Abstract
Chagas disease (CD) is caused by the parasite Trypanosoma cruzi. CD affects people worldwide, primarily in tropical areas. The central nervous system (CNS) is an essential site for T. cruzi persistence during infection. The protozoan may pass through the blood–brain barrier and may cause motor and cognitive neuronal damage. Once in the CNS, T. cruzi triggers immune responses that the purinergic system can regulate. Treatment for CD is based on benznidazole (BNZ); however, this agent has negative side-effects and is toxic to the host. For this reason, we investigated whether resveratrol (RSV), a potent antioxidant and neuroprotective molecule, would modulate purinergic signaling and RSV alone or in combination with BNZ would prevent changes in purinergic signaling and oxidative damage caused by T. cruzi. We infected mice with T. cruzi and treated them with RSV or BNZ for 8 days. Increases in ATP and ADP hydrolysis by NTPDase in the total cortex of infected animals were observed. The treatment with RSV in infected group diminished ATP, ADP, and AMP hydrolysis compared to infected group. The combination of RSV + BNZ decreased AMP hydrolysis in infected animals compared to the INF group, exerting an anti-inflammatory effect. RSV acted as a neuroprotector, decreasing adenosine levels. Infected animals presented an increase of P2X7 and A2A density of purine receptors. RSV reduced P2X7 and A2A and increased A1 density receptors in infected animals. In addition, infected animals showed higher TBARS and reactive oxygen species (ROS) levels than control. RSV diminished ROS levels in infected mice, possibly due to antioxidant properties. In short, we conclude that resveratrol could act as a neuroprotective molecule, probably preventing inflammatory changes caused by infection by T. cruzi, even though the mice experienced high levels of parasitemia.
Keywords: ATP, A1R, Resveratrol, Cellular stress, T. cruzi
Introduction
Chagas disease is caused by the Trypanosoma cruzi parasite that affects the liver, heart, gastrointestinal tract, and central nervous system (CNS). There is evidence to suggest that the parasite has a tropism for the CNS [1, 2]. Trypomastigote forms are often found in sympathetic and parasympathetic ganglia, where they target glial and other supporting cells for intracellular parasite proliferation. After completing its cycle, the parasite breaks out of these cells, releasing newly produced trypomastigote forms [3–6]. Once into the CNS, the parasite activates a cascade of inflammation responses with the recruitment of macrophages, NK cells, and lymphocytes [1, 2].
Chagas CNS manifestations include mental dysfunction, neurological deficits, and ataxia [7]. A study showed that parasitic infections caused by other parasites such as Trypanosoma evansi [8, 9] and Toxoplasma gondii [10] could negatively alter mice’s behavior interfering with neuroinflammatory responses.
In this context, purinergic signaling is an essential checkpoint in immune cell activation that allows immune cells to adjust their functional responses based on the host's extracellular cues. Extracellular nucleotides adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP), and nucleoside adenosine (ADO) [11], as well as their purinergic receptors (P1 and P2) [12], are directly involved in parasite controlling and host immune responses. ATP binds to P2 receptors that are divided into ionotropic P2X and metabotropic P2Y subtypes [13]. Among the receptors, we highlight one of the P2 receptors, P2X7, for being directly involved in response to inflammatory reactions against intracellular parasites such as M. tuberculosis and T. gondii and modulate host immune responses against the parasites through ATP [14] binding. Although there is little information about the P2X7R associated by T. cruzi-infection in brain, P2X7receptor can mediate the immune response, regulating the activation of T lymphocytes, consequently the production and release of pro-inflammatory cytokines [15]. P2X7 also has been reported to control the levels of intracellular Leishmania in macrophages [16]. Furthermore, other receptors are involved in brain disorders such as P2X4, modulating the inflammatory response after stroke [17]. P2X4 and P2X2 are important in bacterial infections; the receptors are involved in prodcution of NO and ROS in systemic polymicrobial sepsis in mouse model [18–21]. In addition to its more general involvement in cellular metabolism, specific actions of adenosine in the CNS as neuro‐effector are believed to be mediated through specific receptors that have been cloned and classified as A1, A2A, A2B, and A3 receptors. The A2A receptor subtype has been implicated in the modulation of inflammation into CNS [22] during brain injury, while A1R is related to neuroprotection [22].
Once the infection has been established in the vertebrate host, the parasite migrates to organs such as the liver, spleen, brain, intestine, and heart. To survive, the parasite exploits mechanisms involving NTPDase enzymes, especially E-NTPDase-1 [23]. The parasite increases its virulence through cell adhesion, modulation of the immune system, and increases its intracellular survival in the host [24]. The enzyme in the parasite interferes with extracellular ATP signals and interrupts purinergic signaling, inhibiting host defenses [24–26].
Currently, the specific treatment of CD involves benznidazole (BNZ) and nifurtimox; however, in the chronic phase, the treatment is palliative and carries negative side-effects. For these reasons, new therapeutic targets should be investigated. Resveratrol (RSV, 3, 4′, 5-trihydroxy-trans-stilbene), a natural polyphenol found in wine and grapes, possesses antioxidant, anti-inflammatory, and neuroprotector activities. Studies have reported that RSV reversed the adverse effects caused by Toxoplasma gondii on neural progenitor cells [10]. RSV also showed trypanocidal effects [27] and minimized CNS injury in mice embryos [28] during infection with T. cruzi.
Given the potential damage caused by T. cruzi infection, if immune responses were uncontrolled, it is probable that other immunomodulatory pathways may have evolved in response to damage caused by the parasite. Understanding these molecular mechanisms of immune response preconditioning regulation would be essential for the development of therapies. Therefore, in this study, we determined whether the purinergic system would change during the acute phase of infection by T. cruzi in the mouse cerebral cortex. We also investigated whether RSV alone or in combination with benznidazole would bolster the purinergic signaling pathway.
Material and methods
Animal infection and treatment
Four female Swiss mice were infected with T. cruzi (strain Y) for later infection of animals of experimental groups. After confirmation of infection, the animals were euthanized, and the blood was used to infect experimental groups. Mice were infected with 1 × 104 trypomastigote forms by intraperitoneal injection, and animals were divided into seven groups of five mice, each according to infection and treatment. Animals were kept in light/dark cycles (12 h) with controlled temperature and humidity (25 °C and 70%, respectively). Before initiation of treatment and at 24-h intervals, quantification of trypomastigotes in total blood was performed, according to another study [29]. The Ethics Committee on Animal Experimentation of the UFSM approved all animal procedures under protocol number 3060040517/17.
After confirming of infection, the mice received RSV (C14H12O3; molecular weight 228.25 g/mol; purity of > 98%) at 100 mg/kg or BNZ (C12H12N4O3 – LAFEPE) at 100 mg/kg. The treatments were orally administered over 7 days, as previously reported [28].
