Abstract
Respiratory syncytial virus (RSV) is the leading cause of respiratory viral infection in infants and children. However, little is known about the contribution of monocytes to antiviral responses against RSV infection. We identified the IFN-β production of monocytes using IFN-β/YFP reporter mice. The kinetic analysis of IFN-β-producing cells in in vivo RSV-infected lung cells indicated that monocytes are recruited to the inflamed lung during the early phase of infection. These cells produced IFN-β via the myeloid differentiation factor 88-mediated pathway, rather than the TLR7- or mitochondrial antiviral signaling protein-mediated pathway. In addition, monocyte-ablated mice exhibited decreased numbers of IFN-γ-producing and RSV Ag-specific CD8+ T cells. Collectively, these data indicate that monocytes play pivotal roles in cytotoxic T-cell responses and act as type I IFN producers during RSV infection.
Keywords: Respiratory syncytial virus, Type I interferon, Interferon beta, Monocyte
INTRODUCTION
Respiratory syncytial virus (RSV), first recovered from infants with lower respiratory tract illness, is a leading cause of respiratory diseases including pneumonia, bronchiolitis, otitis media, and apnea in young children (1). This viral infectious disease remains a considerable burden to global health because most infants are exposed to RSV at least once within the first two years of life, and severe RSV infection might become a long-lasting risk factor for asthma and bronchiolitis (2,3). In addition to infants and young children, adults older than 65 years have an opportunistic risk for RSV infection.
Type I IFNs play a central role in initiating and regulating antiviral responses through several mechanisms such as inhibiting viral replication in infected cells, activating Ag presentation, and contributing to adaptive immunity for memory responses (4). RSV is known to poorly induce type I IFNs, and the limited production has been speculated to restrict the antiviral response (3). Nevertheless, these cytokines are also critical for the antiviral response to RSV infection, and several studies have shown that RSV infection can induce type I IFN production by various cell types including endothelial cells, macrophages, and dendritic cells (DCs) (5,6,7,8,9).
Type I IFNs can be produced by recognizing pathogen-associated molecular patterns via pattern recognition receptors, such as TLRs, retinoic acid inducible gene I (RIG-I)-like receptors, or nucleotide-binding oligomerization domain-like receptors (10). Previously, we have shown that mucosal RSV infection can induce IFN-β production of macrophages, DCs, and plasmacytoid dendritic cells (pDCs) via the myeloid differentiation factor 88 (MyD88)-dependent pathway and that pDCs, not macrophages or DCs, require TLR7 to produce IFN-β (6,8). In addition, monocytes can produce type I IFNs against various RNA virus infections through TLR7 and TLR8 signaling (11). However, it remains unclear whether RSV infection can also induce monocytes to produce type I IFNs.
This study examined the IFN-β production of monocytes in the lung tissue of mice infected with RSV. Here, we report that monocytes are a cellular source of IFN-β in RSV infection, require MyD88 pathway to produce IFN-β, and regulate the adaptive T-cell immune response.
MATERIALS AND METHODS
Animals
IFN-β/YFP reporter (12), MyD88−/− (13), IFNαR1−/− (14), TLR7−/− (15), and mitochondrial antiviral-signaling protein (MAVS)−/− (B6;129-Mavstm1Zjc/J) (16) mice have been reported previously. TLR7−/− and MAVS−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). IFN-β/YFP reporter mice were crossed with TLR7−/−, MyD88−/−, IFNαR1−/−, or MAVS−/− mice. All mice were housed in a specific pathogen-free facility of KAIST Laboratory Animal Resource Center. All procedures involving animals were in accordance with the guidelines and policies for rodent experimentation provided by the Institutional Animal Care and Use Committee of KAIST. In addition, the study protocol was approved by this committee (KA2013-55). Gender- and age-matched mice (8–12 weeks of age) were used for the experiments.
RSV infection in vivo and in vitro
The A2 strain of RSV was grown on HEp-2 cells and titrated for infectivity as described elsewhere (17). Mice were anaesthetized with intraperitoneal ketamine (80 mg/kg) and xylazine (16 mg/kg) before intranasal inoculation with 1.0×107 plaque-forming units of RSV.
Bone marrow cells in mice were isolated according to the published procedures (6). Total bone marrow cells were stimulated with live or heat-inactivated RSV at a multiplicity of infection value of 3 in complete media for 18 h.
RSV titers in the lung
According to published procedures (18), RSV-infected mice were euthanized, and the lungs were collected in PBS. The tissues were then processed through 70-μm cell strainers to obtain single-cell suspensions. The supernatants were collected, and RSV titres in the supernatants were measured using a plaque assay on HEp-2 cell monolayers.
