Abstract
Interferon-alpha (rIFNα) is the only disease-modifying treatment for polycythemia vera (PV), but whether or not it prolongs survival is unknown. This large single center retrospective study of 470 PV patients compares the myelofibrosis-free survival (MFS) and overall survival (OS) with rIFNα to two other primary treatments, hydroxyurea (HU) and phlebotomy-only (PHL-O). The median age at diagnosis was 54 years (range 20–94) and the median follow-up was 10 years (range 0–45). Two hundred and twenty-nine patients were women (49%) and 208 were high-risk (44%). The primary treatment was rIFNα in 93 (20%), HU in 189 (40%), PHL-O in 133 (28%) and other cytoreductive drugs in 55 (12%). The treatment groups differed by ELN risk score (p<0.001). In low-risk patients, 20-year MFS for rIFNα, HU, and PHL-O was 84%, 65% and 55% respectively (p<0.001) and 20-year OS was 100%, 85% and 80% respectively (p=0.44). In high-risk patients, 20-year MFS for rIFNα, HU, and PHL-O was 89%, 41% and 36% respectively (p=0.19) and 20-year OS was 66%, 40%, 14% respectively (p=0.016). In multivariable analysis, longer time on rIFNα was associated with a lower risk of myelofibrosis (HR: 0.91, p<0.001) and lower mortality (HR: 0.94, p=0.012). In conclusion, this study supports treatment of PV with rIFNα to prevent myelofibrosis and potentially prolong survival.
Introduction
Polycythemia vera (PV), a myeloproliferative neoplasm (MPN) characterized by clonal erythrocytosis, has the propensity to progress to myelofibrosis (MF), a condition associated with significant morbidity and mortality1,2. Recombinant interferon-alpha (rIFNα) has been shown to induce complete and durable histomorphologic responses in PV3–10 and reverse marrow fibrosis9,11. However, no prospective or retrospective study has determined whether rIFNα improves myelofibrosis-free survival (MFS) or overall survival (OS) compared to the other primary treatments, hydroxyurea (HU) or phlebotomy-only (PHL-O). This is important because PHL-O remains widely used for low-risk patients and HU for high-risk patients, although rIFNα is also recommended for treating high-risk PV by national and international guidelines12,13.
Clinical trials comparing rIFNα to HU have demonstrated effectiveness of both as initial cytoreductive therapy for high-risk PV patients14–16, but these studies do not have the long-term follow-up required to assess differences in MFS and OS. Moreover, the hematologic and molecular response endpoints used in these studies are not validated surrogates of post-polycythemia vera myelofibrosis (PPVMF) or mortality. Accordingly, we developed a research data repository (RDR) to aggregate longitudinal clinical information from all of our PV patients for analysis of outcomes. We developed automated tools to capture multiple rich datasets to integrate into the RDR. In this study, we compare the MFS and OS of the PV patients in our RDR who were treated with rIFNα, HU, or PHL-O and determine the effect of time on therapy on PPVMF and mortality outcomes.
Methods:
Study criteria and data collection
This single-center retrospective study at Weill Cornell Medicine (WCM) was approved by the WCM institutional review board (IRB). Patients were identified by querying the Observational Medical Outcomes Partnership (OMOP) Common Data Model for patients by the International Classification of Diseases, code versions 9 and 10 or by the Systemized Nomenclature of Medicine – Clinical Terms for the diagnosis of PV. The diagnosis of PV and the date of diagnosis were all confirmed by manual review of records. The study included all patients diagnosed with PV by either the PVSG criteria (1974–2007)17, WCM criteria (2008–2016)18, or WHO 2016 criteria (2016–2019)19. Structured query language was used for data extraction and facilitated the collection of patient age, sex, race, ethnicity, diagnosis date, cardiovascular risk factors (diabetes mellitus, hyperlipidemia, hypertension, smoking history)20, laboratory values, bone marrow pathology findings, gene mutations, treatments, adverse events, dates of progression to PPVMF and transformation to acute myeloid leukemia (AML), follow-up dates and survival status. Data requiring manual annotation and verification were collected and hosted on the Research Electronic Data Capture at WCM.
The European LeukemiaNet (ELN) risk category at diagnosis was determined for each patient; high-risk patients included those who were 60 years of age or older and/or had a history of thrombosis at diagnosis; low-risk patients were younger than 60 years and had no prior history of thrombosis at diagnosis13. The rIFNα treatment included recombinant interferon alpha-2a, recombinant interferon alpha-2b, and pegylated interferon alpha-2a. Treatment with other cytoreductive drugs and combinations was classified as “Other”. Other cytoreductive treatment included ruxolitinib, anagrelide, imatinib, dasatinib, busulfan or combinations thereof. PHL-O PV patients, by definition, had no cytoreductive treatment. Treatment toxicity was classified into organ system categories according to Common Terminology Criteria for Adverse Events version 5 21. Progression to PPVMF, established by IWG-MRT criteria, required documenting bone marrow fibrosis (grade 2–3 on 0–3 scale), and two additional criteria including anemia, leukoerythroblastosis, splenomegaly or strictly defined constitutional symptoms22. Transformation to AML was established based on WHO criteria for the diagnosis of blast phase MPN19.
Design and statistical analysis
An intention to treat (ITT) analysis of MFS and OS classified patients to rIFNα, HU, PHL-O, or other cytoreductive regimens. Patients were classified by the first cytoreductive treatment they received for at least one year. Treatment was PHL-O if patients did not receive cytoreductive drugs. Patients with follow-up of less than one year (n=30) were classified according to the treatment they received for the longest period. Differences in demographic and clinical characteristics between two treatment groups were determined using the chi-squared test for categorical variables, analysis of variance for continuous variables, and two sample t-test or Wilcoxon rank-sum test for mean or median values, respectively. MFS was determined from the date of PV diagnosis to the date of progression to PPVMF, or censored at the date of death or last follow-up. OS was determined from the date of PV diagnosis to the date of death, or censored at the date of last follow-up. Both MFS and OS were estimated using Kaplan-Meier (KM) methods and compared between ITT groups using the log-rank test. This analysis was performed for both low-risk and high-risk patients. The 20-year MFS and OS instead of median MFS and OS is reported because the median was not always reached and was influenced by smaller numbers of patients followed beyond 20 years. The group of patients allocated to other treatment regimens was excluded from the KM analysis because the treatment heterogeneity disables a clinically meaningful interpretation of MFS and OS.
