DNAm occurs in CpG-rich regions within the gene promoter [7]. Such gene-expression changes were suggested to be one central mechanism by which the individual’s physical and emotional systems adapt to stressors like CM [8]. CM-associated changes in the epigenetic regulation of gene products related to physiological stress responses were already reported [9–16]. In contrast, the effects of CM on emotion dysregulation seem to be buffered by the oxytocinergic system [17] via its key players, the neuropeptide oxytocin (OXT) and its receptor (OXTR).
The oxytocinergic system is considered a buffering factor of the long-term consequences of CM on psychological and physical health for several reasons: First, OXT activity is involved in fostering interpersonal relationships (e.g. reproductive behavior, mother–child bonding; [18]). Second, patients with higher OXT plasma levels show fewer symptoms of major depression, post-partum depression, and anxiety symptoms [19, 20]. Third, alterations in oxytocin were also reported in the central nervous system. For example, women with a history of child abuse showed less OXT concentrations in cerebrospinal fluid [21]. On a biomolecular level, we previously reported that OXT levels modulate the physiological impact of CM on immunocellular bioenergetics and telomere length stability [22, 23]. Moreover, the oxytocinergic system might buffer the negative impact of adverse early life experiences and CM on the maladaptive changes in HPA-axis functioning [23]. Finally, OXT has immune-modulating effects (reviewed in [24]). There is cumulating evidence for the impact of CM on the immunoregulatory functions of the oxytocinergic system. For example, the OXTR density in peripheral blood mononuclear cells (PBMC) was reduced with increasing severity of CM experiences [25]. In the context of DNAm, higher methylation of the OXTR gene was associated with depression [19, 26, 27] and early life adversity [28, 29], suggesting that DNAm of the OXTR might contribute to the pathophysiological transition from chronic stress (i.e., CM) to stress-related pathology. Taken together, these results demonstrate a complex relationship between the oxytocinergic system and early life stress, which might affect OXTR regulation not only on an epigenetic level but also on a physiological and behavioral level.
In the last years, traumatic stress research work has focused on the investigation of biological and physiological associations and the potential mechanisms for direct, intergenerational transmission of CM-associated biological changes. If such transmission occurs, inherited changes can impact the development, health, behavior, as well as the psychopathological risk of the non-affected offspring. Based on intergenerational observations, the long-neglected evolutionary theory of Lamarckian inheritance is currently being re-discussed. Epigenetic modifications, and the consequent regulation of the gene expression, offer a plausible mechanism for acquired biological traits and behavioral adaptations to endure throughout generations [30]. Here, the oxytocinergic system has been proposed as a possible mediator of these CM-related intergenerational transmission effects, mainly because it is a key modulator of parenting behavior, attachment, and risk of post-partum depression [31–33]. The associations of CM-linked methylation changes in the OXTR gene with translational consequences on maternal behavior, maternal agression, and mother–child bonding may contribute to an intergenerational transmission or direct inheritance of CM-related effects (for a review see ref. [34]). Indeed, children of mothers with CM have a higher risk of emotional and behavioral problems [35]; and newborns of mothers who had been socially isolated during the second pregnancy trimester exhibited less methylated OXTR in cord blood cells [36].
Stress research also started to address the relevance of genetic variance for the intergenerational transmission of CM consequences; especially focusing on gene×environment (G×E) interactions, which occur when two or more genotypes respond to environmental challenges with different sensitivity, specificity, or physiological kinetics [17, 34, 37]. Depending on the individual genotype of genes contributing to the oxytocin-signaling pathway, which affects the release of OXT into the bloodstream and the binding efficacy of OXT to its receptor (OXTR), CM experiences might affect maternal oxytocin-related signaling differently. This phenomenon is further discussed in the “differential susceptibility” hypothesis, which suggests that genotypes that predispose to pathology in the general population when associated with adverse events can exert protective effects when associated with favorable environmental conditions, particularly in relation to positive parenting. This is different from the concept of resilience, where genotypes are largely unresponsive to environmental contingencies [38, 39]. This theory explains why genotypes associated with susceptibility have remained throughout evolution. In the context of epigenetic modifications, some single nucleotide polymorphisms (SNPs) in oxytocinergic genes may, in combination with CM, be associated with molecular changes of the oxytocinergic system regulation that might confer adaptation in such challenging early life environment (reviewed in [40]). Similarly, the transmission of biological associations of maternal CM experiences may occur depending on the genotype susceptibility of their offspring. On the behavioral scale, biological alterations might lead to changes in parenting and bonding towards their newborns [41]. Therefore, it is of importance to additionally account for allelic variations in the context of intergenerational studies [38].
Research on oxytocin-signaling pathway genes has mostly focused on the sites rs53576 (G/A) and rs2254298 (G/A), both located within intron 3 of the OXTR gene, and on rs2740210 (A/C), located within the OXT gene. These three SNPs are involved in several G×E interactions that modulate the risk for psychopathology (reviewed in [42]). The GG genotype of OXTR rs53576 in combination with CM was linked to emotion dysregulation [17] and higher levels of internalizing symptoms [18]. Among adolescent girls, the rs2254298 genotype interacts with familiar psychopathology leading to a higher risk of depression and anxiety symptoms [43, 44]. Finally, the rs2740210 polymorphism in the OXT gene interacts with early life adversity to predict depressive symptoms [45].
In a cohort of women who recently gave birth to a child, we aimed to investigate whether maternal CM exposure accounts for differences in OXTR gene methylation (across regions exon 1 to exon 3) and OXTR gene expression. Also, we hypothesized that CM-associated alterations in OXTR methylation are modulated by allelic variants of the OXTR gene (rs53576 and rs2254298) as well as the OXT gene (rs2740210). To address the intergenerational aspects, we first hypothesized that maternal CM experiences are associated with DNAm changes in their newborns. We further assessed associations between the OXTR-methylation profile in immune cells collected from mothers and their newborns. By addressing DNAm in immune cells isolated from cord blood from newborns, our study approach allowed investigating the intergenerational perspective minimizing the influence of post-partum parenting behaviors. Finally, we tested for G×E interactions between maternal CM and child’s SNP variants to investigate the possible role of rs53576, rs2254298, and rs2740210 on the intergenerational transmission of epigenetic consequences of CM.
Materials and methods
Study participants
In total, 533 women who gave birth at the maternity ward of Ulm University Hospital between October 2013 and December 2015 were included in the My Childhood–Your Childhood study (See Supplemental Information [SI], section 1 for more detail). After receiving full information about the study, mothers who agreed to participate and did not meet any exclusion criteria (i.e., age under 18 years, insufficient knowledge of the German language, and severe health problems of mother or child during pregnancy or labor), gave written informed consent and provided basic sociodemographic data (N = 533). Directly after birth, a maximum of 45 mL fetal blood was collected from the umbilical cord. Within one week following parturition, venous blood was taken from mothers. Blood samples were collected into CPDA-buffered tubes (Sarstedt S-Monovette, Nürmbrecht, Germany) and transported to the laboratory of the Ulm University Department of Clinical & Biological Psychology for the isolation of peripheral blood mononuclear cells (PBMC) from mothers and umbilical blood mononuclear cells (UBMC) from the newborns, respectively. The study protocol and all the procedures were approved by the Ulm University Ethics Committee and was conducted in accordance with the Declaration of Helsinki (2013). All biological material was immediately discarded if mothers did not provide informed consent to study participation.
