Abstract
Background:
Prenatal vitamin D3 supplementation has been linked to reduced risk of early life asthma/recurrent wheeze. This protective effect appears to be influenced by variations in the 17q21 functional SNP rs12936231 of the child, which regulates the expression of ORMDL3, and for which the high-risk CC-genotype is associated with early-onset asthma. However, this does not fully explain the differential effects of supplementation. We investigated the influence of maternal rs12936231 genotype variation on the protective effect of prenatal vitamin D3 supplementation against offspring asthma/recurrent wheeze.
Methods:
We determined the rs12936231 genotype of mother-child pairs from two randomized-controlled trials: the Vitamin D Antenatal Asthma Reduction Trial (VDAART, n=613) and the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010, n=563) to examine the effect of maternal genotype variation on offspring asthma/recurrent wheeze at age 0–3 years between groups who received high-dose prenatal vitamin D3 supplementation versus placebo.
Results:
Offspring of mothers with low-risk GG-genotype or GC-genotype who received high-dose vitamin D3 supplementation had a significantly reduced risk of asthma/recurrent wheeze when compared to the placebo group (hazard ratio [HR], 0.54; 95% confidence interval [CI], 0.37–0.77; P<0.001 for VDAART and HR, 0.56; 95% CI, 0.35–0.92; P=0.021 for COPSAC2010), whereas no difference was observed among the offspring of mothers with high-risk CC-genotype (HR, 1.05; 95% CI, 0.61–1.84; P=0.853 for VDAART and HR, 1.11; 95% CI, 0.54–2.28; P=0.785 for COPSAC2010).
Conclusion:
Maternal 17q21 genotype has an important influence on the protective effects of prenatal vitamin D3 supplementation against offspring asthma/recurrent wheeze.
INTRODUCTION
Asthma represents a globally significant disease burden affecting over 300 million people worldwide [1] and results in substantial childhood morbidity as measured by school absenteeism, emergency department visits, and hospitalizations [2]. The origins of asthma have been linked to fetal development and prenatal exposures are thought to play a key role in disease pathogenesis [3]. In particular, exposure to vitamin D has been linked to fetal lung and immune system development [4]. Therefore, we conducted two independent randomized controlled trials that evaluated the potential of high-dose prenatal vitamin D3 supplementation to reduce offspring asthma/recurrent wheeze: the Vitamin D Antenatal Asthma Reduction Trial (VDAART) [5] and the Copenhagen Prospective Studies on Asthma in Childhood 2010 (COPSAC2010) [6]. A meta-analysis of these two trials showed that high-dose prenatal vitamin D3 supplementation reduced early life asthma/recurrent wheeze among offspring by 26% [7].
17q12-21 is the most replicated risk locus for childhood asthma, and overexpression of ORMDL3 and GSDMB at this locus has been linked to increased risk of childhood-onset asthma and recurrent wheeze [8, 9]. One of the key regulators of ORMDL3 expression is a functional single nucleotide polymorphism (SNP) rs12936231 located at the ZPBP2 intronic region in the 17q21 locus. A G-to-C change at this SNP independently alters ORMDL3 expression by switching the binding site of the CCCTC-binding factor (CTCF) from the ZPBP2 to the ORMDL3 intronic region in T cells [10, 11]. We previously showed that the protective effect of prenatal vitamin D3 supplementation against early life asthma/recurrent wheeze in VDAART and COPSAC2010 seemed to be influenced by the child’s rs12936231 genotype [12]. However, as the supplementation is given to the mothers and genetic factors may explain inter-individual variability of metabolic response to nutrient intake [13], it is possible that maternal genotype may also influence the effects of the supplementation. Furthermore, a supplementation that is dependent on the maternal genotype could be utilized for precision prevention purposes
In the present study, we evaluated the influence of variation in the maternal rs12936231 genotype on the protective effect of high-dose prenatal vitamin D3 supplementation against offspring asthma and recurrent wheeze in VDAART and COPSAC2010. The primary endpoint was asthma/recurrent wheeze in the child’s first three years of life.
MATERIAL AND METHODS
Study subjects
The VDAART (clinicaltrials.gov identifier: NCT00920621) and COPSAC2010 (clinicaltrials.gov identifier: NCT00856947) trial designs and populations have been described in detail previously [5, 6]. Both trials randomized pregnant women (at 10–18 gestational weeks in VDAART and at 22–26 gestational weeks in COPSAC2010) to receive either high-dose vitamin D3 supplementation (4000 IU/d in VDAART and 2400 IU/d in COPSAC2010) or placebo in addition to regular prenatal vitamin D3 supplementation (400 IU/d). A subset of the mothers in COPSAC2010 were additionally randomized to receive prenatal fish oil supplementation in a factorial 2×2 design [14]. VDAART recruited US mothers who had a history of asthma, eczema, or allergic rhinitis, or whose partners had a history of any of these diseases. COPSAC2010 is a Danish population-based mother-child cohort.
Clinical endpoint
Asthma/recurrent wheeze in the child’s first 3 years of life was assessed based on the predefined criteria of both trials. For VDAART, the definition was based on parental report of physician-diagnosed asthma at age 0–3 years or parental report of recurrent wheeze satisfying at least one of the following five conditions ascertained from quarterly questionnaires since birth: 1) wheeze after the child’s second birthday, preceded by wheeze before the second birthday; 2) asthma control medication use after the second birthday, preceded by wheeze before the second birthday; 3) at least two episodes of wheeze after the second birthday; 4) at least one episode of wheeze and asthma control medication use at distinct visits after the second birthday; or 5) two distinct reports of the asthma control medication use after the second birthday[5]. For COPSAC2010, asthma/recurrent wheeze was defined as meeting all of the following four criteria captured in daily symptom diaries from birth: 1) at least five episodes of troublesome lung symptoms within six months, each lasting at least three consecutive days; 2) typical symptoms of asthma; 3) intermittent bronchodilator use; and 4) response to a three-month inhaled corticosteroid trial and relapse upon cessation [6].
Genotype
The Illumina Infinium HumanOmniExpressExome Bead chip was used to determine the 17q21 genotype of SNP rs12936231 for the mothers and children in both cohorts. Only mother-child pairs with maternal genotype data were included in the present study. The G allele was considered the dominant low-risk allele and the C allele the recessive high-risk allele [12].