Preparation of lung single-cell suspension
To obtain single lung cell suspensions, isolated lung samples were prepared according to published procedures (19). Pure single-cell suspensions were obtained using a Percoll density gradient (GE Healthcare, Amersham, UK) following RBC lysis. The resulting cells were used for flow cytometric analysis.
Flow cytometry
Single-cell suspensions were pretreated with anti-CD16/32 (clone: 2.4G2) Ab to block Fc receptors. Then, they were stained with the following antibodies: anti-CD11b (clone: M1/70), anti-Ly6C (clone: AL-21), anti-Ly6G (clone: 1A8), anti-Siglec-F (clone: E50-2440), anti-F4/80 (clone: BM8), anti-CD4 (clone: GK1.5), anti-CD8α (clone: 56-6.7), or anti-CD45.2 (clone: 104). Leukocytes were gated based on forward- and side-scatter properties, and live cells were gated based on DAPI (Invitrogen, Waltham, MA, USA) exclusion. Multiple color samples were acquired on a flow cytometer (LSR Fortessa or Calibur; BD Biosciences, San Jose, CA, USA).
Leukocytes from the lungs of immunized mice were cultured in the presence of 5 µg/ml M187-195 peptide (NAITNAKII) and 50 ng/ml brefeldin A (Sigma-Aldrich) for 5 h. Then, cells were surface stained with anti-CD4 (clone: GK1.5), anti-CD44 (clone: IM7), and anti-CD8α (clone: 53-6.7) and fixed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions. Allophycocyanin (APC)–labelled anti-mouse IFN-γ Ab (clone: XMG1.2; eBioscience, San Diego, CA, USA) was used for intracellular staining. The frequency of IFN-γ-secreting T cells was analyzed by flow cytometry (Calibur; BD Bioscience).
H-2Db tetramers specific for RSV M187-195 peptide (NAITNAKII) were prepared with streptavidin-APC per the protocol of the NIH Tetramer Core Facility. Single-cell suspensions from the lungs of immunized mice were pretreated with anti-CD16/32 (clone: 2.4G2) Ab to block Fc receptors and then stained with anti-CD8α (clone: 53-6.7) and anti-CD3ε (clone: 145-2C11). Then, APC-labelled tetramer staining was performed. The frequency of RSV M187-195 peptide-specific CD8+ T cells was analyzed by flow cytometry (Calibur; BD Bioscience). Final analysis and graphical output were obtained using FlowJo software (Tree Star, Inc., Ashland, OR, USA).
Cell sorting
Lung single-cell suspensions were prepared as described above. Cells were stained with Ly6G and Ly6C and incubated for 30 min at 4°C in the presence of anti-CD16/32 Ab to block Fc receptors. Stained cells were sorted using a MoFlo XDP cell sorter (Beckman Coulter, Brea, CA, USA) and analyzed with an LSR Fortessa. The sorting strategy of monocytes (DAPI−, Singlet, Ly6C+, Ly6G−) is shown in Fig. 1C.
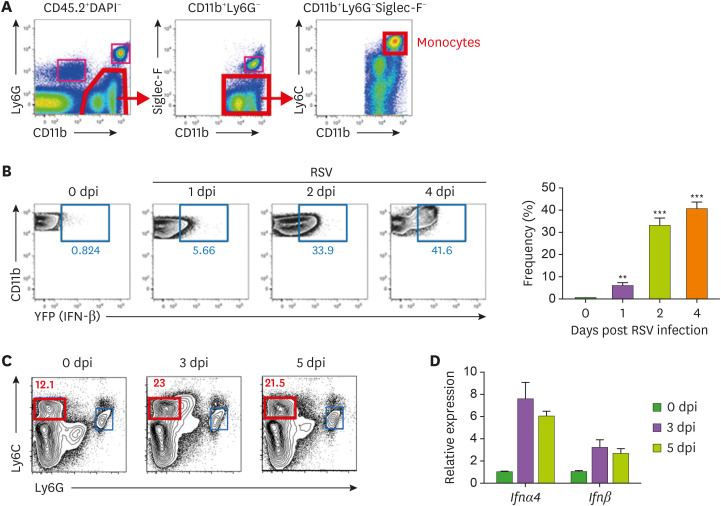
Figure 1. Monocytes in the lungs produce IFN-β post RSV infection. (A) Representative dot plots of flow cytometric gating strategy for monocytes in lung tissue showing monocytes in the CD11b+Ly6G−Siglec-F−Ly6Chi population gated on CD45.2+DAPI−. (B) Representative dot plot and bar graph showing monocytes with IFN-β expression by days post infection (dpi). Monocytes were collected from IFN-β/YFP reporter mice intranasally infected with RSV (1.0×107 pfu). (C) Monocytes (DAPI−, Ly6C+, Ly6G−) in the mice lung at 0, 3, and 5 days post RSV infection sorted with cell sorter. (D) Ifnα4 and Ifnβ mRNA expression measured with real-time quantitative PCR. Hprt was used as an internal control, and results are shown as the fold difference relative to mock control mice. The error bars show the SEM. The results are representative of 3 experiments.