The German short version of the Childhood Trauma Questionnaire (CTQ; [46]) was used to retrospectively assess CM experiences. Using the mild cut-off criteria from Bernstein and Fink (1998; [47]), mothers who reported at least mild CM experiences in at least one CTQ subscale (emotional, physical or sexual abuse, and emotional or physical neglect) were categorized as CM+, and mothers without a history of CM were categorized as CM−. DNAm analyses were conducted in a subset of study participants due to limitations in the availability of biomaterials as described in detail in the SI section 1. The final cohort consisted of 117 mothers (n = 59 CM−, and n = 58 CM+) and 113 infants. See Table 1 for a summary of the demographic and clinical characteristics of the study cohort. CM+ and CM− mothers did not differ in maternal sociodemographic (i.e. age, origin, academic education, stable relation status) or pregnancy characteristics (i.e. primipara status, smoking status during pregnancy, cesarean section), and none of the characteristics displayed in Table 1 were associated with changes in OXTR methylation levels (all p-values > 0.05).
Table 1.
Demographic and biological characteristics.
| Total cohort | CM+ | CM− | ||
|---|---|---|---|---|
| (N = 117) | (n = 58) | (n = 59) | Statisticsa | |
| Mean age in years (SD) | 32.9 (4.4) | 32.8 (4.6) | 33.1 (4.2) | t(115) = 0.36, p = 0.72 |
| N Caucasian maternal ethnicity b (%) | 115 (98.3) | 56 (96.6) | 59 (100) | χ2 (1) = 2.07, p = 0.47 |
| N academic educationc (%) | 63 (53.8) | 37 (48.3) | 31 (59.3) | χ2(1) = 0.52, p = 0.47 |
| N female sex of infant d (%) | 52 (45.2) | 28 (48.3) | 24 (40.7) | χ2(1) = 0.68, p = 0.41 |
| Mean birth weight (SD) in grams | 3387 (497) | 3460 (474) | 3312 (512) | t(115) = 1.62, p = 0.11 |
| Mean gestational age (SD) in weeks | 39.5 (1.4) | 39.7 (1.1) | 39.3 (1.6) | W = 1978.5, p = 0.13 |
| N cesarean section e (%) | 34 (29.1) | 21 (36.2) | 13 (23.6) | χ2(1) = 2.03, p = 0.15 |
| N smokers during pregnancy (%) | 11 (8.7) | 6 (8.5) | 5 (10.3) | χ2(1) = 0, p = 0.98 |
| N primiparae mothers (%) | 58 (49.6) | 23 (39.7) | 35 (59.3) | χ2(1) = 3.77, p = 0.50 |
| N in a stable relationship (%) | 116 (99.1) | 58 (100) | 58 (98.3) | χ2(1) = 0, p = 1 |
| OXTR rs53576 allelic distribution | ||||
| N mother A allele carrier (%) | 73(62.3) | 37 (63.8) | 36 (61.0) | χ2(1) = 0.14, p = 0.90 |
| N children A allele carrier (%) | 59 (52.2) | 30 (54.5) | 29 (50.0) | χ2(1) = 0.87, p = 0.77 |
| OXTR rs2254298 allelic distribution | ||||
| N mother A allele carrier (%) | 21 (17.9) | 10 (17.2) | 11 (18.6) | χ2(1) = 0, p = 1 |
| N children A allele carrier (%) | 24 (21.2) | 12 (21.8) | 12 (20.7) | χ2(1) = 0, p = 1 |
| OXT rs2740210 allelic distribution | ||||
| N mother C allele carrier (%) | 61 (52.1) | 28 (48.3) | 33 (55.9) | χ2(1) = 0.41, p = 0.52 |
| N children C allele carrier (%) | 66 (58.4) | 37 (67.3) | 29 (50.0) | χ2(1) = 1.0, p = 0.32 |
| Childhood maltreatment | ||||
| Mean CTQ sum score (SD) | 33.6 (10.8) | 40.2 (12.1) | 27.1 (1.9) | W = 103.5, p < 0.001 |
| Emotional abuse f (N (%)) | – | 22 (37.9) | ||
| Physical abuse f (N (%)) | – | 16 (27.6) | ||
| Sexual abuse f (N (%)) | – | 16 (27.6) | ||
| Emotional neglect f(N (%)) | – | 40 (69.0) | ||
| Physical neglect f (N (%)) | – | 10 (17.2) | ||
Group differences calculated with chi-square tests for binomial and t-tests for continuous variables.
SD standard deviation, CM childhood maltreatment, CTQ childhood trauma questionnaire, CTQ sum score childhood maltreatment load.
aMain effect of the CTQ classification (t-tests or chi-square tests).
bOne study participant of Brazilian origin and one of North American origin.
cThe education information from one CM+ mother was missing.
dFor gestational and children characteristics, only mother–infant dyads were included: nCM−=58; nCM+=55.
eIncluded planned (nCM− =16, nCM+=12) and emergency (nCM− =4, nCM+=1) forms of cesarean section.
fAmount of women with at least mild experiences in the given CTQ subscale.
DNA methylation analyses
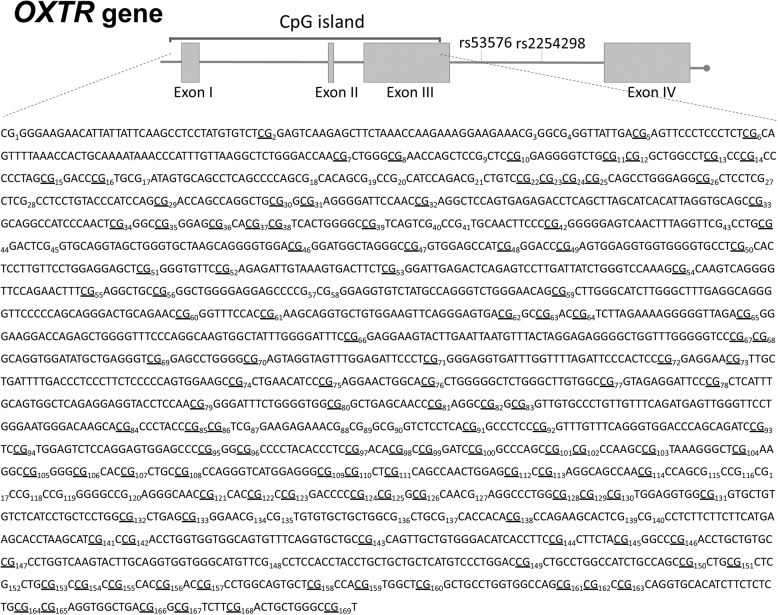
For a detailed description of the isolation of (U)PBMC from mothers and newborns and of DNA preparation for mass array please see SI, sections 2 and 3, respectively. The EpiTYPER assay (Sequenom Inc, USA) was used to quantitatively measure the DNAm levels at individual CpG sites. The analyzed region of the OXTR gene spans across exons 1, 2, and 3 as well as introns 1 and 2 (Fig. 1). For each CpG unit, the percentage of methylated CpG sites over the sum of methylated and unmethylated CpG sites of the pre-analytical PCR products was used for statistical analyses. As previously reported [9], two quality criteria were applied for data processing: First, CpG units with missing values in more than 30% of the samples were excluded from analyses (Fig. 1). Second, samples with missing values in more than 50% of the CpG units were also excluded, which resulted in the exclusion of one newborn sample only. Thus, the final EpiTYPER dataset consisted of samples from 117 mothers and 112 infants. For statistical analyses of DNAm, the mean percentage of methylation across all remaining CpG sites after data processing was calculated for each individual. Samples were measured blinded to the experimenter.