To account for treatment cross-over and duration on the analysis of outcomes, a univariable and multivariable analysis of PPVMF and mortality using Cox proportional-hazards model was performed. Time on treatment variables were included for rIFNα, HU and other cytoreductive treatment. Additional variables including age, sex, thrombosis history, CV risk factors, diagnosis year, and PV bone marrow fibrosis grade were also incorporated.
All p-values were derived from two-tailed statistical analyses; an alpha level of 0.05 was used. The analysis was performed using RStudio software v 1.1.423.
Results:
The automated query identified 480 patients of whom 10 were excluded after manual review because they did not meet specified criteria for the diagnosis of PV. The median age at diagnosis of the 470 PV patients was 54 years (range 20–94); 229 (49%) patients were women; and 208 (44%) were high-risk. The median follow-up was 10 years (range 0–45). The majority of the follow-up was at WCM (78%; 4149 of 5319 patient-years); 204 patients (43%) were followed exclusively at WCM from diagnosis, and only 30 (6.4%) had a single consultation. The majority of patients (n=290, 62%) were under the care of four senior hematologists at WCM.
The first cytoreductive treatment was rIFNα in 93 patients (20%), HU in 189 (40%) and other drugs in 55 (12%); the median time to the cytoreductive treatment, from diagnosis, was 1 year (range 0–15), 1 year (range 0–26) and 1 year (range 0–30) respectively (Table 1). One hundred thirty-three patients were treated with PHL-O (28%). The treatment groups rIFNα, HU and PHL-O differed by age at diagnosis: median age was 50, 58 and 54 years respectively (p <0.001); ELN risk score: high-risk comprised 24%, 56% and 42% respectively (p<0.001); and cardiovascular (CV) risk factors: present in 27%, 26% and 10% respectively (p=0.002) (Table 1). Allocation to treatment with rIFNα, HU or PHL-O was physician-biased (p < 0.001).
Table 1:
Demographics and clinical characteristics of PV patients
| Variable | Overall (n = 470) | PHL (n = 133) | IFN (n = 93) | HU (n = 189) | Other (n = 55) | P |
|---|---|---|---|---|---|---|
| Age (years), median (range) | 54 (20–94) | 54 (20–94) | 50 (26–82) | 58 (22–84) | 52 (20–91) | <0.001 * |
| Sex: female, n (%) | 229 (49) | 55 (41) | 43 (46) | 102 (54) | 29 (53) | 0.14 |
| Race, n evaluable = 404, n (%) | 0.13 | |||||
| White | 361 (86) | 99 (88) | 81 (93) | 141 (82) | 40 (83) | |
| Asian, Black or Hispanic | 43 (10) | 11 (10) | 4 (5) | 24 (14) | 4 (8) | |
| Other minority | 15 (4) | 3 (3) | 2 (2) | 6 (4) | 4 (8) | |
| Cardiovascular (CV) risk factors, n (%) | 101 (21) | 13 (10) | 25 (27) | 50 (26) | 13 (24) | 0.002 * |
| Thrombosis history, n (%) | 65 (14) | 15 (11) | 11 (12) | 31 (16) | 8 (15) | 0.55 |
| ELN risk: high, n (%) | 208 (44) | 56 (42) | 22 (24) | 105 (56) | 25 (45) | <0.001 * |
| JAK2V617F positive, n evaluable = 361, n (%) | 355 (98) | 76 (96) | 83 (99) | 151 (99) | 45 (100) | 0.36 |
| Cytogenetic risk, n evaluable = 231, n (%) | 0.04 | |||||
| Low | 207 (90) | 46 (88) | 60 (95) | 79 (85) | 22 (96) | |
| Intermediate | 18 (8) | 2 (4) | 3 (5) | 12 (13) | 1 (4) | |
| High | 6 (3) | 4 (8) | 0 (0) | 2 (2) | 0 (0) | |
| Co-occurrent mutations present, n evaluable = 105, n (%) | 51 (49) | 12 (55) | 11 (37) | 20 (62) | 8 (38) | 0.14 |
| Diagnosis date (year), median (range) | 2005 (1966–2019) | 2008 (1986–2019) | 2005 (1982–2019) | 2004 (1966–2018) | 2004 (1986–2018) | 0.009 * |
| Follow-up duration (years), median (range) | 10 (0–45) | 5 (0–32) | 13 (1–35) | 11 (0–45) | 9 (1–32) | <0.001 |
| Time to cytoreductive treatment (years), median (range) | 1 (0–30) | NA | 1 (0–15) | 1 (0–26) | 1 (0–30) | 0.21 |
| Treatment start (year), median (range) | 2008 (1982–2019) | 2008 (1986–2019) | 2009 (1982–2019) | 2007 (1983–2019) | 2008 (1997–2019) | 0.39 |
| Treatment duration (years), median (range) | 4 (0–28) | 4 (0–28) | 5 (1–26) | 5 (0–26) | 3 (1–15) | 0.005 * |
p-values comparing IFN to PHL, IFN to HU, HU to PHL by:
Age = 0.006, <0.001, 0.01
ELN risk = 0.006, <0.001, 0.02
CV risk factors = 0.001, ns, <0.001
Diagnosis date = 0.01, ns, 0.001
Treatment duration = ns, ns, ns
Of the low-risk group (n=262), treatment was rIFNα, HU or PHL-O in 71 (27%), 84 (32%) and 77 (29%) patients respectively. Indications for initiating rIFNα or HU treatment in low-risk patients, when present, included the occurrence of thrombosis and/or aging over 60 in 12 (17%) and 20 (24%) respectively; thrombocytosis and/or leukocytosis in 12 (17%) and 24 (24%) respectively; symptoms and splenomegaly in 17 (24%) and 14 (17%) respectively; and high phlebotomy requirements in 10 (14%) and 7 (8.3%) respectively. Of the high-risk group (n=208), treatment was rIFNα, HU or PHL-O in 22 (11%), 105 (50%) and 56 (27%) respectively. The high-risk patients treated with PHL-O were not on cytoreductive therapy because of physician’s choice (n=24, 43%), before the establishment of risk-adapted treatment guidelines; patient’s choice (n=6, 11%); patient’s intolerance to single or multiple attempts at treatment with a cytoreductive drug (n=6, 11%); and a short duration of ≤ 1 year from diagnosis (n=18, 32%) during which decision-sharing for cytoreductive treatment was ongoing.
