Abstract
The World Health Organization (WHO) recommends tuberculosis preventive treatment (TPT) in people with HIV (PWH), yet implementation remains poor, especially in rural communities. We examined factors influencing TPT initiation in PWH on antiretroviral therapy (ART) in rural South Africa using the Promoting Action on Research Implementation in Health Services (PARiHS) framework to identify contextual factors and facilitation strategies to successfully implement TPT. Patient and clinical factors were extracted from medical records at two primary healthcare clinics (PHCs). Among 455 individuals eligible for TPT, only 263 (57.8%) initiated TPT. Patient-level characteristics (older age and symptoms of fever or weight loss) were significantly associated with TPT initiation in bivariate analysis, but PHC was the only independent correlate of TPT initiation (aOR: 2.24; 95% CI: 1.49–3.38). Clinic-level factors are crucial targets for implementing TPT to reduce the burden of HIV-associated TB. Gaps in knowledge of HCW, staff shortages, and non-integrated HIV/TB services were identified barriers to TPT implementation. Evidence-based strategies for facilitating TPT implementation that might be under-prioritized include ongoing reprioritization, expanding training for primary care providers, and quality improvement strategies (organizational changes, multidisciplinary teams, and monitoring and feedback). Addressing contextual barriers through these facilitation strategies may improve future TPT implementation in this setting.
Keywords: Implementation, HIV, rural health, tuberculosis, prevention
INTRODUCTION
Globally, tuberculosis (TB) is the leading cause of death for people with HIV (PWH) (World Health Organization, 2020). In 2019, the global incidence of TB increased to 10 million, and among incident TB cases, 8% were co-infected with HIV. South Africa experiences the highest global burden of HIV/TB co-infection, with an HIV prevalence of 58% among incident TB cases in 2019 (World Health Organization, 2020). The South African province of KwaZulu-Natal is substantially affected by the HIV/TB co-epidemic with 65–85% of newly diagnosed TB patients being co-infected with HIV (Jacobson et al., 2015).
In 2008, the World Health Organization (WHO) endorsed the strategy of Three I’s—Intensive Case Finding, Isoniazid Preventive Therapy (IPT), and Infection Control—to reduce the incidence of HIV-associated TB (World Health Organization, 2008). IPT, independent of antiretroviral therapy (ART), effectively addresses the reservoir of latent TB and reduces the risk of developing active TB by 33–62% and decreases mortality (Badje et al., 2017; Golub et al., 2011; Rangaka et al., 2015; World Health Organization, 2008). Shortly after the WHO endorsed IPT, South Africa innovatively incorporated IPT into its national guidelines in 2010 and has been responsible for the majority of IPT implementation occurring globally (Republic of South Africa Department of Health., 2010; World Health Organization, 2018). Recent studies have offered more options for TB preventive therapy (TPT) that the WHO has endorsed, many of which are shorter in duration than IPT (Sterling et al., 2011; Swindells et al., 2019; World Health Organization, 2020). Despite international and South African guidelines recommending TPT in PWH, implementation has lagged in recent years (Alsdurf et al., 2016; Bristow et al., 2012; Chehab, et al., 2012; Kufa T et al., 2014; Page-Shipp et al., 2012). Few studies have examined the implementation factors that might influence clinical practice and scale-up of TPT in a rural primary care setting using quantitative data. These include strategies such as quality improvement, defined as the enhancement of organizational structures and the incorporation of problem-solving techniques, using multidisciplinary teams to monitor problems and provide feedback (Pantoja et al., 2017).
Implementation Assessment
The Promoting Action on Research Implementation (PARiHS) framework is used to outline the factors leading to successful implementation of evidence-based practices in clinical settings (Harvey et al., 2002; Kitson et al. 1998; Kitson et al., 2008; McCormack et al., 2002; Rycroft-Malone et al., 2004). This framework emphasizes the interplay between evidence, context, and facilitation in the implementation process. Evidence is defined as the research, clinical experience, patient experience, and local data supporting an evidence-based practice. The context focuses on the external factors, including the environment, culture, leadership, and evaluation affecting implementation at the site of interest. Facilitation refers to the processes and players that enable the incorporation of evidence-based policy into clinical practice.
We used this theoretical framework to assess critical elements of the healthcare system affecting the evidence-based practice of TPT. Given the importance of TPT in reducing morbidity and mortality among PWH, we sought to examine the state of TPT implementation after incorporation into national policy by determining the frequency of TPT initiation among PWH receiving ART and identifying correlates of TPT initiation in KwaZulu-Natal, South Africa. Then, we explored the contextual factors potentially impacting TPT implementation and proposed future implementation strategies that may lead to sustained changes in evidence-based practices in the future (Stetler et al., 2011). Findings from this study will support future implementation science research and policy decisions related to TPT in rural, resource-limited, high burden HIV/TB settings.
DESIGN
Study Setting
KwaZulu-Natal has one of the worst HIV/TB co-epidemics in South Africa with a TB incidence of 1100/100,000, antenatal HIV prevalence greater than 30%, and 80% of TB patients co-infected with HIV (Grant et al., 2005; Jacobson et al., 2015). The Msinga subdistrict of KwaZulu-Natal is home to 180,000 traditional Zulu people. People living in Msinga seek healthcare at the Church of Scotland Hospital (COSH), a 350-bed provincial government district hospital, or at one of 16 nurse-managed primary healthcare clinics (PHCs) (Jacobson et al., 2015). HIV and TB prevention and treatment services are offered through COSH and PHCs. First line ART was comprised of lamivudine, tenofovir, and efavirenz. TB therapy for drug susceptible TB included isoniazid, rifampin, ethambutol, pyrazinamide for the first two months, and isoniazid and rifampin for the subsequent four months.
Study Design/Data Collection
We conducted a retrospective chart review using medical records of HIV patients in two PHCs: Clinics A and B. These clinics were chosen due to their large patient volume relative to other PHCs. All patients ≥18 years old who initiated ART between January 2015 and June 10, 2016 were eligible for inclusion. TPT eligibility was defined as having a negative TB symptom screen, or having a positive symptom screen with active TB excluded through clinical and microbiological evaluation, including sputum culture. Sampling in each clinic differed because of different medical record filing systems; Clinic A’s filing system was organized by birth year while Clinic B’s system was organized alphabetically. Random selection in Clinic A was performed to include 10 patients per birth year, while in Clinic B, 20 patients per letter in the alphabet (last name) were selected. Demographic (age, gender, and pregnancy status) and clinical data (history of previous TB, CD4 count, hepatic transaminases and renal function, and symptoms of TB) were abstracted from medical records. We examined TPT initiation as a binary outcome; follow up data assessing TPT adherence and completion were beyond the scope of this review.
