Abstract
Nucleotide signaling is a key element of the neutrophil activation pathway. Neutrophil recruitment and migration to injured tissues is guided by purinergic receptor sensitization, mostly induced by extracellular adenosine triphosphate (ATP) and its hydrolysis product, adenosine (ADO), which is primarily produced by the CD39-CD73 axis located at the neutrophil cell surface. In inflammation unrelated to cancer, neutrophil activation via purinergic signaling aims to eliminate antigens and promote an immune response with minimal damage to healthy tissues; however, an antagonistic response may be expected in tumors. Indeed, alterations in purinergic signaling favor the accumulation of extracellular ATP and ADO in the microenvironment of solid tumors, which promote tumor progression by inducing cell proliferation, angiogenesis, and escape from immune surveillance. Since neutrophils and their N1/N2 polarization spectrum are being considered new components of cancer-related inflammation, the participation of purinergic signaling in pro-tumor activities of neutrophils should also be considered. However, there is a lack of studies investigating purinergic signaling in human neutrophil polarization and in tumor-associated neutrophils. In this review, we discussed the human neutrophil response elicited by nucleotides in inflammation and extrapolated its behavior in the context of cancer. Understanding these mechanisms in cancerous conditions may help to identify new biological targets and therapeutic strategies, particularly regarding tumors that are refractory to traditional chemo- and immunotherapy.
Keywords: Human neutrophils, Purinergic activation, Neutrophil migration, Purinergic signaling, Neutrophil modulation, Activation spectrum, N1/N2 profile
Introduction
Normal tissues are composed of different types of cellular, molecular, and microenvironmental signals that work together to ensure homeostasis and proper tissue functioning [1]. In nonphysiological conditions such as infection, tissue damage, or inflammatory processes, the initiation, triggering, or recruitment of innate immune cells and plasma proteins occurs at the sensitized site [2]. Although tissues are resistant to many disorders, the tumorigenesis process is capable of disrupting homeostasis to the point of no possible restoration [1].
The origins of solid cancer are not completely understood; however, its functional relationship with inflammation has been widely discussed [3, 4]. Chronic inflammation contributes to tumor stabilization based on the release of cytokines into the microenvironment. Tumor growth is sustained by the presence of immune cells, growth and angiogenesis factors, and DNA damage-promoting agents [4–6]. Cell proliferation caused by tissue regeneration after injury increases until tissue repair [7]. In contrast, cells continue to grow and develop in a chronic inflammatory microenvironment, establishing irreparable lesions. The power of inflammatory cells in tumor progression is undeniable as they promote neoplastic processes and provide an attractive tumor microenvironment (TME) [4–7]. The immune system is a regulated and integrated cellular network that preserves and restores homeostasis, and purinergic signaling helps to adjust the functions of immune cells [8].
Neutrophils, which are part of the polymorphonuclear (PMN) leukocyte family, have a major role during the early stages of the inflammatory response. They are the first leukocytes recruited to the injured site within a few hours of the damage. In addition, pathogens are eliminated through a variety of mechanisms such as degranulation, necrosis, and phagocytosis [9–11]. Specific chemokines and exogenous ligands are common mechanisms of neutrophil recruitment to injured sites [11, 12]. However, promoters of early migration of PMN cells to distant sites of metastasis in the absence of detectable inflammation are not yet defined [13]. In this regard, neutrophils can be associated with a quick response to any disturbance.
Stressed cells release ATP as a danger and “find me” signal, guiding the migration of phagocytes such as neutrophils [8, 14, 15]. Indeed, extracellular nucleotides and nucleosides, such as ATP and adenosine (ADO), are potent signaling molecules that, through activation of purinergic receptors (P2 and P1, respectively), modulate proliferation, differentiation, cell death, and immune/inflammatory responses [16, 17]. Each of these nucleotides is capable of signaling through distinct purinergic receptors. The P2 receptors are further classified as ionotropic P2X (P2X1-7) receptors and metabotropic P2Y (P2Y1, 2, 4, 6, 11, 12, 13, 14), which are G-protein-coupled receptors. While P2X receptors are exclusively activated by ATP, P2Y receptor responses are triggered by ATP, adenosine diphosphate (ADP), uridine triphosphate (UTP), uridine diphosphate (UDP), and UDP-glucose [18]. Metabotropic P1 receptors are activated by ADO. There are four P1 receptors in humans, A1, A2a, A2b, and A3, which exhibit differential affinity for ADO [19] (Table 1).
Table 1.
Purinergic receptors involved in neutrophil physiology
| Receptor | Agonist | Mechanism | Action on human neutrophils | References |
|---|---|---|---|---|
| A1 |
High ADO affinity (EC50 0.2-0.5 μM) |
Inhibition of cAMP formation by Gi/o-coupled protein |
↑ Adhesion ↑ Chemotaxis ↓ Neutrophils extravasation ↑ A2 upregulation |
[19, 20] |
| A2a | Low ADO affinity (EC50 0.6-0.9 μM) | Promotion of cAMP formation by Gs-coupled protein |
↓ Chemotaxis ↓ Adhesion ↓ ROS production ↓ Degranulation |
[20, 21] |
| A2b |
Very low ADO affinity (EC50 16-64 μM) |
Promotion of cAMP production by Gs-coupled protein |
↓ Release of VEGF ↓ Transendothelial migration ↓ Oxidative burst ↓ NET formation |
[22] |
| A3 |
High ADO affinity (EC50 0.2-0.5 μM) |
Inhibition of cAMP production and stimulation of IP3 production by Gi/o and Gq-coupled proteins |
↓ Migration ↑ Chemotaxis Regulation of directional movement and migration speed |
[23, 24] |
| P2Y2 | ATP | Gq protein increases cytosolic Ca2+ through interaction with the actin cytoskeleton |
↑ Chemotaxis ↑ Orientation in chemoattractant gradients ↑ Migration ↑ Superoxide production |
[23–26] |
| P2Y6 | UDP |
Gq protein stimulation causes PLCβ activation, Ca2+ mobilization, and IP3 formation |
Suppression of HNP1-mediated apoptosis Regulator of neutrophil IL-8-mediated chemotaxis ↓ Phagocytosis ↓ ROS production ↑ NET formation induced by gout-associated MSU |
[27, 28] |
| P2Y11 | ATP and NAD+ | Promotion of cAMP production by Gs protein | ↓Apoptosis | [29, 30] |
| P2X1 | ATP | Ion channels permeable for Na+, K+, and Ca2+ | ↓ Chemotaxis in response to LPS-induced autocrine ATP release | [8, 31] |
| P2X7 | ATP | Ion channels permeable for Na+, K+, and Ca2+ | ↑ Local immune responses by mediating ATP-induced NLRP3 inflammasome and IL-1β secretion | [32] |
Abbreviations: A2: α-2 adrenergic G-protein-coupled receptor; ADO: adenosine; ADP: Adenosine diphosphate; ATP: Adenosine triphosphate; cAMP: cyclic adenosine monophosphate; HNP1: human neutrophil peptide; IL-1β: interleukin 1 beta; IL-8: interleukin; IP3: inositol triphosphate; LPS: lipopolysaccharide; MSU: monosodium urate crystals; NAD+: nicotinamide adenine dinucleotide oxidized; NET: neutrophil extracellular traps; NLRP3: NOD-, LRR- and pyrin domain-containing protein 3; PLCβ: phospholipase C; ROS: reactive oxygen species; UDP: uridine diphosphate
Due to its proinflammatory actions, extracellular ATP is considered to be a damage-associated molecular pattern (DAMP) [14, 15, 33]. Conversely, extracellular ADO, which is mainly generated by the hydrolysis of ATP by ectonucleotidases, triggers immunosuppressive and immunomodulatory responses [19, 34]. There are two major ectonucleotidases responsible for the control of ATP and ADO levels in the bloodstream and at the surface of leukocytes, the ecto-nucleoside triphosphate diphosphohydrolase-1 (NTPDase1/CD39) and the ecto-5’-nucleotidase (CD73). The CD39-CD73 axis is also present on the surface of tumor cells. Together, these two enzymes convert extracellular ATP to ADO in a sequential manner [35] (Fig. 1).