The median MFS and OS for all patients was 23.8 and 26.3 years respectively and the 20-year MFS and OS was 61% and 69% respectively (Figure 1A, B). In low-risk patients; 20-year MFS for all was 66%, and for rIFNα, HU, and PHL-O was 84%, 65% and 55% respectively (p<0.001); 20-year OS for all was 87%, and for rIFNα, HU, and PHL-O was 100%, 85% and 80% respectively (p=0.44) (Figure 1C–F). In high-risk patients; 20-year MFS for all was 47%, and for rIFNα, HU, and PHL-O was 89%, 41% and 36% respectively (p=0.19); and 20-year OS for all was 34%, and for rIFNα, HU, and PHL-O was 66%, 40%, 14% respectively (p=0.016) (Figure 1C, D, G, and H).
Figure 1: Myelofibrosis-free survival (MFS) and overall survival (OS) of PV patients.
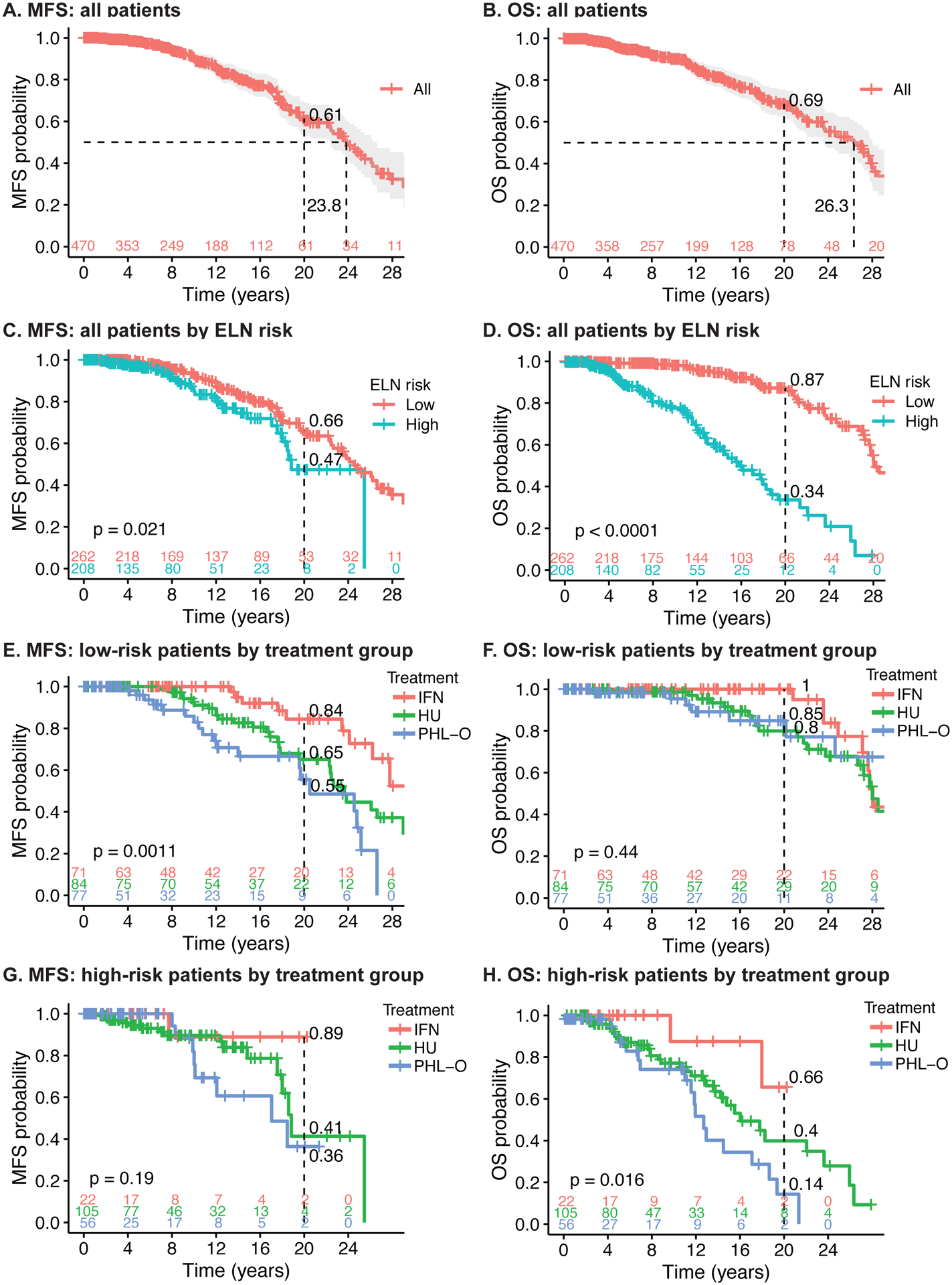
A MFS of all patients, B OS of all patients, C MFS of all patients by ELN risk, D OS of all patients by ELN risk, E MFS of low-risk patients by treatment group, F OS of low-risk patients by treatment group, G MFS of high-risk patients by treatment group, H OS of high-risk patients by treatment group.
The degree of marrow fibrosis was graded in 526 bone marrow biopsies from 242 patients. At 8–14 years from the initial biopsy, the percentage of patients with MF2–3 fibrosis was significantly lower for the rIFNα group compared to the HU and PHL-O groups (Figure 2).