Clinic Description
The 16 PHCs within the Msinga subdistrict are allocated based on regional population and location along main roads. Clinic A was staffed by 6 prescribing nurses and provided clinical care to 4,250 patients monthly. Clinic B was staffed by 5 nurses and provided clinical care to 2,850 patients monthly. The hours of operation were similar at both clinics, operating from 7–5pm five days per week.
Data Analysis
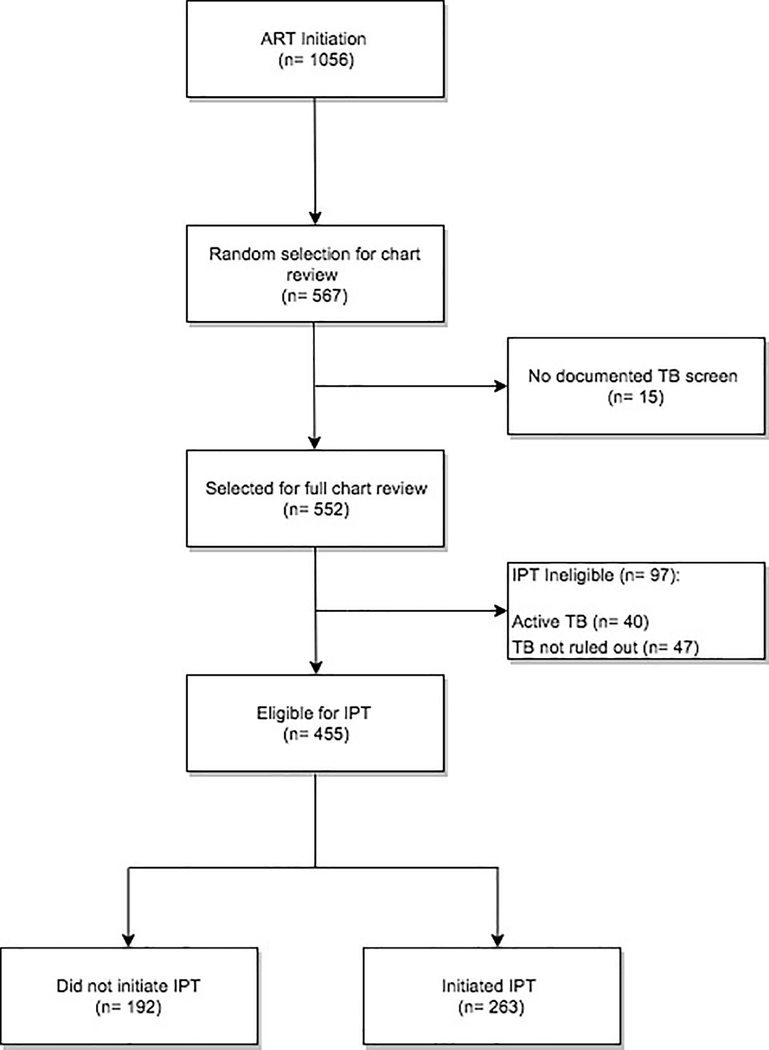
The disposition of ART-initiating HIV patients is depicted in Figure 1, leaving 455 patients eligible for TPT. The primary outcome was TPT initiation, represented as a binary variable. Descriptive statistics were obtained for covariates within demographic and clinical domains. Bivariate analyses were conducted using the Pearson Chi-Square/Fisher’s Exact tests for categorical variables and the Wilcoxon Rank Sum test for continuous variables. Covariates significant at the p= 0.1 level in bivariate analyses were included in the logistic regression multivariable model. Backward selection was used to arrive at the most parsimonious multivariable model. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SAS 9.4.
Figure 1.
Flowchart of obtaining final analytic sample
Ethical Approval
The Institutional Review Boards (IRBs) at Yale University School of Medicine and the South African Medical Association approved the study.
RESULTS
Between January 1, 2015 and June 10, 2016, 1,056 PWH initiated ART at Clinics A and B. Using our random selection strategy, 567 (53.7%) medical records were reviewed. Due to incomplete TB screenings, 15 (2.6%) records were excluded, resulting in a sample of 552 (52.3%) patients who initiated ART. A comparison of the study sample to the total patient population initiating ART showed no significant differences in age (p= 0.72), gender (p= 0.65), and baseline CD4 counts (p= 0.20), suggesting that the study sample is demographically representative of the overall HIV patient population attending Clinics A and B.
Among 552 ART initiators, 395 (71.6%) were female, the median age was 30 years (IQR= 25–38), and 118 (31.1%) were pregnant (Table 1). Overall, 47 (8.9%) patients previously had TB, and 40 (7.9%) patients were currently receiving treatment for active TB at the time of ART initiation. The median CD4 count prior to ART initiation was 317 (IQR= 191–433), and the most recent median CD4 count after ART initiation was 316 (IQR= 181–440). On initial TB screening, 63 (12.4%) patients reported cough, 33 (6.6%) reported fever, 120 (23.4%) reported weight loss, and 47 (9.2%) reported night sweats. Among ART initiators, 305 (55.3%) attended Clinic A and 247 (44.8%) attended Clinic B.
Table 1.
Baseline characteristics of patients that initiated ART between January 2015 and June 10, 2016
| ART Initiators (n= 552) | |
|---|---|
|
| |
| Demographic Characteristics (n, %) | |
| Age a | 30 (25 – 38) |
| Female | 395 (71.6) |
| Pregnant b, c | 118 (31.1) |
| Clinical Characteristics (n, %) b | |
| Prior history of TB | 47 (8.9) |
| Active TB | 40 (7.9) |
| Baseline CD4 (cells/μL) a | 317 (191 – 433) |
| Recent CD4 (cells/μL) a | 316 (181 – 440) |
| ALT (U/L) a | 16 (12 – 25) |
| Creatinine (μmol/L) a | 56 (46 – 68) |
| Hemoglobin (g/dL) a | 11.9 (11 – 13) |
| Initial TB Symptom Screening (n, %) b | |
| Cough | 63 (12.4) |
| Fever | 33 (6.6) |
| Weight Loss | 120 (23.4) |
| Night Sweats | 47 (9.2) |
| Clinic (n, %) | |
| Clinic A | 305 (55.3) |
| Clinic B | 247 (44.8) |
Data are presented as median (interquartile range) where appropriate.