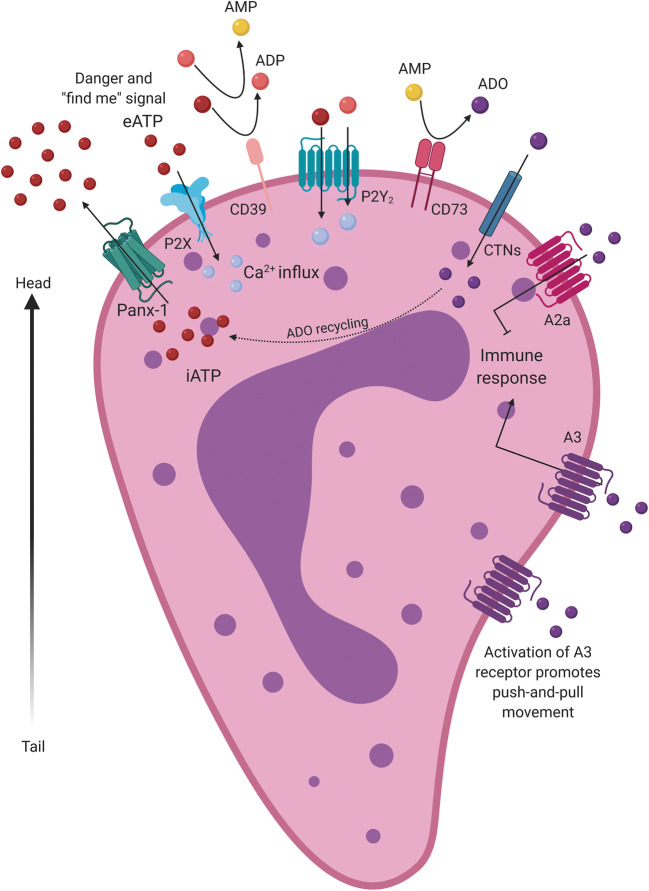
Fig. 1.
Neutrophil migration through purinergic pathway activation. Pannexin-1 (PNX-1) releases ATP (red balls), a danger, and “find me” signal. The increase in extracellular ATP potentiates neutrophil migration. ATP is hydrolyzed to ADP (pink balls) and ADP to AMP (yellow balls) by CD39. CD73 hydrolyzes AMP to adenosine (ADO) (purple balls). ATP recognizes P2X receptors and ATP/ADP/UTP/UDP binds P2Y2 receptors in neutrophils, inducing cell activation via intracellular Ca2+ release. Besides, extracellular adenosine binds to two main receptors: A2a and A3. The responses elicited by ATP and adenosine generate a movement of “push and pull” that regulates neutrophil phenotype and orients it migration
The neutrophil activation spectrum, classified as antitumor (N1) and pro-tumor (N2) neutrophil phenotypes, is similar to that proposed for macrophage polarization. However, these studies are preliminary, and there are no distinctive markers of maturation, activation, or polarization states of neutrophils; the effector mechanisms that modulate the leukocyte functional behavior and its role in disease perpetuation are not completely understood as well [6, 7, 12].
In line with the complexity of neutrophil polarization in solid tumors, some studies have shown that tumor-associated neutrophils (TANs) exhibit mixed characteristics of N1/N2 polarized cells [36]. Additionally, recent evidence points to arginase-1 and LOX-1 to be hallmarks of PMN myeloid-derived suppressor cells (PMN-MDSCs) [37]. MDSCs are a heterogeneous group of immature myeloid cells related to either the neutrophil (PMN-MDSCs) or monocyte (M-MDSC) differentiation pathways, which promote tumor growth by suppressing immune surveillance [38].
PMN-MDSCs, also recently proposed by some authors as neutrophils with proven immunosuppressive activity or, alternatively, as pathologically activated neutrophils [38, 39], inhibit T cell function, myeloid, and natural killer (NK) cells; enhance angiogenesis through the production of metalloproteinase-9 (MMP-9), prokineticin 2, and vascular endothelial growth factor (VEGF); and promote tumor metastasis [39]. The effects of extracellular purines on immunosuppressor cells have raised interest. For example, MDSCs overexpress P2X7 receptor that upon ATP binding induces arginase-1, reactive oxygen species (ROS), and transforming growth factor β (TGF-β) release, resulting in unexpected ATP immunosuppressive activity [40]. Additionally, ADO promotes the expansion of the MDSC population by engaging A2b receptors that are expressed on myeloid precursor cells [41]. It is important to note that the discovery of LOX-1 and arginase-1 as hallmarks of PMN-MDSCs may facilitate the understanding of immunosuppressive mechanisms of neutrophils in TME as well as its tumor-promoting role [37].
Neutrophil polarization to a pro-tumor phenotype may also be dependent on purinergic signaling. The increase in neutrophil activation and oxidative burst also depends on the autocrine mechanism previously described [23, 24, 42]. In addition, the extracellular ATP levels of non-self-source, such as the microenvironment or the injured site, could result in P2X and P2Y stimulation in a paracrine manner [8, 43]. Interestingly, the P2X7 expression and function also directly impacts on ATP content in the TME, which further determine the behavior of tumor-infiltrated immune cells [44]. Under desensitization conditions, such as prolonged stimulation with high ATP levels present in the TME, P2Y2 receptors expressed by neutrophils are useful for sustaining the signaling, due to the lower response, retaining cellular viability, which has already been demonstrated in macrophages [45]. Purinergic signaling is widely studied because it is found on the surface of most cells and is a fundamental component of the immune/inflammatory response. Recent studies explored the role of purinergic signaling in cancer-infiltrating immune cells, including macrophages, CD4+/CD8+ lymphocytes, NK, and MDSC cells. These investigations point that the modulation of purinergic signaling in tumor-associated immune cells supports proliferation, chemotaxis, and cytokine release [44, 46–49]. Although the well-known participation of purinergic signaling in neutrophil function regulation, little was investigated about how this pathway affects the neutrophil behavior in the TME. Here, we have reviewed which purinergic receptors contribute to neutrophil functions, in light of their diversity and plasticity. The discussion is focused on studies performed on human neutrophils, in view of the high heterogeneity in neutrophils, including immature, mature, aged neutrophils, PMN-MDSCs, and the lack of specific markers to define these subsets. Purinergic signaling in neutrophils under both acute and chronic inflammatory diseases has been explored and further extrapolated in the context of cancer-related inflammation.
Driving license: purinergic regulation of neutrophil migration and chemotaxis
Neutrophil migration depends on a frontal excitatory and a back inhibitory signal on the cell surface. ATP release via Pannexin-1 (PNX-1) induces the chemotaxis of neutrophils at the front edge by autocrine stimulation of the P2Y2 receptor. Subsequently, ADO is recognized by P1 type receptors, localized on the neutrophil tail, such as A2a, which blocks chemoattractant signaling and alternatively binds to A3 receptors, which stimulate immune migration. This purinergic feedback loop promotes neutrophil movement toward the chemoattractant source [8, 23]. The rapid conversion of extracellular ATP into ADO by neutrophils allows the activation of A2a receptors, providing an important counterpoint to the stimulation of P2Y2 and A3 receptors. Suppressive actions of A2a receptors provide a limiting mechanism for the main functions of neutrophils [50]. In summary, P2Y2 and A2a receptors mainly provide excitatory and inhibitory responses, respectively, producing a push-pull movement, thereby allowing neutrophil migration [23, 43]. As neutrophils are recruited in response to different stimuli, including bacterial products, complement proteins (C5a), immune complexes, chemokines, and cytokines [10], a phenotypic adaptation to these different microenvironments is inevitable [12].