Fig. 1. Schematic view of the targeted sequence of the OXTR gene.
All CpG units located within the targeted sequence (GRCh37/hg19, chr3:8809305-8811438, sequence shown 3' to 5') are numbered consecutively. Underlined are the CpG sites that were included for analyses after data cleaning for the analyses in mothers. Thereafter, 133 CpG sites remained from maternal samples and 142 from children (in contrast to maternal analyses, the CpG 21, CpG 40.41, and CpG 115.116.117.118.119.120 remained for children’s analyses after applying the quality criteria).
Gene-expression analyses
Total RNA was purified from freshly thawed (U)PBMC using the Qiagen RNeasy Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The RNA yield was quantified with the Qubit RNA broad range (BR) assay in combination with a Qubit spectrophotometer (Life Technologies). Samples were stored in RNase free water (Life Technologies) at −20 °C for a maximum of 7 days prior to cDNA transcription (see SI, section for further details). As previously reported [9] we selected Succinate dehydrogenase complex, subunit A, flavoprotein variant (SDHA) and Importin 8 (IPO8) as gene expression references. Detailed information about the gene expression analyses and the selection procedure of appropriate house-keeping genes for human (U)PBMC is given in the SI, section 4.
Allelic discrimination of the SNPs rs53576, rs2254298, and rs2740210
Taqman assays for SNP genotyping are based on the qPCR melting curve approach, which was performed on a QuantStudio 6 platform (Life Technologies, USA). See SI, section 5, for the conditions of thermal cycling. In line with previous research [44, 48–52], we used a dominant model contrasting A-allele carriers (AA/AG genotype) versus G homozygotes (GG) for rs53576 as well as for rs2254298. Regarding OXT rs2740210, we dichotomized the AA genotype versus C-allele carriers due to the low frequency of the CC genotype (only 17 mothers were CC carriers). Among the complete cohort, all three SNPs were distributed following the Hardy Weinberg equilibrium (rs53576: χ2(2) = 0.014, p = 0.90; rs2254298: χ2(2) = 1.14, p = 0.29; rs2740210: χ2(2) = 2.77, p = 0.10).
Data pre-processing and statistical analyses
Data pre-processing and statistical analyses were conducted with the statistical software R, version 3.2.3. Shapiro–Wilk tests were used to test the normal distribution of model residuals. Descriptive differences between groups were analyzed using χ2-tests and Student’s t tests in case of normally distributed data; otherwise, Mann–Whitney U-tests were used. All statistical models included potential confounders for DNAm measurements. The following covariates for the analyses of maternal data were all included in one model: age, percentages of monocytes and lymphocytes in whole blood as separate covariates and days between parturition and isolation of PBMC. For the analyses of infants’ OXTR DNAm, sex, smoking during pregnancy, mode of delivery, and gestational age (in weeks) of the infant were included as covariates. For the models testing associations between maternal and infant’s OXTR DNAm, maternal age, sex of the infant, and gestational age in weeks were included as covariates. Complete data for all covariates were available for 107 women and 112 infants. None of the covariates showed a main effect on OXTR DNAm (all p-values > 0.05). Maternal OXTR DNAm residuals were distributed normally. Here, ANCOVA and multiple linear regressions were used. Newborns’ DNAm and maternal and newborns’ gene expression residuals were not normally distributed. Thus, non-parametric permutation tests [53] of the Student’s t tests for group comparisons, and of linear regression models for regressional analyses were used for: (1) all tests including maternal gene expression data, (2) all tests including infant’s DNAm data, (3) all tests including infant’s gene expression data, and (4) interaction tests. Standardized β coefficients are reported. For single CpG methylation analyses, the False Discovery Rate (FDR; [54]) was used to correct for multiple testing. All tests were performed two-tailed with a significance threshold of alpha ≤ 0.05 and a CI of 95%.
Results
Association of CM experiences and OXTR methylation in mothers
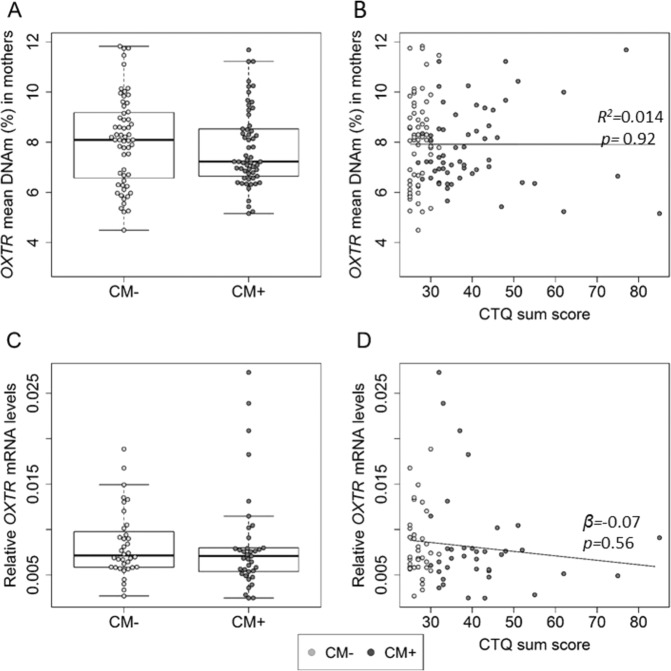
Across the targeted sequence of the OXTR, mean DNAm did not differ significantly between CM+ (M ± SD = 7.7% ± 1.5%) and CM− (M ± SD = 8.1% ± 1.8%; N = 107, F(1,101) = 0.34, p = 0.56; Fig. 2A) and did not correlate with CM load (R2 = 0.014, p = 0.92; Fig. 2B), represented by the CTQ sum score. Finally, none of the CTQ subscales showed associations with OXTR DNA methylation (all p-values > 0.05.
Fig. 2. Association between CM and OXTR epigenetic regulation in mothers.
A OXTR DNAm did not differ between mothers with childhood maltreatment experiences (CM+; marked with dark points) and without (CM−; marked with light points) (N = 107, F(1,101) = 0.34, p = 0.56). B The severity of CM experiences (measured as the CTQ sum score) did not correlate with maternal OXTR DNAm (R2 = 0.014, p = 0.92). C, D The relative OXTR-gene expression did not differ between CM+ and CM− mothers (N = 73, β = −0.01, p = 0.93) and did not correlate with the severity of CM (N = 73, β = −0.07, p = 0.56). Whiskers indicate variability outside the upper and lower quartiles.
For analyses on a single CpG level, we used permutation analysis of the linear regression between CM and DNA methylation of all CpG units (a total of 76). Fifteen CpG units were significantly associated with CM status (see Table S1 in SI for results). After adjusting for multiple comparisons by FDR, 4 CpG remained significant: CpG 169, CpG 2, CpG 5, and CpG 6. Methylation levels of the CpG 169 were significantly higher in CM+ compared to CM− (β = 0.29, padj = 0.04). CpG 2 (β = −0.41, padj < 0.0001), CpG 5 (β = −0.34, padj = 0.01), and CpG 6 (β = −0.40, padj < 0.0001) was significantly less methylated in CM+ compared to CM−. There were no associations between methylation levels of the targeted CpG units and CM load (all padj > 0.05). See SI, Table S1 for detailed information on single CpG results.