Denominator does not equal total sample size due to missing data.
Denominator includes only females.
Of 552 patients who initiated ART, 455 (82.4%) patients had active TB ruled out and were eligible for TPT and comprised the final analytical sample. Among 97 (17.5%) patients not eligible for TPT, 40 were on treatment for active TB disease and 47 had no documentation ruling out active TB disease (Figure 1). Of those eligible for TPT, however, only 263 (57.8%) initiated TPT. Compared to patients not initiating TPT, those who did (Table 2) were significantly older (p= 0.03) and more likely to be symptomatic with fever (p= 0.01) and weight loss (p= 0.03). From a structural perspective, patients were significantly more likely to initiate TPT at Clinic B rather than Clinic A (p < 0.001).
Table 2.
Characteristics of patients eligible for TPT, by TPT initiation status
| No TPT Initiation (n=192) | TPT Initiation (n=263) | P-value† | |
|---|---|---|---|
|
| |||
| Demographic Characteristics (n, %) | |||
| Age a | 28 (23 – 35) | 29 (24 – 39) | 0.03 |
| Female | 145 (75.5) | 204 (77.6) | 0.61 |
| Male | 47 (24.5) | 59 (22.4) | 0.61 |
| Pregnant b, c | 45 (31.9) | 70 (35.7) | 0.47 |
| Clinical Characteristics (n, %) b | |||
| Prior history of TB | 14 (7.7) | 18 (7.1) | 0.84 |
| Baseline CD4 (cells/μL) a | 340.5 (200 – 426) | 329 (232 – 461) | 0.23 |
| CD4 <200 (cells/μL) | 38 (22.4) | 59 (22.9) | 0.90 |
| CD4 <350 (cells/μL) | 87 (51.1) | 134 (51.9) | 0.88 |
| CD4 <500 (cells/μL) | 151 (88.8) | 219 (84.9) | 0.24 |
| Recent CD4 (cells/μL) a | 345 (207 – 430) | 334.5 (222 – 461) | 0.45 |
| ALT (U/L) a | 15 (10 – 23) | 15 (12 – 22) | 0.32 |
| Creatinine (μmol/L) a | 55.5 (45 – 67) | 53 (45 – 67) | 0.58 |
| Hemoglobin (g/dL) a | 12 (10 – 13) | 12 (11.00 – 13.30) | 0.34 |
| Initial TB Symptom Screening (n, %) b | |||
| Cough | 7 (4.1) | 16 (6.4) | 0.30 |
| Fever | 0 (0.0) | 10 (4.0) | 0.01 |
| Weight Loss | 14 (8.0) | 38 (15.1) | 0.03 |
| Night Sweats | 3 (1.7) | 11 (4.4) | 0.17 |
| Clinic (n, %) | < .001 | ||
| Clinic A | 135 (70.3) | 125 (47.5) | |
| Clinic B | 57 (29.7) | 138 (52.5) | |
Data are presented as median (interquartile range) where appropriate.
Denominator does not equal total sample size due to missing data.
Denominator includes only females.
P-value for analysis of Wilcoxon Rank Sum Test (continuous variable) or χ2test/Fisher’s Exact (categorical variable).
Among those eligible for TPT (Table 3), 195 (42.9%) attended Clinic B and 260 (57.1%) received care at Clinic A. Clinic B differed significantly from Clinic A by having a patient population with higher prevalence of pregnancy (p= 0.03), higher median creatinine levels (p= 0.001) and more symptoms of TB, including cough (p= 0.01), fever (p= 0.03), and weight loss (p < 0.001).
Table 3.
Patient characteristics of individuals eligible for TPT, by clinic
| Clinic A (n= 260) | Clinic B (n= 195) | P-value† | |
|---|---|---|---|
| Demographic Characteristics (n, %) | |||
| Age a | 28 (23 – 37) | 30 (25 – 37) | 0.07 |
| Female | 203 (78.1) | 146 (74.9) | 0.42 |
| Male | 57 (21.9) | 49 (25.1) | 0.42 |
| Pregnant b, c | 59 (29.5) | 56 (40.9) | 0.03 |
| Clinical Characteristics (n, %) b | |||
| History of TB | 20 (7.9) | 12 (6.6) | 0.62 |
| Baseline CD4 (cells/μL) a | 324.5 (220 – 428) | 348.5 (214 – 467) | 0.46 |
| CD4 <200 (cells/μL) | 54 (21.7) | 43 (24.0) | 0.57 |
| CD4 < 350 (cells/μL) | 132 (53.0) | 89 (49.7) | 0.50 |
| CD4 < 500 (cells/μL) | 216 (86.8) | 154 (86.0) | 0.83 |
| Recent CD4 (cells/μL) a | 328 (215 – 438) | 351 (207 – 461) | 0.99 |
| ALT (U/L) a | 15 (11 – 23) | 15 (11 – 21) | 0.701 |
| Creatinine (μmol/L) a | 53 (44 – 63) | 60 (48 – 70) | 0.001 |
| Hemoglobin (g/dL) a | 12 (11 – 13) | 12 (11 – 14) | 0.30 |
| Initial TB Symptom Screening (n, %) b | |||
| Cough | 7 (3.0) | 16 (8.6) | 0.01 |
| Fever | 2 (0.9) | 8 (4.3) | 0.03 |
| Weight Loss | 15 (6.3) | 37 (19.6) | < .001 |
| Night Sweats | 5 (2.1) | 9 (4.8) | 0.13 |
| Initiated IPT (n, %) | 125 (48.1) | 138 (70.8) | < .001 |
Data are presented as median (interquartile range) where appropriate.
Denominator does not equal total sample size due to missing data.
Denominator includes only females.
P-value for analysis of Wilcoxon Rank Sum Test (continuous variable) or χ2 test/Fisher’s Exact (categorical variable).
The multivariable model (Table 4) demonstrates that the clinic attended was the only independent predictor of TPT initiation; after adjusting for age and weight loss, patients attending Clinic B had a 2.24-fold (95% CI: 1.49–3.38) higher odds of initiating TPT compared to patients attending Clinic A.
Table 4.