On day 8 post-infection (PI), the mice were anesthetized using isofluorane in a controlled inhalation box and were euthanized by cardiac puncture. The brains were removed, and cerebral cortexes were isolated and stored at –30 °C until analysis.
Protein determination
Protein content was determined using the Coomassie blue method according to Bradford [30] using bovine serum albumin as standard. The protein supernatants (S1) of tissue were maintained at 1.0 mg/mL.
Nucleotide and nucleoside hydrolysis assays
For enzymatic assays, cortex tissues were homogenized in saline solution and centrifuged for 5 min at 200 × g to yield supernatants for all analyses. Twenty microliters of S1 (0.9–1.0 mg/mL protein) were added to the reaction mixture of NTPDase or 5′-nucleotidase for a final volume of 200 μL and were pre-incubated for 10 min at 37 °C according to the method Lanzeta et al. [31]. The reaction was started by adding ATP or ADP as substrate at a final concentration of 1.0 mM. E-5′-nucleotidase was determined using the method described by Heymann et al. [32]. Phosphate released by ATP, ADP, and AMP hydrolysis was measured using KH2PO4 as the standard. The results were reported as ɳmol Pi released/min/mg of protein.
ADA activity was estimated spectrophotometrically as described by previous research [33] as the measurement of ammonia produced when adenosine deaminase acts in excess of adenosine. For the assay, 50 μL of S1 reacted for 60 min with 21 mmol/L of adenosine, pH 6.5, at 37 °C. The reaction was stopped by adding a solution of 106.2 mM phenol and 167.8 nM sodium nitroprusside, and a hypochlorite solution. Ammonium sulfate at 75 µM was used as the ammonium solution. The amount of ammonia produced was measured at 620 nm, and the results were expressed in units per milligram (U/mg).
Western blotting receptors assay
Samples of the total cortex were homogenized in ice-cold radioimmunoprecipitation assay buffer (RIPA buffer) with 1 mM protease and phosphatase inhibitors (DTT 1 M (1:1000), NaF 1 M (1:1000), Na3VO4 1 M (2:1000), PMSF 220 mM (1:1000), aprotinin 1 mg/ml (1:1000), and pepstatin 1 mg/ml (1:1000) Sigma-Aldrich, EUA) and centrifuged at 12.000 rpm at 4 °C for 10 min. The protein concentration was determined using the BCA Protein Assay Kit (Sigma-Aldrich, EUA). The diluted samples were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Biosciences, UK). After blocking, the membranes were incubated overnight at 4 °C with primary antibodies: P2X7R (1:800 Santa Cruz Biotechnology), A1R (1:500 Santa Cruz Biotechnology), and A2AR (1:500, Santa Cruz Biotechnology, CA, USA), followed by incubation with secondary antibody (Thermo Fisher scientific 1:10,000) for 90 min at room temperature. The membranes were incubated with an enhanced chemifluorescent substrate (Amersham Biosciences) and were analyzed using an Amersham Imager 600 (GE Healthcare Life Sciences, EUA). The membranes were reprobed and tested for β-actin immunoreactivity as a control for protein concentration, as previously described by Rebola et al. [34].
Lipid peroxidation and reactive species
Lipid peroxidation was measured as TBARS levels and was expressed in terms of malondialdehyde (MDA) content. MDA, an end-product of fatty acid peroxidation, reacts with TBA to form a colored complex. The TBARS was analyzed in serum as described previously [35]. The results were expressed as ɳmoles of malondialdehyde/mg of protein.
Reactive oxygen species (ROS) were measured using 2′-7′-dichlorofluorescein (DCFH) fluorescence levels as an index of peroxide production by cellular components according to as described [36]. Cortex tissue protein (0.8 μg) was added to a medium containing Tris–HCl buffer (10 mM; pH 7.4) and DCFH (1 mM). The mixture medium was incubated in the dark for 1 h until the fluorescence measurement procedure (excitation at 488 nm and emission at 525 nm, and both slit widths were 1.5 nm). The results were expressed as U DCF/mg protein.
Statistical analysis
Results are expressed as mean ± standard errors of the mean (SEM). Statistical analysis was performed by two-way ANOVA using Tukey as the post hoc test with the GraphPad Prism (Version 6.0) software. *p < 0.05 was considered statistically significant.
Results
Course of infection
To confirm T. cruzi infection, parasitemia was evaluated over 8 days (Fig. 1). Blood smear analysis confirmed the presence of trypomastigote forms from 3 days PI to day 8 PI. The treatment with RSV (100 mg/kg) in infected animals reduced trypomastigote forms after 6 days of infection compared to infected mice (p < 0.05). The combination of RSV + BNZ reduced parasitemia at 5 day PI. As expected, treatment with BNZ in infected animals exhibited an effect per se by reducing the number of trypomastigotes 4 days after infection compared to the INF and untreated groups.
Fig. 1.
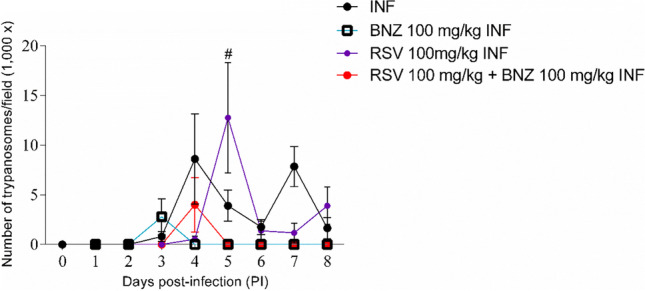
Time course of Trypanosoma cruzi infection. T. cruzi parasitemia over time in mice treated with or without BNZ and RSV (INF: infected group, BNZ: benznidazole group, RSV: resveratrol group). There was a significant increase in trypomastigote counts on the fourth day PI. The animals were euthanazed on day 8 PI. The data represent mean ± SEM analyzed with two-way ANOVA with post hoc Tukey test. #p < 0.05. (#T. cruzi vs other groups)
Nucleotide and nucleoside hydrolysis in the cortex
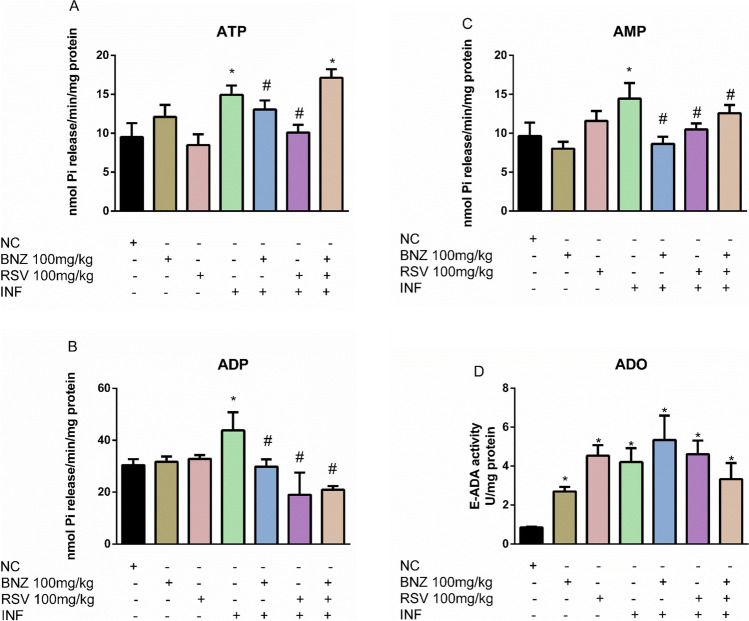
To investigate the capacities of infected animals to hydrolyze nucleotides and nucleosides, we measured ecto NTPDase, ecto 5′-nucleotidase, and adenosine deaminase activities in the total cortex (Fig. 2).
