Highlights
-
•
This ESMO Clinical Practice Guideline provides key recommendations for end-of-life care for patients with advanced cancer.
-
•
It details care that is focused on comfort, quality of life and approaching death of patients with advanced cancer.
-
•
All recommendations were compiled by a multidisciplinary group of experts.
-
•
Recommendations are based on available scientific data and the authors’ collective expert opinion.
Key words: end of life, cancer patient, total care, death and dying, palliative care, clinical practice guideline
Introduction
In palliative care (PC), the term ‘end of life’ (EoL) is commonly used but inconsistently defined.1 This European Society for Medical Oncology (ESMO) Clinical Practice Guideline (CPG) refers to EoL as care for people with advanced disease once they have reached a point of rapid physical decline, typically the last few weeks or months before an inevitable death as a natural result of a disease. This guideline will only consider the last weeks and days of life for the adult patient with advanced cancer.
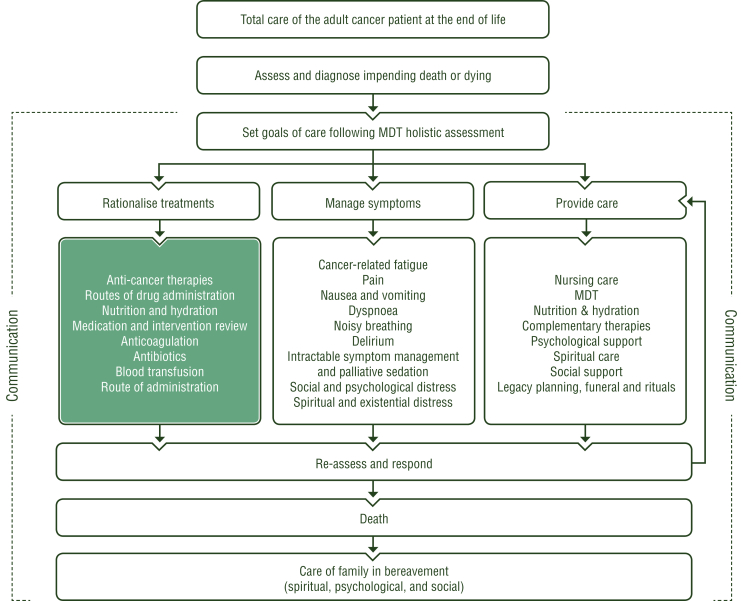
At EoL, the goal of care is focused towards comfort, offering a tailored and individualised management of quality of life (QoL) and approaching death of the patient. Thus, early integration of supportive care and PC in oncology is essential.2 Comfort care is holistic and person-centred, focusing on the interrelationship between physical, psychosocial and spiritual issues (Figure 1). This warrants the cessation of cancer-modifying treatments and disproportionate interventions, focusing on symptom relief and ‘whole person’ or ‘total care’. It is well documented that PC teams improve symptom control, satisfaction and psychological support for patients and families in hospitals, hospices and community settings, particularly at EoL.3 In the absence of multiprofessional PC teams, and to enhance better collaboration, oncologists need skills to intervene beyond oncological therapies. Communication with the patient and their family becomes a priority to ascertain the therapeutic choices available. Furthermore, the care setting at EoL needs careful evaluation so that monitoring of overall well-being enables the best QoL and a dignified death. EoL provides specific challenges in clinical management of oncology patients, which can be addressed through a multiprofessional and collaborative approach. Oncologists have a responsibility to ensure the smooth transition of the patient and family from living to dying and to coordinate the necessary resources for effective and timely intervention.
Figure 1.
Total care of the adult cancer patient at the end of life.
Turquoise: combination of treatments or other systemic treatments; white: other aspects of management. MDT, multidisciplinary team.
Communication and the family
‘Family’, as understood by the patient, is the unit of care at EoL. Identifying key people in the patient's life is vital.
Patients, families and carers rate effective communication and shared decision making as having primary importance, ahead of other EoL domains such as expert, respectful and compassionate care and trust and confidence in clinicians.4 Moreover, patients often express a preference for involving family and friends in EoL discussions.5 An anticipatory approach, assessing patient level of knowledge of their diagnosis, wishes around disclosure and acknowledging emotional responses, is essential.6 Some patients may choose not to confront their own mortality, or lack capacity to do so.
The treating physician should engage in discussion about approaching EoL, treatment options and goals of care, place of care and place of death, and address legal or personal matters. The potential for patients to die in their preferred location is increased if oncologists are aware of their preference.7
Tailored information should be shared in clear, manageable and jargon-free language to avoid misinterpretation, in a location that ensures privacy. Professional interpretation services should be used if families do not share a common language with the clinician.6,8
Patient and family understanding should always be confirmed. Pacing and staging of information-sharing, aligned with stage of illness or deterioration and paraphrasing of the patient’s own words, are important communication strategies.9 Question prompt lists may assist relatives to overcome barriers and encourage involvement in decision making.9 Advance care planning with the patient can identify substitute decision makers, or prepare family members for death, a possible protective factor against later distress.10 Identifying relevant cultural customs, spiritual beliefs and practices around death and dying is equally important, as is access to spiritual care and/or faith representatives.11
The family's and carer's needs for support and information should be assessed separately from those of the patient and referral for intervention to other experts within the multidisciplinary team should be considered. High levels of pre-death distress and low levels of preparedness for death are associated with adverse bereavement outcomes, in addition to spousal status, pre-death depressive symptoms, low educational level and economic disadvantage.12,13
A family meeting or conference can explore understanding of prognosis and assess preparedness for death. It may also benefit family carers by identifying and addressing unmet needs and concerns.