Correlates of IPT initiation in two South African Primary Care Clinics
| TPT Initiation | ||
|---|---|---|
|
| ||
| Unadjusted OR* (95% CI) | Adjusted OR* (95% CI) | |
| Clinic | ||
| A | 1.00 | 1.00 |
| B | 2.62 (1.77 – 3.87) | 2.24 (1.49 – 3.38) |
| Initial TB Symptom Screening | ||
| Cough | 1.61 (0.65 – 4.01) | -- |
| Fever | N/A | -- |
| Weight Loss | 2.05 (1.08 – 3.91) | 1.56 (0.80 – 3.06) |
| Night Sweats | 2.60 (0.71 – 9.45) | |
| Demographic Characteristics | -- | |
| Age | 1.02 (1.00 – 1.04) | 1.02 (1.00 – 1.04) |
| Female | 1.12 (0.72 – 1.74) | -- |
| Pregnant | 1.19 (0.75 – 1.88) | -- |
| Clinical Characteristics | ||
| History of TB | 0.93 (0.49 – 1.91) | -- |
| Baseline CD4 (cells/μL) | 1.00 (1.00 – 1.003) | -- |
| CD4 <200 (cells/μL) | 1.03 (0.65 – 1.64) | -- |
| CD4 <350 (cells/μL) | 1.03 (0.70 – 1.52) | -- |
| CD4 <500 (cells/μL) | 0.71 (0.39 – 1.27) | -- |
| Recent CD4 (cells/μL) | 1.00 (1.00 – 1.00) | -- |
| ALT (U/L) | 1.00 (0.99 – 1.01) | -- |
| Creatinine (μmol/L) | 1.00 (0.99 – 1.01) | -- |
| Hemoglobin (g/dL) | 1.07 (0.97 – 1.18) | -- |
OR: Odds Ratios
DISCUSSION
We evaluated the implementation of TPT in two rural South African PHCs by determining the proportion of eligible individuals initiating TPT and the correlates of TPT initiation among PWH who initiated ART over an 18-month period. The findings indicate that overall TPT initiation at these PHCs was low, with only 57.8% of eligible patients initiating TPT in our study sample. Low TPT uptake is consistent with data from other settings, as indicated by a recent meta-analysis where only 62.3% (CI: 52–72) of eligible patients initiated an isoniazid-based treatment for latent TB (Alsdurf et al., 2016). Despite robust evidence regarding the efficacy of TPT (Menzies et al., 2018; Sterling et al., 2016; Sterling et al., 2011; Swindells et al., 2019), implementation in TB endemic settings lags. Our analysis demonstrates that poor implementation of TPT, as represented by a low proportion of TPT initiation, is potentially attributable to health system factors that differ by clinic. Though South Africa has been a global leader in integrating TPT into national policy, this evidence suggests that the national guidelines are not being optimally implemented in rural PHCs due to structural factors. Using the PARiHS theoretical framework, which assesses the quality of the evidence, unique characteristics about the context, and the use of active and acceptable facilitation strategies, we further explored the structural factors contributing to the findings observed in this study and how to guide future clinic-level interventions for scaling up TPT.
Evidence:
Systematic reviews and meta-analyses confirm the efficacy of TPT, and both international guidelines (WHO) and the Department of Health of the Republic of South Africa strongly recommend its use in all PWH (Menzies et al., 2018; Republic of South Africa Department of Health., 2010; Sterling et al., 2016; Sterling et al., 2011; Swindells et al., 2019; World Health Organization, 2020).
Context:
Several structural components in the context of the PHCs, related to healthcare provider knowledge, clinic staffing shortages, and degree of HIV/TB service integration, have been found to influence low TPT initiation (Charles et al., 2016; Heaman et al., 2015; Moolphate et al., 2013; Munthali et al., 2015; Singh et al., 2017; Teklay et al., 2016; Wilunda et al., 2017).
The knowledge and attitudes of healthcare workers (HCWs) towards TPT is one structural factor potentially driving our study findings (Aït-Khaled et al., 2009; Lester et al., 2010; Moolphate et al., 2013; Rutherford et al., 2012; Singh et al., 2017). Specific to South Africa, clinic staff have been found to lack knowledge about TPT efficacy and guidelines (Lester et al., 2010). HCWs fear, without supportive data, that TPT, specifically the isoniazid monotherapy evaluated in our study, would contribute to drug resistance (Lester et al., 2010). Concerns about drug resistance are especially relevant in South Africa, where there is a high prevalence of multi-drug and extensively-drug resistant TB (Lester et al., 2010). Apprehension that TPT will contribute to drug resistance has been reported in several other high TB burden settings as well (Aït-Khaled et al., 2009; Lester et al., 2010; Moolphate et al., 2013; Rutherford et al., 2012; World Health Organization, 2013). However, studies show that there is no increased risk of resistance associated with IPT/TPT (Balcells et al., 2006; World Health Organization, 2020; Van Halsema et al., 2010).
Clinic staffing levels may also explain the low rates of TPT initiation observed. Our findings are consistent with other South African studies that have found associations between lower provider-patient ratios and poor HIV outcomes, including unsuppressed viral load and a higher risk of ART default (Charalambous et al., 2016; Vella et al., 2010). In our rural setting, the nurse to patient ratio per month for Clinics A and B were 1:708 and 1:570, respectively. Compared to Clinic B, the lower nurse to patient ratio for Clinic A is a potential explanation for the lower TPT initiation rates observed.
Differential models of HIV and TB service delivery between the PHCs are an important consideration for how TPT is implemented within rural primary care settings. In our study, Clinic B has an integrated model of service delivery compared to Clinic A. At Clinic B, one nurse provides both HIV and TB services, including ART initiation, TB treatment, and TPT; this method of service delivery means patients seeking ART and TPT only need to visit one provider and stand in one queue within the clinic to receive services. At Clinic A, however, patients will receive ART and TPT from different nurses at various locations throughout the clinic. Multiple studies have shown that HIV and TB service integration facilitates access to clinical care, including TPT uptake, whereas lack of integration can lead to poor TB treatment compliance and TPT adherence (Bajunirwe F et al., 2016; Charles et al., 2016; Fox et al., 2016; Gupta et al., 2014; Munro et al., 2007; Suthar et al., 2014; Szakacs et al., 2006). Lack of service integration can inhibit HCWs’ ability to properly coordinate dual HIV and TB clinical care, leading to reduced access to providers, longer waiting times and queues, and medication stock-outs (Bajunirwe F et al., 2016; Munro et al., 2007; Szakacs et al., 2006). Moreover, when services are integrated, there is a common shared knowledge among providers about multiple conditions which increases skills and knowledge about treatment (Sylla et al., 2007). Furthermore, integration of TB-HIV services may result in greater attention to TB symptoms among PWH. Without integrated HIV and TB service delivery, Clinic A may have experienced these challenges that would have undermined coordination of TB clinical care among PWH, including the implementation of TPT. We speculate that clinics with integrated HIV and TB services will improve rates of TPT uptake and adherence.