Fig. 2.
The effect of RSV on nucleotide and nucleoside hydrolysis during acute T. cruzi infection. A—ATP hydrolysis; B—ADP hydrolysis; C—AMP hydrolysis; D—E-ADA activity. (NC: negative control, INF: infected group, BNZ: benznidazole group, RSV: resveratrol group). The data represent mean ± SEM analyzed with two-way ANOVA with post hoc Tukey test. *p < 0.05 (*significant differences compared to the control group) (# significant differences compared to the infected group)
T. cruzi-infected animals presented high NTPDase activity when ATP (Fig. 2A) and ADP (Fig. 2B) were used as a substrate when compared to CN group. Also, an increment in 5′-NT was observed in the INF group using AMP (Fig. 2C) as a substrate in comparison to CN group (p < 0.05). Notorious significant differences in ATP, but not in ADP and AMP hydrolysis, were observed when BNZ (100 mg/kg) was administered as a treatment in healthy animals compared to the CN group or in infected animals compared to the INF group.
RSV (100 mg/kg) treatment did not alter ATP (Fig. 2A), ADP (Fig. 2B), or AMP (Fig. 2C) hydrolysis when compared to the CN group. However, RSV administration reduces NTPDase and 5′-NT activity in infected animals when ATP (Fig. 2A), ADP (Fig. 2B), and AMP (Fig. 2C) were used as the substrate in comparison to the INF group (p < 0.05). Furthermore, the combination of RSV and BNZ augmented NTPDase activity when ATP was used as substrate and reduces ADP hydrolysis in infected animals when compared to the INF group (Fig. 2A) (p < 0.05).
In addition, ADO hydrolysis was measured by E-ADA activity in the total cortex (Fig. 2D). The data reveals an increment of E-ADA in T. cruzi-infected animals compared to the CN group (p < 0.05). BNZ and RSV isolated also increase E-ADA in the cortex of healthy animals compared to the CN group. However, no significant differences were observed in E-ADA activity when RSV or BNZ combinate were administered in infected animals compared to the INF group (p > 0.05).
Expression of purine receptors in the cortex
Considering the alterations in ectonucleotidase activity by T. cruzi infection, P2X7, A1, and A2A purinergic receptor subtype expression patterns were determined using western blot (Fig. 3). Concerning P2X7 receptor expression (Fig. 3A), the RSV group (100 mg/kg) showed greater expression in P2X7 receptors in healthy animals when compared to the CN group (p < 0.05). P2X7 expression was greater during acute T. cruzi infection. However, treatments with BNZ, RSV, and the combination RSV + BNZ in the infected group diminished overexpression compared to the INF group.
Fig. 3.
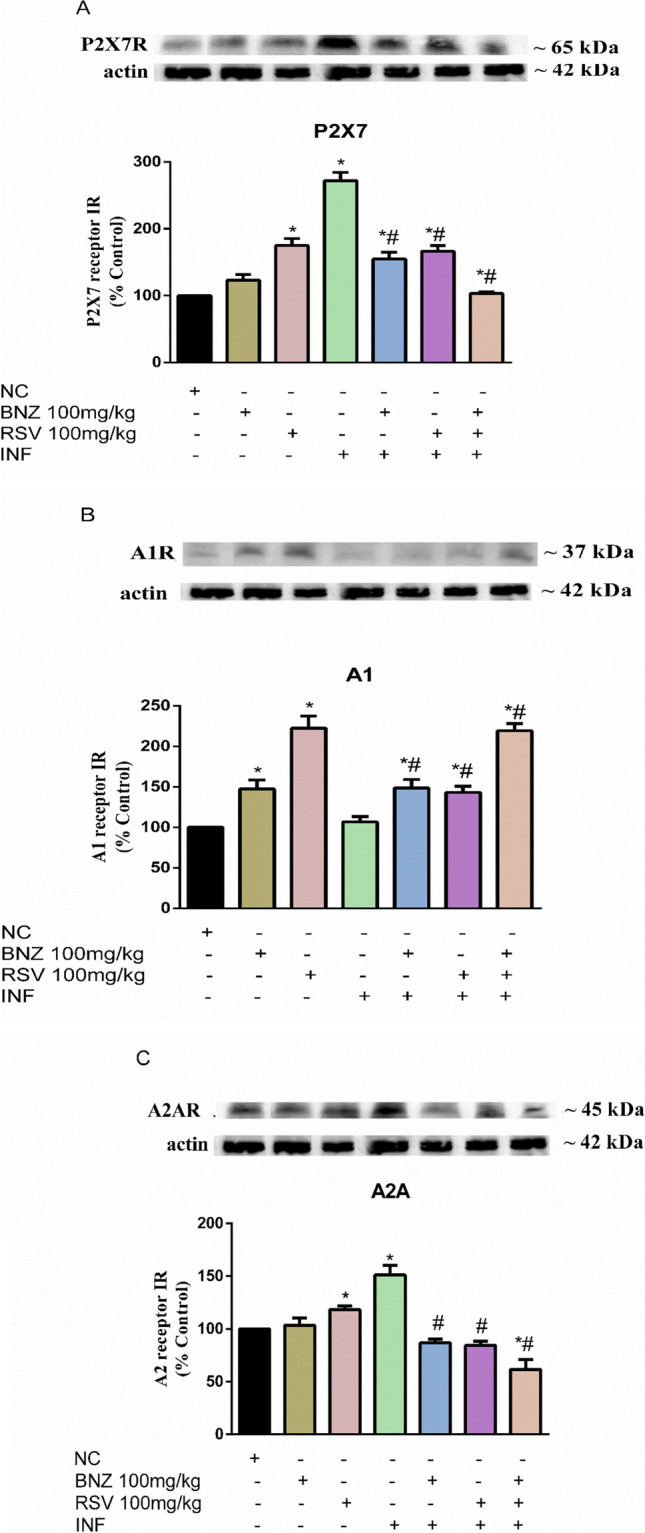
RSV modulates the increment of purine receptors during acute T. cruzi infection. A—P2X7 receptor; B—A1 receptor; C–A2A receptor (NC: negative control, INF: infected group, BNZ: benznidazole group, RSV: resveratrol group). The data represent mean ± SEM analyzed using two-way ANOVA with post hoc Tukey test. *p < 0.05 (*significant differences compared to the control group) (# significant differences compared to the infected group)
The purine receptor A1 (Fig. 3B) was overexpressed (p < 0.05) in the BNZ (100 mg/kg) and RSV (100 mg/kg) groups when compared to the CN group. No significant differences were observed in the INF group in comparison to the CN group. By contrast, the administration of BNZ, RSV, and the combination RSV + BNZ groups increased A1 receptor density in infected animals compared to the INF group. In addition, we measured A2A receptor density (Fig. 3C). Our results reveal that RSV (100 mg/kg) increased the expression of A2A, the receptor, when compared to CN in healthy animals (p < 0.05). A2A receptor density was greater in the INF group than in the CN group (p < 0.05). The treatments with BNZ, RSV, or combination RSV + BNZ significantly reduced A2A receptor density compared to the INF group.