Patients who are parents of young children
Parents/guardians need clear prognostic information as well as support and guidance from professionals in order to prepare children for the death of their parent, although professionals may be reluctant to engage because of concerns about lack of time and of expertise.14,15 Parental death during childhood is associated with increased mortality in early adulthood from all causes. The ability of the surviving parent to support and nurture healthy emotional expression of grief by children is the most significant protective factor in limiting negative outcomes.16 Appropriate support and advice about communicating with children should be offered before and after death to include, where possible, family, friends and social networks, trying to maintain structures and routines for the children.14
Recommendations
-
•
Effective communication and shared decision making are essential at EoL [II, A].
-
•
Strategic preparation of patient and family, respecting personal wishes and beliefs, is critical to reducing adverse bereavement outcomes [III, A].
Nursing considerations
Nurses provide a critical role in the management of patients and families in palliative and EoL care. One qualitative systematic review proposed that nurses act as coordinators of care by virtue of the range of nursing activities required in PC, proximity to the patient and response to need.17,18
Core nursing roles include managing physical deterioration of the body (managing tissue viability and oral, bowel and bladder care), guiding and supporting families in the adjustment to the process of dying, recognition of death and managing the moment of death and its aftermath. So far, however, evidence is largely based on qualitative data and/or expert opinion, particularly in relation to nursing roles in managing patient care and comfort.19 One recent study demonstrates the importance of nurses’ recognition in predicting early signs of physical deterioration.17 Nurses also have a role in addressing family burden and, given the proximity to patient and family, advising on changing goals and settings of care. The ability to appropriately diagnose dying is critical to sensitively guide family members from the point of deterioration to death.
Nurses need specific education about knowledge and attitudes to work in EoL care. The impact of education on improving nursing care is demonstrated through the End-of-Life Nursing Education Consortium (ELNEC) core curriculum, a cascade method to extend nursing knowledge at EoL.20 Attitudes of oncology and PC nurses towards dying would appear to be an indicator of confidence in care.21 Strategies to embed and integrate the principles of palliative and EoL care into nursing practice, and their benefits to multiprofessional caregiving and family support, are recognised.22
Recommendations
-
•
The nursing role is considered vital to the care of patients and families at EoL [V, C].
-
•
The role of PC teams is critical to the care of patients and families at EoL [II, A].
Prognostic factors in advanced cancer
Prognostic estimates may elicit important patient goals or unfinished business and preferences about site and style of EoL care.
Physician prediction of survival is inaccurate and often overly optimistic. Probabilistic estimates are more accurate. Objective factors associated with short prognosis include deteriorating performance status (PS) and presence of symptoms including dyspnoea, dysphagia, xerostomia, anorexia and cognitive impairment.23 Laboratory findings associated with systemic inflammatory response, e.g. elevated C-reactive protein (CRP) levels, reduced albumin levels and leucocytosis, are also associated with poor prognosis.24
Prognostic models incorporating physician prediction of survival and clinical and laboratory factors improve the accuracy of clinical prediction.25 Validated, most frequently-studied tools able to predict survival are: the Palliative Performance Scale, the Palliative Prognostic Score, the Palliative Prognostic Index and the Glasgow Prognostic Score. Of these, the Glasgow Prognostic Score is considered the most favourable as it uses only two objective parameters (CRP levels and decreased albumin concentration) and has prognostic value complementary to PS.25
Diagnosis of dying
Advanced cancer patients demonstrate signs predictive of the last weeks and days of life.26 Early signs, present >1 week before death, may include reduced conscious state, Palliative Performance Scale ≤20% (i.e. bed-bound, completely dependent, food intake minimal-to-none) and dysphagia for liquids. Late signs increasing likelihood of death within 3 days include non-reactive pupils, pulselessness of the radial artery, urine output <100 ml in 12 hours, inability to close eyelids, Cheyne-Stokes respiration and vocal fold grunting.23,26 A prognostic model utilising PS and drooping of nasolabial folds has been developed; when both are present, 3-day mortality is 94%.23 Unfortunately, although these indicators are highly suggestive of short prognosis, their absence does not rule out the possibility of impending death.
Recommendations
-
•
Clinicians need to be watchful for objective physical symptoms indicating prognosis of days to weeks, especially declining PS [I, A].
-
•
Poor prognosis is associated with declining PS and onset/worsening of symptoms such as dyspnoea, dysphagia, weight loss, xerostomia, anorexia and cognitive impairment [I, A].
-
•
Routine use of prognostic tools may improve accuracy of physician predictions [III, B].
Rationalising treatments
Discontinuation of treatments must be individualised and influenced by patient and family preferences, goals of care, patient prognosis and risk–benefit assessment of the treating physician.
Anticancer therapies
Chemotherapy (ChT) in the last month of life is associated with adverse outcomes including poor quality of care, emergency department attendance, cardiopulmonary resuscitation, mechanical ventilation and with dying in an intensive care unit.27 Radiotherapy (RT) offers limited benefit for patients with poor PS [e.g. European Cooperative Oncology Group (ECOG) grade 4] and is not recommended in the last month of life.28,29 Single-fraction RT may provide effective symptomatic relief for metastatic bone pain within 2 weeks, or tumour-related bleeding within 2 days.30 Use of immunotherapy at EoL is associated with increased risk of dying in hospital and potential for significant financial hardship.31 Immune checkpoint inhibitors should not be used at the EoL.32
Recommendations
-
•
ChT and immunotherapy should not be used in the last weeks of life [IV, D].
-
•
RT may have symptomatic benefit for pain or bleeding but is not recommended in the last days of life [III, D].
Routes of drug administration
As death approaches, the oral route may be unsuitable due to generalised weakness, swallowing dysfunction, altered consciousness, nausea, vomiting or bowel obstruction. Intermittent or continuous administration of medication either intravenously (i.v.) or subcutaneously (s.c.) is effective, taking into account opioid and other medication dose conversion ratios from the oral route.33 Gastrostomy or nasogastric tubes should not be initiated, but if in situ may be of benefit. If the gastrostomy tube is dislodged, or the i.v. central access is lost, reinsertion may be inappropriate. Placement of s.c. access and transdermal patches on the trunk or abdomen may ensure more effective absorption and distribution of medication. Transdermal delivery systems (e.g. fentanyl or buprenorphine patches) may be continued but initiation or increase in dose of transdermal treatment is not recommended because efficacy may be unpredictable due to decreasing peripheral perfusion, sweating or acute absorption from fever, causing adverse effects.