P2Y receptors have been shown to be major influencers of neutrophil activation. Indeed, the release of IL-8 a major chemokine for neutrophils is regulated by P2 receptors sensitization. In this regard, P2Y6 induces IL-8 secretion from human monocytes, which in turn controls in vitro neutrophil migration [51]. Moreover, P2 receptor activation, particularly P2Y2, is required for IL-8-induced neutrophil chemotaxis [52]. Finally, Kukulski and colleagues demonstrated with a transwell apparatus that P2Y2 receptor activation is necessary for TLR4-induced in vitro transendothelial neutrophil migration, which was potentiated by UTP, a P2Y2 agonist. However, in opposite to which was expected, this phenomenon was mostly regulated by Rho kinase pathway, rather than IL-8 release [53]. Therefore, extracellular nucleotides participate of crosstalk among immune and endothelial cells, orchestrating the neutrophil responses.
Interestingly, Gabl and colleagues argued that P2Y2 downregulation in neutrophils probably originates from inside by a novel cytoskeleton-dependent mechanism [25]. They demonstrated that receptors occupied by their ligands undergo an agonist-induced conformational change, which elevates intracellular Ca2+ levels by coupled G-protein signaling. The authors also proposed that the P2Y2 receptor blockade inhibits the NADPH oxidation signaling pathway [25].
Neutrophil chemoattraction involves chemokines, lipids, anaphylatoxins from the complement system (C5a-C3a), and platelet activation factor (PAF), but IL-8 promotes a more potent binding with CXCR1 and CXCR2. There are also additional mediators that can work as recruiters of neutrophils [48, 52, 53]. Thus, the purinergic system also plays an important role in activating neutrophil migration. Besides, this pathway contains signaling molecules that modulate differentiation and proliferation and that are able to control inflammatory events, which might be responsible for neutrophil activation.
The release of microenvironment chemokines plays an essential role in tumor progression. Considering that in glioblastoma cells the purinergic signaling is active, a study from our group showed that the spontaneous and lipopolysaccharide (LPS)-mediated IL-8 release by tumor cells is dependent on P2Y6 and P2X7, which in turn promotes glioma cell proliferation [54] and may induce in vivo neutrophil recruitment. In addition, the neutrophil expression of CD39 may facilitate the molding of the immune response [55]. Indeed, IL-8 production is controlled by the activity of CD39 expressed by human neutrophils. Interestingly, ATP is a potent stimulus for IL-8 release by neutrophils only upon CD39 inhibition, suggesting that at physiological condition neutrophils remain unresponsive to nucleotide stimulation due to its intrinsic CD39 activity [55]. Neutrophil participation in tumor progression has been investigated in preclinical studies of animals. However, few studies have correlated tumors, neutrophils, and purinergic signaling in humans.
The purinergic cascade produces a very important metabolite in neutrophil activation and migration control, the ADO. A study showed that ADO promotes chemotaxis while inhibiting the activation and the consequent release of ROS [56]. Hence, neutrophils migrate to the site of infection without damaging healthy tissues along their path.
CD39 hydrolyzes ATP to AMP in a sequential manner; a second enzyme, CD73, hydrolyzes AMP to ADO. Therefore, these enzymes profoundly influence immune response [35]. The abnormal activity of CD39 and CD73 produces high amounts of ADO and may favor an immunosuppressive environment, which reinforces cancer development by impairing immune surveillance [57]. Maintaining the harmony between inflammatory and anti-inflammatory responses prevents exacerbated immunosuppression or uncontrolled inflammation, as the CD39-CD73 axis can promote the self-tolerance mechanism. The outcome of the activity escalation of these enzymes generates elevated levels of extracellular immunosuppressive ADO [35, 57].
Survival of the dead: neutrophil modulation through purinergic activation
Neutrophils are commonly believed to remain viable in circulation for approximately 4 days, followed by apoptosis [12, 58]. Understanding the mechanisms that affect the life span of neutrophils may help to identify new therapeutic targets. The following paragraphs highlight the importance of the neutrophil life span, in view of the fact that nucleotides affect neutrophil apoptosis.
Neutrophils have few mitochondria in their cytosol and therefore produce energy mainly through glycolysis. Thus, mitochondria rarely participate in ATP formation [42, 50]. However, for a higher level of intracellular ATP production, the cell relies on the tricarboxylic acid cycle (TCA), probably by the activation of the mTOR pathway, an important metabolic pathway that regulates biological and physiological processes such as proliferation, growth, cell survival, and autophagy. This allows the flow of Ca2+ into the neutrophil mitochondria, which is related to the activation of the P2Y2 receptor, leading to the production of intracellular ATP. The ATP produced is externalized by PNX-1, which impacts the P2Y2 receptors in an autocrine manner, potentiating neutrophil migration [42].
P2Y2 overstimulation is unfavorable under different circumstances. An investigation showed that increased levels of systemic ATP in sepsis impair neutrophil functions by disrupting the endogenous purinergic signaling mechanisms that regulate cell activation and chemotaxis mediated by P2Y2. The authors proposed that targeting systemic ATP may improve neutrophil function and host defenses, as a new therapeutic strategy for sepsis treatment [43].
Upon activation, TCA significantly increases the production of ATP. In this scenario, the NADH produced is oxidized in the respiratory chain reaction, ATP is synthesized, and NAD+ returns to the cycle. A study observed that increased NAD+ levels are directly related to neutrophil aging, probably because of increased energy demand. Moreover, intracellular ATP levels are not consistent with expectations. It is argued that there may be an increase in ATP synthesis but also an increase in consumption, and therefore the final energy balance is lower [59]. Thus, ATP levels are decreased in aged neutrophils. Considering that intracellular ATP is produced by activating purinergic signaling and the few existing mitochondria, the decrease in ATP may be caused by increased energy demand or decreased production [59].
Another P2Y receptor, P2Y6, has been drawing attention for its relationship to neutrophil apoptosis inhibition. The study conducted by Nagaoka and colleagues evaluated the interaction between the P2Y6 antagonist (MRS2578) and apoptotic behavior [27]. The authors observed that apoptosis was reactivated in the presence of the P2Y6 antagonist. The P2Y6 ligand, UDP, induced suppression of programmed cell death when bound to the receptor. MRS2578 also prevented the binding of P2Y6 to its ligand, allowing neutrophil apoptosis, suggesting that the induction by HNP-1 downregulated pro-apoptotic and upregulated the anti-apoptotic activities by Bcl-xl, which in turn inhibited apoptosis. The mitochondrial membrane potential and caspase-3 activity resulted in decreased pro-apoptotic signals through the P2Y6 signaling pathway [27].
In cancer, increased survival of TAN has been proposed to play an important role in the development and growth of tumor mass [60]. Therefore, further studies on the life span of TAN in cancer and the purinergic pathway in neutrophil activation and migration, as well as its connection with cellular death, may help in identifying new molecular targets for cancer therapeutics.
Suicide squad: extracellular nucleotide levels in inflammation and cancer
Injured tissues, whether inflamed or infected, secrete neutrophil recruitment chemokines that signal the attack site to peripheral blood circulating neutrophils. Neutrophils are the first immune cells to reach the damaged tissue. It can be said that these cells are the infantry of our immune system. Upon arrival at the injured site, neutrophils begin the process of receptor-mediated respiratory burst and degranulation, leading to neutrophil apoptosis [61].