OXTR was not differentially expressed in PBMC depending on CM status or CM load (Figs. 2C, D). OXTR methylation and OXTR gene expression were not significantly associated in the mothers (N = 73, β = 0.01, p = 0.92).
G×E interactions on OXTR methylation and expression among mothers
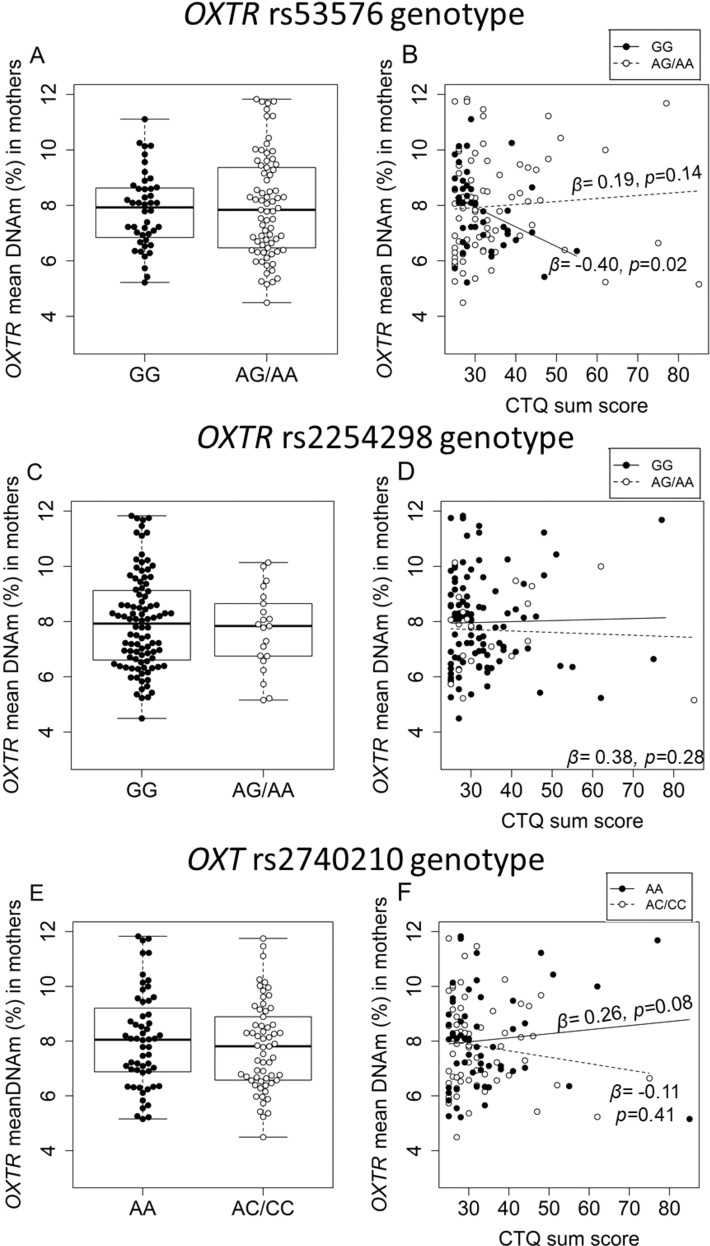
Even though none of the SNP genotypes exerted main effects on OXTR DNAm (Fig. 3A, C, E), the OXTR rs53576 genotype interacted with CM load in modulating the mean OXTR methylation levels (β = 1.24, p = 0.01; Fig. 3B). Subsequent allele-specific analyses revealed that only mothers with the rs53576 GG genotype show a negative association between CM load and OXTR mean methylation (β = −0.40, p = 0.02), but not A-allele carriers (β = 0.19, p = 0.14). There was also an interaction between OXT rs2740210 genotype and CM load (β = −0.84, p = 0.02) that modulated OXTR methylation levels (Fig. 3F), but not between the OXTR rs2254298 and CM load (β = 0.38, p = 0.28; Fig. 3D). Only women with the AA genotype (rs2740210) showed a tendency for a positive association between the CM load and the OXTR DNAm (β = 0.26, p = 0.08).
Fig. 3. Interaction of rs53576, rs2254298, and rs2740210 with childhood maltreatment (CM) load on maternal OXTR DNAm among mothers.
A OXTR mean DNAm did not differ significantly between mothers with the GG genotype for the OXTR rs53576 (n = 41) and A-allele carrier mothers (n = 66) (β = 0.03, p = 0.78). B The OXTR rs53576 genotype modulated OXTR-methylation levels in interaction with the severity of CM experiences (N = 107, β = 1.24, p = 0.01). Further analyses revealed that only mothers with the GG genotype of the rs53576 showed a negative association between the CTQ sum score and OXTR methylation (n = 41, β = −0.40, p = 0.02), while A-allele carriers did not (n = 66, β = 0.19, p = 0.14). C The OXTR DNAm did not differ between mothers carrying at least one A allele of the OXTR rs2254298 polymorphism (n = 18) and GG-homozygous mothers (n = 89, β = −0.04, p = 0.70). D The rs2254298 genotype did not interact with the severity of CM experiences in predicting maternal OXTR DNAm (N = 107, β = 0.38, p = 0.28). E Mothers carrying a C-allele of the OXT-rs2740210 SNP (N = 56) did not differ from AA-homozygous mothers (n = 51, β = −0.05; p = 0.59) with regard to OXTR methylation. F However, the rs2740210 modulated DNAm levels in interaction with CM severity (measured as the CTQ sum score) (N = 107, β = −0.84, p = 0.02). While AA-homozygous women exhibited a trend of higher OXTR DNAm with higher CM severity (n = 51; β = 0.26, p = 0.08), no such association was observed among women carrying a C allele (n = 56; β = −0.11, p = 0.41).
OXTR gene expression was not modulated by the interaction between any of the SNPs and CM status (N = 67; CM ⨯ rs53576: β = −0.15, p = 0.52; CM ⨯ rs2254298: β = −0.20, p = 0.19; CM ⨯ rs2740210: β = 0.33, p = 0.11).
