Abstract
Background:
This study was designed to explore the levels of serum moleculartumor markers carbohydrate antigen 125 (CA125), human epididymis protein 4 (HE4), and carcinoembryonic antigen (CEA) in patients with primary epithelial ovarian cancer, and their correlation with the progression of the cancer.
Methods:
A total of 222 people were enrolled in this study admitted to Jining Maternal and Child Health and Family Planning Service Center from January 2016 to December 2017. There were 122 patients with primary epithelial ovarian cancer (ovarian cancer group), 50 patients with benign ovarian diseases (benign control group), and 50 healthy individuals (normal control group). The levels of serum CA125, CEA, and HE4 were detected by the electrochemical luminescence method and ELISA.
Results:
The levels of serum CA125, HE4, and CEA in the ovarian cancer group were significantly higher than those in patients with the benign control group and the normal control group (P<0.01). The levels of serum CA125, HE4, and CEA in the high clinical staging group (stage III and stage II), low differentiation group, comorbid ascites group, metastasis group, and recurrence group were significantly higher than those in the low clinical staging group (stage I and stage II), high + moderate differentiation group, non-ascites group, non-metastasis group, and non-recurrence group, respectively (all P<0.05), and the levels of serum CA125, HE4, and CEA decreased significantly after treatment (P<0.01).
Conclusion:
The levels of serum CA125, HE4, and CEA are closely related to the development and progression of epithelial ovarian cancer, and combined detection of CA125, HE4 and CEA is of great significance for early diagnosis, disease development monitoring, and prognosis evaluation of epithelial ovarian cancer.
Keywords: Epithelial ovarian cancer, Carbohydrate antigen, Protein
Introduction
Ovarian cancer (OC) is a common malignant tumor in gynecology, with the incidence rate ranking the third after cervical cancer and corpus carcinoma. Epithelial ovarian cancer (EOC) accounts for 90% of malignant ovarian tumors, characterized with high recurrence rate, high metastasis rate, and low survival rate. In addition, EOC shows the highest mortality among gynecologic malignant tumors (1), which poses a serious threat to women's life. The ovary is located in the deep part of human pelvic cavity, and its early lesions are insidious, so early symptoms of ovary-related disease are not obvious.
According to statistics, about 65% of patients with OC are already at the advanced stage at the first time of diagnosis (2), which deprives them of optimal treatment timing. The 5-year survival rate of patients with advanced OC is only 20–30% (3), and early diagnosis and treatment of the disease can increase the 5-year survival rate to 90%, so early diagnosis and early clinical intervention are essential for improving the survival rate of patients.
Traditional examination for OC is mainly based on imaging, but the value of imaging is not high in evaluating early epitheliogenic malignant ovarian tumor or epitheliogenic malignant ovarian tumor with a small lesion range. So exploring a novel method to improve the coincidence rate of diagnosis of early OC and to monitor its development is a hot research topic today. At present, with the rapid development of molecular biology and the continuous progress of laboratory detection technology, increasing molecular tumor markers are found to be closely related to the biological behaviors of OC, and they are also found in the development and progression of OC.
We selected serum molecular tumor markers CA125, HE4 and CEA for combined dynamic detection and explored their correlations with the development and progression of EOC, aiming at providing laboratory basis for constructing a risk model for predicting the cancer.
Materials and Methods
General data
A total of 122 patients with EOC admitted to Jining Maternal and Child Health and Family Planning Service Center, Jining City, China from January 2016 to December 2017 were enrolled in the OC group. They were confirmed with EOC by postoperative pathology, and had complete data of serum CA125, HE4, and CEA, and follow-up information. They were between 25 and 81 years old, with a median age of 56.82 years, treated for the first time, without any radiotherapy, chemotherapy and endocrinotherapy before. They received cytoreductive surgery for OC, and were not accompanied with other primary malignant tumors. In terms of histopathological type, the 122 patients included 79 patients with ovarian serous cystadenocarcinoma, 28 patients with ovarian mucinous cystadenocarcinoma, and 15 patients with ovarian endometrioid carcinoma; in terms of clinical staging according to the criteria released by the International Federation of Gynecology and Obstetrics (FIGO), there were 10 patients at stage I, 36 patients at stage II, 64 patients at stage III, and 12 patients at stage IV; in terms of differentiation according to grading criteria released by the WHO, there were 15 patients with high differentiation, 33 patients with moderate differentiation, and 74 patients without differentiation. In addition, 50 patients with benign ovarian diseases admitted to our hospital during the same period were enrolled into a benign control group, all of which had no canceration according to histopathology, including ovarian serous cystadenocarcinoma, ovarian mucinous cystadenoma, and ovarian cyst. They were between 23 and 82 years old, with a median age of 55.87 years. Moreover, 50 healthy individuals were enrolled into a normal control group, all of which were confirmed without any major organ diseases and were between 24 and 84 years old, with a median age of 56.12 years.