**p<0.01; ***p<0.001 as calculated by Student's t-test.
Real-time quantitative PCR
Cell lysis and reverse transcription were conducted using SuperPrep Cell Lysis & RT Kit for qPCR (TOYOBO, Osaka, Japan). Ifnα4, Ifnβ, and Hprt mRNA expression were measured in 96-well optical plates (Thermo Scientific, Waltham, MA, USA) using SYBR GREEN PCR Master Mix (TOYOBO) and the CFX96 Real-Time PCR Systems (Bio-Rad, Hercules, CA, USA). Amplification of endogenous Hprt was used as an internal control, and results were determined as the fold difference relative to mock control mice.
Monocyte depletion in mice
Clodronate-liposomes and PBS-liposomes were obtained from ClodronateLiposomes.org and provided by Dr. Nico van Rooijen. As published (20), to deplete circulating monocytes in vivo, mice were inoculated with 200 µl clodronate-liposomes via the tail vein. Twenty-four hours later, the mice were subjected to further study.
CD4+ and CD8+ T-cell responses
RSV-specific T-cell responses were analyzed. At 8 days post infection, CD4+ or CD8+ T cells were isolated from the spleens of infected mice using anti-CD4 or anti-CD8 microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Then, CD4+ or CD8+ T cells were restimulated with the indicated amounts of heat-inactivated RSV virions or RSV M187-195 peptides for 72 h at 37°C. IFN-γ production in supernatants was measured by ELISA (eBioscience).
Statistical analysis
The data are presented as the means ± SEM. Statistical significance was evaluated with two-tailed unpaired Student's t-tests using Prism software (GraphPad; GraphPad, San Diego, CA, USA). Differences were considered statistically significant at p-values less than 0.05.
RESULTS
Mucosal RSV infection induces monocytes to produce IFN-β
To determine IFN-β production of monocytes in respiratory mucosa during RSV infection in vivo, we intranasally inoculated IFN-β/YFP reporter mice with RSV and analyzed CD11b+Ly6Chi monocytes in lung tissue (Fig. 1A). At the early phase of RSV infection, monocytes were rapidly recruited to the RSV-infected lungs and began producing IFN-β 1 day post infection; their production rapidly increased until 4 days post infection (Fig. 1B). To confirm the capacity of type I IFN production in wild-type (WT) mice against RSV infection, we isolated Ly6G−Ly6Chi monocytes from the lung tissues of RSV-infected WT mice using a cell sorter (Fig. 1C) and measured type I IFN gene expression. The expression of Ifnβ, as well as Ifnα4, was upregulated in monocytes at 3 and 5 days post infection (Fig. 1D). These results demonstrate that monocytes act as one of cellular sources of IFN-β during mucosal RSV infection.
Monocytes from bone marrow require the MyD88-mediated pathway, not the TLR7-mediated pathway, to produce IFN-β during RSV infection
Little is known about how monocytes recognize RSV and drive the type I IFN response. TLRs are representative receptors for viral infection, leading to the initiation of antiviral responses. In particular, TLR7 and TLR8, which recognize single-stranded RNA, are important to sense RNA virus infection (21). In addition, MAVS is the downstream of intracellular RNA sensor RIG-I/melanoma-differentiation-associated gene 5 (MDA5), which are important to sense RSV infection and initiate innate immune responses (22).