Figure 2: Fibrosis grade MF2-3 over time on treatment.
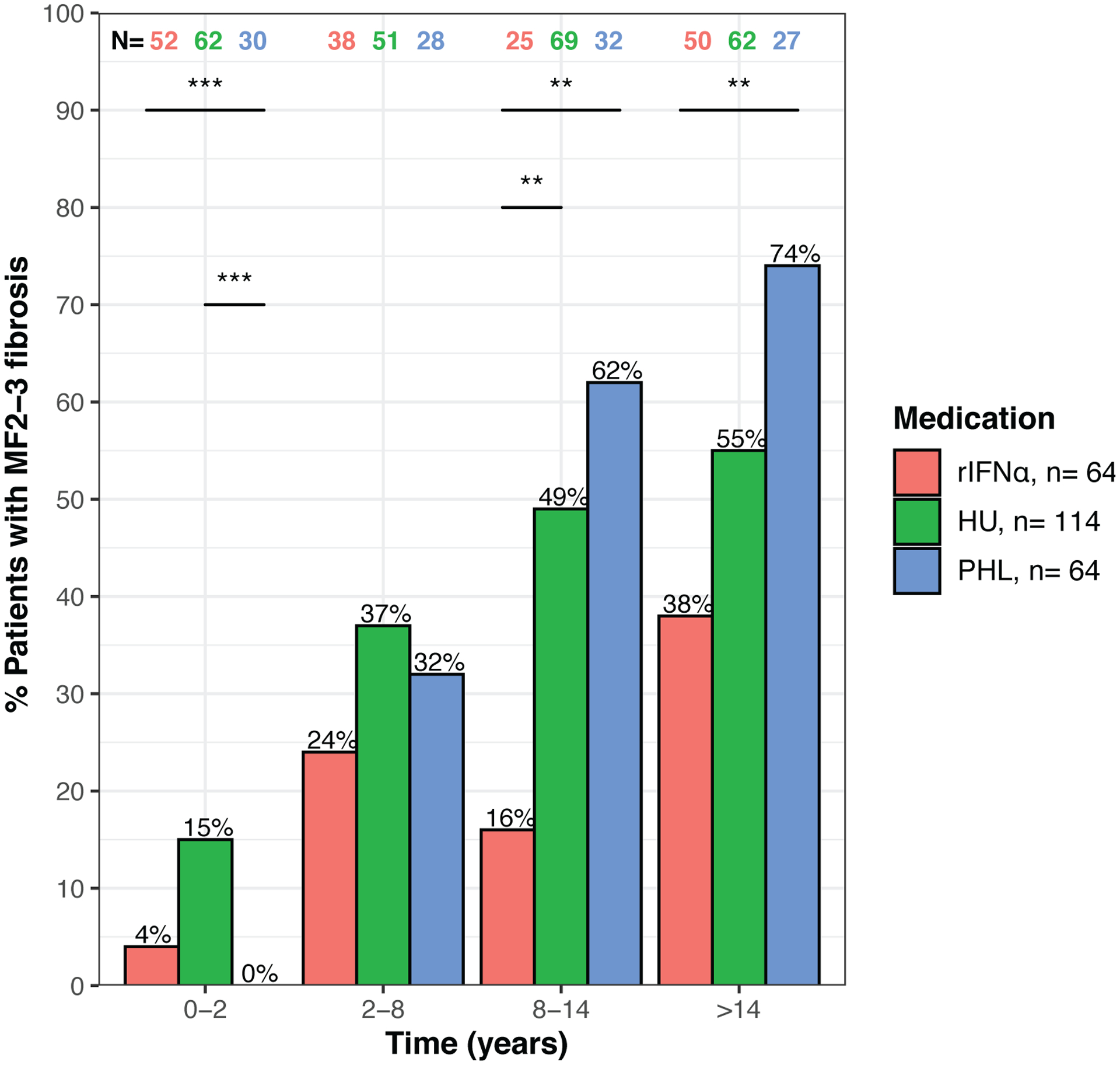
significant differences by chi-squared test are noted at p<0.01(**) and p<0.001 (***)).
Treatment cross-over occurred in 18 of 93 (19%) rIFNα treated patients and 50 of 189 (26%) HU treated patients (Figure 3). Excluding patients on “Other” treatment, 199 patients received rIFNα at any time for a cumulative duration of 1137 patient-years, whereas 285 patient received HU at any time for cumulative duration of 1671 years. To account for treatment cross-over and duration in the analysis of PPVMF and mortality, time on treatment univariable and multivariable analyses were performed. The multivariable analysis demonstrated that longer time on rIFNα was associated with a lower risk of PPVMF (HR 0.91, 95%CI 0.87–0.95, p<0.001) and all-cause mortality (HR 0.94, 95%CI 0.9–0.99, p=0.012) independent of age, sex, thrombosis history, CV risk factors, diagnosis year and PV fibrosis grade (Tables 2–3). Longer time on HU was not associated with a significantly lower risk of PPVMF or all-cause mortality. The multivariable analysis also showed that older age of diagnosis was associated with increased mortality (HR 1.1, 95%CI 1.07–1.12, p<0.001) and women had decreased mortality (HR 0.54, 95%CI 0.36–0.83, p=0.005).
Figure 3: Cytoreductive treatment timeline of PV study population ordered by first treatment and its duration.