Oxidative stress in the cerebral cortex of infected animals
To evaluate oxidative parameters, reactive oxygen species (ROS) and TBARS levels were measured in the cerebral cortex (Fig. 4). There were greater ROS levels in the INF group than in the CN group (p < 0.05). RSV treatment decreased ROS levels in infected animals when compared to the INF group.
Fig. 4.
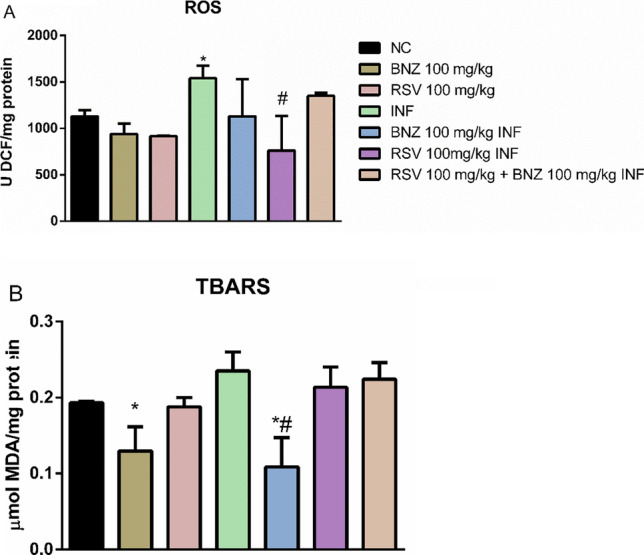
T. cruzi-infection promotes oxidative stress in the cortex. A—ROS levels; B–TBARS levels (NC: negative control, INF: infected group, BNZ: benznidazole group, RSV: resveratrol group). The data represent mean ± SEM analyzed using two-way ANOVA with post hoc Tukey test. *p < 0.05 (*significant differences compared to the control group) (# significant differences compared to the infected group)
In terms of lipid peroxidation, TBARS levels decreased in the BNZ (100 mg/kg) group (Fig. 4B) compared to the CN group. This effect was also observed in infected animals treated with BNZ compared to the INF group (p < 0.05). The treatments with RSV alone or in combination with BNZ did not affect TBARS levels in infected animals compared to the INF group (p > 0.05).
Discussion
This study aimed to investigate the effects of BNZ and RSV alone and in combination on the course of infection and as a modulator of purinergic signaling and the influence of these agents on oxidative stress during acute T. cruzi infection in the brain. As expected, BNZ at 100 mg/kg reduced the number of trypomastigotes in the blood of infected animals. The treatments with RSV-free and BNZ combination did not directly affect parasitemia in infected mice (Fig. 1). According to other studies, RSV may promote the survival of trypomastigote forms by interaction with Sirt genes [37, 38]. Thus, RSV does not appear to be a therapeutic target to reduce trypomastigotes forms during acute phase T. cruzi infection.
Nevertheless, RSV is a potent anti-inflammatory, antioxidant, and neuroprotector molecule that activates several intracellular mechanisms to prevent intense inflammatory and oxidative processes in the brain during CD. In addition, RSV crosses the blood–brain barrier [39] and attenuates the intracellular formation of reactive molecules, decreasing cell damage [40]. In this context, we investigated whether RSV would modulate the purinergic pathway through ectonucleotidase activities and P2X7, A1, and A2A purinergic receptors in an experimental model of acute infection by T. cruzi.
We found that NTPDase and 5′-NT enzymes in the cortex were affected by T. cruzi (Fig. 2). During acute T. cruzi infection, increased ATP, ADP, and AMP hydrolysis were observed in infected animals. It is believed that this increased activity is related to the enzymatic modulation in the presence of high grade stimulation by T. cruzi during CD infection, leading to a high release of ATP by cells. Once into extracellular medium, ATP acts to mediate events such as stimulate astrocyte proliferation and differentiation, cytokine release, and the formation of reactive nitrogen and oxygen species [24]. These events triggered by extracellular ATP as a danger signal can protect the host from T. cruzi and induce apoptosis.
Some studies have reported the involvement of nucleotides and nucleosides in CD. A study conducted by Do Carmo et al. [41] showed an increase of seric ATP and ADP levels in infected animals suggesting consequences on the pro-inflammatory response of host against parasite contributing to immunomodulation response. In another study with patients naturally infected by T. cruzi, Souza et al. [42] showed alterations in ATP, AMP, and ADO levels indicating an agreement with the immune response against T. cruzi infection.
In our study, we observed RSV-mediated on ectonucleotidases induced by T. cruzi (Fig. 2). RSV is an anti-inflammatory compound that modulates several molecular pathways dependent on silent information regulator-1 (SIRT1). RSV plays an essential role in neuronal protection as it regulates reactive oxygen species (ROS), nitric oxide (NO), and proinflammatory cytokine production [43, 44].
BNZ, a choice theraphy to CD, acts through the formation of free radicals or electrophilic metabolites, affecting all macromolecules of the parasite [45]; moreover, a study revealed that BNZ is distributed systemically in the brain, kidneys, and lung, among others. Although they are not the target organs of the parasite, the wide distribution of the drug prevents parasitic proliferation [46], consequently leading to host apoptosis with ATP release.
