Abstract
Introduction
There is a paucity of information regarding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients undergoing maintenance hemodialysis. We aimed to estimate the cumulative attack rate of SARS-CoV-2 in hemodialysis patients in China using a serological test.
Methods
We enrolled all hemodialysis patients from 8 hemodialysis facilities in Honghu and Jingzhou of Hubei province and Guangzhou and Foshan of Guangdong province in China. We screened these patients for SARS-CoV-2 infection by both a reverse transcriptase polymerase chain reaction (RT-PCR) test for viral RNA and a serological test for IgG and IgM antibodies. Data on demographics and clinical characteristics were collected by means of case report forms. We also enrolled the health care workers (HCWs) from the participating hospitals and compared the seropositive rate between hemodialysis patients and HCWs in the same region.
Results
Among 1542 hemodialysis patients, 5 (0.32%) and 51 (3.3%) tested positive by the RT-PCR test and the serological test, respectively. The seropositive rate in Hubei (3.6%) was higher than that in Guangdong (2.8%), although the difference was not statistically significant (P = 0.5). Most of the seropositive patients were asymptomatic. Independent risk factors for SARS-CoV-2 infection were age greater than 65 years, a manifestation of lung infection on imaging examinations, and a lower level of serum albumin. In comparison, the seropositive rate in 3205 health care workers was 1.2%, which was significantly lower than that observed in the hemodialysis patients (P < .001).
Conclusion
The cumulative rate of SARS-CoV-2 infection in hemodialysis patients in China was high, at 3.3%. The serological test detected 10 times more cases of SARS-CoV-2 infection than the RT-PCR test, and should be the preferred tool for estimating the prevalence of coronavirus disease 2019 (COVID-19).
Keywords: antibody, COVID-19, hemodialysis, RT-PCR
Graphical abstract
Novel COVID-19 is a highly contagious disease caused by SARS-CoV-2.1, 2, 3 The World Health Organization declared it a global pandemic on 12 March 2020.4 As of 6 June 2020, about 6.8 million cases of COVID-19 had been reported in 188 countries, with nearly 400,000 deaths.5
COVID-19 has been reported in all ages, including children.6 Pregnant women, elderly individuals, and patients with comorbidities are more susceptible to the infection and are likely to experience more severe illness.7 Patients with end-stage kidney disease, particularly those on in-facility hemodialysis, are potentially at risk for COVID-19. Patients on hemodialysis are characterized by dysfunction in both innate and adaptive immune systems, which predisposes them to an increased risk of infections and a diminished vaccine response.8 To date, there is a paucity of information regarding SARS-CoV-2 infection in patients undergoing maintenance hemodialysis.
Detection of SARS-CoV-2 from asymptomatic subjects9,10 suggests that subclinical SARS-CoV-2 infection is possible and could be seeding new outbreaks. Currently, the reported cases of COVID-19 are in individuals with clinical symptoms, in close contact with individuals with confirmed cases, or with a history of travel to the epidemic regions, and rely on a reverse transcriptase polymerase chain reaction (RT-PCR) test that is sensitive to the method and the timing of specimen collection.6,7 A large number of subclinical infection cases are believed to be undetected. Therefore, it is crucial to assess the rates of subclinical SARS-CoV-2 infection, particularly in vulnerable populations, and to understand their infectivity and future immunity to COVID-19.11,12
The serological test for antibodies against SARS-CoV-2 should provide a more accurate assessment of the scope of the COVID-19 epidemic in a population compared with the RT-PCR test. However, the serological test has not been available until very recently. We conducted a screening survey on SARS-CoV-2 infection in patients from 8 hemodialysis facilities in 2 cities (Jingzhou and Honghu, Hubei province) close to Wuhan, the epicenter of China, and another 2 cities (Guangzhou and Foshan, Guangdong province), far from the epicenter. The aim of the study was to estimate the cumulative rates of SARS-CoV-2 infection detected by both the RT-PCR test and the serological test in patients on maintenance hemodialysis and to compare with that in health care workers in the same regions.