Similar health system challenges in implementing different preventive services have been reported in other resource poor settings. Perceived low quality of care, distance to health facilities, cost of treatment, perceived judgmental and lack of culturally competent healthcare providers, staff shortages, long wait times, stock-outs of supplies, inadequate space, lack of female providers, and intermittent availability of screening services were barriers to antenatal care and cervical cancer screening (Heaman et al., 2015; Munthali et al., 2015; Wilunda et al., 2017). Whether these challenges are specific to implementing preventive services in a resource poor setting or are broadly applicable to any newly established health program remains unknown but are worthy of future assessment to guide facilitation strategies. Nevertheless, these studies underscore the influence that structural barriers can have on the implementation and utilization of important preventive services.
Facilitation:
These potential health system challenges highlight the need for innovative implementation strategies for a critical WHO-endorsed preventive service that significantly reduces the risk of incident TB and TB-associated mortality among PWH (Badje et al., 2017; Churchyard et al., 2003; Grant et al., 2005). Accounting for the evidence and contextual factors documented in the literature, we propose implementation strategies that can facilitate improved TPT uptake in rural primary care settings. Facilitation approaches include reprioritization of TPT, improving HCW training, and integrating routine quality improvement surveillance (Pantoja et al., 2017; Powell et al., 2015). While reprioritization of TPT is a passive facilitation process, HCW training and quality improvement practices are active and ongoing and increase clinic-wide awareness.
Reprioritize TPT Nationally
A low rate of TPT initiation potentially suggests that TB prevention has declined among healthcare priorities. Consequently, public health officials reprioritizing TPT nationally could be an important step to improving TPT implementation at the primary care level. Re-emphasizing the wealth of evidence supporting TPT at the national level would highlight to HCWs that TPT is a valuable and effective intervention that should be performed routinely. A reprioritization strategy should utilize policy entrepreneurs, who are well connected to both scientific and policy communities and can bridge the gaps between, evidence, policy, and implementation (Hutchinson et al., 2011). The use of such policy entrepreneurs was found to be highly important in the successful implementation of co-trimoxazole prophylaxis among PWH in Zambia, Malawi, and Uganda (Hutchinson et al., 2011). South Africa has made strides in this regard through the development of a TB Think Tank to facilitate innovation in TB programs (White et al., 2018). Reprioritization by key stakeholders, particularly healthcare officials, may improve attention to TPT and increase rates of TPT prescriptions. To be successful, they are optimized when combined with repeated assessments and setting benchmarks for coverage. Downstream effects of reprioritization might involve increased funding and/or resources that would lead to health system changes at PHCs, including integration of HIV and TB services and increased provider to patient staffing ratios, to ensure that TPT becomes and remains a sustainable clinical practice. Alternatively, a reprioritization practice with known benefits elsewhere is the implementation of pay-for-performance incentives, often linked to pre-defined benchmarks (Lee et al., 2015; Schuster et al., 2018; Witter et al., 2012).
Healthcare Worker Training
HCWs in South Africa and other high burden TB settings often have limited knowledge about TPT, despite incorporation into national guidelines (Aït-Khaled et al., 2009; Lester et al., 2010; Moolphate et al., 2013; Rutherford et al., 2012; Singh et al., 2017). Interventions aimed at improving knowledge about the importance of TPT, how to prescribe TPT according to South African guidelines, and dispelling misconceptions (ex. that TPT leads to drug resistance) are important strategies for increasing implementation. Educational interventions that have been studied in low-income settings targeting HCWs include simple education materials, internet-based learning, educational meetings and workshops (Pantoja et al., 2017). Of these strategies, educational meetings and workshops show the strongest evidence in improving patient outcomes (Pantoja et al., 2017; Sunguya et al., 2013). In comparable sub-Saharan African settings, training interventions including simple education showed success in improving provider knowledge, TB screening practices, and initiation of TPT (Tadesse et al., 2013; Zaeh et al., 2013). Ongoing coaching using tele-education models, such as Project ECHO, should also be considered (Zurawski et al., 2016). There is evidence that ongoing tele-educational interventions that use a collaborative training method have demonstrated success in increasing access to clinical education and training in resource-constrained regions of KwaZulu-Natal, South Africa (Mars, 2014). Lastly, these strategies of education, training, and mentoring should be implemented in an engaging and participatory environment, where HCWs thoughts and considerations regarding TPT implementation are taken into account (Van Ginderdeuren et al., 2019). The revamping of HCW training programs for TPT may itself not be sufficient for improving TPT uptake. However, given the rural and under-resourced nature of our setting, improving training is a potential cost-effective strategy for improving HCWs knowledge and skills related to TPT implementation that should be utilized alongside other facilitation strategies (Azadi et al., 2014).
Routine Quality Improvement
Quality improvement (QI) aims to monitor healthcare service delivery in the dimensions of effectiveness, equity, efficiency, patient-centered care, safety, and access in order to achieve desired health outcomes (Institute of Medicine Committee on Quality of Health Care in America., 2001). QI activities often involve the collection and analysis of healthcare metrics to monitor and observe changes in a clinical policy or practice. Routine audits of PHCs’ clinical databases, periodic onsite observers, and conducting surveys among healthcare providers and patients are all examples of strategies to monitor TPT implementation (together with other healthcare initiatives). Currently, South Africa’s National TB Control Program (NTCP) uses the Electronic TB register (ETR.Net) system, an existing surveillance framework already integrated into South African PHCs, to document patients initiating treatment for TB. While the PHCs have the ability to record and document TPT treatment through this system, it is not being routinely done. If TPT administration was better integrated into this register, it would facilitate QI activities related to TPT implementation, such as audits to track trends in TPT initiation and completion (German et al., 2001; Mlotshwa et al., 2017). To supplement this quantitative data, NTCP representatives could also conduct qualitative interviews with HCWs and patients to gain a more complete perspective on TPT implementation (German et al., 2001). At the time of our study’s data collection, there was minimal TPT surveillance in the PHCs. Routine QI activities across the health system, distinct from monitoring and evaluation efforts within the national TB programme, can provide the appropriate external oversight needed to hold health facilities accountable to national TPT policy.