Sphingolipid metabolites
In a subset of children in VDAART, five metabolites from the sphingolipid pathway (sphingosine-1-phosphate, sphingosine, sphinganine-1-phosphate, sphinganine, and phosphoethanolamine) were measured using untargeted metabolomic profiling (Metabolon, Inc., NC) from plasma samples that were drawn at ages 1 and 3 years as described previously [12].
Statistical analyses
Chi-squared test or one-way analysis of variance were used to evaluate differences in baseline characteristics by maternal rs12936231 genotype. Cox proportional hazards regression and Kaplan-Meier survival curves were used to evaluate the effect of prenatal vitamin D3 supplementation on asthma/recurrent wheeze at age 0–3 years among subgroups according to maternal rs12936231 genotype. Multivariable logistic regression models were used to analyze interactions between maternal rs12936231 genotype and vitamin D3 supplementation on the risk of asthma/recurrent wheeze at age 0–3 years. The interaction models for COPSAC2010 were adjusted for fish oil supplementation, as a subset of the mothers were additionally randomized to fish oil supplementation. Comparisons between different rs12936231 genotypes were performed using 1) an additive model comparing GG-genotype, GC-genotype, and CC-genotype; and 2) a dominant model comparing GG/GC-genotype and CC-genotype. R Version 3.6.0 was used for all statistical analyses and two-tailed tests with a confidence level of 95% were applied.
RESULTS
Baseline characteristics
Table 1 illustrates the baseline characteristics of the study subjects. In total, 613 mother-child pairs from VDAART and 563 mother-child pairs from COPSAC2010 were included in the present study; all of whom had information on maternal rs12936231 genotype. Child rs12936231genotype information was available for 565 subjects in VDAART and 502 in COPSAC2010. The serum 25-hydroxyvitamin D levels at randomization did not differ between mothers with different rs12936231 genotypes in either of the cohorts, but mothers in VDAART had significantly lower 25-hydroxyvitamin D levels at randomization than those in COPSAC2010 (mean 23.2 ng/ml for VDAART and 30.5 ng/ml for COPSAC2010, P<0.001). Vitamin D insufficiency (25-hydroxyvitamin D level of <30 ng/ml) was observed in 471 mothers (77%) in VDAART and in 269 mothers (48%) in COPSAC2010 at randomization. Insufficiency was especially prevalent among African American subjects in VDAART (238/260 [92%]). There was no difference in the number of subjects in the vitamin D3 intervention arms by genotype in either of the cohorts. Within COPSAC2010, which additionally supplemented a subset of women with fish oil, no difference in genotype frequency was observed between any of the intervention arms (Table E1).
Table 1.
Baseline characteristics in VDAART and COPSAC2010.
| VDAART | COPSAC2010 | |||||||
|---|---|---|---|---|---|---|---|---|
| Mother rs12936231 genotype | P value | Mother rs12936231 genotype | P value | |||||
| GG | GC | CC | GG | GC | CC | |||
| N | 132 | 299 | 182 | 152 | 263 | 148 | ||
| Vitamin D3 intervention, N (%) | 60 (46%) | 158 (53%) | 84 (46%) | 0.223 | 71 (47%) | 136 (52%) | 77 (52%) | 0.559 |
| Maternal race, N (%) | 0.009 | N.A. | ||||||
| African American | 67 (51%) | 115 (39%) | 80 (44%) | 0 | 0 | 0 | ||
| Caucasian | 55 (42%) | 131 (44%) | 65 (36%) | 152 (100%) | 263 (100%) | 148 (100%) | ||
| Other | 10 (8%) | 53 (18%) | 37 (20%) | 0 | 0 | 0 | ||
| Maternal asthma, N (%) | 49 (37%) | 114 (38%) | 85 (47%) | 0.121 | 39 (26%) | 59 (22%) | 51 (35%) | 0.025 |
| Maternal baseline* serum 25-hyroxyvitamin D level (ng/ml, mean (SD)) | 23.0 (9.6) | 23.7 (10.8) | 22.5 (10.6) | 0.457 | 30.7 (10.5) | 29.7 (9.3) | 31.7 (10.7) | 0.167 |
| Child rs12936231 genotype, N (%) | <0.001 | <0.001 | ||||||
| GG | 68 (51%) | 55 (21%) | 0 | 70 (51%) | 62 (26%) | 0 | ||
| GC | 64 (49%) | 137 (52%) | 82 (49%) | 68 (49%) | 116 (49%) | 67 (52%) | ||
| CC | 0 | 74 (28%) | 85 (51%) | 0 | 57 (24%) | 62 (48%) | ||
| Child sex (male, N (%)) | 63 (48%) | 160 (54%) | 101 (55%) | 0.377 | 74 (49%) | 135 (51%) | 78 (53%) | 0.775 |
| Child asthma/recurrent wheezing at 0–3 years, N (%) | 34 (26%) | 93 (31%) | 50 (28%) | 0.467 | 25 (16%) | 45 (17%) | 30 (20%) | 0.640 |
Before intervention, i.e. at 10–18 gestational weeks for VDAART and at 22–26 gestational weeks for COPSAC2010.
The prevalence of maternal asthma was higher in the maternal high-risk CC-genotype relative to GC-genotype and GG-genotype in COPSAC2010 (35% vs. 22% vs. 26%, respectively, P=0.025); with a similar but nonsignificant trend in VDAART (47% vs. 38% vs. 37%, respectively, P=0.121). However, we observed no significant differences in offspring asthma/recurrent wheeze between different maternal rs12936231 genotypes in either cohort.
Maternal 17q21 genotype and vitamin D3 intervention
In both VDAART and COPSAC2010, high-dose prenatal vitamin D3 supplementation resulted in a significantly reduced risk of asthma/recurrent wheeze in the children of mothers with the low-risk GG-genotype or GC-genotype, but not in the high-risk CC-genotype (VDAART: hazard ratio [HR], 0.54; 95% confidence interval [CI], 0.37–0.77; P<0.001 for GG/GC-genotype compared to HR, 1.05; 95% CI, 0.61–1.84; P=0.853 for CC-genotype and COPSAC2010: HR, 0.56; 95% CI, 0.35–0.92; P=0.021 for GG/GC-genotype and HR, 1.11; 95% CI, 0.54–2.28; P=0.785 for CC-genotype) (Table 2 and Figure 1). Additional sensitivity analyses in the COPSAC2010 excluding subjects receiving fish oil supplementation also demonstrated a significant protective effect of vitamin D3 supplementation among the offspring of mothers with GG-genotype or GC-genotype, but not in mothers with CC-genotype (Table E2). In VDAART, a clear genotype-specific protective effect of the supplementation was observed among mothers with insufficient vitamin D levels at randomization but not among those with sufficient levels (Table E3). However, there was no clear pattern in COPSAC2010.