The preferred route of administration for most patients is continuous s.c. infusion, considered safe, flexible, broadly feasible and non-burdensome. Leaving an s.c. cannula in situ for a maximum of 7 days (usually 3-5 days) secured with clear adhesive dressing is safe practice. The sub-clavicular and abdominal areas are suitable s.c. sites; easily accessible and appropriate even if peripheral circulation is diminished. Rescue or breakthrough immediate-release medications should be prescribed s.c., sublingually (s.l.) or rectally.34
In the last days of life, many patients are confused and unable to express their needs. Thus, regular dosing or prescribing orders, such as ‘offer, may refuse’, may be a better option than ‘as needed’ or ‘per request’.
Recommendations
-
•
In the absence of a central venous catheter, continuous s.c. infusion is the preferred route, being effective, feasible, safe and inexpensive [V, B].
-
•
In the last days of life, s.c. cannulae should be placed on the trunk or abdomen, rather than extremities, due to potentially diminished peripheral perfusion [V, B].
Nutrition and hydration
In patients with an expected survival of less than a few months and not receiving anticancer treatment, nutritional interventions with low risks/burdens for the patient (counselling, oral nutritional supplements) are preferred. Very few trials compare different modes or amounts of nutritional support. In a study randomising patients with severely compromised food intake and limited survival of 1-4 months, supplemental parenteral nutrition or oral feeding did not improve QoL or survival, but increased adverse events.35
Therefore, patients with expected survival of days-to-weeks are unlikely to benefit from enteral and parenteral artificial nutrition.36,37 Each individually focused decision requires intensive clinical and ethical consideration and discussion.38 Before considering use of artificial nutrition, therapeutic goals should be explicit.
If the patient is able to swallow, they should be encouraged to take liquids and preferred foods by mouth, using small-volume meals spaced throughout the day or at request of the patient rather than large quantities of food at scheduled times. For patients in the last weeks of life nutritional interventions are rarely indicated, and care needs to primarily target alleviation of hunger (if present), thirst and other distressing symptoms.36,37
Pharmaceutical agents to stimulate appetite are few and limited by lack of evidence of efficacy. A short trial of progesterone analogues or corticosteroids may be considered depending on treatment goals and individual risk–benefit analysis.37
Nutrition, however, may be less important when approaching the last weeks and days of life, as reduced intake of food and liquids caused by anorexia–cachexia, dysphagia, delirium and reduced desire for food is part of a natural process of dying.
Concerns about whether the patient’s impending death is due to starvation or if it is possible to stimulate patient desire to eat with or without pharmacological treatments and artificial feeding tubes should be explored. Family members and caregivers often experience a high level of distress when food and fluids are no longer taken orally. The decision to hydrate cancer patients by i.v. or s.c. administration at EoL remains controversial.39 Artificial hydration given through i.v. or s.c. routes in the last days of life has not been demonstrated to prevent or relieve symptoms of thirst, dehydration or delirium in randomised trials.40 Moreover, hydration may worsen oedema, ascites and respiratory secretions. For these reasons each clinical case must be carefully evaluated and hydration tailored according to patient needs. Impeccable mouth care should always be provided.
Recommendations
-
•
In patients with an expected survival of less than a few weeks or days, the invasiveness of nutritional interventions should be decreased and dietary counselling and oral supplements should be provided [V, B].
-
•
In patients with an expected survival of less than a few weeks, comfort-directed care is the recommended approach, including alleviating thirst, eating-related distress and other debilitating symptoms [V, B].
-
•
Artificial nutrition should not be initiated in the last weeks of life [V, D].
-
•
Artificial hydration does not improve or prevent symptoms of thirst [V, C].
Medication and intervention review
Polypharmacy is common, but undesirable, at EoL.41 Physiological changes such as impaired swallowing and worsening renal or hepatic function affect medication management. Continued therapy should be based on risk–benefit analysis of each medication for the specific patient situation. Potential withdrawal symptoms and drug–drug interactions should be considered. Tools have been developed to assist clinicians with discontinuing medications at EoL.42
Preventive medications, e.g. lipid-lowering agents, antiplatelet agents and proton pump inhibitors with limited benefit at EoL should be discontinued.41 Antiseizure medications should be continued if the patient has a history of seizures. S.c. benzodiazepines (e.g. midazolam or clonazepam) offer an appropriate substitute. Diabetes medications may be contraindicated due to impaired organ function and decreased food intake. Blood glucose should be managed to a target of 5-15 mmol/l to minimise symptoms. Bisphosphonates or denosumab to prevent skeletal-related events should not be initiated, and previously commenced administration should be discontinued if life expectancy is short.43 Zoledronic acid may be considered for alleviation of delirium caused by malignant hypercalcaemia.44
Anticoagulation
Therapeutic anticoagulation should be evaluated based on risk–benefit analysis, where benefit may be diminished and bleeding risks increased at EoL. Therefore, thromboprophylaxis, generally recommended for hospitalised cancer patients, may be inappropriate at EoL, when risk of major bleeding is increased.45,46
Antibiotics
Antibiotics may provide symptomatic benefit, especially for urinary tract infection, less so for respiratory tract infection or resolution of fever and minimal benefit for bacteraemia.47 Symptomatic benefit versus possible adverse effects should be considered.
Blood transfusion
Anaemia, presenting as fatigue or dyspnoea, is common in advanced cancer and often multifactorial. Red blood cell transfusion may provide short-term benefit and improve QoL.48 Blood transfusion is not associated with improved PS.48 Potential harms, though infrequent, include allergy, haemolysis and fluid overload.