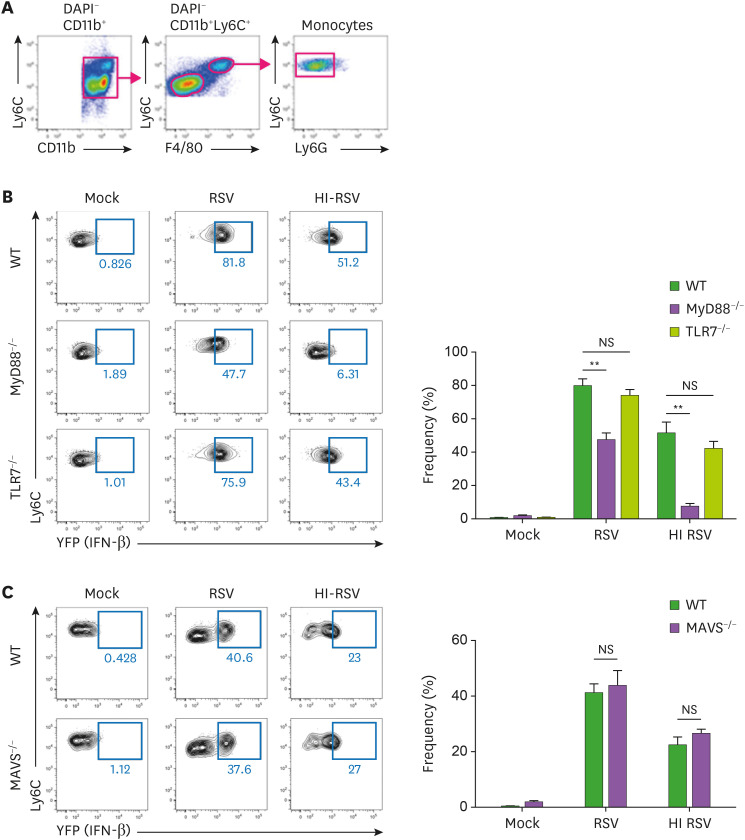
To determine which signaling pathway is activated during RSV infection to drive type I IFN production, we crossed IFN-β/YFP reporter mice with MyD88-, TLR7-, or MAVS-deficient mice and tracked IFN-β production of CD11b+Ly6Chi monocytes from bone marrow (Fig. 2A). MyD88 protein, which is activated by TLR stimulation, was critical for monocytes to produce IFN-β (Fig. 2B). However, TLR7 deficiency did not affect the production of IFN-β by monocytes. In addition, the MAVS-mediated pathway was not required for IFN-β production in RSV-infected monocytes (Fig. 2C). These results suggest that RSV infection induces monocytes to produce IFN-β via MyD88 signaling, not via TLR7 and MAVS signaling.
Figure 2. IFN-β production is dependent on the MyD88-mediated pathway in monocytes from bone marrow. (A) Representative dot plots of flow cytometric gating strategy for monocytes in bone marrow showing monocytes in the Ly6ChiLy6G−F4/80+ population gated on DAPI−CD11b+. (B, C) Bone marrow cells from IFN-β YFP/reporter (WT), MyD88-deficient IFN-β/YFP reporter (MyD88−/−), TLR7-deficient IFN-β/YFP reporter (TLR7−/−), or MAVS-deficient IFN-β/YFP reporter (MAVS−/−) mice were infected with RSV at a multiplicity of infection value of 3. After 18 h stimulation, these cells were harvested and analyzed for IFN-β expression by flow cytometry. The error bars are the mean of 3 mice ± SEM. The error bars show the SEM. The results are representative of 3 experiments.
**p<0.01 as calculated by Student's t-test.
MyD88- or IFNαR1-deficiency impairs IFN-β production of lung monocytes during mucosal RSV infection
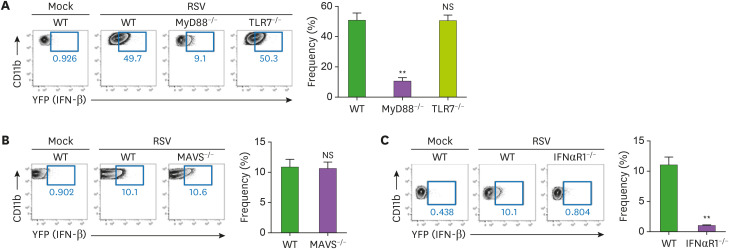
To confirm the signaling pathway driving IFN-β production in lung monocytes during RSV infection in vivo, we used the crossbred IFN-β/YFP reporter mice described above. In response to mucosal RSV infection, TLR7-deficient or MAVS-deficient monocytes had a similar frequency of YFP+ cells producing IFN-β compared to WT monocytes; however, MyD88-deficient monocytes had much less YFP+ cells (Fig. 3A and B). In addition, similar to the in vitro results from bone marrow cells, in vivo RSV infection induced monocytes to produce IFN-β via MyD88 signaling but not via TLR7 and MAVS signaling. Furthermore, MyD88-deficient mice showed the exacerbation of viral clearance at 4 day post mucosal RSV infection (Supplementary Fig. 1A).