Table 2.
The effect of prenatal vitamin D3 supplementation on the development of asthma/recurrent wheeze by age 0–3 years stratified by maternal 17q21 genotype.
| Mother rs12936231 genotype | VDAART | COPSAC2010 | ||||
|---|---|---|---|---|---|---|
| Cases/Total | HR (95% CI) | P value | Cases/Total | HR (95% CI) | P value | |
| GG/GC* | 127/431 | 0.54 (0.37–0.77) | <0.001 | 70/415 | 0.56 (0.35–0.92) | 0.021 |
| GG | 34/132 | 0.39 (0.18–0.83) | 0.015 | 25/152 | 0.51 (0.22–1.18) | 0.117 |
| GC | 93/299 | 0.58 (0.39–0.88) | 0.011 | 45/263 | 0.59 (0.33–1.08) | 0.086 |
| CC | 50/182 | 1.05 (0.61–1.84) | 0.853 | 30/148 | 1.11 (0.54–2.28) | 0.785 |
Combined maternal GG-genotype and GC-genotype.
Analyses were performed using Cox proportional hazard regression. G is considered as the dominant low-risk allele and C the recessive high-risk allele.
Figure 1.
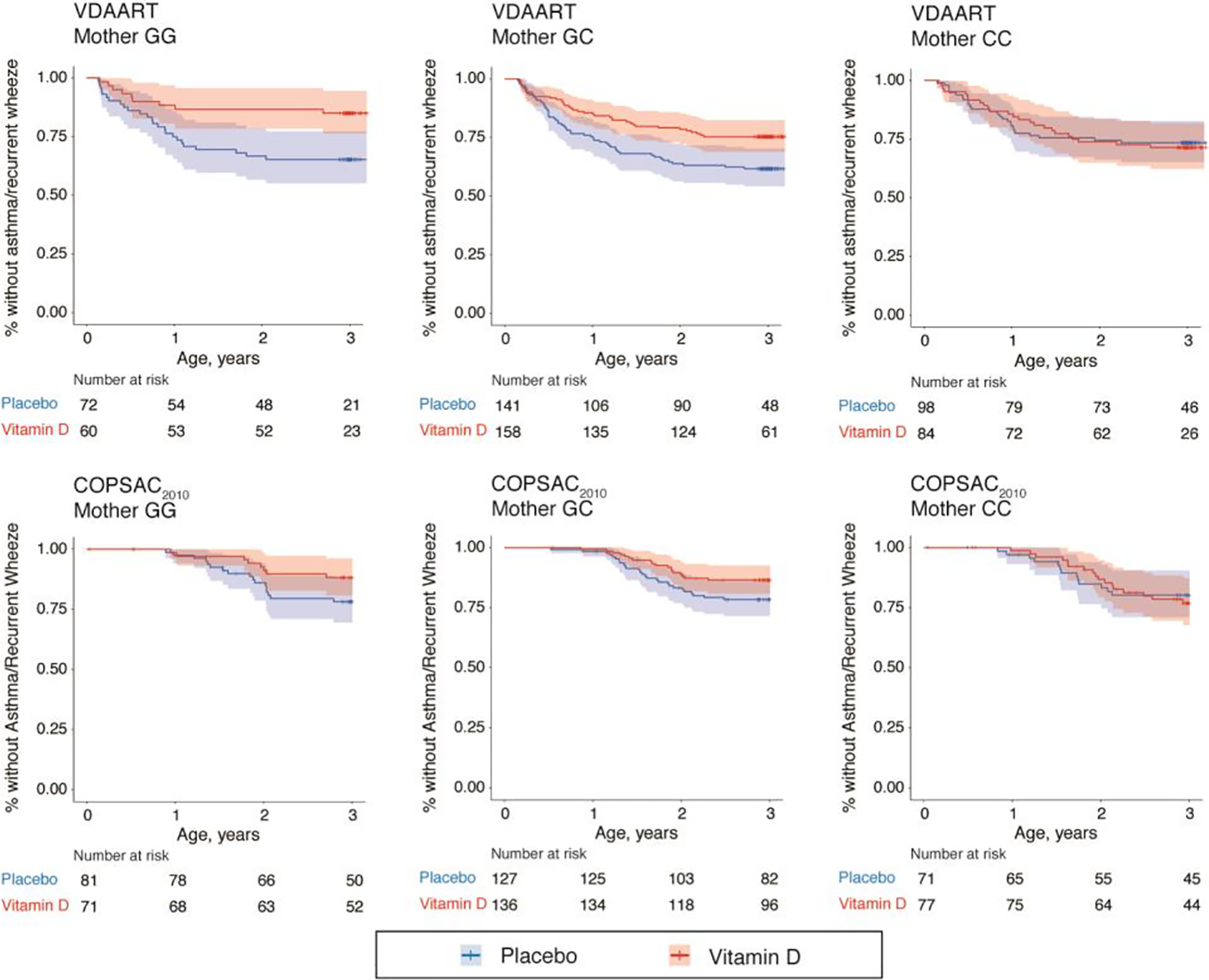
Kaplan-Meier survival curves for the effect of high-dose prenatal vitamin D3 supplementation on the development of asthma/recurrent wheeze at age 0–3 years stratified by maternal 17q21 functional SNP rs12936231 genotype in the VDAART and COPSAC2010 trials. *P<0.05 in the Cox proportional hazard regression model.