Platelet transfusion for thrombocytopaenia may assist symptomatic bleeding although benefits are limited by the short half-life of platelets.49 Other strategies utilised to control bleeding include local measures and oral, i.v. or topical fibrinolysis inhibitors (e.g. tranexamic acid).
The ESMO CPG on the management of anaemia and iron deficiency in patients with cancer provides detailed recommendations for treatment of anaemia and iron deficiency.50 Currently, there are no robust studies to guide decision making.49 For this reason, therapy must be personalised according to expected symptomatic benefit, risk–benefit assessment and the patient's hopes.
Recommendations
-
•
Prophylactic anticoagulation should not be used at EoL [IV, D].
-
•
Red blood cell and platelet transfusions may have limited benefits in the last weeks of life [IV, D].
Symptom management
Cancer-related fatigue
Cancer-related fatigue (CRF) is defined as a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion, related to cancer or cancer treatment and disproportionate to recent activity, that interferes with usual functioning.51
The intensity of CRF increases in the last months of life, with a prevalence of 88% in the last 1-2 weeks of life52 and up to 98% during the last days of life.53 The detailed principles of diagnosis and management of CRF (not considering specifically EoL) are reported in a recently published ESMO CPG.51
Most pharmacological treatments are not recommended in CRF, except short-term use of dexamethasone or methylprednisolone.51 The use of corticosteroids is contraindicated in patients with delirium or approaching imminent death.44
Pain
As indicated in an existing ESMO CPG, pain is reported in 30%-75% of patients in the last days of life.34 Although prevalence and average intensity of pain often decreases in the last days of life, some patients may experience significant pain exacerbations.54
Assessment of pain should be carried out regularly and consistently and should refer to the quality and intensity, pathophysiology, trigger and relieving factors, concomitant symptoms, efficacy and tolerability of analgesics currently and previously used, clinical situation, impact on patient daily functioning, pain-related psychosocial and spiritual distress, alcohol or substance abuse and patient needs.34 Standardised scales for assessment of pain intensity, e.g. visual analogue scale (VAS), verbal rating scale (VRS), numerical rating scale (NRS) and observational scales based on clinical assessment in the presence of lowered consciousness, delirium or palliative sedation should be used.55
Principles of treatment
As death approaches, ongoing pain management is usually required, with morphine and other opioids being the mainstay of treatment.34 Non-pharmacological management that promotes comfort measures and psychological support may be beneficial.
Reversible causes of pain should be considered e.g. urinary retention, a common cause of pain and agitation in the last days of life.
Choice of medication
The process of dying includes progressive renal failure with oliguria and anuria. Opioids and their metabolites, excreted renally, may accumulate, causing confusion, drowsiness and hallucinations. Careful review of continuing need should be made, and reduced doses or extended dosing intervals considered.34 Fentanyl and methadone have inactive metabolites and only small amounts of unaltered drug are excreted renally. Buprenorphine has a dual route of elimination. Dose modification is not required.
If a pain crisis occurs in the last few days of life, immediate-release s.l., s.c. or i.v. opioids ensure faster pain control.34
The fear of opioid toxicity or hastening death may affect use of opioids in the last days or hours of life.56 The medical use of opioids may be associated with a shortened survival in advanced cancer patients, but cause and effect relationship remains uncertain and decisions should be guided by goals of care, patient prognosis and risk–benefit assessment of the treating physician.57,58
There is only low-level evidence to guide use of nonsteroidal anti-inflammatory drugs (NSAIDs) in the last weeks of life.59
Other orally administered adjuvant drugs, e.g. gabapentin, pregabalin or serotonin-norepinephrine re-uptake inhibitors, should be withdrawn. Early and gradual weaning of these medications prevents a withdrawal syndrome.
Nausea and vomiting
At EoL, possible causes of nausea and vomiting (NV) include: emetogenic medications (e.g. opioids), recent ChT or RT, psychological causes (anxiety, fear), biochemical causes (e.g. hypercalcaemia, hyponatraemia, uraemia, raised creatinine), increased intracranial pressure, vestibular dysfunction, excessive cough, ascites, gastroparesis and malignant bowel obstruction.60
Treating potentially reversible causes of NV should be considered in the context of prognosis and patient goals. Appropriate hydration and correction of electrolyte abnormalities should be considered in patients with NV.
Largely poor quality or uncontrolled trials and case studies are available to guide clinical prescribing judgement. The level of evidence in most studies is low. Antiemetic therapy is tailored to potential mechanism of action, although empirical therapy is effective.60 The drug of choice for managing NV in advanced cancer is metoclopramide titrated to effect. Alternative options include haloperidol, levomepromazine or olanzapine. For opioid-induced NV, no recommendation can be made.60
Evidence supports switching opioid, or administration route from oral to s.c. to reduce opioid-induced NV.61 According to the 2016 updated Multinational Association of Supportive Care in Cancer (MASCC)/ESMO consensus recommendations,60 weak evidence suggests drugs with an antidopaminergic mode of action (e.g. haloperidol, metoclopramide) and serotonin 5-hydroxytryptamine (5-HT3) receptor antagonists (e.g. ondansetron, palonosetron) are effective in patients with opioid-induced emesis.34,60 The evidence for effectiveness of cannabinoids in treatment of non-ChT-associated NV is too weak to recommend its use in the last days of life.62
According to the 2016 updated MASCC/ESMO consensus recommendations, the drug recommended in malignant bowel obstruction is octreotide, with or without an antiemetic such as haloperidol.60 For NV associated with malignant bowel obstruction, continuous nasogastric suction is an uncomfortable intervention and is suggested only as a short-term option.