Figure 3. The MyD88-mediated pathway is required to produce IFN-β in lung monocytes. (A-C) Lung monocytes from IFN-β/YFP reporter (WT) and MyD88-deficient IFN-β/YFP reporter (MyD88−/−) mice or TLR7− deficient IFN-β/YFP reporter (TLR7−/−) mice (A) or MAVS-deficient IFN-β/YFP reporter (MAVS−/−) mice (B) or IFNαR1-deficient IFN-β/YFP reporter (IFNαR1−/−) mice (C) infected intranasally with 1.0×107 pfu of RSV. After 2 days post infection, lung cells were isolated from mice and analyzed for IFN-β expression by flow cytometry. Representative dot plot and bar graph showing monocytes with IFN-β expression. Monocytes in lung tissue were gated as the CD11b+Ly6G−Siglec-F−Ly6Chi population gated on CD45.2+DAPI− as Figure 2A. The error bars are the mean of 3 mice ± SEM. The error bars show the SEM. Similar results were obtained from 3 separate experiments.
**p<0.01 as calculated by Student's t-test.
To address the role of IFNαR feedback in type I IFN production during mucosal RSV infection, we infected IFNαR1-deficient IFN-β/YFP reporter mice with RSV and then analyzed the RSV-infected lung cells. Lung monocytes in IFNαR1-deficient RSV-infected mice did not produce IFN-β in vivo (Fig. 3C). Thus, in mucosal RSV infection, IFNαR feedback is required for the induction of proper IFN-β responses in monocytes.
Circulating monocytes contribute to cytotoxic T-cell responses against mucosal RSV infection
To determine the role of the adaptor MyD88 in adaptive immunity against RSV infection, mice deficient in MyD88 were infected with RSV. Then, RSV-primed CD8+ T cells were restimulated with inactivated H-2Db-restricted M187-195 peptides. Following RSV infection, the numbers of interferon-γ-producing CD8+ T cells and RSV-specific CD8+ T cells decreased in the lungs of MyD88-deficient mice compared with those in WT mice (Supplementary Fig. 1B-D).
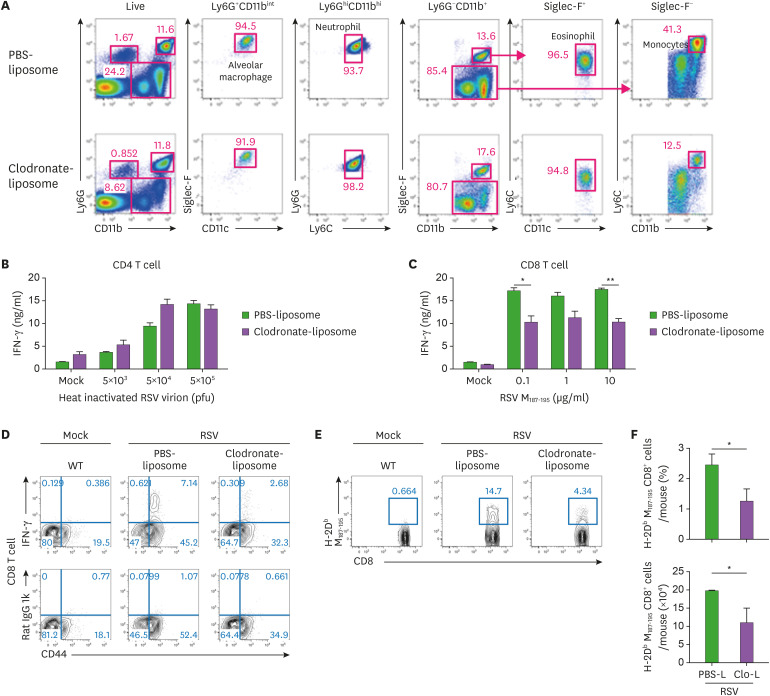
To further understand the importance of monocytes in adaptive immunity against RSV infection, we depleted circulating monocytes by treatment with intravenous clodronate-liposomes. The clodronate-liposome treatment decreased the population of monocytes, without affecting other cell populations including alveolar macrophage, neutrophils, and eosinophils (Fig. 4A). No difference in CD4+ T-cell response was found between circulating monocyte–deficient mice and PBS-liposome-treated mice (Fig. 4B). Unexpectedly, cytotoxic T-cell response was weaker in circulating monocyte–deficient mice than in PBS-liposome-treated mice (Fig. 4C). In addition, the lungs of circulating monocyte-deficient mice exhibited less IFN-γ-producing CD8+ T cells and RSV-specific CD8+ T cells (Figs. 4D, E, and F). Taken together, these results suggest that circulating monocytes support cytotoxic CD8+ T-cell responses against mucosal RSV infection.