Effects of maternal CM experiences on newborns’ OXTR methylation and gene expression
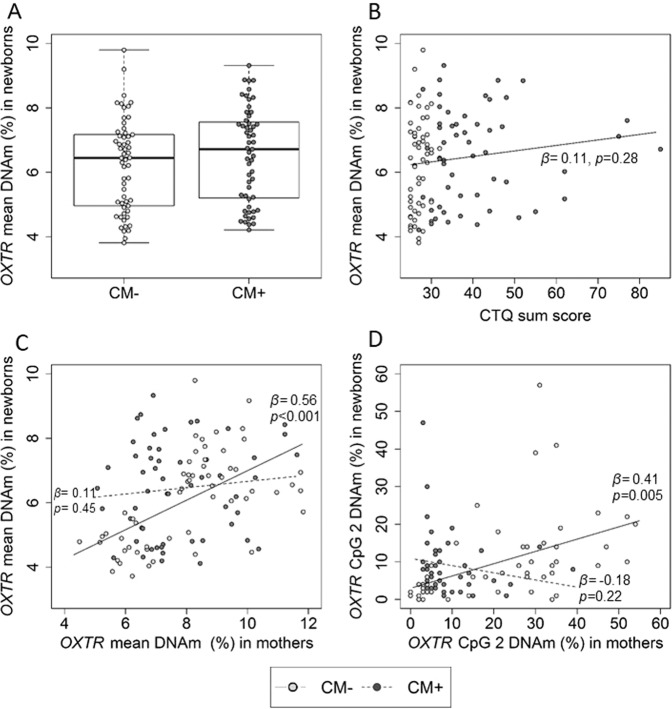
There were no differences in OXTR DNAm between boys or girls (N = 112; W = 1427; p = 0.46). OXTR DNAm levels did not differ (N = 112, β = 0.07, p = 0.45; Fig.4A) between infants of CM+ mothers (M ± SD = 6.5 ± 1.5%) infants of CM− mothers (M ± SD = 6.2 ± 1.4%). Among the complete cohort, the severity of maternal CM experiences (CM load) was not associated with children OXTR methylation levels (N = 112, β = 0.11, p = 0.28, Fig. 4B), also independently of the sex. Single CpG unit analyses showed that newborns’ DNAm levels at specific CpG sites were not associated with maternal CM experiences for any of the analyzed CpGs (all p-values > 0.05).
Fig. 4. Effects of maternal childhood maltreatment (CM) experiences on infant’s OXTR DNAm levels.
The severity of maternal CM experiences was not associated with alterations in the DNA methylation among newborns. A DNAm did not differ between infants from CM+ (n = 55) and CM− mothers (n = 57) (β = 0.07, p = 0.45). B The severity of maternal CM experiences was not associated with newborns’ OXTR DNAm (β = 0.11, p = 0.28). C Mean OXTR DNAm of mothers and their offspring correlated positively (N = 112, β = 0.34, p < 0.001). There was an interaction effect between maternal OXTR methylation and maternal CM on newborn’s OXTR methylation (N = 112, β = −0.95, p = 0.03). Further analyses confirmed a positive association between maternal and newborns OXTR methylation specifically for CM− mothers (β = 0.56, p < 0.001) but not CM+ mothers (β = 0.11, p = 0.45). D At OXTR CpG 2, maternal DNAm was linked to infants’ DNAm depending on maternal CM: Maternal and infant’s CpG 2 DNAm levels were positively correlated only among dyads with CM− mothers (β = 0.41, p = 0.005), but not among dyads with CM+ mothers (β = −0.18, p = 0.22).
Overall mean OXTR DNAm levels of mothers and their offspring were positively correlated (N = 112, β = 0.34, p < 0.001; Fig. 4C). Permutation tests of the linear regression model showed an interaction effect between OXTR methylation levels among mothers and their CM status on infant’s OXTR methylation levels (N = 112, β = −0.95, p = 0.03). Further analyses confirmed that only CM− mother–infant dyads showed a positive association between their mean OXTR DNAm levels (n = 57, β = 0.56, p < 0.001), but not CM+ mother–infant dyads (n = 55, β = 0.11, p = 0.45) (Fig. 4C). When we stratified by sex, again only CM− dyads showed the mother–infant positive association between their mean OXTR DNAm levels (Girls: CM− dyads: n = 24, β = 0.45, p = 0.02; CM+ dyads: n = 28, β = 0.08, p = 0.76/Boys: CM− dyads: n = 33, β = 0.71, p < 0.001; CM+ dyads: n = 27, β = 0.10, p = 0.43).
We further analyzed the associations between the OXTR DNAm of mothers and their newborns on a single CpG unit level. To this end, we correlated maternal and infant’s DNAm only for those CpG candidates for which an effect of CM exposure was found among mothers (CpG 2, CpG 5, CpG 6, and CpG 169). Across the complete cohort, CpG 2 DNAm levels were positively associated between mothers and infants (N = 98, β = 0.27, p = 0.008). Further analyses showed an interaction between maternal CpG 2 DNAm and the CM status on the infant´s CpG 2 DNAm (N = 98, β = −0.36, p = 0.02; Fig. 4D).
Relative OXTR RNA levels did not differ in the UBMC of infants of CM+ mothers compared to those of CM− mothers (N = 38, β = 0.01, p = 0.96) and were not associated with maternal CM load (N = 38, β = 0.06, p = 0.76). OXTR methylation and OXTR gene expression were not significantly associated in infants (N = 38, β = 0.12, p = 0.51).
G×E interactions on OXTR methylation and expression among infants
None of the infants’ SNPs interacted with maternal CM status on infants’ methylation levels (N = 112; rs53576: β = −0.12, p = 0.46; rs2254298: β = −0.17, p = 0.23; rs2740210: β = −0.19; p = 0.24), nor did maternal SNP variants (all p-values > 0.05). Moreover, maternal and infants’ genotypes of the analyzed SNPs did not modulate the association between infants’ and maternal OXTR DNAm levels (all p-values > 0.05). Finally, OXTR gene expression did not depend on the interaction between the analyzed SNPs and maternal CM experiences (N = 34; all p-values > 0.05).
Discussion
This study aimed at investigating the intra- and intergenerational effects of CM on the epigenetic regulation of the OXTR gene. The most important finding is that maternal and newborns mean OXTR DNAm levels were positively associated in mothers without a history of CM, but not in mothers with CM experiences. While CM load had no main effect on maternal mean DNAm of the OXTR gene, we observed a CpG-specific effect of CM on the hypomethylation of CpG 169 and hypermethylation of CpG 2, CpG 5, and CpG 6 within the OXTR gene of mothers. Finally, OXTR rs53576 and OXT rs2740210 polymorphisms interacted with the severity of CM experiences on OXTR mean DNAm in mothers: only women with the GG genotype at rs53576 showed a negative correlation between the CTQ sum score and OXTR methylation, and only women with at least one C-allele in the rs2740210 SNP showed a tendency for a positive association between CM load and OXTR methylation.
Our results suggest that the intergenerational transmission of the epigenetic regulation of the oxytocinergic system differs in mothers with CM experiences and their newborns when compared to controls, independently of the sex of the offspring. These results can be interpreted from several perspectives: From an evolutionary neo-Lamarckian point of view, our results suggest that maternal epigenetic adaptations might only be perpetuated across CM− dyads, and not across CM+. In CM− dyads, this transmission may prepare the next generation to deal with stress. However, in CM+ dyads it might not be evolutionary adaptive to transmit the maternal adaptations, which were presumably acquired to deal with severe, detrimental experiences and thus do not provide evolutionary fitness under normal circumstances [55]. Accordingly, a recent meta-analysis discusses the role of natural selection in the developmental programming of oxytocinergic after early life adversity and CM [39]. From a physiological perspective, given the modulating role of the OXT-system on the immune system, and our results in immune cells, one could speculate that this is one of the multiple mechanisms explaining why offspring from CM+ mothers are at increased risk for psychological and physical health [56, 57]. In line with our results, previous literature highlighted the regulatory role of the oxytocinergic system on the impact of stress across generations. For example, the total number of maternal critical life events up to 2 years before the second trimester predicted OXTR DNAm of newborns’ cord blood cells [36]. Moreover, it has been suggested that the OXT pathway starts preparing for parenting behavior already during pregnancy [58]. With regards to the effects on single CpG sites, of special interest is the CpG 2, as its methylation correlated between mothers and their infants only in the CM-free dyads. Regarding the role of the SNPs rs53576, rs2254298, and rs2740210, in our study the CM-associated changes on the comparability of OXTR DNAm between mothers and infants occurred independently of the maternal or newborn’s genotype. However, previous findings suggested that maternal transmission of psychopathology depends on the OXTR rs2254298 genotype of the daughters [44].