Breathlessness
Breathlessness is the most distressing symptom in cancer patients at any stage of disease. It is highly prevalent, affecting between 20% and 70% of cancer patients and is associated with poor prognosis.63 The evaluation of breathlessness should identify all contributing causes that are amenable to treatment. The gold standard for breathlessness assessment is based on patient self-report at rest and on moving.63 In patients unable to self-report breathlessness, vital signs may assist diagnosis and treatment. Screening for breathlessness should include an assessment of intensity with unidimensional tools (e.g. NRS) and functional impact [e.g. modified Medical Research Council (mMRC) breathlessness scale].63
Principles of treatment
For dyspnoea, the initial focus should be on optimising treatment of a patient’s underlying disease.63,64 Treating potentially reversible causes should be considered. Non-pharmacological treatment could provide short-term benefit but at EoL this may not be tolerated.63
Choice of medication
Most of the evidence comes from studies in chronic obstructive pulmonary disease (COPD) patients. According to the ESMO CPG on breathlessness, preferred treatment of the relief of chronic refractory breathlessness is a systemic, oral or parenteral low-dose opioid that reduces mean chronic breathlessness by ∼20% over baseline with no evidence of significant or clinically-relevant respiratory adverse effects.63
In opioid-tolerant patients, an increase in baseline dose of opioid by 25%-50% may be considered.63 A larger confirmatory randomised trial is currently under way to examine the effect of dexamethasone on breathlessness in cancer patients (clinicaltrials.gov NCT03367156).63 Benzodiazepines are frequently used when breathlessness is associated with anxiety. There is insufficient evidence from clinical trials to support benzodiazepine use for the relief of breathlessness. In individuals suffering from refractory breathlessness, midazolam infusion may be considered as palliative sedation. Randomised, controlled studies with antidepressants have not reported significant improvement of breathlessness. Three case studies reported breathlessness relief by citalopram, sertraline or mirtazapine.63 Supplemental oxygen is indicated only in patients with chronic severe hypoxaemia.63
Noisy breathing
In the last hours of life, accumulation of tracheobronchial secretions, caused by a decreased ability to swallow and reduced reflexive clearing of the oropharynx, may occur as a result of weakness and decreased neurological function. This leads to noisy breathing (NB), gurgling, crackling or rattling sounds with each breath, often referred to as ‘death rattles’.65
NB is relatively common in dying patients. It is an indicator of impending death and present in 12%-80% of patients in last 3 days of life.26,66 The median time of onset is 11-28 hours before death67 and is more frequent in women and weakly associated with lung and brain metastases.65,66
Anticipatory clarification of its nature and meaning should be a key element of EoL care. NB is not associated with dyspnoea. Patients are usually unconscious when it occurs. It is unlikely to cause suffering or be bothersome to the patient, although highly distressing for relatives and those in close vicinity. It may be perceived as indicating untreated dyspnoea, drowning or suffocation.26,66,67
The accumulation of tracheobronchial secretions may be reduced by avoiding fluid overload.67,68 Evidence does not support oropharyngeal suction, which may even exacerbate secretions.67 Most family caregivers report suctioning increases patient discomfort and does not bring lasting improvement. Changing patient position, e.g. head down or lateral, may help but has not been studied for effectiveness.67
Commonly used medications for reducing tracheobronchial secretion, thereby reducing the sound of NB, include hyoscine butylbromide and glycopyrronium bromide, which both weakly cross the blood–brain barrier. Less preferred are hyoscine hydrobromide and atropine, which do cross the barrier. The use of antisecretory medication is controversial and not recommended by the National Institute for Health and Care Excellence (NICE)69 with little evidence to inform clinical practice.67
Antimuscarinic agents may induce dry mouth, urinary retention, delirium, agitation or excessive sedation and require regular monitoring for side-effects.70 If unacceptable side-effects persist, antimuscarinic agents should be stopped. If NB continues, changing or ceasing pharmacological interventions should be considered.
Nursing interventions are very important not only to manage NB, but also to communicate with the family, clarifying the patient’s lack of awareness of the symptom and fostering the family’s ability to remain with the patient, thereby reducing their distress.26,67
Delirium
Delirium has an adverse impact on functional decline, increased length of hospital stay and medical cost, is a significant cause of distress and is associated with short survival. In the last days of life ∼90% of patients suffer from delirium.44 Delirium is characterised by disturbed consciousness with reduced ability to focus, sustain or shift attention, altered cognition or perceptual disturbance and acute onset that occurs over a short period of time and fluctuates throughout the day.71
Non-pharmacological interventions that target delirium risk factors have been recommended for preventing and managing the condition in various CPGs, including guidance for care of inpatients in hospitals.44
Patient reports suggest the presence of family caregivers is beneficial, helps orientate confused patients and protects against fear, anxiety and isolation. Diagnostic tools, pharmacological and non-pharmacological management of delirium in advanced cancer patients is the subject of a recent ESMO CPG.44
Intractable symptom management and palliative sedation
Palliative sedation is considered a treatment of last resort for EoL symptoms refractory to standard therapies and where a patient remains distressed despite all attempts at symptom relief.33 Frequency of palliative sedation varies widely between studies from 10% to 50%. This may be due to inconsistency of definitions and variations in local practice.72 The most frequent symptoms for which sedation is used are refractory delirium, dyspnoea, psychological distress and pain.73 Emergency situations, e.g. catastrophic haemorrhage, convulsions or airway obstruction, may also require rapid sedation to relieve distress. Refractory symptoms are defined when the only available treatment options would (i) not adequately relieve distress, (ii) not provide relief in an appropriate time frame or (iii) be associated with intolerable toxicity or adverse effects.33 Ideally, sedation is only considered after PC consultation and other multidisciplinary input where appropriate and available (e.g. pain specialist, psychiatrist). The decision must be made in discussion with the patient or legal substitute decision-maker if the patient is unable to consent. The consent process must include discussion of alternatives to sedation for symptom relief, the aim and process of sedation and predicted prognosis.33
The intent of therapy is to reduce consciousness and relieve the distress. Ethically, this intention separates the practice from euthanasia or medically-assisted dying in that treatment is proportionate and the intention is reduced consciousness, not death.74 Sedation medication doses are titrated only until required depth of sedation is achieved. Despite concerns that it may hasten death, palliative sedation has not been associated with reduced survival in cohort studies in PC settings.73
Medications used for sedation are usually continuous infusions (s.c. or i.v.) of benzodiazepines (midazolam, lorazepam), antipsychotics (levomepromazine, chlorpromazine) or barbiturates (phenobarbitone) and less commonly anaesthetic agents (propofol).33,75 Other medications for symptom management (e.g. opioid analgesia) are usually continued during sedation, especially if they have been effective. Sedation for existential or spiritual distress remains controversial, as is use of sedation where expected prognosis is >2 weeks.74 In some situations, respite sedation is considered, i.e. the patient is temporarily sedated then awoken after a period of respite from their distress.74
Use of sedation can have emotional impacts on both families and staff and must be considered. Regular communication must be maintained with family and support persons involved, providing information and reassurance regarding the patient’s comfort and expected prognosis.76 Sedation removes the ability to finalise conversations, goodbyes and last wishes with families. Opportunity should be provided for conversations before sedation is initiated.