Table 3 illustrates the results from multivariable logistic regression models for an interaction between maternal rs12936231 genotype and high-dose prenatal vitamin D3 supplementation on offspring risk of asthma/recurrent wheeze. There was a significant interaction in the additive model of VDAART (P=0.048) and a borderline significant interaction in additive model of COPSAC2010 (P=0.070) and the dominant models of both VDAART (P=0.059) and COPSAC2010 (P=0.053). In contrast, no interaction between fish oil supplementation and maternal rs12936231 genotype on offspring risk of asthma/recurrent wheeze was observed in COPSAC2010 (Table E4).
Table 3.
Multivariable models† for the interaction between maternal 17q21 genotype and prenatal vitamin D3 supplementation on the risk of offspring asthma/recurrent wheeze at 0–3 years.
| VDAART (n=613) | COPSAC2010 (n=563) | |||
|---|---|---|---|---|
| Estimate | P value | Estimate | P value | |
| Additive model | ||||
| Mother rs12936231 genotype | −0.03 | 0.321 | −0.02 | 0.514 |
| Vitamin D3 intervention | −0.20 | 0.002 | −0.13 | 0.015 |
| Mother rs12936231 genotype*Vitamin D3 intervention | 0.10 | 0.048 | 0.08 | 0.070 |
| Dominant model | ||||
| Mother rs12936231 genotype | −0.09 | 0.112 | −0.04 | 0.445 |
| Vitamin D3 intervention | −0.14 | 0.001 | −0.09 | 0.016 |
| Mother rs12936231 genotype*Vitamin D3 intervention | 0.15 | 0.059 | 0.14 | 0.053 |
For VDAART, the model was: asthma/recurrent wheeze ~ mother genotype*vitamin D3 intervention + child sex + child race + study site. For COPSAC2010, the model was: asthma/recurrent wheeze ~ mother genotype*vitamin D3 intervention + child sex + fish oil intervention.
The additive model compares maternal genotypes GG vs. GC vs. CC and the dominant model compares maternal genotypes GG/GC vs. CC.
Maternal and offspring 17q21 genotype combinations and vitamin D3 intervention
There was inherently a high correlation between maternal and child rs12936231 genotype (Table 1). Comparison of mother and child rs12936231 genotype combinations demonstrated a protective effect of high-dose prenatal vitamin D3 supplementation when both mother and child had a low-risk GG-genotype or GC-genotype (HR, 0.54; 95% CI, 0.35–0.83; P=0.005 for VDAART and HR, 0.57; 95% CI, 0.31–1.02; P=0.060 for COPSAC2010) (Table 4 and Figure 2). However, no protective effect was seen if the mother had the high-risk CC-genotype, regardless of child genotype. Race-stratified analyses in VDAART demonstrated a clear allele-additive modifying effect among African Americans based on maternal genotype: HR 0.24 (95% CI 0.09–0.65, P=0.005) for the GG-genotype, HR 0.82 (95% CI 0.45–1.50, P=0.520) for the GC-genotype, and HR 1.12 (95% CI 0.52–2.39, P=0.770) for the CC-genotype (Table 5).
Table 4.
The effect of prenatal vitamin D3 supplementation on the development of early life asthma/recurrent wheeze stratified by maternal and offspring 17q21 genotype combinations.
| VDAART | COPSAC2010 | ||||||
|---|---|---|---|---|---|---|---|
| Mother rs12936231 genotype | Child rs12936231 genotype | Cases/Total | HR (95% CI) | P value | Cases/Total | HR (95% CI) | P value |
| GG/GC* | GG/GC* | 91/324 | 0.54 (0.35–0.83) | 0.005 | 47/316 | 0.57 (0.31–1.02) | 0.060 |
| GG | GG | 11/68 | 0.26 (0.06–1.20) | 0.083 | 11/70 | 0.43 (0.11–1.62) | 0.212 |
| GG | GC | 23/64 | 0.44 (0.18–1.06) | 0.068 | 10/68 | 0.68 (0.19–2.42) | 0.554 |
| GC | GG | 11/55 | 0.54 (0.17–1.77) | 0.311 | 7/62 | 0.41 (0.08–2.10) | 0.282 |
| GC | GC | 46/137 | 0.69 (0.39–1.25) | 0.222 | 19/116 | 0.64 (0.26–1.59) | 0.334 |
| GC | CC | 23/74 | 0.60 (0.26–1.36) | 0.218 | 16/57 | 0.65 (0.24–1.74) | 0.391 |
| CC | GC | 23/82 | 1.02 (0.45–2.32) | 0.966 | 10/67 | 0.80 (0.23–2.76) | 0.723 |
| CC | CC | 22/85 | 1.16 (0.50–2.68) | 0.731 | 16/62 | 1.60 (0.58–4.41) | 0.362 |
Combined GG-genotype and GC-genotype.
Analyses were performed using Cox proportional hazard regression. G is considered as the dominant low-risk allele and C as the recessive high-risk allele.
Figure 2.