Limitations
Despite the many important findings, some limitations remain. First, this was a cross-sectional study, and we were unable to make any temporal conclusions from this analysis. We were unable to collect data on TPT completion, ART adherence, follow-up viral loads, and other important longitudinal data points. Second, our sample was drawn from only two PHCs, though these represent the largest PHCs in our region. Evaluating medical records from multiple clinics may have strengthened the finding that health system factors were a critical component to TPT initiation. Third, we restricted our assessment of TPT implementation to initiation of treatment. A more complete assessment of TPT implementation should also evaluate TPT adherence and completion, and we plan to complete such evaluations in the future. A more comprehensive TPT implementation assessment would also address the possibility that patients chose to decline treatment even when offered by a healthcare provider. In the current study, there was no documentation in medical records to support this; in fact, previous studies from our site have shown that patients usually initiate TPT when prescribed because they have strong trust in their healthcare providers (Jacobson et al., 2017). Nevertheless, even if patients actively declined TPT, health system factors likely still play an important role in patients’ individual healthcare decisions and should be addressed in future implementation strategies (Jacobson et al., 2017; Singh et al., 2017). Next, our measurement of health systems factors was limited to assessing the clinics as a proxy. We hope that future studies will directly measure the health system factors we speculate are relevant in this analysis. In this study, where we reviewed medical records that were filed differently at each PHC, we acknowledge the possibility of bias associated with missing data and sampling. While selection bias is also possible, our sample was representative of the source population across age, gender, and CD4 counts. Last, our understanding of the service delivery issues resulting in low TPT initiation could be improved through qualitative data from healthcare providers on their perceived barriers to TPT implementation. We are currently collecting this data to supplement findings from this study. Regardless, the data presented here provide important insights regarding the structural barriers to TPT implementation and directs future research to focus on HCW and facility level interventions in resource-limited settings.
CONCLUSION
Years after incorporation into national policy, TPT implementation lags in rural South African PHCs. Poor TPT initiation in this setting is associated with structural factors.
Contextual factors that may contribute to poor TPT initiation include poor HCW knowledge and attitudes towards TPT, staff shortages, and lack of HIV/TB service integration. Potential strategies to facilitate implementation include reprioritization of TPT, expanding training for HCWs, and QI strategies. Future studies should explore in greater detail the speculated HCW and facility level barriers to TPT implementation. These evaluations can help tailor proposed implementation strategies to overcome health system challenges and improve TPT uptake in rural, resource-limited, high HIV/TB prevalent settings.
ACKNOWLEDGMENTS
We would like to acknowledge the healthcare providers at COSH and the PHCs in Msinga sub-district for their dedication to patient care. We would also like to acknowledge the staff from the nongovernmental organization, Philanjalo, for their continued support of the Msinga community and public health research to improve patient outcomes. The study was funded by Yale University’s Wilbur Downs International Health Fellowship (DC), NIAID K23 K23AI089260 (SS), Gilead Foundation #157201 (SS, AM, LA), CDC #1U01GH000524 (SS, AM, LA), Doris Duke Foundation #2015216 and Irene Diamond Foundation #2006078 (SS, LA).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The spouse of SS worked part time at Amgen Pharmaceuticals October 2015 - October 2018.
REFERENCES
- Aït-Khaled N, Alarcon E, Bissell K, Boillot F, Caminero J, Chiang C, … Dlodlo R (2009). Isoniazid preventive therapy for people living with HIV: public health challenges and implementation issues. International Journal of Tuberculosis and Lung Disease, 13(8), 927–935. [PubMed] [Google Scholar]
- Alsdurf H, Hill PC, Matteelli A, Getahun H, & Menzies D (2016). The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis, 16(11), 1269–1278. doi: 10.1016/s1473-3099(16)30216-x [DOI] [PubMed] [Google Scholar]
- Azadi M, Bishai DM, Dowdy DW, Moulton LH, Cavalcante S, Saraceni V, … Golub JE (2014). Cost-effectiveness of tuberculosis screening and isoniazid treatment in the TB/HIV in Rio (THRio) Study. Int J Tuberc Lung Dis, 18(12), 1443–1448. doi: 10.5588/ijtld.14.0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badje A, Moh R, Gabillard D, Guehi C, Kabran M, Ntakpe JB, … Anglaret X (2017). Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health, 5(11), e1080–e1089. doi: 10.1016/s2214-109x(17)30372-8 [DOI] [PubMed] [Google Scholar]
- Bajunirwe F, Tumwebaze F, Abongomera G, Akakimpa D, Kityo C, & Mugyenyi PN. (2016). Identification of gaps for implementation science in the HIV prevention, qualitative study in 19 districts in Uganda. BMC Research Notes, 9(217). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcells ME, Thomas SL, Godfrey-Faussett P, & Grant AD (2006). Isoniazid preventive therapy and risk for resistant tuberculosis. Emerging infectious diseases, 12(5), 744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow C, Larson E, Vilakazi-Nhlapo A, Wilson M, & KLausner J (2012). Scale-up of isoniazid preventive therapy in PEPFAR-assisted clinical sites in South Africa. International Journal of Tuberculosis and Lung Disease, 16(8), 1020–1022. [DOI] [PubMed] [Google Scholar]
- Charalambous S, Grant AD, Churchyard GJ, Mukora R, Schneider H, & Fielding KL (2016). Clinic-level factors influencing patient outcomes on antiretroviral therapy in primary health clinics in South Africa. AIDS, 30(7), 1099–1109. [DOI] [PubMed] [Google Scholar]
- Charles MK, Lindegren ML, Wester CW, Blevins M, Sterling TR, Dung NT, … Fenner L (2016). Implementation of Tuberculosis Intensive Case Finding, Isoniazid Preventive Therapy, and Infection Control (“Three I’s”) and HIV-Tuberculosis Service Integration in Lower Income Countries. PLoS One, 11(4), e0153243. doi: 10.1371/journal.pone.0153243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab J, Vilakazi-Nhlapo K, Vranken P, Peters A, & Klausner J (2012). Survey of isoniazid preventive therapy in South Africa, 2011. International Journal of Tuberculosis and Lung Disease, 16(7), 903–907. [DOI] [PubMed] [Google Scholar]
- Churchyard GJ, Fielding K, Charalambous S, Day JH, Corbett EL, Hayes RJ, … Grant AD (2003). Efficacy of secondary isoniazid preventive therapy among HIV-infected Southern Africans: time to change policy? AIDS, 17(14), 2063–2070. doi: 10.1097/01.aids.0000076319.42412.70 [DOI] [PubMed] [Google Scholar]