This study was approved by the Medical Ethics Committee of our hospital, and all participants signed informed consent forms and voluntarily participated in the study.
Methods
Four ml fasting venous blood was sampled from each patient in the OC group from 6:00 to 9:00 in the morning before treatment and at 3 months after treatment. Four ml fasting venous blood was also sampled from each participant in the benign control group and the normal control group from 6:00 to 9:00 in the morning. The sampled blood was let to stand for self-coagulation, and then centrifuged to take serum for later analysis. CA125 and CEA in the serum were quantified using the electrochemical luminescence method with Roche ELecsys-2010 instrument and Roche original reagent, and HE4 in the serum was detected by ELISA, with reagent provided by the R&D Company of the United States and a FL-312e automatic microplate reader (Bio-Kinetics Company, the United States) in strict accordance with the operation instructions. Normal reference values of the three indexes: CA125≤35.00U/mL, CEA≤3.40ng/mL, and HE4 ≤140.00 pmol/ml.
Result determination
The level of a serum molecular tumor marker above the normal value indicated a positive result, while the level below the normal value indicated a negative result. One or more positive items in the combination for joint examinations indicated a positive result, while all negative levels indicated a negative result
Evaluation indexes of diagnostic tests
The standard method of diagnostic test evaluation: statistically analyze positive and negative results of patients and non-patients (judged according to the gold standard) obtained using a certain test method, and then calculate and obtain the diagnostic indexes commonly used in diagnostic tests. The test diagnosis results were divided into true positive (a), false positive (b), false negative (c), and true negative (d). The calculation formula: Sensitivity = a/(a+c); specificity = d/(d+b); accuracy = (a+d)/(a+b+c+d); positive predictive value = a/(a+b); negative predictive value =d/(d+c).
Statistical analysis
A database was established using Excel 2007. SPSS17.0 (Chicago, IL, USA), t test, and χ2 test were adopted. Quantitative data about concentration were expressed as the (x̄±s), and mean data were compared between groups using the independent-samples t test. In addition, the rates of enumeration data were compared using the χ2 test. P<0.05 indicates a significant difference.
Results
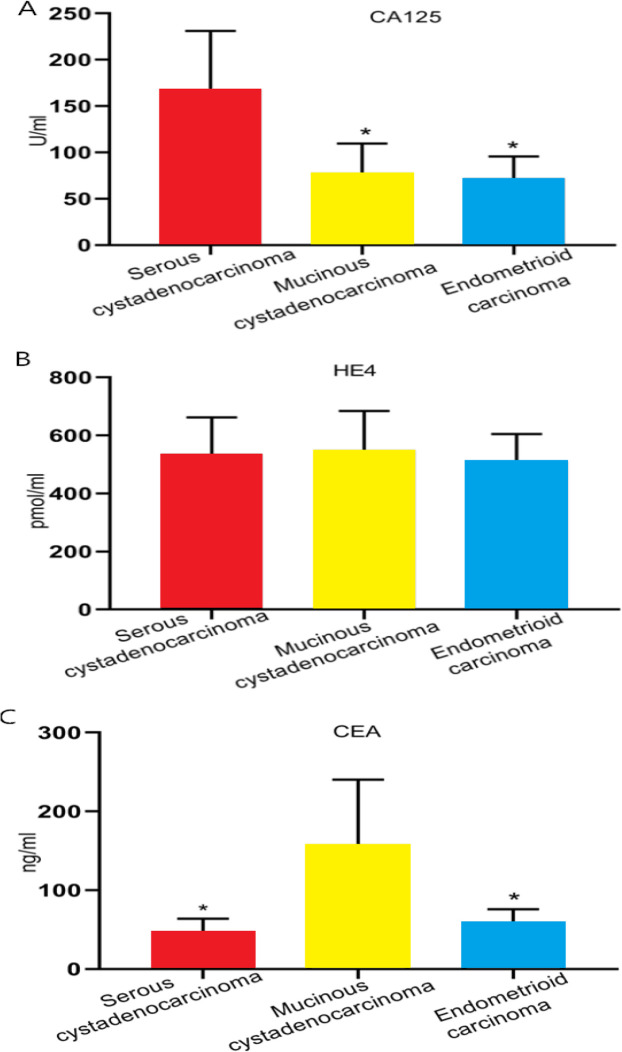
The levels of serum CA125, HE4, and CEA in the OC group were significantly higher than those in the benign control group and the normal control group (both P<0.01), and there was no significant difference between the benign control group and the normal control group in the levels of them (Table 1). The levels of serum CA125, HE4, and CEA in the high clinical staging group (stage III and stage II), low differentiation group, comorbid ascites group, metastasis group, and recurrence group were significantly higher than those in the low clinical staging group (stage I and stage II), high+moderate differentiation group, non-ascites group, non-metastasis group, and non-recurrence group, respectively (all P<0.05), and the levels of serum CA125, HE4, and CEA decreased significantly after treatment as the tumor disappeared or the tumor load decreased (P<0.05) (Table 2). Pathological tissue types of EOC are classified into