Several lines of evidence indicate that adenosine may be an endogenous neuroprotective agent in the CNS [47]. Hence, adenosine-potentiating agents which elevate endogenous adenosine levels by either inhibiting its degradation (adenosine deaminase and kinase inhibitors) or preventing its transport offer protection against damage. A growing body of evidence also supports the role of both A1 and A2A receptors in the neuroprotective mechanisms. It has been suggested that the beneficial effects seen after chronic administration of adenosine antagonists may be due to, e.g., the upregulation of A1 receptors. Here we observed that RSV and BNZ isolated or associated lead to lower ADO levels caused by enhanced ADA activity, which could have similar effects of A2A antagonists that diminish activation of microglial cells and astrocytes [47], so the lower ADO levels caused by enhanced ADA activity in the results may have a similar effect.
In addition, adenosine deaminase (ADA) activity was significantly augmented in infected animals compared to control mice (Fig. 2D). The treatments with BNZ or RSV alone or in combination also stimulated ADO hydrolysis by E-ADA. These results suggest a suitable immunosuppressive effect of RSV during acute T. cruzi infection [47]. Therefore, ADA is related to vital functions of the parasites such as Trypanosoma evansi [48] and Plasmodium falciparum [49], since this enzyme is responsible for degrading adenosine in inosine, which is later used in the purine rescue pathway of these parasites [50]. Thus, our data suggest that treatments with BNZ or RSV modulate ADA activity, reduce ADO levels and convert inosine by E-ADA, and suppress immune responses. It is essential to highlight that activation of various adenosine receptor subtypes has been reported to mediate different effects of endogenous adenosine, as observed in our study.
Once in the extracellular environment, nucleosides and nucleotides activate two families of purinergic receptors, named P1 and P2 receptors [11]. Our results showed that T. cruzi infection stimulated P2X7 receptor expression in the cerebral cortex (Fig. 3A). In response to parasite infection, ATP is released from immune and non-immune cells, which can activate P2X7 receptor. As a consequence, P2X7 receptor activation induces ATP release-chiefly via pannexin hemichannels-boosting inflammation as already mencioned by Savio et al. [51]. Furthermore, continued activation of P2X7 receptors by ATP during chronic infection has been proposed as a mechanism for the elimination of T. cruzi in the thymus [52].
Previous studies conducted by our research group using RSV as treatmed showed that RSV can modulate P2X7 receptors in T. gondii-infected neural precursor cells [53] as alternative therapy to inflect the balance between inflammation and parasite control in CNS. Here the treatments with BNZ, RSV, and RSV + BNZ downregulated P2X7 expression in the cerebral cortex (Fig. 3A). Overall, these data support a role for RSV to modulate ATP-P2X7 receptor in boosting the immune system against the protozoa infections.
The functions of P2X7 in inflammation and cell death have been studied extensively [54]. Here the treatments with BNZ, RSV, and RSV + BNZ combinated downregulated P2X7 expression in the cerebral cortex (Fig. 3A). Previous study by our research group showed the effect of RSV on P2X7 receptors in T. gondii-infected neural precursor cells [53]. Thus, in the presence of physiological amounts of ATP, P2X7 may control microglia proliferation in the CNS while sustained activation may induce cell death.
T. cruzi infection increased A2A but not A1 receptor density in the cortex of infected mice (Fig. 3C). BNZ and RSV, alone and in combination, up- and downregulated A1 and A2A receptor densities. Various endogenous adenosine concentrations may activate adenosine receptors; the levels of endogenous adenosine available to bind to and activate these receptors help control specific physiological responses to adenosine [22].
The A1A subtype is expressed in the CNS, mainly in the cerebral cortex [55, 56]. This broad distribution reflects the wide range of physiological functions regulated by A1AR, spanning neurotransmitter release, dampening of neuronal excitability, control of sleep/wakefulness, and other effects [57]. This positive modulation of the A1 receptor during T. cruzi infection by RSV or BNZ causes a receptor upregulation in protein expression, which could promote chemotaxis and consequently neuroprotection by immune cells [58]. In addition, A2AR has expressed on both pre- and postsynaptic neurons astrocytes, microglia, and oligodendrocytes, where it orchestrates several functions related to excitotoxicity, including neuronal glutamate release, glial reactivity, blood–brain barrier permeability, and peripheral immune cell migration [59].
As already reported, high levels of ATP act as proinflammatory danger signals, activating the inflammasome that processes pro-IL-1β into mature IL-1β [60, 61]. Therefore, it has been suggested that CD39 expression has an essential role in cell proliferation and growth, inflammatory processes, and triggering cellular responses from ATP-induced contribute to apoptosis and host defense [60–64]. Our findings suggest an increase of ADA activity in the total cortex in BNZ, RSV, INF, BNZ + INF, and RSV + INF experimental groups compared to the CTL group. We suggest that this increase in ADA activity could result from the increment in extracellular adenosine (ADO). Once in the extracellular space, ADO binding to A1 or A2A receptors during brain disorder exerts neuroprotective and immunosuppressive capacities, respectively [56]. ADO inhibits neutrophil phagocytosis via activation of A2A receptor and ROS generation by macrophages and neutrophils, improving the VEGF secretion by macrophages [65] and inducing a Th2-like profile in the CNS.
During CD, inflammatory responses involve high ROS levels, nitric oxide production (NO), and promotion of oxidative stress as crucial defense mechanisms against intracellular pathogens. We evaluated oxidative parameters to test our hypothesis whether RSV would reduce oxidative damage in the cerebral cortex and attenuate cellular damage.
In addition, we found that T. cruzi infection increased ROS levels in the cerebral cortex and increased lipid peroxidation in the INF group (Fig. 4). The treatments with BNZ avoid lipid peroxidation by reducing TBARS levels in healthy and infected animals. RSV acted as an antioxidant molecule, reducing ROS levels in infected mice. Previous studies reported that T. cruzi infection led to oxidative stress as a defense mechanism of the host cell to inhibit parasite survival and replication [66–69].
RSV is an antioxidant molecule, probably that decrease ROS levels in host as compensatory mechanism. However, the inflammatory process increases the ROS levels; these imply not only the parasitic action as well as other damage to the host. In T. cruzi infection, these ROS can be produced as a consequence of tissue destruction caused by toxic parasite secretions, immune-mediated cytotoxic reactions, and secondary damage to mitochondria [70]. Thus, the RSV molecule can act by decreasing tissue destruction, as well as decreasing ROS levels, and can prevent cell damage and mitochondrial dysfunction during an acute infection by T. cruzi. RSV also increased the activity of antioxidant enzymes and free radical scavengers, decrease the ROS levels [71]. In addition, it is known that high levels of ROS can impact numerous cell damage such as cancer, inflammation, cardiovascular diseases, and aging [72]. Therefore, keeping the ROS levels low can be a compensatory mechanism that may be related to the reduction of cellular damage caused by the inflammatory and infectious process triggered by the parasite.