Materials and Methods
Study Design and Participants
We enrolled patients from 8 in-hospital hemodialysis facilities in Honghu and Jingzhou of Hubei province and Guangzhou and Foshan of Guangdong province in China. Honghu and Jingzhou are 2 neighboring cities 200 kilometers west of Wuhan, the epicenter of China. Guangzhou and Foshan are 2 neighboring cities 900 kilometers south of Wuhan. There were a total of 1567 patients on maintenance hemodialysis in these facilities, of whom 1542 (98.4%) agreed and 25 (1.6%) refused to participate in the study. We screened patients for SARS-CoV-2 infection by both an RT-PCR test from nasopharyngeal swabs and a serological test for IgG and IgM antibodies against a recombinant antigen of the virus. The study population overlapped with the hemodialysis patients described elsewhere.13 We collected data on demographics, clinical characteristics, computed tomography/radiological examination, and laboratory tests of the patients by means of case report forms filled out by physicians in the participating facilities. We also enrolled 3205 health care workers from the participating hospitals, of whom 2945 and 260 were from Hubei and Guangdong, respectively. The health care workers received the serological test only.
The Medical Ethics Committee of Nanfang Hospital approved the study. All patients signed a consent form.
Laboratory Measurements
Nasopharyngeal swabs of the hemodialysis patients were collected by trained nurses in the local facility and tested for SARS-CoV-2 RNA in a designated virology laboratory in the region using a real-time RT-PCR assay as previously reported.7 The test results were further confirmed by local Center for Disease Control and Prevention.
Serum samples were collected in local facilities. All samples were inactivated at 56 °C for 30 minutes and stored at −20°C before testing. The IgG and IgM antibodies against SARS-CoV-2 were measured at a central laboratory (Kingmed Diagnostic Center, China) using a commercially available Magnetic Chemiluminescence Enzyme Immunoassay kit (Bioscience, Chongqing, China) according to the manufacturer’s instructions.14 Antibody levels were expressed as the ratio of the chemiluminescence signal over the cutoff value (S/CO). An S/CO value of >1.0 for either IgG or IgM was regarded as positive.
We independently validated the serological assay using sera of 447 end-stage kidney disease/hemodialysis patients collected before June 2019 as a negative control and sera of 242 patients with COVID-19 confirmed by the viral nucleic acid test as a positive control.13 In the validation, the serological test had an overall sensitivity of 83% and specificity of 100%. In patients with confirmed COVID-19, the cumulative seropositive rates of IgG and IgM were 56% and 44%, respectively, on day 7 after the onset of symptoms and reached 95% on day 16 and day 20, respectively. Both IgG and IgM levels can persist for at least 28 days.
Statistical Analysis
Among 1567 hemodialysis patients enrolled, 25 missed the case report forms and were excluded from further analyses. Clinical characteristics of the patients were summarized by strata of seropositive status. Continuous variables were presented as mean ± SD, and categorical variables as counts and percentages. A single logistic regression model was used to estimate the effects of age, sex, region, comorbidities, symptoms, computed tomography findings, and laboratory tests on the risk of being seropositive. In the regression, missing values were assigned to the modal category. The difference between groups was tested using the Mann−Whitney test for quantitative variables and a χ2 test for categorical variables. In validation of the serological assays, the cumulative rates of seropositive for IgG and IgM after the onset of symptoms were estimated using the Kaplan−Meier method in patients with COVID-19.
Results
A total of 1542 patients, constituting more than 98% of the patients on maintenance hemodialysis in the participating facilities, were screened by both an RT-PCR test with a nasopharyngeal swab and a serological assay of IgG and IgM antibodies to recombinant antigen of SARS-CoV-2.13 The RT-PCR tests were performed between 21 January and 8 March 2020, whereas the serological tests were performed between 1 and 15 March 2020.