Among mothers, we observed associations of CM with altered DNAm level in four CpG units but not on the mean DNAm of OXTR, suggesting that CM-associated changes of OXTR methylation are CpG-specific. CpG 169 methylation was higher among CM+ mothers, while the methylation of CpG 2, CpG 5, and CpG 6 was lower among CM+ mothers compared to the CM− group. These results showing a CpG-specific methylation pattern indicate a complex epigenetic regulation of the OXTR gene. Previous studies have associated childhood adversity with higher OXTR methylation [29, 59, 60]. However, the correlational directions of the environment on gene methylation have been inconsistent [61]. This inconsistency might result from (i) differences in cohorts, (ii) differences in CM assessment and classification, and (iii) differences in analytical methods used to measure DNAm (e.g. MassARRAY vs. Illumina).
Previous studies suggested a higher sensitivity to the effects of early life stress exposure in GG carriers for the rs53576 OXTR genotype [34, 62, 63]. We found that only GG-homozygous women showed lowered OXTR DNAm with increased CM load. This rs53576-dependent reduction of the DNAm might reflect the differential susceptibility reported in the literature [64], and the body’s attempt to regulate OXTR expression during the post-partum period when parenting behavior becomes especially important. Regarding the rs2740210 SNP, our results complement previous genetic evidence about the predictive role of rs2740210 on individual mothering behavior [45, 65]. We provide the first epigenetic evidence that the level of OXTR methylation in immune cells is regulated by an interaction of rs2740210 × CM exposure. Yet, these results have to be interpreted with caution due to the right-skewed distribution of the data and the low prevalence of severely traumatized individuals in the sample that leads to heteroscedasticity problems. Although we tried to counter this problem by using robust permutation analyses of the linear regression models, the strengths of the association of these findings are relatively weak. Finally, we did not find any associations between rs2254298 and CM-associated OXTR regulation, which is in line with a study that found that rs53576 and rs2254298 do not modulate the associations between CM and depression or anxiety in adulthood [66]. In sum, our findings indicate differential epigenetic adaptation of OXTR-DNAm levels in immune cells to CM exposure depending on the OXTR rs53576, and presumably OXT rs2740210, genotypes.
In this study, the observed methylation changes were not associated with alterations in gene expression. In contrast to our findings, one study using luciferase reporter gene expression assays showed that higher methylation within the same CpG island analyzed in our study resulted in lowered OXTR gene expression [67]. It is important to note that the degree of suppression by DNA methylation was tissue-dependent [67]. Using two house-keeping genes as a reference we here show that, at least in PBMC, alterations in the mean or site-specific DNAm of OXTR might not consequently lead to alterations of its gene expression. In a recent study, we found that CM-affected women showed reduced OXTR protein density in PBMC compared to women without CM [24]. We thus speculate that the OXTR is exposed to an additional level of genetic regulation in immune cells. Indeed, OXTR gene expression is activated via the inflammation regulator NF-kB in human macrophages [68], which might protect immune cells under distress in the presence of external stressors such as CM. In line with this hypothesis, previous studies suggested immune-modulating effects of OXT [69] and higher peripheral OXT levels were associated with a reduction of oxygen consumption of immune cells and shortened telomere length [22, 23]. Therefore, future studies should investigate the potential effects of OXT regulation on stress-associated inflammatory disease outcomes, and extend our results to cell sub-population levels to elucidate the immunological implications of our observations.
Some limitations need to be taken into account when interpreting the findings. First, as sex-specific effects on the OXTR regulation after early life adversities have been reported [29, 60], future studies should also investigate father-newborn dyads. Second, the data were generated with PBMC and cannot be generalized to other tissues such as neurons. While other studies used buccal cells, we used PBMC because they are physiologically more comparable to neurons than any other peripheral tissue. Accordingly, a previous genome-wide association study (GWAS) showed similar patterns of OXTR regulation in immune cells and brain tissue [70]. Third, one significant difference between maternal venous and newborns’ cord blood cells is the presence of CD34+ (embryonic stem cells) in umbilical cord blood. As a consequence, the epigenetic signature from both groups might reflect the different cellular composition. Future studies investigating methylation change on the isolated blood-cell subsets, e.g. T and B cells and CD34+ cells are warranted. Some limitations are related to the special nature of our cohort, which included only puerperal mothers. Pregnancy, and especially delivery, can increase physiological oxytocin and cortisol levels, which usually peak at the end of the third trimester [71–73]. Moreover, we did not address breastfeeding status, lacerations, or whether mothers received exogenous oxytocin during delivery. Delivery wounds, lack of sleep, and perinatal hormonal fluctuations may impact oxytocinergic physiological regulation. However, whether such post-partum factors directly affect methylation of OXTR itself remains uninvestigated. Our results do not show associations between OXTR methylation and other perinatal factors like being primipara mother and type of delivery. With regards to OXT infusions, so far there are inconsistent findings on whether OXT can transfuse through the placenta [74, 75]. Future studies are warranted to investigate implications of breastfeeding, lacerations, postnatal inflammatory status, and OXT infusions during delivery on OXTR methylation. Lastly, our cohort consisted only of healthy women who reported relatively low levels of CM load, which might mask some effects of more severe CM experiences and associated clinical outcomes on the OXTR methylation. Besides negative CM experiences, future studies should also consider alternative scales for CM assessments and especially the protective role of positive parenting experiences.
In conclusion, this exploratory study showed rather specific effects of CM exposure on the OXTR modulation in immune cells of mother–newborn dyads. Most importantly, we found indications that the maternal CM status might interfere with the biological inheritance of the OXTR regulation from the mother to the child—at least on the level of immune cells. Regarding G×E interaction, our results suggest a protective effect of the GG genotype for the rs53576 SNP. We further provide first evidence for a potential modulating role of the OXT rs2740210 polymorphism on the regulation of the oxytocinergic system in women with a history of CM experiences, but these results need future replication. Our findings shed light on the complex regulation of the OXTR gene in the context of CM and highlight the importance of the oxytocinergic system as a potential candidate to further investigate the psychobiological consequences of CM and how maternal CM experiences might disrupt the normal transmission of OXTR from generation to generation.
Supplementary information
Supplemental Material_Ramo et al_CM and OXTR
Acknowledgements
The study My Childhood–Your Childhood was funded by a grant from the German Federal Ministry of Education and Research of Germany (funding number: 01KR1304A). A.B. and A.M.G. were supported by a scholarship of the Konrad Adenauer Foundation and C.B. was supported by a scholarship of the Carl Zeiss Foundation. We thank Traudl Hiller for her contribution to blood processing and PBMC/UBMC isolation as well as Kim Le and Albrecht Fröhlich for their contributions to gene expression and genotyping analyses in the laboratory. We also acknowledge Nico Preising (UPEP, Ulm University) for providing access to the technical resources required for the lyophilization of biomaterial. We acknowledge the general support by Dr. Frank Reister, the whole maternity ward staff at the Ulm University Hospital, and all women who participated in our study. Finally, we would like to thank the whole team of the project My Childhood–Your Childhood for their collaboration on the study coordination. A.Ka. is now at the Department for Clinical Psychology from the University of Innsbruck, Austria. C.W. is now at the Clinic for Psychosomatic Medicine and Psychotherapy, Klinikum Nürnberg, Germany.