An investigation performed by Patel and collaborators [13] observed the chemotactic activity of PMN-MDSCs from cancer patients when compared to that of control neutrophils. The study found that there was less chemotactic activity in PMN-MDSCs, probably due to the lack of extracellular ADO, suggesting that ATP hydrolysis might be slowed down in this situation [13].
Cancer can be characterized as chronic inflammation. ATP levels in cancer are higher than those under physiological conditions, as a result of ATP release from necrotic, stromal, and cancer cells as well as from stress and hypoxia factors [14, 44, 62]. In addition, the mechanism by which ATP is secreted in the extracellular medium is crucial for P2-mediated responses [14]. Extracellular ATP has a dual role in cancer, which includes an antitumor immune response inducing tumor cell death and a pro-tumor response that increases the proliferation and metastasis of cancer cells [63]. Hypoxia is a tumor condition that increases CD39 and CD73 expression, and consequently ADO formation, which is associated with resistance to chemotherapy due to its immunosuppressor effect [34]. Thus, purinergic signaling can modulate cancer progression by activating P2 and P1 receptors expressed by tumors as well as immune-associated cells [40, 41, 54, 63].
During noncancerous inflammation, ATP is released at high concentrations by injured cells as a “danger signal” or DAMP to restore tissue integrity [14, 15, 33, 40]. In this scenario, P2X receptors are upregulated in immune cells including neutrophils, macrophages, and lymphocytes [18]. The P2X7 receptor is particularly involved in inflammation by releasing proinflammatory cytokines such as IL-1β and tumor necrosis factor-α (TNF-α) [32, 64]. In addition, P2X7 promotes PI3K/Akt activation, HIF1α expression, and VEGF secretion, regulating MYCN oncogene which further implicate in cell proliferation and poor overall survival of patients with neuroblastoma [65, 66]. Regarding P1 receptor, A3 receptor plays an important role in the migration of neutrophils to inflammation sites [8].
The need for extracellular ATP for direct migration as well as its modulation in the release of proteolytic enzymes has been discussed in previous studies [13, 23]. On one hand, neutrophil recruitment is necessary for maintaining homeostasis, and on the other hand, neutrophil enzymes lack specificity for necrotic cells, and this causes damage to the adjacent tissues [3]. This nonspecific behavior may be one of the factors influencing cancer progression.
A feature of ADO that deserves attention is its ability to inhibit proinflammatory mediator production by monocytes and dendritic cells (DCs), such as ROS and TNF-α, in addition to the A2a-mediated immunosuppressive function in T-regulatory cells [47]. Moreover, P2X7 is overexpressed in several malignancies as well as in immune cells, where it participates in growth-promoting activity and contributes to TME composition via regulation of cytokine release, including TGF-β and IL-1β [65, 67, 68]. P2X7 antagonism is also related to downregulation of CD39-CD73 axis in CD4+ T-effector cells and DCs, further decreasing the ADO levels in TME [44]. Taken together, these characteristics may be involved in the maintenance of the TME, considering that extracellular ADO has immunosuppressive action while recruiting more leukocytes.
Neutrophil and monocyte modulation may also be related to purinergic signaling. In the case of neutrophil activation by gram-positive pathogens, in vitro a study showed that the inhibition of CD73 decreased the ability of PMN cells to kill bacteria, suggesting that the ablation of enzymes that generates extracellular ADO impairs both the recruitment and bactericidal activity of PMNs [69]. Therefore, ADO affects neutrophil-killing cellular functions.
Changes in the number of neutrophil extracellular traps (NETs) are related to autoimmunity promotion, the presence of vascular diseases, and thrombosis and contribute to tumor progression and metastasis. A relationship between elevated NET production and poor prognosis in human tumors has been shown in previous studies. Indeed, these NETs are capable of catching circulating tumor cells and favor metastatic implant formation [3, 70, 71].
Tumor hustle: neutrophils wrap cancer cells
In solid tumors, the presence of DAMPs and cytokines in the TME may induce differential neutrophil responses [3, 7, 38]. Moreover, the lack of specificity of neutrophil enzymes may contribute to cancer progression, which is especially associated with purinergic pathway activation. ADO is a key molecule with an established role as an immunosuppressive agent, regulating immune cell recruitment, modulating neutrophil-killing features, and promoting cancer progression.
N1- and N2-like neutrophils represent extremes of different molecular phenotypes, which depend on the microenvironment [7]. Considering that purinergic signaling has great influence on neutrophil activation and migration, our hypothesis is that the neutrophil activation spectrum is related to the activation and signaling of purinergic receptors.
Few studies have shown the relationship between these phenotypes and the purinergic signaling. It is known that the immune system plays a fundamental role in tumor progression, although all the mechanisms are not yet well elucidated. In vivo and in vitro studies have found that these PMN leukocytes modulate the TME [5, 6]. The antitumor phenotype is characterized by enhanced expression of TNF-α, CCL3, and ICAM-1, and reduced arginase-1 production, inhibition of angiogenesis, and promotion of antitumor response of T lymphocytes [72, 73]. In contrast, it is discussed that the immunosuppressive phenotype is acquired by the presence of TGF-β, favoring the infiltration of neutrophils with high expression of CXCR4, VEGF-A, and MMP-9 [73]. Neutrophils are the major producers of VEGF-A and delivery high levels of MMP-9, which releases the active form of VEGF-A from the extracellular matrix. However, pro-tumor neutrophils are able to discharge MMP-9 even in the absence of a protease inhibitor, and increased levels contribute to angiogenesis and tissue invasion [74]. P2X7 is described as an important angiogenesis and immunossupressive mediator as its sensitization results in VEGF and TGF-β release in TME [44, 66]. Although the expression of P2X7 on human neutrophils is controversial [32, 75], the soluble factors present in the TME as a consequence of P2X7 activity certainly impact the function and phenotype of TANs.
In cancer, the formation of metastasis is linked to NET release, in which circulating tumor cells are trapped by neutrophils, facilitating their deposition at distant sites of metastasis [76–78]. The quantification of NETs in patients diagnosed with cancer remains challenging; however, the presence of NETs in the tumor niche is associated with a worse prognosis [79] and indirectly links with patient survival [80–82].
The participation of purinergic signaling in NETs formation has been investigated in inflammatory conditions, including deficiency of ADA2 and gout [28, 83, 84]. Indeed, ADO contributes to NETs release via A1 and A3 receptor activation expressed on neutrophils [83], while A2a receptor induces the opposite effect [84]. Regarding P2 receptors, a study performed by Sil and colleagues demonstrated that P2Y6 receptor is essential for regulating neutrophil functions in gout disease. The investigation elucidated that P2Y6/store-operated Ca2+ influx/IL-8 axis participates in MSU crystal-induced NET formation, suggesting P2Y6 as an interesting target to modulate neutrophil function and activation [28]. Therefore, the antagonism of purinergic receptors may be an alternative to debilitate the pro-tumor immune response of neutrophils in TME as well as its over-activation in inflammatory diseases.