Figure 4. Monocytes are required to induce a potent cytotoxic T cell response against mucosal RSV infection. (A-F) Representative dot plot showing lung immune cells isolated from WT mice receiving the corresponding treatments for 24 h before infection. PBS-liposome-treated and clodronate-liposome-treated mice were infected intranasally with 1.0×107 pfu of RSV. (A) Lung cells isolated from WT mice treated with intravenous PBS-liposome or clodronate-liposome for 24 h. Representative dot plots show alveolar macrophages (CD11b+Ly6G+CD11chiSiglec-Fhi), neutrophils (CD11bhiLy6C+Ly6Ghi), eosinophils (CD11b+Ly6G−Siglec-F+), and monocytes (CD11b+Ly6G−Siglec-F−Ly6Chi) gated on CD45.2+ DAPI−. The results are representative of two independent experiments. (B-C) Levels of IFN-γ production from CD4+ T cells (B) and CD8+ T cells (C) was measured by ELISA. At 8 days post infection, CD4+ and CD8+ T cells were isolated from mice spleens and stimulated with the indicated amount of heat-inactivated virions or RSV M peptides for 72 h, respectively. The error bars show the SEM. (D, E) Representative dot plot of IFN-γ-producing CD8+ T cells (D) or RSV M peptide-specific CD8+ T cells (E) were detected in the lungs of mice at 8 days post infection. (F) the frequency and cell number of RSV M peptide-specific CD8+ T cells. The error bars are the mean of 3 mice ± SEM. The results are representative of three experiments.
*p<0.05; **p<0.01 as calculated by Student's t-test.
DISCUSSION
In this study, we investigated IFN-β-producing monocytes in response to RSV infection. Our results showed that mucosal RSV infection induces monocytes to produce IFN-β and that this production increases gradually post infection. In addition to IFN-β expression, IFNα expression in monocytes from lung tissues was elevated, suggesting that RSV infection induces monocytes to produce type I IFN. These results are consistent with the finding that monocytes from bone marrow cells produce IFN-β post in vitro RSV infection. In response to both in vivo and in vitro RSV infection, monocytes required the MyD88 pathway but not the TLR7 or MAVS pathway to produce IFN-β. Furthermore, the depletion of circulating monocytes reduced cytotoxic CD8+ T-cell responses against mucosal RSV infection.
Type I IFNs centrally orchestrate innate immune responses against viral infection, including RSV infection. In viral infection, pDCs have been considered as the major population producing type I IFNs (23,24). Our previous study also demonstrated that pDCs produce type I IFNs, especially IFN-β, via the TLR7-MyD88-mediated pathway during RSV infection (8). In this study, we found that monocytes are another cellular source of IFN-β during RSV infection. Unlike pDCs, the IFN-β production of monocytes was not affected by TLR7 signaling. This result is consistent with a recent study showing that TLR7 does not contributes to type I IFN response of mouse and human CD14+ monocytes against infection with several types of RNA viruses (11). This study also described that TLR7 and TLR8 stimulation differentially induce signaling pathways and TLR8 blockade inhibited the expression of type I IFN genes during RNA virus infections. In this respect, it is possible that the IFN-β production of monocytes is mediated by other TLR signaling, such as TLR4 and TLR8, during RSV infection.
MAVS is an adapter protein required for the signaling of RIG-I/MDA5, which can sense cytosolic viral RNA (25). While one study demonstrated that MAVS is essential for innate immunity against RSV infection (22), another study showed that MAVS deficiency induces increased inflammation and reduced viral clearance (26). Previously, we demonstrated that the MAVS pathway is required for type I IFN production of DCs and macrophages derived from bone marrow cells during RSV infection. These results were consistent with the results of alveolar macrophages in another study (6,27). Although DCs and macrophages are derived from monocytes, monocytes did not require the MAVS pathway to produce IFN-β during RSV infection in this study. A previous study showed that viral infection induces the rapid differentiation of human monocytes into DCs (28). Concerning this, RSV-infected monocytes might differentiate into DCs, requiring the MAVS pathway, but uninfected monocytes might produce IFN-β via other viral sensing receptors.
Alveolar macrophages were shown to produce type I IFNs at early time points (within 1 day post infection) of RSV infection, leading to the recruitment of inflammatory monocytes (27). In this study, we observed an increase in IFN-β-producing monocytes after 2 days post infection. Therefore, we speculate that monocytes recruit in response to type I IFNs produced by alveolar macrophages at the early phase of RSV infection and become the next cellular source of type I IFNs for continuous antiviral responses. Furthermore, this would explain our finding that IFNαR deficiency impairs the IFN-β production of monocytes during RSV infection.