- Fox M, Rosen S, Geldsetzer P, Barnighausen T, Negussie E, & Beanland R (2016). Interventions to improve the rate or timing of initiation of antiretroviral therapy for HIV in sub-Saharan Africa: meta-analyses of effectiveness Journal of the International AIDS Society, 19(1). doi: 10.7448/IAS.19.1.20888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, & Waller MN (2001). Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep, 50(Rr-13), 1–35; quiz CE31–37. [PubMed] [Google Scholar]
- Golub JE, Pronyk P, Mohapi L, Thsabangu N, Moshabela M, Struthers H, & Gray GE (2011). Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS, 23(5), 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Charalambous S, Fielding K, Day J, Corbett E, Chaisson R, … Churchyard G (2005). Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA, 293(22), 2719–2725. [DOI] [PubMed] [Google Scholar]
- Gupta S, Granich R, Date A, Lepere P, Hersh B, Gouws E, & Samb B (2014). Review of policy and status of implementation of collaborative HIV-TB activities in 23 high-burden countries. Int J Tuberc Lung Dis, 18(10), 1149–1158. doi: 10.5588/ijtld.13.0889 [DOI] [PubMed] [Google Scholar]
- Harvey G, Loftus-Hills A, Rycroft-Malone J, Titchen A, Kitson A, McCormack B, & Seers K (2002). Getting evidence into practice: the role and function of facilitation. J Adv Nurs, 37(6), 577–588. doi: 10.1046/j.1365-2648.2002.02126.x [DOI] [PubMed] [Google Scholar]
- Heaman MI, Sword W, Elliott L, Moffatt M, Helewa ME, Morris H, … Cook C (2015). Barriers and facilitators related to use of prenatal care by inner-city women: perceptions of health care providers. BMC Pregnancy Childbirth, 15, 2. doi: 10.1186/s12884-015-0431-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Droti B, Gibb D, Chishinga N, Hoskins S, Phiri S, & Parkhurst J (2011). Translating evidence into policy in low-income countries: lessons from co-trimoxazole preventive therapy. Bull World Health Organ, 89(4), 312–316. doi: 10.2471/blt.10.077743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Quality of Health Care in America. (2001) Crossing the Quality Chasm: A New Health System for the 21st Century. Washington (DC): National Academies Press (US) Copyright 2001 by the National Academy of Sciences. All rights reserved. [Google Scholar]
- Jacobson K, Moll A, Friedland G, & Shenoi S (2015). Successful Tuberculosis Treatment Outcomes among HIV/TB Coinfected Patients Down-Referred from a District Hospital to Primary Health Clinics in Rural South Africa. PLoS One, 10(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KB, Niccolai L, Mtungwa N, Moll AP, & Shenoi SV (2017). “It’s about my life”: facilitators of and barriers to isoniazid preventive therapy completion among people living with HIV in rural South Africa. AIDS Care, 1–7. doi: 10.1080/09540121.2017.1283390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson A, Harvey G, & McCormack B (1998). Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care, 7(3), 149–158. doi: 10.1136/qshc.7.3.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitson AL, Rycroft-Malone J, Harvey G, McCormack B, Seers K, & Titchen A (2008). Evaluating the successful implementation of evidence into practice using the PARiHS framework: theoretical and practical challenges. Implement Sci, 3, 1. doi: 10.1186/1748-5908-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufa T, Chihota V, Charalambous S, Churchyard GJ. (2014). Isoniazid preventive therapy use among patients on antiretroviral therapy: a missed opportunity. International Journal of Tuberculosis and Lung Disease, 18(3), 312–314(313). [DOI] [PubMed] [Google Scholar]
- Lee CY, Chi MJ, Yang SL, Lo HY, & Cheng SH (2015). Using financial incentives to improve the care of tuberculosis patients. Am J Manag Care, 21(1), e35–42. [PubMed] [Google Scholar]
- Lester R, Hamilton R, Charalambous S, Dwadwa T, Chandler C, Churchyard G, & Grant A (2010). Barriers to implementation of isoniazid preventive therapy in HIV clinics: a qualitative study. AIDS, 24(Suppl 5), 45–58. [DOI] [PubMed] [Google Scholar]
- Mars M (2014). Tele-education in South Africa. Front Public Health, 2, 173. doi: 10.3389/fpubh.2014.00173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack B, Kitson A, Harvey G, Rycroft-Malone J, Titchen A, & Seers K (2002). Getting evidence into practice: the meaning of ‘context’. J Adv Nurs, 38(1), 94–104. doi: 10.1046/j.1365-2648.2002.02150.x [DOI] [PubMed] [Google Scholar]
- Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, … Benedetti A (2018). Four Months of Rifampin or Nine Months of Isoniazid for Latent Tuberculosis in Adults. New England Journal of Medicine, 379(5), 440–453. doi: 10.1056/NEJMoa1714283 [DOI] [PubMed] [Google Scholar]
- Mlotshwa M, Smit S, Williams S, Reddy C, & Medina-Marino A (2017). Evaluating the electronic tuberculosis register surveillance system in Eden District, Western Cape, South Africa, 2015. Glob Health Action, 10(1), 1360560. doi: 10.1080/16549716.2017.1360560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolphate S, Lawpoolsri S, Pungrassami P, Sanguanwongse N, Yamada N, & Kaewkungwal J (2013). Barriers to and motivations for the implementation of a treatment programme for latent tuberculosis infection using isoniazid for people living with HIV, in upper northern Thailand. Global Journal of Health Science, 5(4), 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, & Volmink J (2007). Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med, 4(7), e238. doi: 10.1371/journal.pmed.0040238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthali AC, Ngwira BM, & Taulo F (2015). Exploring barriers to the delivery of cervical cancer screening and early treatment services in Malawi: some views from service providers. Patient Prefer Adherence, 9, 501–508. doi: 10.2147/ppa.s69286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-Shipp L, Voss De Lima Y, Clouse K, De Vos J, Evarts L, Bassett J, … Van Rie A (2012). TB/HIV integration at primary care level: a quantitative assessment at 3 clinics in Johannesburg, South Africa. South African Journal of HIV Medicine, 13(3), 138–143. [PMC free article] [PubMed] [Google Scholar]
- Pantoja T, Opiyo N, Lewin S, Paulsen E, Ciapponi A, Wiysonge CS, … Oxman AD (2017). Implementation strategies for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev, 9, Cd011086. doi: 10.1002/14651858.CD011086.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, … Kirchner JE (2015). A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation Science, 10(1), 21. doi: 10.1186/s13012-015-0209-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaka MX, Cavalcante SC, Marais BJ, Thim S, Martinson NA, Swaminathan S, & Chaisson RE (2015). Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet, 386(10010), 2344–2353. doi: 10.1016/s0140-6736(15)00323-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Republic of South Africa Department of Health. (2010). Guidelines for Tuberculosis Preventive Therapy Among HIV Infected Individuals in South Africa.