Concluding remarks
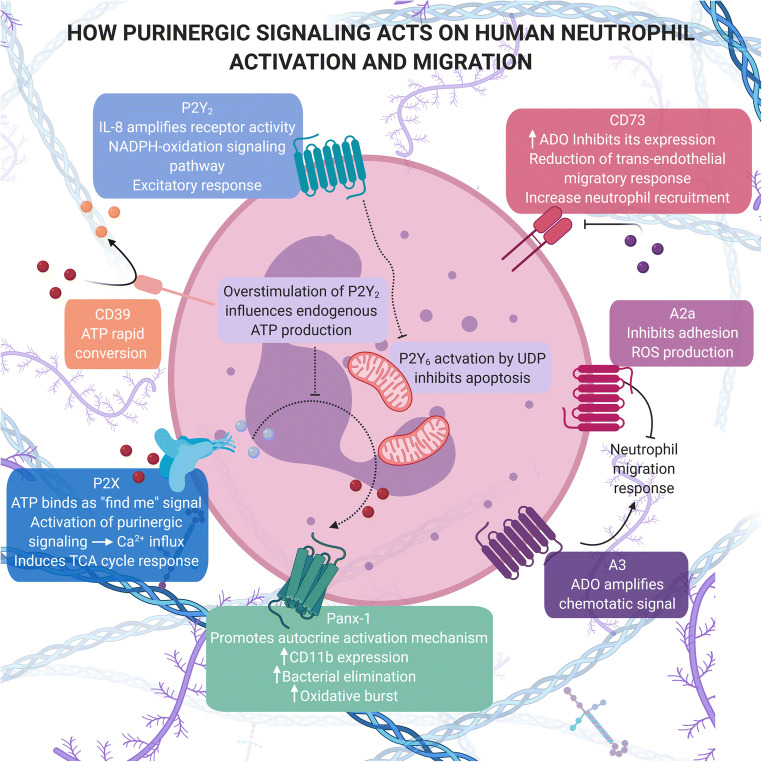
To summarize, as several studies have shown, the purinergic pathway profoundly influences neutrophil features. The driving license of neutrophils has a P2Y2 stamp, seeing that excitatory and inhibitory responses from this receptor and A2a produce a push-pull movement, allowing neutrophil migration. In fact, IL-8 favors ATP-mediated P2Y2 sensitization and regulates neutrophil migration. In addition, P2Y2 activation increases intracellular Ca2+ levels and blocks NADPH oxidation, inducing a conformational change in the cytoskeleton. Nevertheless, high ATP levels and P2Y2 overstimulation can disrupt physiological responses [25, 43, 53].
Although neutrophils are not “zombies,” their aging process has subtle characteristics. Higher NAD+ levels and smaller ATP levels are present, due to the increased energy demand in aging cells. Meanwhile, neutrophils can act like zombies, as P2Y6 pathway stimulation blocks neutrophil apoptosis via mitochondrial membrane potential, caspase-3 activity, and Bcl-xl upregulation, increasing neutrophil life span [27, 59].
Above all, neutrophils are the immune suicide squad, since these cells are the first line of body defense and their attack mechanism results in neutrophil death, contributing to their short life span. In this case, a small change can impair the immune response and allow tumor progression. As shown in this review, the purinergic system plays a crucial role in neutrophil action. For instance, neutrophil activation can lead to extracellular ATP hydrolysis and consequently chemotaxis regulated by P1 receptors. ADO receptors also modulate migration, adhesion, and ROS release, allowing neutrophils to migrate without shattering other tissues [8, 14, 56, 61] (Fig. 2).
Fig. 2.
Overview of purinergic activation in neutrophils
Although preclinical models are a great option for disease research, studies show that there are differences between human and murine immune cells that should be considered [85, 86]. In this regard, it is suggested that at least two different approaches should be used in the in vivo model so that the conclusions reflect the true contribution of neutrophils [87–89]. The worldwide movement around proposals that reduce the number of animals in research reinforces the need to promote actions that use alternatives; thus, the use of human neutrophils, when suitable, comes from a sample that generates little or no stress to the individual.
The literature regarding human neutrophils in a cancer context is interfered by the unclear identification of different subsets and with functions that might be misinterpreted. Understanding how the activation of purinergic receptors generates intracellular responses might be helpful in discovering novel drug targets. Tumor behavior in the presence of immune cells has attracted attention due to the close connection between them. In summary, more studies regarding neutrophil purinergic activation in human tumor sites are needed to provide new therapeutic strategies based in purine targets.
Glossary
- A2
α-2 adrenergic G-protein-coupled receptor.
- A1/A2a/A2b/A3
P1 purinergic receptors sensitized by adenosine.
- ADO
Adenosine is a purine nucleoside that participates in the purinergic system as a form of extracellular signaling, modulating proliferation, differentiation, cell death, and control of inflammatory response events, acting mainly as an immunosuppressive/immunomodulatory molecule via P1 receptor sensitization.
- ADP
Adenosine diphosphate is a nucleotide that also participates in the purinergic system as a form of extracellular signaling, inducing platelet aggregation and microglial migration via P2Y12 sensitization.
- AMP
Adenosine monophosphate is a nucleotide formed in the extracellular environment mainly via ATP hydrolysis mediated by NTPDase1/CD39 enzyme activity. Until now, no purine-receptor has been described to be activated by this nucleotide.
- ATP
Adenosine triphosphate is a purine nucleotide involved in complex signaling pathways, including driving energy to the cells and being a precursor to DNA and RNA. In this case, it participates in the purinergic system, a form of extracellular signaling via the P2 receptor agonist.
- NTPDase1/CD39
Ecto-nucleoside triphosphate diphosphohydrolase-1 is an enzyme located at the cell surface of immune cells and some cancer cells that hydrolyze the P2 receptor ligands ATP, ADP, UTP, and UDP to the respective monophosphate-nucleosides by removing one phosphate at a time.
- CD73
Ecto-5’-nucleotidase is an enzyme present on the cell surface of a large number of tissues that is responsible for converting AMP to ADO in the purinergic system. It also acts as a cell-cell and cell-matrix protein, important for cell communication and migration, by potentiating EGFR/Akt and VEGF/Akt pathways. In addition, it promotes invasion, migration, and adhesion of tumor cells.
- HIF1α
Hypoxia-inducible factor 1-alpha. It is a transcriptional regulator of cellular and developmental response to hypoxia.
- HNP-1
Human neutrophil peptide 1 belonging to the α-defensin family of antimicrobial peptides.
- IL-8
Interleukin-8, a chemokine released by macrophages and other cells of the innate immune response that attracts neutrophils and other immune cells to the tumor or infection site. It is also involved in angiogenesis, cell proliferation, and tissue remodeling.
- LPS
Lipopolysaccharide is a large molecule made of a lipid and a polysaccharide that occurs in the membrane of Gram-negative bacteria, acting as a trigger for the innate immune system; it is classified as a PAMP (pathogen-associated molecular pattern).
- N1
Neutrophils with antitumor activities.
- N2
Neutrophils with pro-tumor activities.
- NAD+
The oxidized form of nicotinamide adenine dinucleotide, a cofactor involved in redox reactions, transporting electrons from one substrate to another.
- NADH
The reduced form of NAD+, a cofactor involved in redox reactions.
- NADP+
A coenzyme called nicotinamide adenine dinucleotide phosphate, acting as a cofactor in anabolic metabolism.
- NETs
Neutrophil extracellular traps are a defense mechanism, where neutrophils release chromatin to form an extracellular fibril matrix, which traps pathogens.
- P2 receptors (P2Y1, P2Y2, P2Y6, P2X1, P2X7)
Receptors that are activated by purines (e.g. ATP, ADP) or pyrimidines (e.g. UTP, UDP).
- PNX-1
Pannexin-1 is a large transmembrane channel in the plasmatic membrane, allowing the passage of ions and small molecules, such as ATP.
- TLR4
Toll-like receptor 4 is a cell surface receptor activated by LPS derived from Gram-negative bacteria or by endogenous ligands such as HMGB1, which elicit potent innate immune responses in several cells such as macrophages, dendritic cells, and neutrophils.
- UDP-glucose
Uridine diphosphate-glucose is a nucleotide sugar involved in glycosyl-transferase reactions that activates one of the P2 purinergic receptors.
- VCAM-1
Vascular cell adhesion molecule-1 is a cell adhesion molecule expressed by the vascular endothelium.