serous cystadenocarcinoma, mucinous cystadenocarcinoma, and endometrioid carcinoma. The level of serum CA125 in patients with ovarian serous cystadeno-carcinoma was significantly higher than that in patients with one of the other two types, and there was no significant difference among patients with the three types in the level of serum HE4. In addition, the level of serum CEA in patients with ovarian mucinous cystadenocarcinoma was also significantly higher than that in patients with one of the other two types (Fig. 1).
Table 1:
Comparison of serum CA125, HE4, and CEA levels among the OC group, the benign control group, and the normal control group (x̄±s)
| Group | The number of patients | CAl25 (U/ml) | t | P | HE4 (pmol /ml) | t | p | CEA (ng/ml) | t | P |
|---|---|---|---|---|---|---|---|---|---|---|
| The OC group | 122 | 135.69±30.12a | 536.14±81.62a | 75.29±14.92a | ||||||
| The benign control group | 50 | 20.32±4.56b | 3.774 | 0.009 | 102.35±19.38b | 5.171 | 0.002 | 2.04±0.71b | 4.905 | 0.003 |
| The normal control group | 50 | 18.97±3.65 | 3.824 | 0.009 | 98.76±20.39 | 5.199 | 0.002 | 2.01±0.63 | 4.908 | 0.003 |
Note:
indicates P<0.01 vs. the benign control group and the normal control group;
indicates P>0.05 vs. the normal control group
Table 2:
Comparison of serum CA125, HE4, and CEA levels among patients with difference biological behaviours of OC (x̄±s)
| Group | The number of patients | CA125 (U/mL) | t | P | HE4 (pmol/m) | t | P | CEA (ng/ml) | t | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical staging | ||||||||||
| Stage I–II | 46 | 64.58±11.62 | 2.654 | 0.038 | 318.54±55.06 | 2.936 | 0.026 | 21.29±4.63 | 3.445 | 0.014 |
| Stage III–IV | 76 | 178.35±41.27c | 668.23±105.61c | 108.73±24.96c | ||||||
| Differentiation | ||||||||||
| High and moderate differentiation | 48 | 57.47±9.48 | 2.895 | 0.028 | 276.88±44.29 | 3.617 | 0.011 | 18.19±4.36 | 0.029 | |
| Low differentiation | 74 | 186.23±43.46c | 705.31±109.85c | 112.34±32.61c | ||||||
| Ascites | ||||||||||
| No | 55 | 71.57±12.11 | 2.723 | 0.034 | 298.36±41.41 | 3.641 | 0.011 | 34.32±5.69 | 3.544 | 0.012 |
| Yes | 67 | 188.96±41.37c | 732.25±111.7c | 109.37±20.39c | ||||||
| Lymph node metastasis | ||||||||||
| No | 57 | 73.58±10.30 | 2.572 | 0.042 | 370.17±44.10 | 2.854 | 0.029 | 38.51±6.87 | 2.953 | 0.026 |
| Yes | 65 | 190.75±44.31c | 682.35±100.11c | 108.76±22.78c | ||||||
| Distant metastasis | ||||||||||
| No | 110 | 110.32±17.31 | 2.945 | 0.026 | 472.32±57.70 | 3.856 | 0.008 | 47.47±7.41 | 3.989 | 0.007 |
| Yes | 12 | 372.02±84.01c | 1123.27±158.64c | 265.09±54.05c | ||||||
| Recurrence | ||||||||||
| No | 93 | 35.87±4.81 | 3.127 | 0.020 | 145.25±23.59 | 6.390 | 0.001 | 4.63±1.18 | 5.834 | 0.001 |
| Yes | 29 | 325.36±92.44c | 986.37±129.51c | 230.39±38.68c | ||||||
| Treatment | ||||||||||
| Before treatment | 122 | 135.69±30.12c | 3.442 | 0.014 | 536.14±74.51c | 4.920 | 0.003 | 75.29±14.92 | 5.139 | 0.002 |
| After treatment | 122 | 38.29±6.67 | 152.36±23.06 | 5.01±1.25c |
Note:
indicates P<0.05 in terms of intro-group comparison
Fig. 1:
Comparison of serum CA125, HE4, and CEA in patients with difference pathological tissue types of EOCA, Comparison of serum CA125 in patients with difference pathological tissue types of EOC. B, Comparison of serum HE4 in patients with difference pathological tissue types of EOC. C, Comparison of serum CEA in patients with difference pathological tissue types of EOC.