- Rutherford M, Hill P, Triasih R, Sinfield R, van Crevel R, & Graham S (2012). Preventive therapy in children exposed to mycobacterium tuberculosis: problems and solutions. Tropical Medicine & International Health, 17(10), 1264–1273. [DOI] [PubMed] [Google Scholar]
- Rycroft-Malone J, Seers K, Titchen A, Harvey G, Kitson A, & McCormack B (2004). What counts as evidence in evidence-based practice? J Adv Nurs, 47(1), 81–90. doi: 10.1111/j.1365-2648.2004.03068.x [DOI] [PubMed] [Google Scholar]
- Schuster RC, de Sousa O, Reme AK, Vopelak C, Pelletier DL, Johnson LM, … Young SL (2018). Performance-Based Financing Empowers Health Workers Delivering Prevention of Vertical Transmission of HIV Services and Decreases Desire to Leave in Mozambique. Int J Health Policy Manag, 7(7), 630–644. doi: 10.15171/ijhpm.2017.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AR, Kharate A, Bhat P, Kokane AM, Bali S, Sahu S, … Kumar AM (2017). Isoniazid Preventive Therapy among Children Living with Tuberculosis Patients: Is It Working? A Mixed-Method Study from Bhopal, India. J Trop Pediatr, 63(4), 274–285. doi: 10.1093/tropej/fmw086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling TR, Scott NA, Miro JM, Calvet G, La Rosa A, Infante R, … Villarino ME (2016). Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS, 30(10), 1607–1615. doi: 10.1097/qad.0000000000001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, … Chaisson RE (2011). Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med, 365(23), 2155–2166. doi: 10.1056/NEJMoa1104875 [DOI] [PubMed] [Google Scholar]
- Stetler CB, Damschroder LJ, Helfrich CD, & Hagedorn HJ (2011). A Guide for applying a revised version of the PARIHS framework for implementation. Implementation Science, 6(1), 99. doi: 10.1186/1748-5908-6-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunguya BF, Poudel KC, Mlunde LB, Shakya P, Urassa DP, Jimba M, & Yasuoka J (2013). Effectiveness of nutrition training of health workers toward improving caregivers’ feeding practices for children aged six months to two years: a systematic review. Nutr J, 12, 66. doi: 10.1186/1475-2891-12-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar AB, Rutherford GW, Horvath T, Doherty MC, & Negussie EK. (2014). Improving antiretroviral therapy scale-up and effectiveness through service integration and decentralization. AIDS, 28(Suppl 2), 175–185. [DOI] [PubMed] [Google Scholar]
- Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz J, Mwelase N, … Chaisson RE (2019). One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N Engl J Med, 380(11), 1001–1011. doi: 10.1056/NEJMoa1806808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, & Altice FL (2007). Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. Int J Drug Policy, 18(4), 306–312. doi: S0955-3959(07)00085-0 [pii] 10.1016/j.drugpo.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szakacs TA, Wilson D, Cameron DW, Clark M, Kocheleff P, Muller FJ, & McCarthy AE (2006). Adherence with isoniazid for prevention of tuberculosis among HIV-infected adults in South Africa. BMC Infect Dis, 6, 97. doi: 10.1186/1471-2334-6-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse Y, Yesuf M, & Williams V (2013). Evaluating the output of transformational patient-centred nurse training in Ethiopia. Int J Tuberc Lung Dis, 17(10 Suppl 1), 9–14. doi: 10.5588/ijtld.13.0386 [DOI] [PubMed] [Google Scholar]
- Teklay G, Teklu T, Legesse B, Tedla K, & Klinkenberg E (2016). Barriers in the implementation of isoniazid preventive therapy for people living with HIV in Northern Ethiopia: a mixed quantitative and qualitative study. BMC Public Health, 16(1), 840. doi: 10.1186/s12889-016-3525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ginderdeuren E, Bassett J, Hanrahan C, Mutunga L, & Van Rie A (2019). Health system barriers to implementation of TB preventive strategies in South African primary care facilities. PloS one, 14(2), e0212035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Halsema CL, Fielding KL, Chihota VN, Russell EC, Lewis JJ, Churchyard GJ, & Grant AD (2010). Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. Aids, 24(7), 1051–1055. [DOI] [PubMed] [Google Scholar]
- Vella V, Govender T, Dlamini S, Taylor M, Moodley I, David V, & Jinabhai C (2010). Retrospective study on the critical factors for retaining patients on antiretroviral therapy in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr, 55(1), 109–116. [DOI] [PubMed] [Google Scholar]
- White R, Charalambous S, Cardenas V, Hippner P, Sumner T, Bozzani F, … Kimerling M (2018). Evidence-informed policy making at country level: lessons learned from the South African Tuberculosis Think Tank. The International Journal of Tuberculosis and Lung Disease, 22(6), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilunda C, Scanagatta C, Putoto G, Montalbetti F, Segafredo G, Takahashi R, … Betran AP (2017). Barriers to utilisation of antenatal care services in South Sudan: a qualitative study in Rumbek North County. Reprod Health, 14(1), 65. doi: 10.1186/s12978-017-0327-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter S, Fretheim A, Kessy FL, & Lindahl AK (2012). Paying for performance to improve the delivery of health interventions in low- and middle-income countries. Cochrane Database Syst Rev(2), Cd007899. doi: 10.1002/14651858.CD007899.pub2 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2008). WHO Three I’s Meeting [Google Scholar]
- World Health Organization. (2013). Global Tuberculosis Report 2013.
- World Health Organization. (2018). Global Tuberculosis Report 2018.
- World Health Organization (2020). WHO consolidated guidelines on tuberculosis. Tuberculosis preventive treatment. Geneva. [PubMed] [Google Scholar]
- Zaeh S, Kempker R, Stenehjem E, Blumberg HM, Temesgen O, Ofotokun I, & Tenna A (2013). Improving tuberculosis screening and isoniazid preventive therapy in an HIV clinic in Addis Ababa, Ethiopia. Int J Tuberc Lung Dis, 17(11), 1396–1401. doi: 10.5588/ijtld.13.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski A, Komaromy M, Ceballos V, McAuley C, & Arora S (2016). Project ECHO Brings Innovation to Community Health Worker Training and Support. J Health Care Poor Underserved, 27(4a), 53–61. doi: 10.1353/hpu.2016.0186 [DOI] [PubMed] [Google Scholar]