To ensure consistency of practice and ethical application, local protocols should be developed, followed and audited.33,73
Recommendations
-
•
The intensity of pain should be assessed regularly, particularly using validated instruments to include patients with reduced consciousness or cognition [V, C].
-
•
Pain treatment should be personalised and monitored also in the last days of life [V, B].
-
•
Non-oral routes of administration should be used, such as s.c. or i.v., whenever benefits outweigh burden [V, C].
-
•
Concern about hastening death should not influence decisions regarding opioid therapy [IV, B].
-
•
The antiemetic drug of choice in advanced cancer is metoclopramide titrated to effect [III, B].
-
•
Alternative antiemetic options include haloperidol, levomepromazine or olanzapine [V, C].
-
•
There is limited evidence to guide antiemetic use of cyclizine or 5-HT3 receptor antagonists [V, C].
-
•
Metoclopramide should be used with caution in partial bowel obstruction [V, C].
-
•
Metoclopramide should not be used in complete bowel obstruction [V, D].
-
•
Octreotide and haloperidol are recommended for NV in malignant bowel obstruction [II, A].
-
•
Treatment with regular, low-dose, slow release (SR) opioids is recommended for palliating severe chronic breathlessness in advanced disease [II, B].
-
•
In opioid-naive patients, starting daily dose of morphine SR 10-20 mg orally over 24 hours can be used for relief of cancer-related breathlessness [II, B].
-
•
In opioid-tolerant patients, an increase in baseline dose of opioid by 25%-50% may be considered for the relief of cancer-related breathlessness [V, C].
-
•
Corticosteroids may be considered for palliation of cancer-related breathlessness refractory to other treatments [II, C].
-
•
Benzodiazepines may be used with caution in cancer patients for relief of breathlessness with associated anxiety if opioids are not effective [V, C].
-
•
In the last days of life, benzodiazepines may be considered for palliative sedation in patients with refractory breathlessness despite other treatments [IV, C].
-
•
The use of antidepressants for treatment of breathlessness should be limited to clinical trial contexts [V, C].
-
•
Palliative oxygen is not recommended in patients with resting SpO2 ≥90% [II, D].
-
•
The risk of NB may be reduced by avoiding fluid overload [V, B].
-
•
Repositioning of the patient may enable elimination of oropharyngeal secretions [V, B].
-
•
There is no evidence to support the use of anticholinergic medications for NB [V, C].
-
•
Oropharyngeal suction should not be used for NB [V, D].
-
•
Clear communication with the patient’s family about the cause and nature of NB is vital [V, B].
-
•
The evidence is insufficient to recommend routine use of screening tools in making a diagnosis of delirium in cancer patients [III, C].
-
•
Deprescribing is worthwhile in older patients, although there is insufficient data to support this recommendation for all cancer patients from the specific perspective of delirium prevention [V, B].
-
•
Administration of olanzapine may offer benefit in symptomatic management of delirium [III, C].
-
•
Administration of quetiapine may offer benefit in symptomatic management of delirium [V, C].
-
•
Benzodiazepines are effective at providing sedation and potentially anxiolysis in acute management of severe symptomatic distress associated with delirium [II, C].
-
•
Sedation is considered as a treatment of last resort where symptoms are refractory to available treatment [V, C].
Psychological issues
Assessment of social and emotional factors, mood, anxiety and depression are part of the comprehensive assessment of cancer patients’ suffering. The presence of emotional distress and generalised psychological symptoms is common, with mood disorders ranging between 30% and 40% of patients at EoL.77 Patients with pre-existing psychiatric disorders are likely to require more support and assistance. It is important to screen for exacerbation of pre-existing mental health illnesses early at cancer diagnosis, as interventions in the last days and weeks of life may be difficult.
Management
The therapeutic relationship of the treating physician with the patient and family is important in managing psychological conditions. To ensure good patient-centred care, emotional and psychosocial factors should be considered alongside physical symptoms and outcomes.77
Non-pharmacological measures
As EoL approaches, cancer patients may not have the cognitive ability or energy to participate in many types of interventions, leaving life review, relaxation therapies and more general supportive and pharmacological therapies as the only interventions.77 Strategies to enhance a sense of meaning can assist with supporting dying cancer patients.
Pharmacological measures
Without early and comprehensive assessment, pharmacological interventions may not have sufficient time to provide real benefit. This is particularly true for antidepressant medication.78 Anxiety is often better managed with antidepressant medications initially rather than benzodiazepines. However, at EoL, anxiety and stress-related disorders, insomnia, terminal agitation and restlessness are more likely to respond to benzodiazepines.33,63 Resistant anxiety and depression and severe sleep disturbances may benefit from the use of antipsychotics.79 Use of palliative sedation should be considered in these patients at EoL.33
Recommendations
-
•
Assessment and treatment of anxiety and existential distress should be undertaken early in the disease as these are highly prevalent in cancer patients at EoL [I, A].