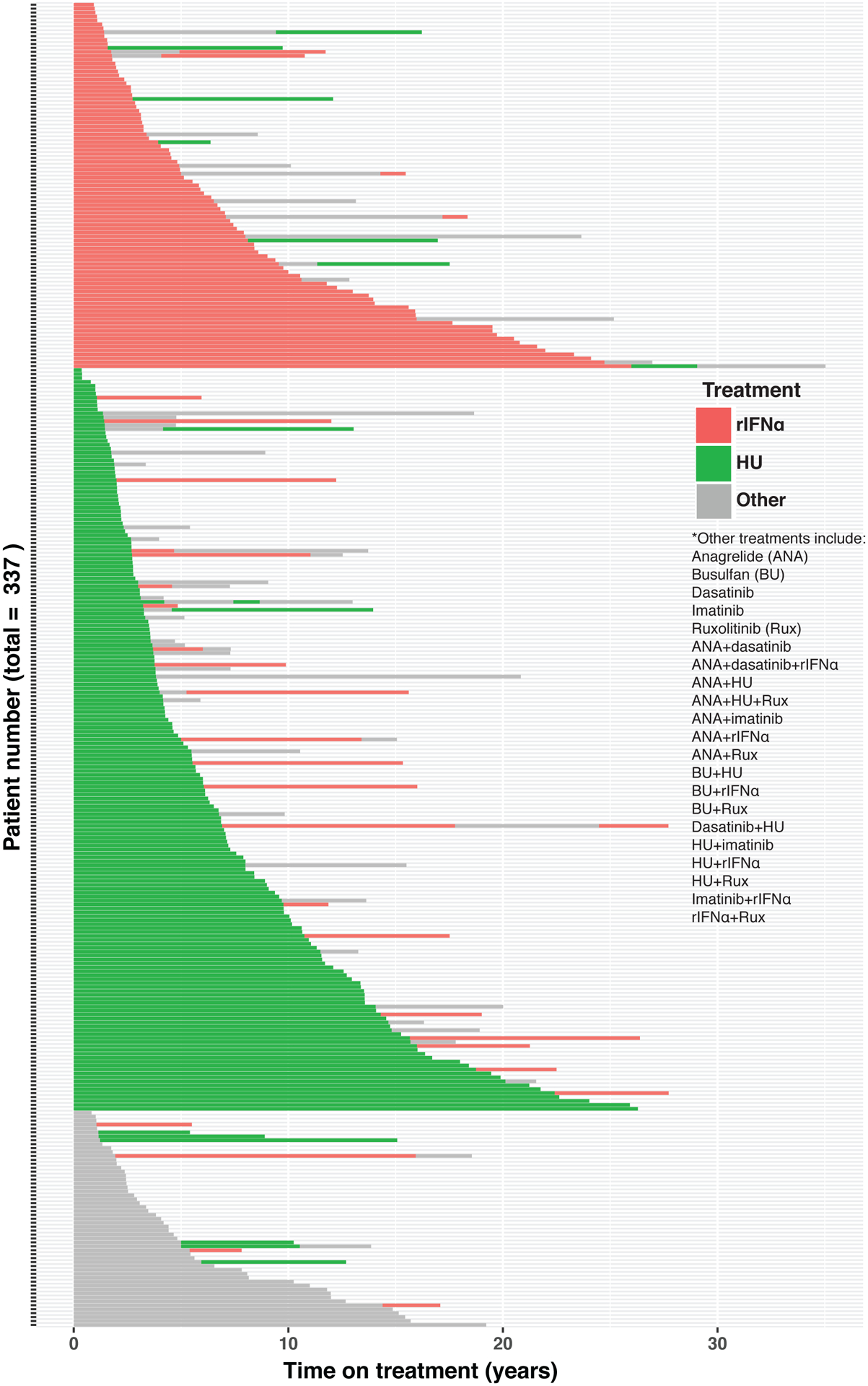
The results are censored at the earlier of last follow up or transformation to PPVMF.
Table 2:
Univariable and Multivariable analyses of PPVMF risk
| Variable | N | Univariable HR (95% CI, p-value) | Multivariable HR (95% CI, p-value) | ||
|---|---|---|---|---|---|
| Age (years) | 470 | 1.03 (1.01–1.05, p=0.001) | 1.01 (0.99–1.03, p=0.462) | ||
| Sex | Male | 241 | - | - | |
| Female | 229 | 0.83 (0.56–1.24, p=0.370) | 0.70 (0.46–1.07, p=0.102) | ||
| Thrombosis history | None | 405 | - | - | |
| Present | 65 | 1.67 (0.92–3.02, p=0.093) | 1.18 (0.63–2.20, p=0.605) | ||
| CV risk factors | None | 369 | - | - | |
| Present | 102 | 0.72 (0.43–1.20, p=0.211) | 0.81 (0.47–1.38, p=0.437) | ||
| Diagnosis date (year) | 470 | 1.12 (1.07–1.16, p<0.001) | 1.11 (1.06–1.15, p<0.001) | ||
| rIFNα (time on therapy) | 470 | 0.92 (0.88–0.96, p<0.001) | 0.91 (0.87–0.95, p<0.001) | ||
| HU (time on therapy) | 470 | 0.98 (0.96–1.01, p=0.215) | 0.98 (0.95–1.01, p=0.250) | ||
| Other (time on therapy) | 470 | 1.00 (0.95–1.05, p=0.979) | 0.99 (0.94–1.05, p=0.810) | ||
| Marrow fibrosis, grade | 0 | 68 | - | - | |
| >=1 | 369 | 1.48 (0.85–2.57, p=0.167) | 1.40 (0.76–2.59, p=0.282) | ||
| - | NA | 33 | - | - | |
Table 3:
Univariable and Multivariable analyses of all-cause mortality risk
| Variable | N | Univariable HR (95% CI, p-value) | Multivariable HR (95% CI, p-value) | |
|---|---|---|---|---|
| Age (years) | 470 | 1.11 (1.09–1.13, p<0.001) | 1.10 (1.07–1.12, p<0.001) | |
| Sex | Male | 241 | - | - |
| Female | 229 | 0.68 (0.45–1.01, p=0.055) | 0.54 (0.36–0.83, p=0.005) | |
| Thrombosis history | None | 405 | - | - |
| Present | 65 | 1.64 (0.94–2.87, p=0.080) | 1.12 (0.61–2.04, p=0.713) | |
| CV risk factors | None | 369 | - | - |
| Present | 102 | 1.28 (0.83–1.97, p=0.265) | 1.06 (0.67–1.68, p=0.794) | |
| Diagnosis date (year) | 470 | 1.14 (1.10–1.19, p<0.001) | 1.07 (1.02–1.13, p=0.007) | |
| rIFNα (time on therapy) | 470 | 0.92 (0.88–0.96, p<0.001) | 0.94 (0.90–0.99, p=0.012) | |
| HU (time on therapy) | 470 | 0.99 (0.96–1.01, p=0.288) | 0.97 (0.94–1.00, p=0.074) | |
| Other (time on therapy) | 470 | 0.98 (0.93–1.04, p=0.531) | 1.00 (0.94–1.06, p=0.892) | |
| Marrow fibrosis, grade | 0 | 68 | - | - |
| >= 1 | 369 | 2.21 (1.14–4.30, p=0.019) | 1.75 (0.84–3.63, p=0.133) | |
| - | NA | 33 | - | - |
Transformation to AML occurred in 18 patients (4%) at a rate of 0.34 per 100 patient-years, but the relative infrequency of this event was insufficient to identify significant group differences among rIFNα (n=2, 2%), HU (n=7, 4%), and PHL-O patients (n=6, 5%).