Note: * indicates P < 0.05
The sensitivity, accuracy, and negative predictive value of the detection of combined serum CA125, HE4 and CAE in the diagnosis of EOC were significantly improved compared with single detection or pairwise detection (Table 3).
Table 3:
Comparison of diagnostic value of different combination modes of serum CA125, HE4 and CEA for EOC [% (n)]
| Detection indexes | Sensibility | Specificity | Accuracy | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|
| CA125 | 59.02 (72/122) | 98.00 (49/50) | 70.35 (121/172) | 98.63 (72/73) | 49.49 (49/99) |
| HE4 | 63.11 (77/122) | 96..00 (48/50) | 72.67 (125/172) | 97.47 (77/79) | 51.61 (48/93) |
| CEA | 51.64 (63/122) | 94.00 (47/50) | 63.95 (110/172) | 95.45 (63/66) | 44.34 (47/106) |
| CA125+HE4 | 77.05 (94/122) | 92.00 (46/50) | 81.40 (140/172) | 95.92 (94/98) | 62.16 (46/74) |
| HE4+CEA | 75.41 (92/122) | 88.00 (44/50) | 79.07 (136/172) | 93.88 (92/98) | 59.46 (44/74) |
| CA125+CEA | 72.95 (89/122) | 90.00 (45/50) | 77.91 (134/172) | 94.68 (89/94) | 57.69 (45/78) |
| CA125+HE4+CEA | 95.90 (117/122)h | 86.00 (43/50) | 93.02 (160/172)h | 94.35 (117/124) | 89.58 (43/48)h |
Note:
indicates P<0.05 in terms of comparison with detection of separate one or detection of pairwise markers
Discussion
Molecular tumor markers are substances secreted by tumor cells or produced by the interaction between tumor and host in the process of canceration of tissue cells. Their occurrence or changes in amount reflect the existence and growth of tumor. There is very little production or even no production of the markers in normal tissues and tissues with benign lesions. These substances exist in tumor cells and tissues, and can also enter blood or other body fluids, and their levels increase significantly with the development and progression of tumors. In addition, changes in their amounts can be used for early diagnosis and evaluation of biological behaviors of malignant tumors, such as clinical staging, recurrence, and metastasis (4).