Dominique S. Rubenich
is bachelor in biomedicine from the Federal University of Health Sciences of Porto Alegre (UFCSPA), and Master in Biosciences from UFCSPA. During her master's degree, she worked at the Research Laboratory of the University Hospital of the University of Laval (CHUL) in Quebec, CA, under the supervision of Dr. Jean Sevigny. Currently, she is CNPq doctoral candidate at Biosciences Graduation Course at UFCSPA with guidance of Dr. Elizandra Braganhol. Co-creator of the science publicity Instagram @ciencia.traduzada.

Funding
The authors would like to thank the Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS; process number 19/2551-0000663-2), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; code 001), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq –process numbers 312187/2018-1; 400882/2019-1), Hospital de Clínicas de Porto Alegre (FIPE process number 2019–0446), Santa Casa de Misericórdia de Porto Alegre, and Universidade Federal de Ciências da Saúde de Porto Alegre. D.S. Rubenich, P.O. de Souza, N. Omizzollo, G.S. Lenz, and E. Braganhol are recipients of UFCSPA, FAPERGS, CAPES, and CNPq fellowships.
Data availability
It is not applicable.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
All authors are in agreement with the content of the manuscript and with the submission.
Conflict of interest
The authors declare no competing interests.
Footnotes
Highlights
1. Activation and upregulation of the purinergic system could favor pro-tumor neutrophil activity.
2. Purinergic receptors P2Y2, A2a, and A3 guide neutrophil migration through an ATP concentration gradient, TLR4 stimulation, or IL-8 secretion.
3. Neutrophil migration to injured sites is impaired by the decrease in extracellular adenosine levels mediated by CD73 inhibition.
4. Extracellular adenosine plays a key role in NET production via A1 and A3 receptor sensitization.
5. P2Y6 signaling upregulates the Bcl-xl-mediated anti-apoptotic pathway and inhibits neutrophil apoptosis.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Basanta D, Anderson ARA. Homeostasis Back and Forth: An Ecoevolutionary Perspective of Cancer. Cold Spring Harb Perspect Med. 2017;7:a028332. doi: 10.1101/cshperspect.a028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 3.Lecot P, Sarabi M, Pereira Abrantes M, Mussard J, Koenderman L, Caux C, Bendriss-Vermare N, Michallet M-C (2019) Neutrophil Heterogeneity in Cancer: From Biology to Therapies. Front Immunol 10. 10.3389/fimmu.2019.02155 [DOI] [PMC free article] [PubMed]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200–207. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Grecian R, Whyte MKB, Walmsley SR. The role of neutrophils in cancer. Br Med Bull. 2018;128:5–14. doi: 10.1093/bmb/ldy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Chen D (2018) Purinergic Regulation of Neutrophil Function. Front Immunol 9. 10.3389/fimmu.2018.00399 [DOI] [PMC free article] [PubMed]
- 9.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil Function: From Mechanisms to Disease. Annu Rev Immunol. 2012;30:459–489. doi: 10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 12.Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159–2167. doi: 10.1182/blood-2018-11-844548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel S, Fu S, Mastio J, Dominguez GA, Purohit A, Kossenkov A, Lin C, Alicea-Torres K, Sehgal M, Nefedova Y, Zhou J, Languino LR, Clendenin C, Vonderheide RH, Mulligan C, Nam B, Hockstein N, Masters G, Guarino M, Schug ZT, Altieri DC, Gabrilovich DI. Unique pattern of neutrophil migration and function during tumor progression. Nat Immunol. 2018;19:1236–1247. doi: 10.1038/s41590-018-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dosch M, Gerber J, Jebbawi F, Beldi G. Mechanisms of ATP Release by Inflammatory Cells. Int J Mol Sci. 2018;19:1222. doi: 10.3390/ijms19041222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moesta AK, Li X-Y, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol. 2020;20:739–755. doi: 10.1038/s41577-020-0376-4. [DOI] [PubMed] [Google Scholar]
- 17.Boison D, Yegutkin GG. Adenosine Metabolism: Emerging Concepts for Cancer Therapy. Cancer Cell. 2019;36:582–596. doi: 10.1016/j.ccell.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnstock G. Introduction to Purinergic Signaling. Methods Mol Biol. 2041;2020:1–15. doi: 10.1007/978-1-4939-9717-6_1. [DOI] [PubMed] [Google Scholar]
- 19.Peleli M, Fredholm BB, Sobrevia L, Carlström M. Pharmacological targeting of adenosine receptor signaling. Mol Asp Med. 2017;55:4–8. doi: 10.1016/j.mam.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Dianzani C, Brunelleschi S, Viano I, Fantozzi R. Adenosine modulation of primed human neutrophils. Eur J Pharmacol. 1994;263:223–226. doi: 10.1016/0014-2999(94)90547-9. [DOI] [PubMed] [Google Scholar]
- 21.Fredholm BB, Zhang Y, van der Ploeg I. Adenosine A2A receptors mediate the inhibitory effect of adenosine on formyl-Met-Leu-Phe-stimulated respiratory burst in neutrophil leucocytes. Naunyn Schmiedeberg's Arch Pharmacol. 1996;354:262–267. doi: 10.1007/BF00171056. [DOI] [PubMed] [Google Scholar]
- 22.Bazzichi L, Trincavelli L, Rossi A, De Feo F, Lucacchini A, Bombardieri S, Martini C. A2B adenosine receptor activity is reduced in neutrophils from patients with systemic sclerosis. Arthritis Res Ther. 2005;7:R189–R195. doi: 10.1186/ar1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP Release Guides Neutrophil Chemotaxis via P2Y2 and A3 Receptors. Science (80-) 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 24.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside Triphosphate Diphosphohydrolase 1 (E-NTPDase1/CD39) Regulates Neutrophil Chemotaxis by Hydrolyzing Released ATP to Adenosine. J Biol Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabl M, Winther M, Welin A, Karlsson A, Oprea T, Bylund J, Dahlgren C, Forsman H. P2Y2 receptor signaling in neutrophils is regulated from inside by a novel cytoskeleton-dependent mechanism. Exp Cell Res. 2015;336:242–252. doi: 10.1016/j.yexcr.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Önnheim K, Christenson K, Gabl M, Burbiel JC, Müller CE, Oprea TI, Bylund J, Dahlgren C, Forsman H. A novel receptor cross-talk between the ATP receptor P2Y2 and formyl peptide receptors reactivates desensitized neutrophils to produce superoxide. Exp Cell Res. 2014;323:209–217. doi: 10.1016/j.yexcr.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Nagaoka (2010) Evaluation of the effect of α-defensin human neutrophil peptides on neutrophil apoptosis. Int J Mol Med 26. 10.3892/ijmm_00000544 [DOI] [PubMed]
- 28.Sil P, Hayes CP, Reaves BJ, Breen P, Quinn S, Sokolove J, Rada B. P2Y6 Receptor Antagonist MRS2578 Inhibits Neutrophil Activation and Aggregated Neutrophil Extracellular Trap Formation Induced by Gout-Associated Monosodium Urate Crystals. J Immunol. 2017;198:428–442. doi: 10.4049/jimmunol.1600766. [DOI] [PubMed] [Google Scholar]