Of 199 patients who were treated with rIFNα at any time, 25 (13%) discontinued treatment due to toxicity over a follow-up of 1137 patient-years (discontinuation rate of 2.2 per 100 patient-years) (Table 4). Of 285 patients treated with HU at any time, 46 (16%) discontinued treatment due to toxicity over 1671 patient-years of follow-up (incidence rate of 2.8 per 100 patient-years). The most common symptoms limiting rIFNα treatment were musculoskeletal and constitutional symptoms, whereas the treatment limiting toxicities of HU were primarily hematologic, gastrointestinal, and skin and soft tissue (Table 4).
Table 4:
Toxicities leading to discontinuation of treatment
| rIFNα (n = 199) | HU(n = 285) | |
|---|---|---|
| Time on therapy (patient-years) | 1137 | 1671 |
| Organ system adverse events, n (%) | ||
| Hematologic | ||
| Anemia | 2 (1.0) | 3 (1.0) |
| Neutropenia | 0 (0.0) | 1 (0.4) |
| Thrombocytopenia | 1 (0.5) | 7 (2.5) |
| Other | 2 (1.0) | 7 (2.5) |
| Allergy & Immunology | ||
| Allergic response | 2 (1.0) | 1 (0.4) |
| Autoimmune disorder | 2 (1.0) | 0 (0.0) |
| Musculoskeletal | ||
| Arthralgia | 4 (2.0) | 0 (0.0) |
| Myalgia | 3 (1.5) | 0 (0.0) |
| Other | 1 (0.5) | 2 (1.0) |
| Gastrointestinal | ||
| Mucositis oral | 0 (0.0) | 3 (0.7) |
| Nausea & Vomiting | 0 (0.0) | 5 (1.8) |
| Other | 1 (0.5) | 2 (0.7) |
| Neuropsychiatric | ||
| Agitation | 1 (0.5) | 0 (0.0) |
| Depression | 1 (0.5) | 0 (0.0) |
| Peripheral neuropathy | 3 (1.5) | 4 (1.4) |
| Other | 1 (0.5) | 1 (0.4) |
| Constitutional | ||
| Fatigue & Malaise | 5 (2.5) | 3 (0.7) |
| Fever | 1 (0.5) | 1 (0.4) |
| Other | 2 (1.0) | 1 (0.4) |
| Cardiopulmonary | ||
| Cardiomyopathy | 1 (0.5) | 0 (0.0) |
| Heart failure | 1 (0.5) | 0 (0.0) |
| Pericarditis | 1 (0.5) | 0 (0.0) |
| Skin & soft tissue | ||
| Rash | 1 (0.5) | 1 (0.4) |
| Skin ulceration | 1 (0.5) | 9 (3.2) |
| Other | 0 (0.0) | 6 (2.1) |
| Total number of events, n | 37 | 57 |
| Total of patients, n (%) | 25 (13) | 46 (16) |
Discussion
Randomized controlled trials comparing rIFNα to HU in high-risk PV, and rIFNα to PHL-O in low-risk PV are ongoing and some have been recently published14–16,23. However, the follow-up of patients on these clinical trials such as the DALIAH14, PROUD-PV and CONT- PV15, MPD-RC 11216, and low-PV23 remains limited to less than 5 years. This does not yet permit a proper assessment of long-term outcomes such as PPVMF (a poor prognostic sign1) and mortality. Moreover, clinical endpoints such as hematologic and molecular responses have not been validated as surrogates of MFS or OS. Retrospective studies, although non-randomized, may have the large number of patients and long follow-up necessary to determine whether rIFNα improves MFS or OS6,24.
This study has unique strengths despite its inherent limitations as a non-randomized retrospective study. First, rigorous diagnostic criteria18,19 were used for inclusion, assuring uniformity of patient selection. These were based upon isotopic techniques, marrow biopsy, and molecular testing as soon as it became available25,26. Second, the majority of the follow-up duration was at WCM (78%); 204 patients (43%) were followed exclusively at WCM from diagnosis and only 30 (6.4%) had a single consultation. The majority of patients (62%) were under the care of only four senior hematologists. Third, our center’s historic introduction and adoption of rIFNα for long-term treatment of PV4,27,28 enabled a powered analysis of survival outcomes with rIFNα. Fourth, we demonstrate that long-term rIFNα treatment may reduce MF risk and may prolong survival in PV patients compared to HU and PHL-O.
In our study of 470 PV patients, long term follow-up enabled analysis of MFS and OS in ELN defined low-risk and high-risk PV patients based on treatment (rIFNα, HU and PHL-O). A relatively large proportion of low-risk PV patients received cytoreductive drugs and a relatively large proportion of high-risk PV patients were treated with PHL-O because there was no risk-adapted treatment standard prior to the establishment of the 2011 ELN recommendations29. In addition to physician’s opinion, indications for starting cytoreductive treatment in low-risk patients included constitutional symptoms, extreme thrombocytosis, and high phlebotomy requirements. Reasons for high-risk patients to receive PHL-O treatment included physician’s opinion, patient’s choice, and prior intolerance to cytoreductive drugs.