Detection of serum tumor markers is advantageous due to convenient specimen collection, small trauma, simplicity, accuracy, and low cost, so it is currently a hot spot in the diagnosis of malignant tumors. Serological screening can provide screening reference for early lesions with small lesions (5), and can also provide laboratory basis for screening of early EOC, thus guiding the diagnosis and treatment of early EOC (6). Correctly understanding the biological behaviors of OC in its development and progression and monitoring the prognosis of patients with OC are conducive to the formulation of individualized treatment plans and efficacy tests plans, and can improve the survival outcome of patients (7). CA125 is a glycoprotein from members of the sugar chain family. Originated from coelomic epithelium during embryonic development, CA125 can bind to monoclonal antibody OC125. It is controlled by MUC 16 gene code, showing no expression or low expression in normal ovarian tissues or cases with benign lesions, but high expression in EOC tissues (8). At present, CA125 is the most commonly used classical marker in clinical diagnosis of OC, especially the preferred marker for diagnosing serous OC, and it also has an important value in monitoring the condition and prognosis of OC (9). In this study, the level of serum CA125 in the OC group was significantly higher than that in the benign control group and the normal control group, which is consistent with the above reports. As a fragment of cell membrane surface released in the process of cancer cell proliferation, CA125 can further promote the proliferation of tumor cells and exacerbate the disease by binding to glycoprotein receptors (10,11). In this study, the level of serum CA125 increased significantly with the metastasis and recurrence of OC, and CA125 could be used as an indicator for monitoring the disease. Although CA125 is a favorable marker for diagnosing OC, it may also be found in cases with other malignant tumors as a broad-spectrum tumor marker, and its expression increases transiently in cases with some benign lesions such as ovarian cyst, pelvic inflammatory disease, and endometriosis (12), so it is essential to detect CA125 along with other markers at the same time to improve the diagnostic accuracy of OC.
HE4 is a precursor of epididymal secretory protein E4, with a relative molecular weight of 20–25KD. It is a novel OC-related tumor marker discovered in recent years, and has important potential in diagnosis of early OC (13). HE4 shows a certain expression in the transitional epithelium of the reproductive system of pregnant women during the embryonic period, but its expression after delivery is low, and it has no expression in normal ovarian tissues. In the process of malignant transformation of ovarian tissue cells, the level of HE4 in peripheral blood significantly increases due to gene mutation, abnormal gene transcription regulation and continuous transcription and translation of HE4 gene (14). In this study, the level of serum HE4 was closely related to the biological behaviors of OC, including the development and progression. The levels of serum HE4 in the high clinical staging group, low differentiation group, comorbid ascites group, metastasis group, and recurrence group were significantly higher than those in the low clinical staging group, high+moderate differentiation group, non-ascites group, non-metastasis group, and non-recurrence group, respectively. In addition, the level of serum HE4 decreased after treatment. Therefore, dynamic detection of peripheral blood HE4 is helpful to disease progress monitoring and prognosis evaluation. However, the sensitivity and accuracy of single detection of HE4 are still unsatisfactory, but the detection of HE4 combined with other markers can improve the diagnostic value.
CEA is an acid glycoprotein originating from embryonic tissues, with a relative molecular weight of (2–10)×105. Its level usually increases in pregnant women in the first 6 months of pregnancy, and its level is very low after delivery. CEA was usually used in the diagnosis of digestive system malignant tumors at first, but it is found to be a broad-spectrum tumor marker and is highly expressed in cases with malignant tumors of the reproductive system, especially in cases with mucinous EOC, so it can be used as one of the diagnostic markers of OC (15). In addition, CEA has a good predictive value in monitoring disease condition and evaluating therapeutic effect, especially in monitoring tumor recurrence and metastasis (16). In this study, CEA in the peripheral blood of patients in the recurrent group and the metastasis group showed a sharp rise, so the changes of CEA level can be adopted to judge prognosis and guide treatment. Because the positive rate of CEA in patients with serous EOC is low, and the sensitivity of CEA in diagnosing EOC was only 51.64% in this study, it is necessary to detect CEA with other markers to reduce missed diagnosis.
To sum up, serum molecular tumor markers, CA125, HE4, and CEA, are strongly linked to the development and progression of EOC. The levels of them in the OC group were significantly higher than those in the benign control group and the normal control group, so they can be used as diagnostic indexes for OC. In addition, the serum levels of them are closely related to different clinicopathological factors (clinical staging, histological type, recurrence, metastasis, etc.). However, detection of one single marker is not sensitive and accurate enough. The sensitivity of CA125, HE4, and CEA in diagnosing OC was 59.02%, 63.11%, and 51.64%, respectively, and the accuracy was 70.35%, 72.67%, and 63.95%, respectively, which is far fromthe clinical requirements, and the combined detection of the two markers is still not sensitive and accurate enough. In this study, the sensitivity, accuracy, and negative predictive value of the detection of combined serum CA125, HE4, and CAE in the diagnosis of EOC were 95.90%, 93.02% and 89.58%, respectively, which were significantly improved compared with single detection or pairwise detection, with reduced missed diagnosis and improved diagnosis accuracy. The serum levels of the three markers were closely related to clinical and pathological features, and dynamic detection of them can help to judge disease condition, monitor disease recurrence and metastasis, guide disease treatment and follow up patients’ prognosis. Therefore, molecular tumor markers, CA125, HE4, and CEA are closely related to the development and progression of EOC.