T-cell response against viral infections is critical to mediate viral clearance and induce memory response (29,30). In addition to its Ag-presenting cell-dependent manner, type I IFNs can directly affect T-cell function (31). CD8+ T cells deficient in type I IFN receptors were found to have an exacerbated capacity to expand and generate memory cells in response to viral infection (32). Another study showed that inflammatory monocytes activate memory CD8+ T cells via type I IFN signaling during microbial invasion (33). In this study, we found that the depletion of circulating monocytes or the MyD88 deficiency yielded less IFN-γ-producing and viral Ag-specific CD8+ T cells during RSV infection. This suggests that type I IFNs produced by monocytes via MyD88 pathway affect the antiviral capacity of CD8+ T cells in response to RSV infection, resulting in memory formation.
In conclusion, our study demonstrates that monocytes producing MyD88-dependent IFN-β are required in the development of adaptive immune responses to RSV infection. These findings have significant implications in the design of vaccines and the management of RSV infection. Stimulants of monocytes may be an ideal adjuvant candidate in RSV vaccines that confer protective immune responses.
ACKNOWLEDGEMENTS
Authors would like to thank Dr. Jun Chang at Ewha Womans University for the discussion and technical help. This study was supported by the National Research Foundation of Korea (NRF-2019R1A2C2087490, NRF-2021M3A9D3026428, and NRF-2021M3A9H3015688) funded by the Ministry of Science and ICT of Korea. The authors also thank Drs. Akiko Iwasaki, Suk-Jo Kang, Myoung Ho Jang, and Mi-Na Kweon for mice and reagents.
Abbreviations
- APC
allophycocyanin
- DC
dendritic cell
- HI-RSV
heat-inactivated respiratory syncytial virus
- MAVS
mitochondrial antiviral signaling protein
- MDA5
melanoma-differentiation-associated gene 5
- MyD88
myeloid differentiation factor 88
- NS
not significant
- pDC
plasmacytoid dendritic cell
- pfu
plaque-forming units
- RIG-I
retinoic acid-inducible gene I
- RSV
respiratory syncytial virus
- WT
wild-type
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Kim TH, Kim CW, Oh DS, Lee HK.
- Data curation: Kim TH, Kim CW, Oh DS.
- Formal analysis: Kim TH, Kim CW, Oh DS, Jung HE, Lee HK.
- Funding acquisition: Lee HK.
- Investigation: Kim TH, Kim CW, Oh DS, Jung HE, Lee HK Methodology.
- Project administration: Lee HK.
- Resources: Lee HK.
- Supervision: Lee HK.
- Validation: Lee HK.
- Writing - original draft: Kim TH, Kim CW, Lee HK.
- Writing - review & editing: Lee HK.
SUPPLEMENTARY MATERIAL
The MyD88-mediated pathway plays a key role in viral clearance and CTL responses against mucosal RSV infection. (A) MyD88-deficient mice were infected intranasally with 1.0×107 pfu of RSV. The levels of viral replication in the lungs were determined by plaque assay on day 4 post infection. The results are shown as the mean ± SEM from three to five mice per each group. (B-E) WT or MyD88-deficient were infected intranasally with 1.0×107 pfu of RSV. (B) At 8 days post-infection, CD8+ T cells were isolated from spleens and stimulated with irradiated APCs with the indicated amount of RSV M peptides for 72 h. IFN-γ production from CD8+ T cells was measured by ELISA. The error bars show the SEM. (C) IFN-γ-producing CD8+ T cells were detected in the lung at 8 days post-infection by intracellular staining. (D) RSV M peptide-specific CD8+ T cells were detected in the lung at 8 days post-infection. The error bars are the mean of 3 mice ± SEM. The results are representative of three experiments.