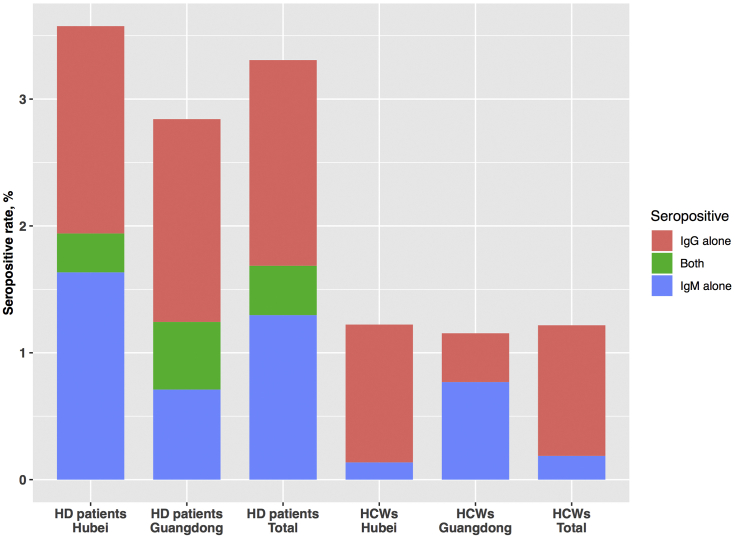
Among the hemodialysis patients in Hubei and Guangdong, 5 (0.5%) and 0 (0%) tested positive for viral RNA (Table 1), and 35 (3.6%) and 16 (2.8%) tested positive for antibodies against SARS-CoV-2, respectively (Figure 1). The levels of IgG and IgM antibodies in hemodialysis patients are shown in Supplementary Figure S1.
Table 1.
Clinical characteristics and prognosis of the hemodialysis patients with confirmed COVID-19 (n = 5)
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age, yr | 74 | 76 | 48 | 69 | 35 |
| Sex | Male | Female | Female | Male | Male |
| Exposure to source of transmission | Yes | Yes | No | No | No |
| Comorbidity | |||||
| Diabetes | No | No | No | No | No |
| Hypertension | Yes | Yes | Yes | Yes | Yes |
| Coronary heart disease | No | No | No | No | No |
| Malignant tumor | No | No | No | No | No |
| Etiology of ESKD | GN | GN | GN | HTN | HTN |
| Laboratory findings | |||||
| White blood cells | 3.27 | 5.02 | 6.57 | 6.95 | 6.36 |
| Platelets | 133 | 311 | 207 | 230 | 167 |
| Lymphocytes | 0.43 | 0.58 | 1.33 | 0.86 | 0.98 |
| C-reactive protein | 8.35 | 63.0 | 7.38 | 6.03 | - |
| Clinical symptoms | |||||
| Fever | Yes | No | No | No | No |
| Cough | No | No | No | No | No |
| Dyspnea | No | No | No | No | No |
| Radiologic findings for pneumoniaa | |||||
| Chest CT scan | Yes | Yes | Yes | Yes | Yes |
| Vasoactive drugs | No | No | No | No | No |
| Mechanical ventilation | No | No | No | No | No |
| Need for intensive care | No | No | No | No | No |
| In-hospital death | No | No | No | No | No |
COVID-19, coronavirus disease 2019; CT, computed tomography; ESKD, end-stage kidney disease.
Including mottling ground-glass opacity, local or bilateral patchy shadowing.
Figure 1.
Seropositive rate of hemodialysis (HD) patients and healthcare workers (HCWs).
Demographic and clinical characteristics of the patients stratified by the results of serological test are summarized in Table 2. More than 95% of all serology-positive patients were without any symptoms. Serology-positive patients were on average 8 years older than serology-negative patients and were more likely to have manifestations of lung infection on chest computed tomography and lower levels of serum albumin (P < 0.01 for all). Otherwise, the 2 groups shared similar clinical features, including the etiology of end-stage kidney disease, presence of comorbidities, and leukocyte and lymphocyte counts. Although the observed infection rate in the patients from Hubei seemed to be higher than that from Guangdong, the differences were not statistically significant (P = 0.5). In our regression analysis, age greater than 65 years, manifestation of lung infection on imaging examinations, and lower serum albumin were independent risk factors for SARS-CoV-2 infection, with corresponding odds ratios (95% confidence intervals) of 2.17 (1.22−3.87), 2.96 (1.65−5.33), and 3.37 (1.53−7.40), respectively (Table 3).
Table 2.