Conclusion
We outlined the molecular effects of RSV on purinergic signaling and oxidative status during acute T. cruzi infection. Notably, the RSV molecule could not decrease parasites; however, RSV treatment had subtle effects on enzymes that hydrolyze extracellular nucleotides and nucleosides. We observed subtle positively regulated purinergic receptors as a compensatory mechanism to eliminate the parasite and oxidative damage in the cerebral cortex of infected mice. In summary, the association between RVS + BNZ appears to be beneficial concerning the inflammatory damage caused by parasitic infection. Nevertheless, further studies are needed to determine possible associations between traditional pharmacotherapy with BNZ and the RSV molecule.
Author contribution
Fracasso M. and Da Silva A.S. contributed to the design and implementation of the research, to the analysis of the results. Bottari N.B., Monteiro, S.G., and Schetinger M.R.C. helped in the elaboration of the project and its execution and financing. Fracasso M., Bottari N.B., Reichert K., and Silva A.D. participated in the execution of the experiment, collection of samples and data, and laboratory analysis. All authors discussed the results and contributed to the final manuscript.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, process number [88887.212883/2018–00] and [23038.004173/2019–93] and Conselho Nacional de Desenvolvimento Científico e Tecnológico [CNPq–Edital Universal 2016].
Data availability
All data and materials used in the experiment are available and are ready to be provided if needed.
Declarations
Ethics approval
Animal experiments were approved by the Ethics Committee on Animal Experimentation of the Universidade Federal de Santa Maria (UFSM), under protocol number 3060040517/17.
Consent to participate
All names in author list have been involved in various stages of experimentation or writing.
Consent for publication
All authors agree with submit the paper for publication in the Purinergic Signalling.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mateus Fracasso, Email: mateus.fracasso1@gmail.com.
Aleksandro Schafer da Silva, Email: aleksandro.silva@udesc.br.
References
- 1.Campos JV, Tafuri WL. Chagas enteropathy Gut. 1973;14:910–919. doi: 10.1136/gut.14.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adad SJ, Cancado CG, Etchebehere RM, Teixeira VP, Gomes UA, Chapadeiro E, et al. Neuron count reevaluation in the myenteric plexus of chagasic megacolon after morphometric neuron analysis. Virchows Arch. 2001;438:254–258. doi: 10.1007/s004280000319. [DOI] [PubMed] [Google Scholar]
- 3.Meyer H, Machado RD, Cintra WM. On the cultivation of Trypanosoma cruzi in tissue cultures of the spinal and sympathetic ganglion from the chick embryo. An Acad Bras Cienc. 1982;54:739–742. [PubMed] [Google Scholar]
- 4.Tanowitz HB, Brosnan C, Guastamacchio D, Baron G, Raventos-Suarez C, Bornstein M, et al. Infection of organotypic cultures of the spinal cord and dorsal root ganglia with Trypanosoma cruzi. Am J Trop Med Hyg. 1982;31:1090–1097. doi: 10.4269/ajtmh.1982.31.1090. [DOI] [PubMed] [Google Scholar]
- 5.Lenzi HL, Oliveira DN, Lima MT, Gattass CR. Trypanosoma cruzi: paninfectivity of CL strain during murine acute infection. Exp Parasitol. 1996;84:16–27. doi: 10.1006/expr.1996.0086. [DOI] [PubMed] [Google Scholar]
- 6.Manning-Cela R, Cortes A, Gonzalez-Rey E, Van Voorhis WC, Swindle J, Gonzalez A. LYT1 protein is required for ficiente in vitro infection by Trypanosoma cruzi. Infect Immun. 2001;69:3916–3923. doi: 10.1128/IAI.69.6.3916-3923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chacko G. Parasitic diseases of the central nervous system. Semin Diagn Pathol. 2010;27:167–185. doi: 10.1053/j.semdp.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Wolkmer P, Silva CB, Paim FC, Duarte MMMF, Castro V, Palma HE, et al. Pre-treatment with curcumin modulates acetylcholinesterase activity and proinflammatory cytokines in rats infected with Trypanosoma evansi. Parasitol Int. 2013;62:144–149. doi: 10.1016/j.parint.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira C, Da Silva A, Souza V, Costa M, Jaques J, et al. NTPDase activity in lymphocytes of rats infected by Trypanosoma evansi. Parasitology. 2012;139:232–236. doi: 10.1017/S0031182011001879. [DOI] [PubMed] [Google Scholar]
- 10.Bottari NB, Pillat MM, Schetinger MR, et al. Resveratrol-mediated reversal of changes in purinergic signaling and immune response induced by Toxoplasma gondii infection of neural progenitor cells. Purinergic Signal. 2019;15:77–84. doi: 10.1007/s11302-018-9634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G. Introduction to the special issue on purinergic receptors. Adv Exp Med Biol. 2017;64:445–446. doi: 10.1007/5584_2017_12. [DOI] [PubMed] [Google Scholar]
- 13.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 14.Petit-Jentreau L, Tailleux L, Coombes JL. Purinergic signaling: a common path in the macrophage response against Mycobacterium tuberculosis and Toxoplasma gondii. Front Cell Infect Microbiol. 2017;7:347. doi: 10.3389/fcimb.2017.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbracchio, MP, Burnstock, G, Gabel, CA (2007) P2 purinergic receptor modulation of cytokine production. Purinergic Signal 3:27–38 [DOI] [PMC free article] [PubMed]
- 16.Chaves, SP, E.C. Torres-Santos, EC, Marques, C, Figliuolo, VR, Persechini, PM, Coutinho-Silva, R, Rossi-Bergmann B (2009) Modulation of P2X (7) purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination 10–11:842–9. doi: 10.1016/j.micinf.2009.05.001 [DOI] [PubMed]
- 17.Verma R, Cronin CG, Hudobenko J, Venna VR, McCullough LD, Liang BT. Deletion of the P2X4 receptor is neuroprotective acutely, but induces a depressive phenotype during recovery from ischemic stroke. Brain Behav Immun. 2017;66:302–312. doi: 10.1016/j.bbi.2017.07.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csóka, B, Németh, ZH, Szabó, I, Davies, DL, Varga, ZV, Pálóczi, J, Falzoni, S, Di Virgilio, F, Muramatsu, R, Yamashita, T, Pacher, P, Haskó G (2018) Macrophage P2X4 receptors augment bacterial killing and protect against sepsis JCI Insight, 3:1–18, [DOI] [PMC free article] [PubMed]