References
- 1.Karron RA. Preventing respiratory syncytial virus (RSV) disease in children. Science. 2021;372:686–687. doi: 10.1126/science.abf9571. [DOI] [PubMed] [Google Scholar]
- 2.Efstathiou C, Abidi SH, Harker J, Stevenson NJ. Revisiting respiratory syncytial virus's interaction with host immunity, towards novel therapeutics. Cell Mol Life Sci. 2020;77:5045–5058. doi: 10.1007/s00018-020-03557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hijano DR, Vu LD, Kauvar LM, Tripp RA, Polack FP, Cormier SA. Role of type I interferon (IFN) in the respiratory syncytial virus (RSV) immune response and disease severity. Front Immunol. 2019;10:566. doi: 10.3389/fimmu.2019.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murira A, Lamarre A. Type-I interferon responses: from friend to foe in the battle against chronic viral infection. Front Immunol. 2016;7:609. doi: 10.3389/fimmu.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol. 2005;79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh DS, Kim TH, Lee HK. Differential role of anti-viral sensing pathway for the production of type I interferon β in dendritic cells and macrophages against respiratory syncytial virus A2 strain infection. Viruses. 2019;11:62. doi: 10.3390/v11010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS, Jr, Bakaletz LO, Durbin RK, Flaño E, Durbin JE. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo . J Virol. 2007;81:9790–9800. doi: 10.1128/JVI.00530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim TH, Oh DS, Jung HE, Chang J, Lee HK. Plasmacytoid dendritic cells contribute to the production of IFN-β via TLR7-myd88-dependent pathway and CTL priming during respiratory syncytial virus infection. Viruses. 2019;11:730. doi: 10.3390/v11080730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-α induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 10.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 11.de Marcken M, Dhaliwal K, Danielsen AC, Gautron AS, Dominguez-Villar M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci Signal. 2019;12:eaaw1347. doi: 10.1126/scisignal.aaw1347. [DOI] [PubMed] [Google Scholar]
- 12.Scheu S, Dresing P, Locksley RM. Visualization of IFNbeta production by plasmacytoid versus conventional dendritic cells under specific stimulation conditions in vivo. Proc Natl Acad Sci U S A. 2008;105:20416–20421. doi: 10.1073/pnas.0808537105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 14.Seo SU, Kwon HJ, Ko HJ, Byun YH, Seong BL, Uematsu S, Akira S, Kweon MN. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Oh DS, Oh JE, Jung HE, Lee HK. Transient depletion of CD169+ cells contributes to impaired early protection and effector CD8+ T cell recruitment against mucosal respiratory syncytial virus infection. Front Immunol. 2017;8:819. doi: 10.3389/fimmu.2017.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Joo DH, Lee JB, Shim BS, Cheon IS, Jang JE, Song HH, Kim KH, Song MK, Chang J. Dual role of respiratory syncytial virus glycoprotein fragment as a mucosal immunogen and chemotactic adjuvant. PLoS One. 2012;7:e32226. doi: 10.1371/journal.pone.0032226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunderkötter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 21.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 22.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, Mejias A, Gomez AM, Jafri H, Ramilo O, et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 24.Bencze D, Fekete T, Pázmándi K. Type I interferon production of plasmacytoid dendritic cells under control. Int J Mol Sci. 2021;22:4190. doi: 10.3390/ijms22084190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Refolo G, Vescovo T, Piacentini M, Fimia GM, Ciccosanti F. Mitochondrial interactome: a focus on antiviral signaling pathways. Front Cell Dev Biol. 2020;8:8. doi: 10.3389/fcell.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demoor T, Petersen BC, Morris S, Mukherjee S, Ptaschinski C, De Almeida Nagata DE, Kawai T, Ito T, Akira S, Kunkel SL, et al. IPS-1 signaling has a nonredundant role in mediating antiviral responses and the clearance of respiratory syncytial virus. J Immunol. 2012;189:5942–5953. doi: 10.4049/jimmunol.1201763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goritzka M, Makris S, Kausar F, Durant LR, Pereira C, Kumagai Y, Culley FJ, Mack M, Akira S, Johansson C. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J Exp Med. 2015;212:699–714. doi: 10.1084/jem.20140825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou W, Gibbs JS, Lu X, Brooke CB, Roy D, Modlin RL, Bennink JR, Yewdell JW. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119:3128–3131. doi: 10.1182/blood-2011-09-379479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt ME, Varga SM. The CD8 t cell response to respiratory virus infections. Front Immunol. 2018;9:678. doi: 10.3389/fimmu.2018.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The MyD88-mediated pathway plays a key role in viral clearance and CTL responses against mucosal RSV infection. (A) MyD88-deficient mice were infected intranasally with 1.0×107 pfu of RSV. The levels of viral replication in the lungs were determined by plaque assay on day 4 post infection. The results are shown as the mean ± SEM from three to five mice per each group. (B-E) WT or MyD88-deficient were infected intranasally with 1.0×107 pfu of RSV. (B) At 8 days post-infection, CD8+ T cells were isolated from spleens and stimulated with irradiated APCs with the indicated amount of RSV M peptides for 72 h. IFN-γ production from CD8+ T cells was measured by ELISA. The error bars show the SEM. (C) IFN-γ-producing CD8+ T cells were detected in the lung at 8 days post-infection by intracellular staining. (D) RSV M peptide-specific CD8+ T cells were detected in the lung at 8 days post-infection. The error bars are the mean of 3 mice ± SEM. The results are representative of three experiments.