Demographic and clinical characteristics of patients on hemodialysisa
| Characteristics | Serological test for
SARS-CoV-2 antibody |
|
|---|---|---|
| Negative, n = 1490 | Positive, n = 51 | |
| Age, yrs, n (%) | ||
| <40 | 197 (13.2) | 0 (0) |
| 40−64 | 794 (53.3) | 23 (45.1) |
| ≥65 | 499 (33.5) | 28 (54.9) |
| Sex, n (%) | ||
| Male | 844 (56.7) | 30 (58.8) |
| Female | 646 (43.3) | 21 (41.2) |
| Smoking, n (%) | ||
| Never | 843 (63.4) | 21 (48.8) |
| Current | 162 (12.2) | 6 (14.0) |
| Former | 325 (24.4) | 16 (37.2) |
| Region, n (%) | ||
| Hubei province | 943 (63.3) | 35 (68.6) |
| Guangdong province | 547 (36.7) | 16 (31.4) |
| Etiology of ESKD, n (%) | ||
| Glomerulonephritis | 376 (25.2) | 11 (21.6) |
| DKD | 200 (13.4) | 8 (15.7) |
| Hypertension | 325 (21.8) | 11 (21.6) |
| Obstructive nephropathy | 43 (2.9) | 2 (3.9) |
| Hereditary renal disease | 55 (3.7) | 2 (3.9) |
| Other | 86 (5.8) | 2 (3.9) |
| Unknown | 405 (27.2) | 15 (29.4) |
| Dialysis yrs, mean (SD) | 3.6 (6.6) | 3.4 (3.5) |
| Comorbidities, n (%) | ||
| Hypertension | 1104 (74.1) | 36 (70.6) |
| Diabetes | 268 (18.0) | 12 (23.5) |
| Coronary heart disease | 121 (8.1) | 7 (13.7) |
| Malignant tumor | 19 (1.3) | 0 (0) |
| CT findings, n (%) | ||
| Signs of lung infection | 296 (30.6) | 24 (58.5) |
| No signs of lung infection | 672 (69.4) | 17 (41.5) |
| Respiratory symptoms, n (%) | ||
| Yes | 21 (1.6) | 2 (4.7) |
| No | 1329 (98.4) | 41 (95.3) |
| Serum albumin, g/l, n (%) | ||
| <35 | 115 (12.4) | 11 (32.4) |
| ≥35 | 810 (87.6) | 23 (67.6) |
| Hemoglobin, g/l, n (%) | ||
| <90 | 318 (22.3) | 9 (18.0) |
| ≥90 | 1109 (77.7) | 41 (82.0) |
| Leukocyte count, 109/l, n (%) | ||
| >10 | 64 (4.5) | 3 (6.1) |
| 4–10 | 1172 (82.3) | 38 (77.6) |
| <4 | 188 (13.2) | 8 (16.3) |
| Lymphocyte count, 109/l, n (%) | ||
| ≥1 | 788 (55.5) | 21 (42.9) |
| <1 | 632 (44.5) | 28 (57.1) |
| C-reactive protein, mg/l, n (%) | ||
| ≥10 | 147 (20.4) | 8 (24.2) |
| <10 | 575 (79.4) | 25 (75.8) |
CT, computed tomography; DKD, diabetic kidney disease; ESKD, end-stage kidney disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Signs of lung infection on CT included ground-glass opacity, local or bilateral patchy shadowing. Symptoms included fever, cough, sputum, and dyspnea. For some clinical features, the sample size of all subgroups may not sum up to the total sample size because of missing values.
One participant was excluded from analysis due to missing data on sex, age, and comorbidities.
Table 3.
Logistic regression model for SARS-CoV-2 infection in hemodialysis patients.
| Variable | Frequency, % | Univariable
model |
Multivariable
modela |
||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Province: Hubei | 63.5 | 1.27 | 0.70-2.31 | 0.44 | 1.59 | 0.80-3.16 | 0.18 |
| Sex: female | 43.3 | 0.91 | 0.52-1.61 | 0.76 | 0.92 | 0.52-1.65 | 0.79 |
| Age: >65 yrs | 34.2 | 2.42 | 1.38-4.24 | 0.002 | 2.17 | 1.22-3.87 | 0.009 |
| Respiratory symptom: yes | 1.5 | 2.86 | 0.65-12.5 | 0.16 | 2.46 | 0.50-12.1 | 0.27 |
| CT sign of lung infection: yes | 20.8 | 3.58 | 2.04-6.30 | 9x10-6 | 2.96 | 1.65-5.33 | 0.0003 |
| Lymphocyte count: <1 × 109/L | 42.8 | 1.65 | 0.94-2.90 | 0.08 | 1.47 | 0.82-2.63 | 0.19 |
| CRP: >10 mg/l | 10.1 | 1.70 | 0.78-3.68 | 0.18 | 1.05 | 0.45-2.46 | 0.91 |
| Hemoglobin: <90 g/l | 21.1 | 0.79 | 0.38-1.64 | 0.53 | 0.46 | 0.21-1.03 | 0.058 |
| Serum albumin: <35 g/l | 8.2 | 3.29 | 1.64-6.58 | 0.0008 | 3.36 | 1.53-7.39 | 0.003 |
| Comorbidity: yes | 76.2 | 0.74 | 0.40-1.37 | 0.34 | 0.76 | 0.40-1.45 | 0.40 |
CI, confidence interval; CRP, C-reactive protein; CT, computed tomography; OR, odds ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Multivariable logistic regression model included all variables listed in table as independent variables.