This study demonstrates, for the first time, that initial treatment with rIFNα of low-risk PV is associated with a significantly higher MFS when compared to PHL-O or HU (log-rank p=0.0011). High-risk PV patients treated initially with rIFNα (n=22), a minority, had a higher OS compared to HU or PHL-O (log-rank p=0.016). The results of the ITT analysis support initial treatment with rIFNα over the standard of care, PHL-O, for low-risk patients, and over the standard of care, HU, for high-risk patients. However, there are limitations to this analysis that we acknowledge. Treatment allocation was heavily influenced by the treating physician’s opinion. Because this is a non-randomized study, there is heterogeneity among treatment groups pertaining mainly to age, and therefore ELN risk score. Another limitation of the ITT analysis is that it does not account for the effect of crossover to subsequent treatment. Indeed, there was a higher rate of crossover from HU to rIFNα or other treatment (26%) than from rIFNα to HU or other treatment (19%) (Figure 3). The proportion of HU patients later receiving rIFNα was higher than the proportion of rIFNα patients later receiving HU, thereby probably underestimating the survival benefit of rIFNα over HU in ITT analysis.
Multivariable analyses of PPVMF and mortality using time on treatment variables were performed to address some of the limitations of ITT analysis. The analyses account for covariable differences between treatment groups, such as age. They also account for the initial and subsequent duration of treatment. The results show that rIFNα was associated with a 9% PPVMF risk reduction per year of treatment (HR 0.91, p<0.001) (Table 2) and a 6% mortality risk reduction per year of treatment (HR 0.94, p=0.012) (Table 3), independent of other risk factors. Inclusion of age and sex in the multivariable model was important because both older age and male sex in our study were associated with increased mortality, which is consistent with other studies30–34. Unlike rIFNα, HU or other treatment was not associated with a lower risk of PPVMF or all-cause mortality.
When selecting treatments for long-term management of PV, additional long-term considerations include treatment toxicity and risk of AML transformation. Both the incidence of toxicity and of AML were not significantly different between treatments. The low incidence of treatment toxicity and of AML is possibly the consequence of the younger age of patients and/or reflects the close follow-up and monitoring of drug dose and duration expected at a comprehensive MPN center.
This study challenges the current risk-adapted treatment guidelines recommending PHL-O for low-risk patients and favoring HU over rIFNα for high-risk patients12,13. However, we emphasize that a definitive recommendation for treating low-risk patients with rIFNα cannot be made without a randomized clinical trial, which in fact is currently ongoing23. An interim analysis of low-PV clinical trial comparing rIFNα (as ropeginterferon) to PHL-O has shown a superior short-term outcome with rIFNα23. Similarly, a definitive recommendation for treating high-risk patients with rIFNα over HU cannot be made until the results of ongoing clinical trials mature with longer follow-up, although some of these studies are already demonstrate evidence of superiority of rIFNα over HU14–16.
In conclusion, the results of this study may support the use of rIFNα in the early treatment of well-defined PV in order to prevent PPVMF and possibly prolong survival. Randomized clinical trials are required to confirm these findings.
Acknowledgements:
We acknowledge Paul J Christos, PhD for his review of the statistical methodology and results.
This study was supported by grants from the David L. Johns Family of the Cancer Research & Treatment Fund (CR&T), the Myeloproliferative Neoplasms Research Foundation (MPN-RF), the Clinical & Translational Science Center (CTSC) of Weill Cornell and the National Center for Advancing Translational Sciences (NCATS) (grant # 1 UL1 TR002384-04)
Data sharing statement
Data will not be publicly available due to HIPAA regulations. Similarly, individual patient data will not be shared. However, a request for sharing de-identified data can be made to the corresponding author and approval will be sought from the IRB.
Footnotes
presented in part at the annual meetings of the American Society of Hematology in Atlanta, GA, 2018; Orlando, FL, 2019, and San Diego, CA, 2020.
Conflicts of interest:
GAZ – Consultancy, PharmaEssentia. SK, TC, GH, DJ, NS, CS, EKR, and JMS – No conflicts of interest to disclose. RTS – Speaker bureau and consultancy, PharmaEssentia.
References:
- 1.Passamonti F, Giorgino T, Mora B, Guglielmelli P, Rumi E, Maffioli M et al. A clinical-molecular prognostic model to predict survival in patients with post polycythemia vera and post essential thrombocythemia myelofibrosis. Leukemia 2017; 31: 2726–2731. [DOI] [PubMed] [Google Scholar]
- 2.Spivak JL. Myeloproliferative Neoplasms. N Engl J Med 2017; 376: 2168–2181. [DOI] [PubMed] [Google Scholar]
- 3.Masarova L, Yin CC, Cortes JE, Konopleva M, Borthakur G, Newberry KJ et al. Histomorphological responses after therapy with pegylated interferon α−2a in patients with essential thrombocythemia (ET) and polycythemia vera (PV). Exp Hematol Oncol 2017; 6: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer 2006; 107: 451–8. [DOI] [PubMed] [Google Scholar]
- 5.Kiladjian J-J, Cassinat B, Chevret S, Turlure P, Cambier N, Roussel M et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood 2008; 112: 3065–3072. [DOI] [PubMed] [Google Scholar]
- 6.Silver RT, Hasselbalch HC. Optimal therapy for polycythemia vera and essential thrombocythemia: Preferred use of interferon therapy based on phase 2 trials. Hematology 2016; 21: 387–391. [DOI] [PubMed] [Google Scholar]
- 7.Tashi T, Swierczek S, Kim SJ, Salama ME, Song J, Heikal N et al. Pegylated interferon Alfa-2a and hydroxyurea in polycythemia vera and essential thrombocythemia: differential cellular and molecular responses. Leukemia 2018; 32: 1830–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacchi S, Leoni P, Liberati M, Riccardi A, Tabilio A, Tartoni P et al. A prospective comparison between treatment with phlebotomy alone and with interferon-alpha in patients with polycythemia vera. Ann Hematol 1994; 68: 247–250. [DOI] [PubMed] [Google Scholar]
- 9.Silver RT, Vandris K, Goldman JJ. Recombinant interferon-α may retard progression of early primary myelofibrosis: A preliminary report. Blood 2011; 117: 6669–6672. [DOI] [PubMed] [Google Scholar]
- 10.Silver RT, Kiladjian JJ, Hasselbalch HC. Interferon and the treatment of polycythemia vera, essential thrombocythemia and myelofibrosis. Expert Rev. Hematol 2013; 6: 49–58. [DOI] [PubMed] [Google Scholar]
- 11.Silver RT, Barel AC, Lascu E, Ritchie EK, Roboz GJ, Christos PJ et al. The effect of initial molecular profile on response to recombinant interferon-α (rIFNα) treatment in early myelofibrosis. Cancer 2017; 123: 2680–2687. [DOI] [PubMed] [Google Scholar]
- 12.Bose P, Deininger MW, Dunbar A, George T, Gojo I, Gundabolu K et al. NCCN Guidelines Panel Disclosures NCCN Guidelines Version 1.2020 Myeloproliferative Neoplasms 2020.