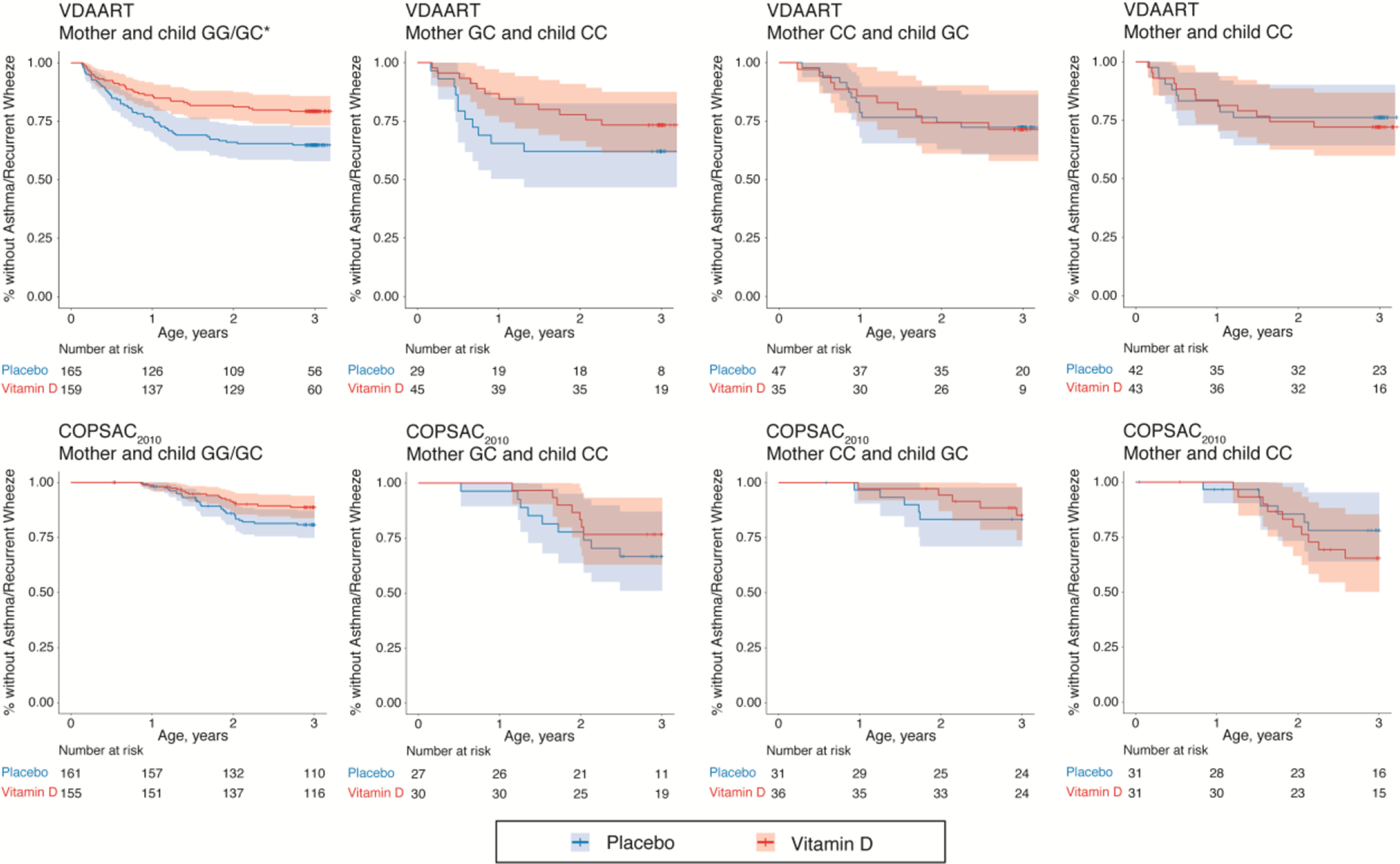
Kaplan-Meier survival curves for the effect of high-dose prenatal vitamin D3 supplementation on the development of asthma/recurrent wheeze at age 0–3 years stratified by mother and child 17q21 functional SNP rs12936231 genotype combinations in the VDAART and COPSAC2010 trials. G is considered as the dominant low-risk allele and C as the recessive high-risk allele. *P=0.005 in the Cox proportional hazard regression model.
Table 5.
The effect of prenatal vitamin D3 supplementation on the development of early life asthma/recurrent wheeze stratified by maternal and child 17q21 genotype in African Americans and in other races from VDAART.
| African American (n=262) | Other races* (n=351) | ||||||
|---|---|---|---|---|---|---|---|
| Cases/Total | HR (95% CI) | P value | Cases/Total | HR (95% CI) | P value | ||
| Mother rs12936231 genotype | |||||||
| GG/GC† | 67/182 | 0.55 (0.33–0.90) | 0.018 | 60/249 | 0.54 (0.33–0.91) | 0.021 | |
| GG | 24/67 | 0.24 (0.09–0.65) | 0.005 | 10/65 | 0.82 (0.23–2.89) | 0.750 | |
| GC | 43/115 | 0.82 (0.45–1.50) | 0.520 | 50/184 | 0.46 (0.26–0.82) | 0.008 | |
| CC | 27/80 | 1.12 (0.52–2.39) | 0.770 | 23/102 | 0.86 (0.37–1.98) | 0.720 | |
| Mother rs12936231 genotype | Child rs12936231 genotype | ||||||
| GG/GC† | GG/GC† | 54/145 | 0.54 (0.31–0.96) | 0.035 | 37/179 | 0.58 (0.30–1.12) | 0.100 |
| GC | CC | 9/25 | 0.89 (0.24–3.33) | 0.870 | 14/49 | 0.47 (0.17–1.35) | 0.160 |
| CC | GC | 13/38 | 1.07 (0.36–3.18) | 0.910 | 10/44 | 0.75 (0.19–2.89) | 0.670 |
| CC | CC | 11/34 | 1.64 (0.48–5.61) | 0.430 | 11/51 | 0.81 (0.25–2.66) | 0.730 |
Other races included: Caucasian (n=251), Asian (n=26), American Indian or Alaska Native (n=8), Native Hawaiian or Other Pacific Islander (n=8), and other races (n=58).
Combined GG-genotype and GC-genotype.
Analyses were performed using Cox proportional hazard regression. G is considered as the dominant low-risk allele and C as the recessive high-risk allele.
Table E5 shows association between child sphingolipid levels and prenatal vitamin D3 supplementation stratified by maternal and child rs12936231 genotype. Consistent increases in the measured sphingolipid levels were seen when both the mother and child had the low-risk GG-genotype or GC-genotype. However, the sample sizes were small for these stratified analyses and we were unable to distinguish the effects of maternal and child genotypes on the changes in child sphingolipid levels.
DISCUSSION
The present study provides evidence that the protective effect of high-dose prenatal vitamin D3 supplementation on early life asthma/recurrent wheeze is dependent on variation in maternal 17q21 functional SNP rs12936231 genotype. Vitamin D3 supplementation significantly reduced the risk of asthma/recurrent wheeze among the offspring of mothers with the low-risk GG-genotype or GC-genotype, whereas no protective effect was seen in the offspring of mothers with the high-risk CC-genotype. The significant effects of maternal rs12936231 genotype variation were observed in two independent cohorts, VDAART and COPSAC2010. Interestingly, the influence of maternal rs12936231 genotype on the vitamin D3-asthma relationship appeared to be even greater than that of the child’s genotype which we reported previously [12]. This is among the first studies to demonstrate the importance of maternal genotype in prenatal interventions which may have important implications for precision prevention.