As a comparison, the seropositive rate was 1.2% (36/2945) and 1.2% (3/260) among health care workers in Hubei and Guangzhou, respectively. When pooled over the regions, the seropositive rate in the hemodialysis patients was significantly higher than that observed in health care workers in the same regions (P < 0.001) (Figure 1).
Discussion
In the hemodialysis population, the positive rate detected by the serological antibody test was high at 3.3% compared with 0.32% detected by the RT-PCR test. More than 90% of the infections detected by the serological test were missed by the RT-PCR test, highlighting the inefficiency of the RT-PCR test as a tool for estimating the prevalence of COVID-19. Detection of asymptomatic or mild SARS-CoV-2 infection is critical for understanding the overall prevalence and the infection potential of COVID-19. Asymptomatic patients may evade current RT-PCR−based surveillance methods, and constitute a significant proportion of the disease population. Many of these cases may be detected only by a serology-based screening method. However, the presence of antibodies indicates a history of SARS-CoV-2 infection, not necessarily an active infection. The fact that asymptomatic infectors are potential sources of SARS-CoV-2 outbreaks10,15 warrants a systematic assessment for the prevalence and transmission dynamics in these individuals.
Herd immunity has been proposed as a strategy for prevention and control of future SARS-CoV-2 infection and outbreak, which is predicted to occur when two-thirds of the population is infected. Our current study populations do not cover the most severe epidemic regions such as Wuhan, and the current cumulative infection rates from our study are lower than that reported in New York City16 and far lower than the two-thirds population threshold for herd immunity. Because of the cross-sectional design of the current study, we are not able to monitor the dynamic changes of antibody titer in the infected patients. It would be important to know how long the antibodies persist in an individual and the quantitative titer of antibodies in an asymptomatic patient that may provide immunity against future infections.
In our hemodialysis population, manifestation of lung infection in imaging studies is an independent predictor for SARS-CoV-2 infection. However, more than 30% of the seronegative patients who underwent chest computed tomography also had manifestation of lung infection, including ground-glass opacity, suggesting that such imaging features are not specific markers for SARS-CoV-2 infection.
The prevalence of COVID-19 confirmed by the RT-PCR test in our dialysis population in Hubei (5 of 978) was at least an order of magnitude higher than that reported in the general populations from the same regions, which was 2.5 per 10,000.17 This difference may be explained partly by the limited and selective testing in the general population, which results in substantial underestimation of the disease prevalence.18 In our study, the cumulative rates of SARS-CoV-2 infection in hemodialysis patients was higher than that in health care workers from the same regions. This may be attributable to multiple factors. First, the hemodialysis population is older than that of health care workers. Second, hemodialysis patients must visit the dialysis facilities regularly, even if an infection has been suspected. Thus, the hemodialysis facilities may become a cesspool environment similar to nursing home facilities. Third, compared with health care workers, dialysis patients may have a lower level of personal protection. Finally, dialysis patients may be more susceptible to SARS-CoV-2 infection because of acquired immunodeficiency. Good management of hemodialysis patients during the epidemic requires aggressive testing in these patients, early isolation of the patients suspected of being infected, intensive disinfection of the dialysis equipment, and adequate personal protection in both health care workers and patients.