- 19.Savio LEB, de Andrade MP, Figliuolo VR, de Avelar Almeida TF, Santana PT, Oliveira SDS, Silva CLM, Feldbrügge L, Csizmadia E, Minshall RD, Longhi MS, Wu Y, Robson SC, Coutinho-Silva R. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. J Hepatol. 2017;67:716–726. doi: 10.1016/j.jhep.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savio LEB, Andrade MGJ, de Andrade MP, Santana PT, Moreira-Souza ACA, Kolling J, Longoni A, Feldbrügge L, Wu Y, Wyse ATS, Robson SC, Coutinho-Silva R. P2X7 Receptor signaling contributes to sepsis-associated brain dysfunction. Mol Neurobiol. 2017;54:6459–6470. doi: 10.1007/s12035-016-0168-9. [DOI] [PubMed] [Google Scholar]
- 21.Larrouyet-Sarto ML, Tamura AS, Alves VS, Santana PT, Ciarlini-Magalhães R, Rangel TP, Siebert C, Hartwig JR, Dos Santos TM, Wyse ATS, Takiya CM, Coutinho-Silva R, Savio LEB. P2X7 receptor deletion attenuates oxidative stress and liver damage in sepsis. Purinergic Signal. 2020;16:561–572. doi: 10.1007/s11302-020-09746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79(3):463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 23.Fietto JL, DeMarco R, Nascimento IP, Castro IM, Carvalho TM, de Souza W, et al. Characterization and immunolocalization of an NTP diphosphohydrolase of Trypanosoma cruzi. Biochem Biophys Res Commun. 2004;316:454–460. doi: 10.1016/j.bbrc.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 24.Santos EC, Novaes RD, Cardoso SA, Oliveira LL. Implication of purinergic signalling pathways in clinical management of Chagas disease. OA Biotechnology. 2013;08:27. [Google Scholar]
- 25.Santos RF, Pôssa MA, Bastos MS, Guedes PM, Almeida MR, Demarco R, et al. Influence of ecto-nucleoside triphosphate diphosphohydrolase activity on Trypanosoma cruzi infectivity and virulence. PLoS Negl Trop Dis. 2009;3:387. doi: 10.1371/journal.pntd.0000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bisaggio DF, Peres-Sampaio CE, Meyer-Fernandes JR, Souto-Padrón T (2003) Ecto-ATPase activity on the surface of Trypanosoma cruzi and its possible role in the parasite–host cell interaction. Parasitol Res. 91273–82. [DOI] [PubMed]
- 27.Vera EAV, Sayé M, Reigada C, Damasceno FS, Silber AM, Miranda MR, Pereira CA. Resveratrol inhibits Trypanosoma cruzi arginine kinase and exerts a trypanocidal activity. Int J Biol Macromol. 2016;87:498–503. doi: 10.1016/j.ijbiomac.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Fracasso M, Bottari NB, da Silva AD, Grando TH, Pillat MM, Ulrich H, Vidal T, de Andrade CM, Monteiro SG, Nascimento LFN, Miletti LC, da Silva AS. Effects of resveratrol on the differentiation fate of neural progenitor cells of mouse embryos infected with Trypanosoma cruzi. Microb Pathog. 2019;132:156–161. doi: 10.1016/j.micpath.2019.04.040. [DOI] [PubMed] [Google Scholar]
- 29.Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop São Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Lanzetta PA, Alvarez LJ, Reinach PS, Candia AO. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979;15:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- 32.Heymann D, Reddington M, Kreutzberg GW. Subcellular localization of 5′-nucleotidase in rat brain. J Neurochem. 1984;43:971–978. doi: 10.1111/j.1471-4159.1984.tb12832.x. [DOI] [PubMed] [Google Scholar]
- 33.Guist G, Galanti B. Colorimetric method. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim: Verlag Chemie; 1984. pp. 315–323. [Google Scholar]
- 34.Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA. Subcellular localization of adenosine A1 receptors in nerve terminals and synapses of the rat hippocampus. Brain Res. 2003;987:49–58. doi: 10.1016/S0006-8993(03)03247-5. [DOI] [PubMed] [Google Scholar]
- 35.Jentzsch AM, Bachmann H, Fürst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radical Bio Med. 1996;20:251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. 4th edn. Oxford University Press.
- 37.Tang Xl, Wang X, Fang G, Zhao Yl, Yan J, Zhou Z, Sun R, Luo Al, Li SY (2021) Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-κB pathway in neonatal mice, The Journal of Nutritional Biochemistry. 90 10.1016/j.jnutbio.2020.108579 [DOI] [PubMed]
- 38.Religa AA, Waters AP. Sirtuins of parasitic protozoa: in search of function (s) Mol Biochem Parasitol. 2012;185(2):71–88. doi: 10.1016/j.molbiopara.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto M, Benfeito S, Fernandes C, Borges F (2020) Chapter 9 - Antioxidant therapy, oxidative stress, and blood-brain barrier: the road of dietary antioxidants, Editor(s): Colin R. Martin, Victor R. Preedy, Oxidative Stress and Dietary Antioxidants in Neurological Diseases, Academic Press. Pages 125–141. 10.1016/B978-0-12-817780-8.00009-8
- 40.Murcia MA, Martínez-Tomé M. Antioxidant activity of resveratrol compared with common food additives. J Food Prot. 2001;64:379–384. doi: 10.4315/0362-028x-64.3.379. [DOI] [PubMed] [Google Scholar]
- 41.do Carmo, G.M., de Sá, M.F., Baldissera, M.D. , et al. Nucleotide and nucleoside involvement in immunomodulation in experimental Chagas disease. Mol Cell Biochem. 2018;447:203–208. doi: 10.1007/s11010-018-3304-1. [DOI] [PubMed] [Google Scholar]
- 42.Souza VCG, Schlemmer KB, Noal CB, Jaques JAS et al (2012) E-NTPDase and E-ADA activities are altered in lymphocytes of patients with indeterminate form of Chagas’ disease. Parasitol Int 61:690–696. 10.1016/j.parint.2012.07.008 [DOI] [PubMed]
- 43.Ye J, Liu Z, Wei J, Lu L, Huang Y, Luo L, Xie H. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neurosci Lett. 2013;553:72–77. doi: 10.1016/j.neulet.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Anoopkumar-Dukie S, Arora D, Davey AK. Review of the anti-inflammatory effect of SIRT1 and SIRT2 modulators on neurodegenerative diseases. Eur J Pharmacol. 2020;867:172847. doi: 10.1016/j.ejphar.2019.172847. [DOI] [PubMed] [Google Scholar]
- 45.Maya JD, Bollo S, Nuñez-Vergara LJ, Squella JA, Repetto Y, Morillo A, Perie J, Chauviere G. Trypanosoma cruzi: effect and mode of action of nitroimidazole and nitrofuran derivatives. Biochem Pharmacol. 2003;65:999–1006. doi: 10.1016/S0006-2952(02)01663-5. [DOI] [PubMed] [Google Scholar]