-
•
Early detection and treatment of psychological distress leads to better adherence to treatment, better communication, reduced patient anxiety and reduced depression [II, A].
Spiritual distress
In 2014, the World Health Assembly passed a resolution which included spiritual care as an essential domain of PC. It was noted in the definition “that it is the ethical duty of health care professionals to alleviate pain and suffering, whether physical, psychosocial or spiritual.”80 Many international guidelines have been developed for addressing spiritual care as part of whole person care.11,81, 82, 83, 84 Spirituality should be addressed as part of well-being and the identification of spiritual distress as part of symptom management. Spirituality is defined broadly as a “dynamic and intrinsic aspect of humanity through which persons seek ultimate meaning, purpose, and transcendence and experience relationship to self, family, others, community, society, nature and the significant or sacred”, with spirituality being “expressed through beliefs, values, traditions and practices”.11
QoL for patients with cancer has been shown to encompass the spiritual as well as physical and psychosocial domains of care, with spiritual well-being as significant as physical well-being. A diagnosis of cancer raises spirituality-related questions and concerns, both existential and religious particularly for a person diagnosed with cancer and at transitions such as end of active treatment.85 Having a strong sense of spirituality helps patients to adjust to and cope with illness.
Spirituality may affect how a patient may cope with the cancer experience and find meaning and peace, and may assist in finding a sense of health in the midst of disease. Spirituality has been demonstrated to impact health outcomes including pain, pain interference, pain catastrophising and QoL and has been associated with decreased mortality and morbidity.86,87 Studies also demonstrate that spiritual well-being in cancer patients has been associated with lower levels of depression, better QoL near death, protection against EoL despair and desire for a hastened death.11,86
The incidence of spiritual distress is high in cancer patients; 73% of cancer patients expressed at least one spiritual need and up to 40% with newly-diagnosed and recurrent cancer reported a significant level of spiritual distress.88 Untreated spiritual suffering may worsen pain experience,89 may be of greater concern than physical symptoms and lead to increased health service utilisation.90 Identifying spiritual distress can have important implications for health outcomes including improved QoL for patients.
Spiritual distress may extend along the continuum of care from ‘common, normal feelings of vulnerability, sadness and fear, to problems that become disabling such as depression, anxiety, panic, social isolation and existential spiritual crises’.89 Spirituality should be an integral component of cancer care, with the recommendation for routine spiritual assessments.91 The National Cancer Comprehensive Network has developed a spiritual distress diagnosis table (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100225).92 Spiritual, religious and existential aspects of care have been identified as one of eight required domains of quality palliative and hospice care.93
Tools for assessing spiritual distress and spiritual concerns include the Faith, belief, meaning, importance and influence, community, address/assessment and plan (FICA) tool, which was validated for cancer patients (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100225).82 A spiritual history should be asked in the context of personal or social history. Spiritual distress and/or spiritual resources should be documented as part of the assessment and plan for each patient.
The practice of spiritual care is based on a generalist-specialist model of care; health care providers address spiritual concerns and work with spiritual care specialists, such as trained chaplains, in treating spiritual distress.11 Thus, all clinicians should address patients’ spirituality, identify and treat spiritual distress and support spiritual resources of strength.
Clinicians can respond to a patient’s sharing of spiritual distress by compassionate listening, offering presence and by sharing a sacred moment. Clinicians should consider referral to spiritual care professionals, such as chaplains, and therapies such as mindfulness, art and narrative94 or music therapy. If available, meaning-oriented therapy,95 and dignity therapy96 are options for care. In-depth spiritual counselling and exploration should be referred to trained spiritual care professionals.92
Recommendations
-
•
Spiritual distress should be assessed as part of routine cancer care [V, A].
-
•
Clinicians can use compassionate listening skills to be present to patients’ suffering and help assess and address spiritual distress [V, C].
-
•
Interventions for spiritual distress include referral to spiritual care professionals, mindfulness, art, narrative and music therapy, meaning-oriented therapy and dignity therapy [II, A].
-
•
For in-depth spiritual assessment and counselling, referral should be to a trained chaplain or spiritual care professional [V, A].
Bereavement care
Bereavement care begins before death of the patient. Bereavement is a universal experience, with grief a normal response to loss. It is a multilayered construct with emotional, physical, cognitive, spiritual and behavioural aspects and responses that vary among those who grieve.
Responses to loss
Most people are resilient and manage changes brought about by the death of a loved one and adjust to loss with the help of family and friends.97, 98, 99 Some will experience a more intense response to loss but adjust within a reasonable time frame, without the need for intervention other than mutual group and/or structured support.100 A small proportion of bereaved individuals experience a complicated grief or prolonged grief disorder and benefit from additional support.101,102 Early identification, before death, of individuals who may need professional help in the future is important, as efficacy of post-death outreach services is not proven.13,102
Assessment of bereavement need
There is currently no single agreed tool to assess for bereavement risk before death.103 Reviews conclude that factors influencing vulnerability to poor outcomes, in terms of complicated grief and post-loss depressive symptoms,104 are: loss of a child, spousal loss at a young age, history of previous multiple losses and life struggles, lack of preparedness for the loss, high levels of caregiver burden pre-death, high levels of pre-death distress and depressive symptoms,13 high levels of family conflict,105 financial distress and poverty12,106 and lower levels of educational attainment.12,106
Treatment-related factors, such as aggressive medical interventions, ambivalence regarding treatment and family conflict regarding treatment may predispose family members to poor outcome in bereavement.107
Risk factors alone are unhelpful in assessment of need as an individual’s coping ability influences outcomes.108,109 Factors that may indicate resilience in people facing loss are connection to family and community, access to practical support, previous experience of coping with loss, acceptance of death, engagement with spiritual beliefs and rituals, ability to make meaning in relation to their experience of loss and high levels of personal resources.110
Resources and services for families
Routine referral for bereavement support is not indicated. Evidence-informed information on grief may assist individuals and families to normalise grief. For the majority, this will be the primary intervention. It is important to signpost appropriate services if family members self-identify a need. A tiered approach to bereavement care,99,101 which requires knowledge of and linkages to local services and referral protocols, may be a useful intervention.