Although the molecular mechanisms underlying prenatal vitamin D3 supplementation and asthma are incompletely understood, vitamin D metabolites are known to exert several effects on lung development and immune system functions that could potentially explain this association. Vitamin D influences fetal lung development [15, 16] and results from the VDAART demonstrated that prenatal vitamin D3 supplementation might have beneficial effects on offspring lung function [17]. Vitamin D also influences several key immune system functions and vitamin D receptors are expressed on a variety of immune cells [4]. Specifically, vitamin D can activate regulatory T cells [18] and increase steroid responsiveness in asthmatic subjects through stimulation of IL-10 production by regulatory T cells [19]. Furthermore, vitamin D can influence the balance between type 1 and 2 helper T cells [4], which is typically altered towards a type 2 T cell predominance in asthma [20].
Several genome-wide association studies have demonstrated a link between the genetic variants in the 17q21 locus and childhood-onset asthma and recurrent wheeze [8, 9]. These risk variants result in cell-specific increases in the expression of ORMDL3 which regulates de novo sphingolipid synthesis by inhibiting the rate-limiting enzyme serine palmitoyl transferase [21] and decreasing levels of sphingolipids [22]. In mice, decreased sphingolipid metabolism has been linked to increased airway hyperreactivity, suggesting that the functional link between the 17q21 locus and asthma susceptibility may be mediated through altered sphingolipid metabolism [23, 24]. However, the causality and exact molecular mechanisms between altered sphingolipid metabolism and asthma have not been verified [25]. Vitamin D metabolites have several modulatory effects on the sphingolipid pathway [26]. Furthermore, vitamin D can alter CTCF recruitment [27]. Therefore, we hypothesized that variation in rs12936231, which alters ORMDL3 expression via changes in the CTCF binding site, may in part explain the differing effects of prenatal vitamin D3 supplementation on offspring asthma/recurrent wheeze. We previously demonstrated that overexpression of ORMDL3 in bronchial epithelial cells inhibits the production of sphingosine-1-phosphate by vitamin D3 and that prenatal vitamin D3 supplementation resulted in increased levels of key sphingolipids in children with the rs12936231 GG or GC-genotype but not in those with the CC-genotype [12]. This supports the hypothesis that the protective effects of vitamin D3 may be mediated through the sphingolipid pathway and explain the lack of effect when ORMDL3 is overexpressed [11]. In the present study, we found that the protective effect of prenatal vitamin D3 supplementation decreased with increasing numbers of maternal rs12936231 risk alleles, suggesting that the risk allele alters key pathways that mediate the effects of vitamin D3 or that vitamin D3 intervention does not affect genetically high-risk subjects. However, we were unable to distinguish the effects of maternal and child genotype on the changes in child sphingolipid levels in response to prenatal vitamin D3 supplementation possibly because of the small sample sizes in the stratified analyses. Therefore, further research is needed to confirm the exact molecular mechanisms underlying the association between vitamin D and asthma, and to clarify whether the genotype-specific effects of vitamin D3 supplementation are mediated via altered maternal sphingolipid metabolism.
Because African Americans have increased risk of asthma and differ in 17q21-associated risk effects and allele frequencies as compared to Caucasians [9], we investigated the effects of maternal genotype separately among African Americans in VDAART. GSDMB has been proposed as the leading candidate gene at the 17q21 locus for childhood-onset asthma in African Americans [28]. Our study demonstrates that the protective effects of prenatal vitamin D3 supplementation against asthma/recurrent wheeze seem to be strongly dependent on maternal rs12936231 genotype, suggesting that in addition to GSDMB, ORMDL3 might play an important role in the gene-environment interactions of asthma at the 17q21 locus among African Americans. In African Americans, rs12936231 is in low linkage disequilibrium with rs2305480 and rs11078927 which have shown the strongest associations with childhood-onset asthma in this population [28], indicating that the effects of the rs12936231 genotype are independent from those seen with rs2305480 or rs11078927. Vitamin D deficiency is extremely common among African Americans [4, 29] and therefore, the genotype-specific protective effects of prenatal vitamin D3 supplementation provide an especially important aspect for precision prevention of asthma among this population.
When studying the individual responses to prenatal interventions, it is essential to distinguish the influence of maternal and offspring genetic characteristics on the studied effect. However, relatedness results in a strong correlation between maternal and offspring genotypes and complicates this separation. Nevertheless, animal studies have demonstrated that maternal genetic effects can influence complex traits such as early life obesity even more than the direct genetic effects of the offspring [30]. We found that vitamin D3 supplementation had a significant protective effect against asthma/recurrent wheeze in the offspring of mothers with the low-risk rs12936231 genotype in both VDAART and COPSAC2010. This effect was even stronger than we previously observed with the child genotype: a risk reduction of 46% was seen in VDAART and 44% in COPSAC2010 among the offspring of mothers with low-risk genotype who received high-dose vitamin D3, whereas a smaller risk reduction of 31% in VDAART and 35% in COPSAC2010 was previously observed among the children with low-risk genotype [12]. The hypothesis of maternal genotype imparting a stronger influence on the prenatal vitamin D3 effects than child genotype was also supported by our findings on the maternal and offspring genotype combinations, although it should be noted that the combination analyses were restricted by relatively small sample sizes. No protective effect was seen if the mother had a high-risk genotype regardless of the genotype of the child. These findings suggest that maternal genotype has an independent influence on child responses to prenatal vitamin D3 supplementation and highlight the need for further research to thoroughly elucidate the role of maternal genetic effects in prenatal exposures and offspring asthma to enable more targeted preventive actions for the disease.
We chose asthma/recurrent wheeze at age 0–3 years as the primary outcome of our study because prenatal supplementation seems to have the strongest influence in early life [17, 31]. However, it should be acknowledged that the diagnosis of asthma before school age is challenging and only a fraction of children with wheezing in early life will continue to have symptoms later in life [32]. As none of the available tests can definitively diagnose asthma in young children, the diagnosis is based on a multifactorial evaluation of symptoms and risk factors [2]. However, even in the absence of asthma diagnosis, wheezing during early life can have long-term effects on lung function and quality of life [33, 34], and results in a substantial economic burden[35].