Author contributions
A.Ka. and I.T.K. designed the study. AKa supervised all biological analyses and the biolaboratory work packages, including the epigenetic and genetic analyses, and I.T.K. supervised all stages of the project from a psychological side as a project coordinator. C.W. financed and supervised the genotyping analyses, together with S.K. and L.R. L.R. performed the selection of OXTR regions to be epigenetically analyzed, with support from A.Ka. and C.B. L.R. supervised and conducted all stages of DNA and RNA/cDNA work. A.M.G. contributed to DNA and RNA isolation and on the genotyping of rs53576, rs2254298, rs2740210. A.B. organized the recruitment of participants, performed screening interviews, and pre-processed clinical data. L.R. performed the statistical analyses and, together with A.Ka. and I.T.K., interpreted the results. L.R. wrote the manuscript with important input from A.Ka. and I.T.K., as well as critical revisions from all co-authors. All authors approved the final version of this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura Ramo-Fernández, Email: laura.ramo.fernandez@gmail.com.
Alexander Karabatsiakis, Email: Alexander.Karabatsiakis@uibk.ac.at.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-021-01546-w.
References
- 1.World Health Organization. Global status report on violence prevention 2014. 2014.
- 2.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry. 2004;65:249–254. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- 3.Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol. Psychiatry. 2014;19:544–554. doi: 10.1038/mp.2013.54. [DOI] [PubMed] [Google Scholar]
- 4.MacMillan HL, et al. Childhood abuse and lifetime psychopathology in a community sample. Am J Psychiatry. 2001;158:1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- 5.Springer KW, Sheridan J, Kuo D, Carnes M. Long-term physical and mental health consequences of childhood physical abuse: results from a large population-based sample of men and women. Child Abus Negl. 2007;31:517–530. doi: 10.1016/j.chiabu.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suglia SF, Clark CJ, Boynton-Jarrett R, Kressin NR, Koenen KC. Child maltreatment and hypertension in young adulthood. BMC Public Health. 2014;14:1149. doi: 10.1186/1471-2458-14-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 8.Lutz PE, Turecki G. DNA methylation and childhood maltreatment: from animal models to human studies. Neuroscience. 2014;264:142–156. doi: 10.1016/j.neuroscience.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramo-Fernández L, et al. The effects of childhood maltreatment on epigenetic regulation of stress-response associated genes: an intergenerational approach. Sci Rep. 2019;9:983. doi: 10.1038/s41598-018-36689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klengel T, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyrka AR, et al. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol. 2015;66:17–20. doi: 10.1016/j.exger.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perroud N, et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: a link with the severity and type of trauma. Transl Psychiatry. 2011;1:e59. doi: 10.1038/tp.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bustamante AC, et al. Glucocorticoid receptor DNA methylation, childhood maltreatment and major depression. J Affect Disord. 2016;206:181–188. doi: 10.1016/j.jad.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carpenter LL, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Voorhees EE, Dennis MF, Calhoun PS, Beckham JC. Association of DHEA, DHEAS, and cortisol with childhood trauma exposure and post-traumatic stress disorder. Int Clin Psychopharmacol. 2014;29:56–62. doi: 10.1097/YIC.0b013e328364ecd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley B, et al. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene. Dev Psychopathol. 2011;23:439–452. doi: 10.1017/S0954579411000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurlemann R, Scheele D. Dissecting the role of oxytocin in the formation and loss of social relationships. Biol Psychiatry. 2016;79:185–193. doi: 10.1016/j.biopsych.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Scantamburlo G, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–410. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Eapen V, et al. Separation anxiety, attachment and inter-personal representations: disentangling the role of oxytocin in the perinatal period. PloS ONE. 2014;9:e107745. doi: 10.1371/journal.pone.0107745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heim C, et al. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2009;14:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- 22.Boeck C, et al. History of child maltreatment and telomere length in immune cell subsets: Associations with stress- and attachment-related hormones. Dev Psychopathol. 2018;30:539–551. doi: 10.1017/S0954579417001055. [DOI] [PubMed] [Google Scholar]
- 23.Boeck C, et al. The association between cortisol, oxytocin, and immune cell mitochondrial oxygen consumption in postpartum women with childhood maltreatment. Psychoneuroendocrinology. 2018;96:69–77. doi: 10.1016/j.psyneuen.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Wang P, et al. Oxytocin-secreting system: A major part of the neuroendocrine center regulating immunologic activity. J Neuroimmunol. 2015;289:152–161. doi: 10.1016/j.jneuroim.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Krause S, et al. Child maltreatment is associated with a reduction of the oxytocin receptor in peripheral blood mononuclear cells. Front Psychol. 2018;9:173. doi: 10.3389/fpsyg.2018.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker KJ, et al. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010;178:359–362. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuen KW, et al. Plasma oxytocin concentrations are lower in depressed vs. healthy. control women and are independent of cortisol. J Psychiatr Res. 2014;51:30–36. doi: 10.1016/j.jpsychires.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumsta R, Heinrichs M. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Curr Opin Neurol. 2013;23:11–16. doi: 10.1016/j.conb.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Unternaehrer E, et al. Childhood maternal care is associated with DNA methylation of the genes for brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) in peripheral blood cells in adult men and women. Stress. 2015;18:451–461. doi: 10.3109/10253890.2015.1038992. [DOI] [PubMed] [Google Scholar]
- 30.Flasbeck V, Brüne M. Association between childhood maltreatment, psychopathology and DNA methylation of genes involved in stress regulation: evidence from a study in Borderline Personality Disorder. PloS ONE. 2021;16(3):e0248514. doi: 10.1371/journal.pone.0248514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Liu H, Sun Z. Lamarck rises from his grave: parental environment-induced epigenetic inheritance in model organisms and humans. Biol Rev Camb Philos Soc. 2017;92:2084–2111. doi: 10.1111/brv.12322. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, et al. Oxytocin and postpartum depression: delivering on what’s known and what’s not. Brain Res. 2014;1580:219–232. doi: 10.1016/j.brainres.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moura D, Canavarro MC, Figueiredo-Braga M. Oxytocin and depression in the perinatal period-a systematic review. Arch Women’s Ment Health. 2016;19:561–570. doi: 10.1007/s00737-016-0643-3. [DOI] [PubMed] [Google Scholar]
- 34.Toepfer P, et al. Oxytocin pathways in the intergenerational transmission of maternal early life stress. Neurosci Biobehav Rev. 2016;73:293–308. doi: 10.1016/j.neubiorev.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosquet Enlow M, Englund MM, Egeland B. Maternal childhood maltreatment history and child mental health: Mechanisms in intergenerational effects. J Clin Child Adolesc Psychol. 2018;47:S47–S62. doi: 10.1080/15374416.2016.1144189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unternaehrer E, et al. Maternal adversities during pregnancy and cord blood oxytocin receptor (OXTR) DNA methylation. Soc Cogn Affect Neurosci. 2016;11:1460–1470. doi: 10.1093/scan/nsw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell AF, et al. Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front Genet. 2015;6:243. doi: 10.3389/fgene.2015.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belsky J, et al. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis BJ, Horn AJ, Carter CS, van IJzendoorn MH, Bakermans-Kranenburg MJ. Developmental programming of oxytocin through variation in early-life stress: Four meta-analyses and a theoretical reinterpretation. Clin Psychol Rev. 2021;86:101985. doi: 10.1016/j.cpr.2021.101985. [DOI] [PubMed] [Google Scholar]
- 40.Brüne M. Does the oxytocin receptor (OXTR) polymorphism (rs2254298) confer ‘vulnerability’ for psychopathology or ‘differential susceptibility’? Insights from evolution. BMC Med. 2012;10:38. doi: 10.1186/1741-7015-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman R, et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 42.Cataldo I, Azhari A, Esposito G. A review of oxytocin and arginine-vasopressin receptors and their modulation of autism spectrum disorder. Front Mol Neurosci. 2018;11:27. doi: 10.3389/fnmol.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hostinar CE, Cicchetti D, Rogosch FA. Oxytocin receptor gene polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Dev Psychopathol. 2014;26:465–477. doi: 10.1017/S0954579414000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36:144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonas W, et al. Genetic variation in oxytocin rs2740210 and early adversity associated with postpartum depression and breastfeeding duration. Genes Brain Behav. 2013;12:681–694. doi: 10.1111/gbb.12069. [DOI] [PubMed] [Google Scholar]
- 46.Bader K, Hänny C, Schäfer V, Neuckel A, Kuhl C. Childhood trauma questionnaire–psychometrische Eigenschaften einer deutschsprachigen Version. Z Klin Psychol Psychother. 2009;38:223–230. [Google Scholar]
- 47.Bernstein DP, Fink L. Childhood trauma questionnaire: a retrospective self-report: manual. San Antonio, TX: Psychological Corporation; 1998.