Conclusion
The levels of serum CA125, HE4, and CEA were closely related to the development and progression of epithelial ovarian cancer, and combined detection of CA125, HE4 and CEA was of great significance for early diagnosis, disease development monitoring, and prognosis evaluation of epithelial ovarian cancer.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
No funding was received in this study.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Kohan-Ivani K, Gabler F, Selman A, et al. (2016). Role of dihydrotestosterone(DHT) on TGF-β1 signaling pathway in epithelial ovarian cancer cells. J Cancer Res Clin Oncol, 142(1): 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li PL, Zhang X, Li TF, et al. (2015). Combined detection of sialic acid and hydroxyproline in diagnosis of ovarian cancer and its comparison with human epididymis protein 4 and carbohydrate antigen 125. Clin Chim Acta, 439: 148–153. [DOI] [PubMed] [Google Scholar]
- 3.Su Z, Graybill WS, Zhu Y. (2013). Detection and monitoring of ovarian cancer. Clin Chim Acta, 415: 341–345. [DOI] [PubMed] [Google Scholar]
- 4.Yang ZJ, Zhao BB, Li L. (2016). The significance of the change pattern of serum CA125 level for judging prognosis and diagnosing recurrences of epithelial ovarian cancer. J Ovarian Res, 9(1): 57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Sandri MT, Bottari F, Franchi D, et al. (2013). Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outeome. Gynecol Oncol, 128(2): 233–238. [DOI] [PubMed] [Google Scholar]
- 6.Al-Musalhi K, Al-Kindi M, Ramadhan F, et al. (2015). Validity of cancer antigen-125 (CA-125) and risk of malignancy index(rmi) in the diagnosis of ovarian cancer. Oman Med J, 30(6): 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narasimhulu DM, Khoury-Collado F, Chi DS. (2015). Radical surgery in ovarian cancer. Curr Oncol Rep, 17(4): 16. [DOI] [PubMed] [Google Scholar]
- 8.Bast RC, Jr, Badgwell D, Lu Z, et al. (2005). New tumor markers: CA125 and beyond. Int J Gynecol Cancer, 15 Suppl 3: 274–281. [DOI] [PubMed] [Google Scholar]
- 9.Zheng LE, Qu JY, He F. (2016). The diagnosis and pathological value of combined detection of HE4 and CA125 for patients with ovarian cancer. Open Med (Wars), 11(1): 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandiera E, Zanotti L, Fabricio AS, et al. (2013). Cancer antigen 125, human epididymis 4, kallikrein 6, osteopontin and soluble mesothelin-related peptide immunocomplexed with immunoglobulin M in epithelial ovarian cancer diagnosis. Clin Chem Lab Med, 51(9): 1815–1824. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, Zhang ZL. (2015). The diagnostic value of transvaginal sonograph (TVS), color doppler, and serum tumor marker CA125, CEA, and AFP in ovarian cancer. Cell Biochem Biophys, 72(2): 353–357. [DOI] [PubMed] [Google Scholar]
- 12.Moore RG, Brown AK, Miller MC, et al. (2008). Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol, 110(2): 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiekema A, Boldingh QJ, Korse CM, et al. (2015). Serum human epididymal protein 4(HE4) as biomarker for the differentiation between epithelial ovarian cancer and ovarian metastases of gastrointestinal origin. Gynecol Oncol, 136(3): 562–566. [DOI] [PubMed] [Google Scholar]
- 14.Jia MM, Deng J, Cheng XL, et al. (2017). Diagnostic accuracy of urine HE4 in patients with ovariancancer:A meta-analysis. Oncotarget, 8(6): 9660–9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Qin Y, Zhang J, et al. (2014). Nipple Discharge of CA15-3, CA125, CEA and TSGF as a New Biomarker Panel for Breast Cancer. Int J Mol Sci, 15(6): 9546–9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Gao SG, Chen JM, et al. (2015). Serum CA242, CA199, CAl25, CEA and TSGF are Biomarkers for the Efficacy and Prognosis of Cryoablation in Pancreatic Cancer Patients. Cell Biochem Biophys, 71(3): 1287–1291. [DOI] [PubMed] [Google Scholar]