This study has several limitations. First, the analysis of interaction between maternal genotype and vitamin D, which remained only borderline significant in most models, is limited by the relatively small sample sizes of both the cohorts. Second, VDAART recruited only parents with asthma or allergies, whereas COPSAC2010 is a population-based cohort. The higher incidence of asthma/recurrent wheeze in VDAART might in part explain the stronger genotype-dependent protective effects of vitamin D3 observed in VDAART. Another possible explanation is racial differences, as the VDAART consists of a multi-ethnic population with predominantly African Americans, whereas COPSAC2010 consists of a more homogeneous Caucasian population. This was further supported by our race-stratified analyses in VDAART which demonstrated strongest genotype-specific protective effects of vitamin D3 supplementation among African Americans. Furthermore, the COPSAC2010 used a lower vitamin D3 dose (2400 IU/d vs. 4000 IU/d) that was started later than in VDAART (22–26 gestational weeks vs. 10–18 gestational weeks), which might in part explain the weaker protective effects seen in COPSAC2010. As lung development begins in the first trimester of pregnancy, it is possible that both trials missed a crucial time window for influencing lung development. Furthermore, recent evidence suggests that alveolarization can continue up to adolescence [36], and therefore postnatal vitamin D sufficiency might be required for maximal effects on lung function. Another limitation of the study is that the interaction between vitamin D3 supplementation and maternal genotype remained only borderline significant in most of the models, which might be due to low statistical power. However, the fact that a significant maternal genotype-dependent protective effect was observed in the two independent trials with substantially different populations and designs increases the confidence in our findings and can also be seen as a strength in terms of generalizability of the findings to other populations.
In conclusion, maternal rs12936231 genotype variation seems to have an important influence on the protective effects of prenatal vitamin D3 supplementation against early life asthma/recurrent wheeze. A significant protective effect was observed in the offspring of mothers with low-risk GG-genotype or GC-genotype, but no protective effect was seen in the offspring of mothers with high-risk CC-genotype. These findings imply that maternal genotype may play an important role in prenatal precision prevention strategies aimed at influencing offspring health.
Supplementary Material
Take home message:
This study demonstrates that maternal 17q21 genotype influences the protective effect of prenatal vitamin D3 supplementation against early life asthma/recurrent wheeze, and this effect appears to be independent of the child’s 17q21 genotype.
ACKNOWLEDGEMENTS
We wish to thank all the participants and study staff involved in the VDAART and COPSAC2010 studies.
Funding
National Heart, Lung, and Blood Institute R01HL091528, UH3OD023268, and R01HL141826. HMK is supported by the Jane and Aatos Erkko Foundation, the Paulo Foundation, and the Pediatric Research Foundation. RSK is supported by K01HL146980 from the National Heart Lung and Blood Institute. PK is supported by the US National Institutes of Health Grant: P01HL132825. BC is funded by an European Research Council Starting grant (no. 946228).
Footnotes
Publisher's Disclaimer: This manuscript has recently been accepted for publication in the European Respiratory Journal. It is published here in its accepted form prior to copyediting and typesetting by our production team. After these production processes are complete and the authors have approved the resulting proofs, the article will move to the latest issue of the ERJ online.
REFERENCES
- 1.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 2017; 5: 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddel HK, Bateman ED, Becker A, Boulet L-P, Cruz AA, Drazen JM, Haahtela T, Hurd SS, Inoue H, de Jongste JC, Lemanske RF, Levy ML, O’Byrne PM, Paggiaro P, Pedersen SE, Pizzichini E, Soto-Quiroz M, Szefler SJ, Wong GWK, FitzGerald JM. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J European Respiratory Society; 2015; 46: 622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet 2015; 386: 1075–1085. [DOI] [PubMed] [Google Scholar]
- 4.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 2007; 120: 1031–1035. [DOI] [PubMed] [Google Scholar]
- 5.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, Sandel M, Iverson RE, Lee-Paritz A, Strunk RC, Bacharier LB, Macones GA, Zeiger RS, Schatz M, Hollis BW, Hornsby E, Hawrylowicz C, Wu AC, Weiss ST. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016; 315: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawes BL, Bønnelykke K, Stokholm J, Vissing NH, Bjarnadóttir E, Schoos A-MM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdóttir S, Arianto L, Hallas HW, Heickendorff L, Brix S, Rasmussen MA, Bisgaard H. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA American Medical Association; 2016; 315: 353–361. [DOI] [PubMed] [Google Scholar]
- 7.Wolsk HM, Chawes BL, Litonjua AA, Hollis BW, Waage J, Stokholm J, Bønnelykke K, Bisgaard H, Weiss ST. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE Public Library of Science; 2017; 12: e0186657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, Berg von A, Bufe A, Rietschel E, Heinzmann A, Simma B, Frischer T, Willis-Owen SAG, Wong KCC, Illig T, Vogelberg C, Weiland SK, Mutius von E, Abecasis GR, Farrall M, Gut IG, Lathrop GM, Cookson WOC. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature Nature Publishing Group; 2007; 448: 470–473. [DOI] [PubMed] [Google Scholar]