- 48.Suchiman HED, et al. Design, measurement and processing of region-specific DNA methylation assays: the mass spectrometry-based method EpiTYPER. Front Genet. 2015;6:287. doi: 10.3389/fgene.2015.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marusak HA, et al. Amygdala responses to salient social cues vary with oxytocin receptor genotype in youth. Neuropsychologia. 2015;79:1–9. doi: 10.1016/j.neuropsychologia.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fujiwara T, et al. Genetic and peripheral markers of the oxytocin system and parental care jointly support the cross-generational transmission of bonding across three generations. Psychoneuroendocrinology. 2019;102:172–181. doi: 10.1016/j.psyneuen.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Krueger F, et al. Oxytocin receptor genetic variation promotes human trust behaviour. Front Hum Neurosci. 2012;6:4. doi: 10.3389/fnhum.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rijlaarsdam J, et al. Prenatal stress exposure, oxytocin receptor gene (OXTR) methylation, and child autistic traits: The moderating role of OXTR rs53576 genotype. Autism Res. 2017;10:430–438. doi: 10.1002/aur.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedman D, Lane D. A nonstochastic interpretation of reported significance levels. J Bus Econ Stat. 1983;1:292–298. [Google Scholar]
- 54.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B: Stat Methodol. 1995;57:289–300. [Google Scholar]
- 55.Skinner MK. Environmental epigenetics and a unified theory of the molecular aspects of evolution: a neo-Lamarckian concept that facilitates neo-Darwinian evolution. Genome Biol Evol. 2015;7:1296–302. doi: 10.1093/gbe/evv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi KW, et al. Maternal depression in the intergenerational transmission of childhood maltreatment and psychological sequelae: testing postpartum effects in a longitudinal birth cohort. Dev Psychopathol. 2019;31:143–156. doi: 10.1017/S0954579418000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madigan S, Wade M, Plamondon A, Maguire JL, Jenkins JM. Maternal adverse childhood experience and infant health: biomedical and psychosocial risks as intermediary mechanisms. J Pediatr. 2017;187:282–289.e1. doi: 10.1016/j.jpeds.2017.04.052. [DOI] [PubMed] [Google Scholar]
- 58.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 59.Smearman EL, et al. Oxytocin receptor genetic and epigenetic variations: association with child abuse and adult psychiatric symptoms. Child Dev. 2016;87:122–134. doi: 10.1111/cdev.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gouin JP, et al. Associations among oxytocin receptor gene (OXTR) DNA methylation in adulthood, exposure to early life adversity, and childhood trajectories of anxiousness. Sci Rep. 2017;7:7446. doi: 10.1038/s41598-017-07950-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cecil C, Zhang Y, Nolte T. Childhood maltreatment and DNA methylation: A systematic review. Neurosci Biobehav Rev. 2020;112:392–409. doi: 10.1016/j.neubiorev.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 62.McInnis OA, McQuaid RJ, Matheson K, Anisman H. The moderating role of an oxytocin receptor gene polymorphism in the relation between unsupportive social interactions and coping profiles: implications for depression. Front Psychol. 2015;6:1133. doi: 10.3389/fpsyg.2015.01133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reiner I, et al. Methylation of the oxytocin receptor gene in clinically depressed patients compared to controls: The role of OXTR rs53576 genotype. J Psychiatr Res. 2015;65:9–15. doi: 10.1016/j.jpsychires.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 64.Flasbeck V, Moser D, Kumsta R, Brüne M. The OXTR single-nucleotide polymorphism rs53576 moderates the impact of childhood maltreatment on empathy for social pain in female participants: evidence for differential susceptibility. Front Psychiatry. 2018;9:359. doi: 10.3389/fpsyt.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mileva-Seitz V, et al. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS ONE. 2013;8:e61443. doi: 10.1371/journal.pone.0061443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tollenaar MS, Molendijk ML, Penninx B, Milaneschi Y, Antypa N. The association of childhood maltreatment with depression and anxiety is not moderated by the oxytocin receptor gene. Eur Arch Psychiatry Clin Neurosci. 2017;267:517–526. doi: 10.1007/s00406-017-0784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kusui C, et al. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochem Biophys Res Commun. 2001;289:681–686. doi: 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- 68.Szeto A, et al. Regulation of the macrophage oxytocin receptor in response to inflammation. Am J Physiol Endocrinol Metab. 2017;312:E183–E189. doi: 10.1152/ajpendo.00346.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol Endocrinol Metab. 2008;295:E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- 70.Gregory SG, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duthie L, Reynolds RM. Changes in the maternal hypothalamic-pituitary-adrenal axis in pregnancy and postpartum: influences on maternal and fetal outcomes. Neuroendocrinology. 2013;98:106–115. doi: 10.1159/000354702. [DOI] [PubMed] [Google Scholar]
- 72.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 73.Prevost M, et al. Oxytocin in pregnancy and the postpartum: relations to labor and its management. Front Public Health. 2014;2:1. doi: 10.3389/fpubh.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malek A, Blann E, Mattison DR. Human placental transport of oxytocin. J Matern Fetal Med. 1996;5:245–255. doi: 10.1002/(SICI)1520-6661(199609/10)5:5<245::AID-MFM3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 75.Patient C, Davison JM, Charlton L, Baylis PH, Thornton S. The effect of labour and maternal oxytocin infusion on fetal plasma oxytocin concentration. Br J Obstet Gynaecol. 1999;106:1311–1313. doi: 10.1111/j.1471-0528.1999.tb08188.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material_Ramo et al_CM and OXTR