Our study is subject to several limitations. First, the relatively small number of seropositive cases prevented us from a more detailed analysis among subgroups. Second, the serological tests were performed at local laboratories using different assay kits. However, all pf these kits were approved by the National Medical Products Administration of China. Third, the sensitivity of the serological test depends on the timing, the level, and the duration of antibody production after SARS-CoV-2 infection. Testing too soon (before the start of antibody production) or too late (after the cessation of antibody production) or in individuals with immunodeficiency may produce a false-negative result. For this reason, the serological test may underestimate the prevalence of SARS-CoV-2 infection.
In summary, we estimated a cumulative rate of 3.6%, and 2.8% for SARS-CoV-2 infection in hemodialysis patients in Hubei and Guangdong, respectively. The serological test detected 10 times more cases of SARS-CoV-2 infection than the RT-PCR test, and should be the preferred tool for estimating the prevalence of COVID-19.
Disclosure
All the authors declare no competing interests.
Acknowledgements
This study was funded by the Clinical Innovation Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR0201003) to FFH, the National Innovation Team Program (81521003 to FFH), and the Major Scientific and Technological Planning Project of Guangzhou (grant No. 201607020004 to XX), Guangzhou Regenerative Medicine and Health Guangdong Laboratory (to FFH). The data used and analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Hou Fan Fan: Supervision, Project administration, Conceptualization, Methodology, Formal analysis, Writing – Review & Editing, Funding acquisition. Xu Xin: Conceptualization, Data curation, Methodology, Formal analysis, Software, Writing – Original Draft, Funding acquisition. Nie Sheng: Conceptualization, Data curation, Methodology, Formal analysis, Software, Writing – Original Draft. Sun Jian: Conceptualization, Data curation, Methodology, Formal analysis, Writing – Review & Editing. Kong Yaozhong: Conceptualization, Resources, Data curation, Methodology, Formal analysis, Writing – Original Draft. Ren Chanjun: Investigation, Resources. Huang Ailong: Validation, Formal analysis. Liang Min: Validation, Formal analysis. Zha Yan: Investigation, Resources. Ma Tean: Investigation, Resources. Li Dongfeng: Investigation, Resources. Gao Shikui: Investigation, Resources. Peng Jiaqing: Investigation, Resources. Peng Gangyi: Investigation, Resources. Shao Yong: Investigation, Resources.
Footnotes
Figure S1. The levels of IgG and IgM antibodies in hemodialysis patients(n=1,542). Each dot represents the level of IgG and IgM antibody level for one patient from Guangdong (red) or Hubei (blue).
Supplementary Material
Figure S1. The levels of IgG and IgM antibodies in hemodialysis patients(n=1,542). Each dot represents the level of IgG and IgM antibody level for one patient from Guangdong (red) or Hubei (blue).
References
- 1.Guan W.J., Ni Z.Y., Hu Y., et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Director-General’s opening remarks at the media briefing on COVID-19-11-March-2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available at:
- 5.Johns Hopkins University Coronavirus Resource Center. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 Available at:
- 6.Xu Y., Li X., Zhu B., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed-Ahmed M., Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:8–15. doi: 10.1053/j.ackd.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Lu X., Zhang L., Du H., et al. Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z., Song C., Xu C., et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Global surveillance for COVID-19 disease caused by human infection with the 2019 novel coronavirus. https://www.who.int/publications-detail/global-surveillance-for-human-infection-with-novel-coronavirus-(2019-ncov) Available at:
- 12.Munster V.J., et al. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 13.Xu X., Sun J., Nie S., et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 14.Long Quanxin, Liu B.Z., Deng H.J., et al. Antibody responses to SARS-CoV-2 in COVID-19 patients: the perspective application of serological tests in clinical practice. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 15.Rothe C., Schunk M., Sothmann P., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins-Dunn H., Breuninger K., Kim J. New York antibody study estimates 13.9% of residents have had the coronavirus, Gov. Cuomo says. https://www.cnbc.com/2020/04/23/new-york-antibody-study-estimates-13point9percentof-residents-have-had-the-coronavirus-cuomo-says.html Available at:
- 17.Health commission of Hubei province. http://wjw.hubei.gov.cn/bmdt/ztzl/fkxxgzbdgrfyyq/xxfb/202003/t20200328_2195436.shtml Available at:
- 18.Li R., Pei S., Chen B., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.