- 9.Stein MM, Thompson EE, Schoettler N, Helling BA, Magnaye KM, Stanhope C, Igartua C, Morin A, Washington C, Nicolae D, Bønnelykke K, Ober C. A decade of research on the 17q12-21 asthma locus: Piecing together the puzzle. J Allergy Clin Immunol 2018; 142: 749–764.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verlaan DJ, Berlivet S, Hunninghake GM, Madore A-M, Larivière M, Moussette S, Grundberg E, Kwan T, Ouimet M, Ge B, Hoberman R, Swiatek M, Dias J, Lam KCL, Koka V, Harmsen E, Soto-Quiros M, Avila L, Celedón JC, Weiss ST, Dewar K, Sinnett D, Laprise C, Raby BA, Pastinen T, Naumova AK. Allele-specific chromatin remodeling in the ZPBP2/GSDMB/ORMDL3 locus associated with the risk of asthma and autoimmune disease. Am. J. Hum. Genet 2009; 85: 377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmiedel BJ, Seumois G, Samaniego-Castruita D, Cayford J, Schulten V, Chavez L, Ay F, Sette A, Peters B, Vijayanand P. 17q21 asthma-risk variants switch CTCF binding and regulate IL-2 production by T cells. Nat Commun Nature Publishing Group; 2016; 7: 13426–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly RS, Chawes BL, Guo F, Zhang L, Blighe K, Litonjua AA, Raby BA, Levy BD, Rago D, Stokholm J, Bønnelykke K, Bisgaard H, Zhou X, Lasky-Su JA, Weiss ST. The Role of the 17q21 Genotype in the Prevention of Early Childhood Asthma and Recurrent Wheeze by Vitamin D. Eur Respir J 2019; : 1900761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Toro-Martín J, Arsenault BJ, Després JP, Vohl MC. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients. 2017;9(8):913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos A-MM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdóttir S, Følsgaard NV, Fink NR, Thorsen J, Pedersen AG, Waage J, Rasmussen MA, Stark KD, Olsen SF, Bønnelykke K. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med Massachusetts Medical Society; 2016; 375: 2530–2539. [DOI] [PubMed] [Google Scholar]
- 15.Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Curr Opin Allergy Clin Immunol 2009; 9: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kho AT, Sharma S, Qiu W, Gaedigk R, Klanderman B, Niu S, Anderson C, Leeder JS, Weiss ST, Tantisira KG. Vitamin D related genes in lung development and asthma pathogenesis. BMC Med Genomics BioMed Central; 2013; 6: 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litonjua AA, Carey VJ, Laranjo N, Stubbs BJ, Mirzakhani H, O’Connor GT, Sandel M, Beigelman A, Bacharier LB, Zeiger RS, Schatz M, Hollis BW, Weiss ST. Six-Year Follow-up of a Trial of Antenatal Vitamin D for Asthma Reduction. N Engl J Med Massachusetts Medical Society; 2020; 382: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol American Association of Immunologists; 2001; 167: 1945–1953. [DOI] [PubMed] [Google Scholar]
- 19.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, Adikibi T, Pridgeon C, Dallman M, Loke T-K, Robinson DS, Barrat FJ, O’Garra A, Lavender P, Lee TH, Corrigan C, Hawrylowicz CM. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J. Clin. Invest American Society for Clinical Investigation; 2006; 116: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambrecht BN, Hammad H. The immunology of asthma. Nature Immunology 2014 16:1 Nature Publishing Group; 2015; 16: 45–56. [DOI] [PubMed] [Google Scholar]
- 21.Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS. Orm family proteins mediate sphingolipid homeostasis. Nature Nature Publishing Group; 2010; 463: 1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ono JG, Kim BI, Zhao Y, Christos PJ, Tesfaigzi Y, Worgall TS, Worgall S. Decreased sphingolipid synthesis in children with 17q21 asthma-risk genotypes. J. Clin. Invest American Society for Clinical Investigation; 2020; 130: 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worgall TS, Veerappan A, Sung B, Kim BI, Weiner E, Bholah R, Silver RB, Jiang X-C, Worgall S. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci Transl Med American Association for the Advancement of Science; 2013; 5: 186ra67–186ra67. [DOI] [PubMed] [Google Scholar]
- 24.Levy BD. Sphingolipids and susceptibility to asthma. Phimister EG, editor. N Engl J Med Massachusetts Medical Society; 2013; 369: 976–978. [DOI] [PubMed] [Google Scholar]
- 25.Debeuf N, Zhakupova A, Steiner R, Van Gassen S, Deswarte K, Fayazpour F, Van Moorleghem J, Vergote K, Pavie B, Lemeire K, Hammad H, Hornemann T, Janssens S, Lambrecht BN. The ORMDL3 asthma susceptibility gene regulates systemic ceramide levels without altering key asthma features in mice. J Allergy Clin Immunol 2019; 144: 1648–1659.e1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Gil M, Pierucci F, Vestri A, Meacci E. Crosstalk between sphingolipids and vitamin D3: potential role in the nervous system. Br. J. Pharmacol John Wiley & Sons, Ltd (10.1111); 2017; 174: 605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seuter S, Neme A, Carlberg C. Characterization of genomic vitamin D receptor binding sites through chromatin looping and opening. Ramagopalan SV, editor. PLoS ONE Public Library of Science; 2014; 9: e96184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ober C, McKennan CG, Magnaye KM, Altman MC, Washington C, Stanhope C, Naughton KA, Rosasco MG, Bacharier LB, Billheimer D, Gold DR, Gress L, Hartert T, Havstad S, Khurana Hershey GK, Hallmark B, Hogarth DK, Jackson DJ, Johnson CC, Kattan M, Lemanske RF, Lynch SV, Mendonca EA, Miller RL, Naureckas ET, O’Connor GT, Seroogy CM, Wegienka G, White SR, Wood RA, et al. Expression quantitative trait locus fine mapping of the 17q12-21 asthma locus in African American children: a genetic association and gene expression study. Lancet Respir Med 2020; 8: 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med American Medical Association; 2004; 158: 531–537. [DOI] [PubMed] [Google Scholar]
- 30.Wolf JB, Leamy LJ, Roseman CC, Cheverud JM. Disentangling prenatal and postnatal maternal genetic effects reveals persistent prenatal effects on offspring growth in mice. Genetics Genetics; 2011; 189: 1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brustad N, Eliasen AU, Stokholm J, Bønnelykke K, Bisgaard H, Chawes BL. High-Dose Vitamin D Supplementation During Pregnancy and Asthma in Offspring at the Age of 6 Years. JAMA American Medical Association; 2019; 321: 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and Wheezing in the First Six Years of Life. N Engl J Med 1995; 332: 133–138. [DOI] [PubMed] [Google Scholar]
- 33.Braig S, Brandt S, Wabitsch M, Florath I, Brenner H, Rothenbacher D, Genuneit J. Age-specific influence of wheezing phenotypes on pre-adolescent and adolescent health-related quality of life. Pediatr Allergy Immunol John Wiley & Sons, Ltd (10.1111); 2014; 25: 781–787. [DOI] [PubMed] [Google Scholar]
- 34.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med 2005; 172: 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens CA, Turner D, Kuehni CE, Couriel JM, Silverman M. The economic impact of preschool asthma and wheeze. Eur Respir J European Respiratory Society; 2003; 21: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 36.Narayanan M, Owers-Bradley J, Beardsmore CS, Mada M, Ball I, Garipov R, Panesar KS, Kuehni CE, Spycher Ben D, Williams SE, Silverman M. Alveolarization Continues during Childhood and Adolescence. Am J Respir Crit Care Med American Thoracic Society; 2012; 185: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