- 13.Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia 2018; 32: 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knudsen TA, Hansen DL, Ocias LF, Bjerrum OW, Brabrand M, El Fassi D et al. Long-Term Efficacy and Safety of Recombinant Interferon Alpha-2 Vs. Hydroxyurea in Polycythemia Vera: Preliminary Results from the Three-Year Analysis of the Daliah Trial - a Randomized Controlled Phase III Clinical Trial. Blood 2018; 132: 580–580. [Google Scholar]
- 15.Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyed M et al. Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol 2020; 7: e196–e208. [DOI] [PubMed] [Google Scholar]
- 16.Mascarenhas J, Kosiorek HE, Prchal JT, Rambaldi A, Berenzon D, Yacoub A et al. Results of the Myeloproliferative Neoplasms - Research Consortium (MPN-RC) 112 Randomized Trial of Pegylated Interferon Alfa-2a (PEG) Versus Hydroxyurea (HU) Therapy for the Treatment of High Risk Polycythemia Vera (PV) and High Risk Essential Thrombocythemia (ET). Blood 2018; 132: 577–577.29954751 [Google Scholar]
- 17.Berlin NI. Diagnosis and classification of the polycythemias. Semin Hematol 1975; 12: 339–51. [PubMed] [Google Scholar]
- 18.Silver RT, Chow W, Orazi A, Arles SP, Goldsmith SJ. Evaluation of WHO criteria for diagnosis of polycythemia vera: a prospective analysis. Blood 2013; 122: 1881–6. [DOI] [PubMed] [Google Scholar]
- 19.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127: 2391–405. [DOI] [PubMed] [Google Scholar]
- 20.Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D et al. Cardiovascular Events and Intensity of Treatment in Polycythemia Vera. N Engl J Med 2013; 368: 22–33. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Institute N. Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events (CTCAE) v5.0 2017https://www.meddra.org/ (accessed 18 May 2019).
- 22.Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the international working group for myelofibrosis research and treatment. Leukemia 2008; 22: 437–438. [DOI] [PubMed] [Google Scholar]
- 23.Barbui T, Vannucchi AM, De Stefano V, Masciulli A, Carobbio A, Ghirardi A et al. Phase II randomized clinical trial comparing ropeginterferon versus phlebotomy in low-risk patients with polycythemia vera. results of the pre-planned interim analysis. Hematologica; 303391; LB.https://library.ehaweb.org/eha/2020/eha25th/303391/tiziano.barbui.phase.ii.randomized.clinical.trial.comparing.ropeginterferon.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dlb2602 (accessed 5 Jul 2020). [Google Scholar]
- 24.Hasselbalch HC, Silver RT. Interferon in polycythemia vera and related neoplasms. Can it become the treatment of choice without a randomized trial? Expert Rev. Hematol 2015; 8: 439–445. [DOI] [PubMed] [Google Scholar]
- 25.Verstovsek S, Silver RT, Cross NCP, Tefferi A. JAK2V617F mutational frequency in polycythemia vera: 100%, >90%, less? Leukemia 2006; 20: 2067. [DOI] [PubMed] [Google Scholar]
- 26.Wang YL, Vandris K, Jones A, Cross NCP, Christos P, Adriano F et al. JAK2 Mutations are present in all cases of polycythemia vera. Leukemia. 2008; 22: 1289. [DOI] [PubMed] [Google Scholar]
- 27.Silver RT. Recombinant interferon-alpha for treatment of polycythaemia vera. Lancet (London, England) 1988; 2: 403. [DOI] [PubMed] [Google Scholar]
- 28.Silver RT. Interferon-α2b: A new treatment for polycythemia vera. Ann Intern Med 1993; 119: 1091–1092. [DOI] [PubMed] [Google Scholar]
- 29.Barbui T, Barosi G, Birgegard G, Cervantes F, Finazzi G, Griesshammer M et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European leukemiaNet. J. Clin. Oncol 2011; 29: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srour SA, Devesa SS, Morton LM, Check DP, Curtis RE, Linet MS et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001–12. Br J Haematol 2016; 174: 382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szuber N, Vallapureddy RR, Penna D, Lasho TL, Finke C, Hanson CA et al. Myeloproliferative neoplasms in the young: Mayo Clinic experience with 361 patients age 40 years or younger. Am J Hematol 2018; 93: 1474–1484. [DOI] [PubMed] [Google Scholar]
- 32.Karantanos T, Chaturvedi S, Braunstein EM, Spivak J, Resar L, Karanika S et al. Sex determines the presentation and outcomes in MPN and is related to sex-specific differences in the mutational burden. Blood Adv 2020; 4: 2567–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol 2005; 23: 2224–2232. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez-Larr an A, Kerguelen A, Hern andez-Boluda JC, erez-Encinas MP, Ferrer-Marín F, arez AB et al. Frequency and prognostic value of resistance/intolerance to hydroxycarbamide in 890 patients with polycythaemia vera. Br J Haematol 2016; 172: 786–793. [DOI] [PubMed] [Google Scholar]