- 29.Pliyev BK, Ivanova AV, Savchenko VG. Extracellular NAD+ inhibits human neutrophil apoptosis. Apoptosis. 2014;19:581–593. doi: 10.1007/s10495-013-0948-x. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, Kassack MU, Bingle CD, Sabroe I, Surprenant A, Whyte MKB. Inhibition of Neutrophil Apoptosis by ATP Is Mediated by the P2Y 11 Receptor. J Immunol. 2007;179:8544–8553. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Qin W, Xu X, Xiong Y, Zhang Y, Zhang H, Sun B. Endotoxin-induced autocrine ATP signaling inhibits neutrophil chemotaxis through enhancing myosin light chain phosphorylation. Proc Natl Acad Sci. 2017;114:4483–4488. doi: 10.1073/pnas.1616752114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat Commun. 2016;7:10555. doi: 10.1038/ncomms10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandolfi F, Altamura S, Frosali S, Conti P. Key Role of DAMP in Inflammation, Cancer, and Tissue Repair. Clin Ther. 2016;38:1017–1028. doi: 10.1016/j.clinthera.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 34.Allard D, Chrobak P, Allard B, Messaoudi N, Stagg J. Targeting the CD73-adenosine axis in immuno-oncology. Immunol Lett. 2019;205:31–39. doi: 10.1016/j.imlet.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Giuliani AL, Sarti AC, Di Virgilio F. Ectonucleotidases in Acute and Chronic Inflammation. Front Pharmacol. 2020;11:619458. doi: 10.3389/fphar.2020.619458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponzetta A, Carriero R, Carnevale S, Barbagallo M, Molgora M, Perucchini C, Magrini E, Gianni F, Kunderfranco P, Polentarutti N, Pasqualini F, Di Marco S, Supino D, Peano C, Cananzi F, Colombo P, Pilotti S, Alomar SY, Bonavita E, Galdiero MR, Garlanda C, Mantovani A, Jaillon S. Neutrophils Driving Unconventional T Cells Mediate Resistance against Murine Sarcomas and Selected Human Tumors. Cell. 2019;178:346–360.e24. doi: 10.1016/j.cell.2019.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si Y, Merz SF, Jansen P, Wang B, Bruderek K, Altenhoff P, Mattheis S, Lang S, Gunzer M, Klode J, Squire A, Brandau S. Multidimensional imaging provides evidence for down-regulation of T cell effector function by MDSC in human cancer tissue. Sci Immunol. 2019;4:eaaw9159. doi: 10.1126/sciimmunol.aaw9159. [DOI] [PubMed] [Google Scholar]
- 38.Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN-MDSC: Their biological role and interaction with stromal cells. Semin Immunol. 2018;35:19–28. doi: 10.1016/j.smim.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Virgilio F, Vuerich M. Purinergic signaling in the immune system. Auton Neurosci. 2015;191:117–123. doi: 10.1016/j.autneu.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic Regulation of the Expansion and Immunosuppressive Activity of CD11b + Gr1 + Cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao Y, Ledderose C, Seier T, Graf AF, Brix B, Chong E, Junger WG. Mitochondria Regulate Neutrophil Activation by Generating ATP for Autocrine Purinergic Signaling. J Biol Chem. 2014;289:26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Kondo Y, Bao Y, Staudenmaier L, Lee A, Zhang J, Ledderose C, Junger WG. Systemic Adenosine Triphosphate Impairs Neutrophil Chemotaxis and Host Defense in Sepsis. Crit Care Med. 2017;45:e97–e104. doi: 10.1097/CCM.0000000000002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Marchi E, Orioli E, Pegoraro A, Sangaletti S, Portararo P, Curti A, Colombo MP, Di Virgilio F, Adinolfi E. The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene. 2019;38:3636–3650. doi: 10.1038/s41388-019-0684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.del Rey A, Renigunta V, Dalpke AH, Leipziger J, Matos JE, Robaye B, Zuzarte M, Kavelaars A, Hanley PJ. Knock-out Mice Reveal the Contributions of P2Y and P2X Receptors to Nucleotide-induced Ca2+ Signaling in Macrophages. J Biol Chem. 2006;281:35147–35155. doi: 10.1074/jbc.M607713200. [DOI] [PubMed] [Google Scholar]
- 46.Adinolfi E, De Marchi E, Orioli E, Pegoraro A, Di Virgilio F. Role of the P2X7 receptor in tumor-associated inflammation. Curr Opin Pharmacol. 2019;47:59–64. doi: 10.1016/j.coph.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709–724. doi: 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 48.Li X-Y, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, Madore J, Lepletier A, Aguilera AR, Sundarrajan A, Jacoberger-Foissac C, Wong C, dela Cruz T, Welch M, Lerner AG, Spatola BN, Soros VB, Corbin J, Anderson AC, Effern M, Hölzel M, Robson SC, Johnston RL, Waddell N, Smith C, Bald T, Geetha N, Beers C, Teng MWL, Smyth MJ. Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity. Cancer Discov. 2019;9:1754–1773. doi: 10.1158/2159-8290.CD-19-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan J, Li X-Y, Roman Aguilera A, Xiao C, Jacoberger-Foissac C, Nowlan B, Robson SC, Beers C, Moesta AK, Geetha N, Teng MWL, Smyth MJ. Control of Metastases via Myeloid CD39 and NK Cell Effector Function. Cancer Immunol Res. 2020;8:356–367. doi: 10.1158/2326-6066.CIR-19-0749. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Yao Y, Sumi Y, Li A, U.K. To. Elkhal A, Inoue Y, Woehrle T, Zhang Q, Hauser C, Junger WG. Purinergic Signaling: A Fundamental Mechanism in Neutrophil Activation. Sci Signal. 2010;3:ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kukulski F, Ben Yebdri F, Lefebvre J, Warny M, Tessier PA, Sévigny J. Extracellular nucleotides mediate LPS-induced neutrophil migration in vitro and in vivo. J Leukoc Biol. 2007;81:1269–1275. doi: 10.1189/jlb.1206758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Lévesque SA, Martín-Satué M, Sévigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46:166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kukulski F, Ben Yebdri F, Bahrami F, Fausther M, Tremblay A, Sévigny J. Endothelial P2Y2 receptor regulates LPS-induced neutrophil transendothelial migration in vitro. Mol Immunol. 2010;47:991–999. doi: 10.1016/j.molimm.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braganhol E, Kukulski F, Lévesque SA, Fausther M, Lavoie EG, Zanotto-Filho A, Bergamin LS, Pelletier J, Bahrami F, Ben Yebdri F, Fonseca Moreira JC, Battastini AMO, Sévigny J. Nucleotide receptors control IL-8/CXCL8 and MCP-1/CCL2 secretions as well as proliferation in human glioma cells. Biochim Biophys Acta Mol basis Dis. 2015;1852:120–130. doi: 10.1016/j.bbadis.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 55.Kukulski F, Bahrami F, Ben Yebdri F, Lecka J, Martín-Satué M, Lévesque SA, Sévigny J. NTPDase1 Controls IL-8 Production by Human Neutrophils. J Immunol. 2011;187:644–653. doi: 10.4049/jimmunol.1002680. [DOI] [PubMed] [Google Scholar]
- 56.Rose FR, Hirschhorn R, Weissmann G, Cronstein BN. Adenosine promotes neutrophil chemotaxis. J Exp Med. 1988;167:1186–1194. doi: 10.1084/jem.167.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrot I, Michaud H-A, Giraudon-Paoli M, Augier S, Docquier A, Gros L, Courtois R, Déjou C, Jecko D, Becquart O, Rispaud-Blanc H, Gauthier L, Rossi B, Chanteux S, Gourdin N, Amigues B, Roussel A, Bensussan A, Eliaou J-F, Bastid J, Romagné F, Morel Y, Narni-Mancinelli E, Vivier E, Paturel C, Bonnefoy N. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Rep. 2019;27:2411–2425.e9. doi: 10.1016/j.celrep.2019.04.091. [DOI] [PubMed] [Google Scholar]
- 58.Németh T, Sperandio M, Mócsai A. Neutrophils as emerging therapeutic targets. Nat Rev Drug Discov. 2020;19:253–275. doi: 10.1038/s41573-019-0054-z. [DOI] [PubMed] [Google Scholar]
- 59.Richer BC, Salei N, Laskay T, Seeger K. Changes in Neutrophil Metabolism upon Activation and Aging. Inflammation. 2018;41:710–721. doi: 10.1007/s10753-017-0725-z. [DOI] [PubMed] [Google Scholar]