- 46.Perin L, Moreira da Silva R, Fonseca KD, Cardoso JM, Mathias FA, Reis LE, Molina I, Correa-Oliveira R, Vieira PM, Carneiro CM (2017) Pharmacokinetics and tissue distribution of benznidazole after oral administration in mice. Antimicrob Agents Chemother. 24;61(4):e02410–16. 10.1128/AAC.02410-16 [DOI] [PMC free article] [PubMed]
- 47.Wardas J. Neuroprotective role of adenosine in the CNS. Pol J Pharmacol. 2002;54:313–326. [PubMed] [Google Scholar]
- 48.Da Silva AS, Bellé LP, Bitencourt PE, Perez HA, Thomé GR, Costa MM, Oliveira CB, Teixeira MM, Moretto MB, Mazzanti CM, Lopes ST, Monteiro SG. Trypanosoma evansi: adenosine deaminase activity in the brain of infected rats. Exp Parasitol. 2011;127(1):173–177. doi: 10.1016/j.exppara.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Ivanov A, Matsumura I. The adenosine deaminases of Plasmodium vivax and Plasmodium falciparum exhibit surprising differences in ligand specificity. J Mol Graph Model. 2012;35:43–48. doi: 10.1016/j.jmgm.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lauri N, Bazzi Z, Alvarez CL, Leal Denis MF, Schachter J, Herlax V, Ostuni MA, Schwarzbaum PJ. ATPe dynamics in protozoan parasites. Adapt or Perish Genes. 2019;10(1):16. doi: 10.3390/genes10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savio LEB, de Andrade MP, da Silva CG, Coutinho-Silva R. The P2X7 receptor in inflammatory diseases: angel or demon? Front Pharmacol. 2018 doi: 10.3389/fphar.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cascabulho CM, Menna-Barreto RFS, Coutinho-Silva R, Persechini PM, Henriques-Pons A. P2X7 modulatory web in Trypanosoma cruzi infection. Parasitol Res. 2008;103(4):829–838. doi: 10.1007/s00436-008-1063-8. [DOI] [PubMed] [Google Scholar]
- 53.Bottari NB, Pillat MM, Schetinger MRC, et al (2019) Resveratrol-mediated reversal of changes in purinergic signaling and immune response induced by Toxoplasma gondii infection of neural progenitor cells. Purinergic Signal. 1577-84. 10.1007/s11302-018-9634-3 [DOI] [PMC free article] [PubMed]
- 54.Kanellopoulos, JM, Delarasse, C (2019). Pleiotropic roles of P2X7 in the central nervous system. Front Cell Neurosci 13: 401. 10.3389/fncel.2019.00401 [DOI] [PMC free article] [PubMed]
- 55.Chen CH, Fiecas M, Gutiérrez ED, Panizzon MS, et al. Genetic topography of brain morphology. Proc Natl Acad Sci. 2013;110(42):17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merighi S, Gessi S, Borea PA (2018) Adenosine receptors: structure, distribution, and signal transduction. In: Borea P., Varani K., Gessi S., Merighi S., Vincenzi F. (eds) The Adenosine Receptors. The Receptors, vol 34. Humana Press, Cham. 10.1007/978-3-319-90808-3_3
- 57.Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K. Pharmacology of adenosine receptors: the state of the art. Physiol Rev. 2018;98:1591–1625. doi: 10.1152/physrev.00049.2017. [DOI] [PubMed] [Google Scholar]
- 58.Aliberti JC, Souto JT, Marino AP, Lannes-Vieira J, Teixeira MM, Farber J, Gazzinelli RT, Silva JS. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am J Pathol. 2001;158:1433–1440. doi: 10.1016/s0002-9440(10)64094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013;61:1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanmarco LM, Ponce NE, Visconti LM, Eberhardt N, Theumer MG, Minguez AR, Aoki MP (2017) IL-6 promotes M2 macrophage polarization by modulating purinergic signaling and regulates the lethal release of nitric oxide during Trypanosoma cruzi infection, Biochimica et Biophysica Acta (BBA) - Molec Basis Dis 1863:857–869. 10.1016/j.bbadis.2017.01.006 [DOI] [PubMed]
- 61.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, Kassam N, Enjyoji K, Robson SC, Strom TB, Gao W. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9:2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garg NJ. Inflammasomes in cardiovascular diseases. Am J Cardiovasc Dis. 2011;1:244–254. [PMC free article] [PubMed] [Google Scholar]
- 63.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 64.Bono MR, Fernández D, Flores-Santibáñez F, Rosemblatt M, Sauma D (2015) CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett 5893454-60.10.1016/j.febslet.2015.07.027 [DOI] [PubMed]
- 65.Ernens I, Léonard F, Vausort M, Rolland-Turner M, Devaux Y, Wagner DR. Adenosine up-regulates vascular endothelial growth factor in human macrophages. Biochem Biophys Res Commun. 2010;392:351–356. doi: 10.1016/j.bbrc.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Munoz-Fernandez MA, Fernandez MA, Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxidedependent mechanism. Immunol Lett. 1992;33(1):35–40. doi: 10.1016/0165-2478(92)90090-B. [DOI] [PubMed] [Google Scholar]
- 67.Cardoni RL, Antunez MI, Morales C, Nantes IR (1997) Release of reactive oxygen species by phagocytic cells in response to live parasites in mice infected with Trypanosoma cruzi. Am J Trop Med Hyg 56329–34 [DOI] [PubMed]
- 68.Melo RC, Fabrino DL, D’Avila H, Teixeira HC, Ferreira AP. Production of hydrogen peroxide by peripheral blood monocytes and specific macrophages during experimental infection with Trypanosoma cruzi in vivo. Cell Biol Int. 2003;27:853–861. doi: 10.1016/S1065-6995(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 69.Alvarez MN, Piacenza L, Irigoin F, Peluffo G, Radi R. Macrophagederived peroxynitrite diffusion and toxicity to Trypanosoma cruzi. Arch Biochem Biophys. 2004;432:222–232. doi: 10.1016/j.abb.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 70.Gupta S, Wen JJ, Garg NJ (2009) Oxidative stress in chagas disease Interdiscipl. Perspect Infect Dis 1-8.10.1155/2009/190354 [DOI] [PMC free article] [PubMed]
- 71.Hung LM, Chen JK, Huang SS, Lee RS, Su ML. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes Cardiovasc. Res. 2000;47:549–555. doi: 10.1016/S0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 72.Lee KW, Lee HJ. Biphasic effects of dietary antioxidants on oxidative stress-mediated carcinogenesis Mech. Ageing Dev. 2006;127:424–431. doi: 10.1016/j.mad.2006.01.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials used in the experiment are available and are ready to be provided if needed.