- 60.Shaul ME, Levy L, Sun J, Mishalian I, Singhal S, Kapoor V, Horng W, Fridlender G, Albelda SM, Fridlender ZG. Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology. 2016;5:e1232221. doi: 10.1080/2162402X.2016.1232221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grassi F. Purinergic Control of Neutrophil Activation. J Mol Cell Biol. 2010;2:176–177. doi: 10.1093/jmcb/mjq014. [DOI] [PubMed] [Google Scholar]
- 62.Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Métivier D, Galluzzi L, Perfettini J-L, Zitvogel L, Kroemer G. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21:79–91. doi: 10.1038/cdd.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campos-Contreras A d R, Díaz-Muñoz M, Vázquez-Cuevas FG. Purinergic Signaling in the Hallmarks of Cancer. Cells. 2020;9:1612. doi: 10.3390/cells9071612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehta VB, Hart J, Wewers MD. ATP-stimulated Release of Interleukin (IL)-1β and IL-18 Requires Priming by Lipopolysaccharide and Is Independent of Caspase-1 Cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 65.Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 receptor: A main player in inflammation. Biochem Pharmacol. 2018;151:234–244. doi: 10.1016/j.bcp.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 66.Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, Raffaghello L, Pistoia V, Varesio L, Adinolfi E. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34:5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 67.De Marchi E, Orioli E, Dal Ben D, Adinolfi E (2016) P2X7 Receptor as a Therapeutic Target. pp. 39–79. 10.1016/bs.apcsb.2015.11.004 [DOI] [PubMed]
- 68.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18:601–618. doi: 10.1038/s41568-018-0037-0. [DOI] [PubMed] [Google Scholar]
- 69.Bou Ghanem EN, Clark S, Roggensack SE, McIver SR, Alcaide P, Haydon PG, Leong JM. Extracellular Adenosine Protects against Streptococcus pneumoniae Lung Infection by Regulating Pulmonary Neutrophil Recruitment. PLoS Pathog. 2015;11:e1005126. doi: 10.1371/journal.ppat.1005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amulic B, Sollberger G. Why Immune Cells Extrude Webs of DNA and Protein. NETS: two faced players in Immunity. Science. 2018;33:44–51. [Google Scholar]
- 71.Sorvillo N, Cherpokova D, Martinod K, Wagner DD. Extracellular DNA NET-Works With Dire Consequences for Health. Circ Res. 2019;125:470–488. doi: 10.1161/CIRCRESAHA.119.314581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hurt B, Schulick R, Edil B, El Kasmi KC, Barnett C. Cancer-promoting mechanisms of tumor-associated neutrophils. Am J Surg. 2017;214:938–944. doi: 10.1016/j.amjsurg.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Galdiero MR, Varricchi G, Loffredo S, Mantovani A, Marone G. Roles of neutrophils in cancer growth and progression. J Leukoc Biol. 2018;103:457–464. doi: 10.1002/JLB.3MR0717-292R. [DOI] [PubMed] [Google Scholar]
- 74.Loffredo S, Borriello F, Iannone R, Ferrara AL, Galdiero MR, Gigantino V, Esposito P, Varricchi G, Lambeau G, Cassatella MA, Granata F, Marone G (2017) Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front Immunol 8. 10.3389/fimmu.2017.00443 [DOI] [PMC free article] [PubMed]
- 75.Martel-Gallegos G, Rosales-Saavedra MT, Reyes JP, Casas-Pruneda G, Toro-Castillo C, Pérez-Cornejo P, Arreola J. Human neutrophils do not express purinergic P2X7 receptors. Purinergic Signal. 2010;6:297–306. doi: 10.1007/s11302-010-9178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, Nakasone ES, Hearn SA, Kuttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg MS, Egeblad M. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016;8:361ra138. doi: 10.1126/scitranslmed.aag1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rayes RF, Mouhanna JG, Nicolau I, Bourdeau F, Giannias B, Rousseau S, Quail D, Walsh L, Sangwan V, Bertos N, Cools-Lartigue J, Ferri LE, Spicer JD (2019) Primary tumors induce neutrophil extracellular traps with targetable metastasis-promoting effects. JCI Insight 4. 10.1172/jci.insight.128008 [DOI] [PMC free article] [PubMed]
- 78.Arpinati L, Shaul ME, Kaisar-Iluz N, Mali S, Mahroum S, Fridlender ZG. NETosis in cancer: a critical analysis of the impact of cancer on neutrophil extracellular trap (NET) release in lung cancer patients vs. mice. Cancer Immunol Immunother. 2020;69:199–213. doi: 10.1007/s00262-019-02474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, Zychlinsky A (2013) A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol 4. 10.3389/fimmu.2013.00048 [DOI] [PMC free article] [PubMed]
- 80.Perisanidis C, Kornek G, Pöschl PW, Holzinger D, Pirklbauer K, Schopper C, Ewers R. High neutrophil-to-lymphocyte ratio is an independent marker of poor disease-specific survival in patients with oral cancer. Med Oncol. 2013;30:334. doi: 10.1007/s12032-012-0334-5. [DOI] [PubMed] [Google Scholar]
- 81.Xiao W-K, Chen D, Li S-Q, Fu S-J, Peng B-G, Liang L-J. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer. 2014;14:117. doi: 10.1186/1471-2407-14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao J, Pan K, Wang W, Chen J, Wu Y, Lv L, Li J, Chen Y, Wang D, Pan Q, Li X, Xia J. The Prognostic Value of Tumor-Infiltrating Neutrophils in Gastric Adenocarcinoma after Resection. PLoS One. 2012;7:e33655. doi: 10.1371/journal.pone.0033655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carmona-Rivera C, Khaznadar SS, Shwin KW, Irizarry-Caro JA, O’Neil LJ, Liu Y, Jacobson KA, Ombrello AK, Stone DL, Tsai WL, Kastner DL, Aksentijevich I, Kaplan MJ, Grayson PC. Deficiency of adenosine deaminase 2 triggers adenosine-mediated NETosis and TNF production in patients with DADA2. Blood. 2019;134:395–406. doi: 10.1182/blood.2018892752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu K, Cooney KA, Shin EY, Wang L, Deppen JN, Ginn SC, Levit RD. Adenosine from a biologic source regulates neutrophil extracellular traps (NETs) J Leukoc Biol. 2019;105:1225–1234. doi: 10.1002/JLB.3VMA0918-374R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mestas J, Hughes CCW. Of Mice and Not Men: Differences between Mouse and Human Immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 86.Wiesner O, Litwiller RD, Hummel AM, Viss MA, McDonald CJ, Jenne DE, Fass DN, Specks U. Differences between human proteinase 3 and neutrophil elastase and their murine homologues are relevant for murine model experiments. FEBS Lett. 2005;579:5305–5312. doi: 10.1016/j.febslet.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 87.Stackowicz J, Jönsson F, Reber LL (2020) Mouse Models and Tools for the in vivo Study of Neutrophils. Front Immunol 10. 10.3389/fimmu.2019.03130 [DOI] [PMC free article] [PubMed]
- 88.Sugawara T, Miyamoto M, Takayama S, Kato M. Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J Pharmacol Toxicol Methods. 1995;33:91–100. doi: 10.1016/1056-8719(94)00062-9. [DOI] [PubMed] [Google Scholar]
- 89.Soroush F, Tang Y, Mustafa O, Sun S, Yang Q, Kilpatrick LE, Kiani MF. Neutrophil-endothelial interactions of murine cells is not a good predictor of their interactions in human cells. FASEB J. 2020;34:2691–2702. doi: 10.1096/fj.201900048R. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
It is not applicable.