Information about grief and bereavement and available support services should be provided through accessible written material including braille, websites, telephone help lines, information events or face-to-face contact with members of the PC team, being aware of culture, faith and language.
Improved outcomes with carer support
A proactive approach may be necessary with carers who have provided care over a prolonged period or who demonstrate high levels of pre-death distress, depressive symptoms or report a history of multiple losses. These factors have been associated with poor outcome in bereavement.12,111 Tools recommended for screening for psychosocial distress are the Distress Thermometer and General Health Questionnaire.89,112 Referral for screening and assessment by psycho-oncology services or other appropriate disciplines or external agencies should be considered for family members/carers where there are concerns about the possibility of poor outcome in bereavement.
Recommendations
-
•
Clinicians should have processes in place to carry out initial screening for psychosocial distress among carers/family members in the pre-death phase [IV, B].
-
•
Referral for intervention and support to psycho-oncology, social work, spiritual care or other appropriate disciplines should be considered for carers with a history of prolonged caring, as they are vulnerable to anxiety, depression and social and financial distress before and after death [III, B].
-
•
Staff need education about grief and loss in order to recognise background factors in carers that may pre-dispose some to poor outcome in bereavement [V, C].
-
•
Assess for pre-death dissatisfaction or conflict about the nature or direction of care or lack of preparedness for death among family members and use targeted communication strategies to address concerns [IV, C].
-
•
Information about normal and adverse grief trajectories and about routes of access to appropriate levels of support should be made available to all families to facilitate help-seeking after death [IV, C].
-
•
Information about, and referral to, specialist services offering evidence-informed interventions are priorities for those who are potentially at risk of complicated or prolonged grief disorder [III, B].
-
•
Teams should review the death from the family perspective and if there were any unexpected difficulties a team member should contact the family in the post-death period [V, C].
Methodology
This CPG was developed in accordance with the ESMO standard operating procedures for Clinical Practice Guidelines development (http://www.esmo.org/Guidelines/ESMO-Guidelines-Methodology). The relevant literature has been selected by the expert authors. Levels of evidence and grades of recommendation have been applied using the system shown in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100225.113 Statements without grading were considered justified standard clinical practice by the experts and the ESMO Faculty. This manuscript has been subjected to an anonymous peer review process.
Acknowledgements
Manuscript editing support was provided by Louise Green and Richard Lutz (ESMO staff).
Funding
No external funding has been received for the preparation of these guidelines. Production costs have been covered by ESMO from central funds.
Disclosure
GBC has received an honorarium from Servier, Australia; TD has received honoraria from Angelini, Bausch, Molteni, Takeda and Teva; PJL has received honoraria for invited speaker fees from Kiowa Kirin and other honoraria as visiting professor from the National Technological University of Norway and institutional research funding as local Principal Investigator from the Pallium Foundation; CIR has received funding from Inpharm, Kyowa Kirin, Amgen Europe and Molteni SpA. AILB, CMP, IM and KH have declared no conflicts of interest.
Supplementary data
References
- 1.Hui D., Nooruddin Z., Didwaniya N. Concepts and definitions for “Actively dying,” “End of life,” “Terminally ill,” “Terminal care,” and “Transition of care”: a systematic review. J Pain Symptom Manage. 2014;47(1):77–89. doi: 10.1016/j.jpainsymman.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan K., Aapro M., Kaasa S. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol. 2018;29(1):36–43. doi: 10.1093/annonc/mdx757. [DOI] [PubMed] [Google Scholar]
- 3.Eagar K., Clapham S.P., Allingham S.F. Palliative care is effective: but hospital symptom outcomes superior. BMJ Support Palliat Care. 2020;10(2):186–190. doi: 10.1136/bmjspcare-2018-001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virdun C., Luckett T., Davidson P.M. Dying in the hospital setting: a systematic review of quantitative studies identifying the elements of end-of-life care that patients and their families rank as being most important. Palliat Med. 2015;29(9):774–796. doi: 10.1177/0269216315583032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waller A., Sanson-Fisher R., Nair B.R. Preferences for end-of-life care and decision making among older and seriously ill inpatients: a cross-sectional study. J Pain Symptom Manage. 2020;59(2):187–196. doi: 10.1016/j.jpainsymman.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Gilligan T., Coyle N., Frankel R.M. Patient-clinician communication: American Society of Clinical Oncology Consensus Guideline. J Clin Oncol. 2017;35(31):3618–3632. doi: 10.1200/JCO.2017.75.2311. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B., Nilsson M.E., Prigerson H.G. Factors important to patients’ quality of life at the end of life. Arch Intern Med. 2012;172(15):1133–1142. doi: 10.1001/archinternmed.2012.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva M.D., Genoff M., Zaballa A. Interpreting at the end of life: a systematic review of the impact of interpreters on the delivery of palliative care services to cancer patients with limited english proficiency. J Pain Symptom Manage. 2016;51(3):569–580. doi: 10.1016/j.jpainsymman.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson R.J., Bloch S., Armstrong M. Communication between healthcare professionals and relatives of patients approaching the end-of-life: a systematic review of qualitative evidence. Palliat Med. 2019;33(8):926–941. doi: 10.1177/0269216319852007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz R., Boerner K., Klinger J. Preparedness for death and adjustment to bereavement among caregivers of recently placed nursing home residents. J Palliat Med. 2015;18(2):127–133. doi: 10.1089/jpm.2014.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puchalski C.M., Vitillo R., Hull S.K. Improving the spiritual dimension of whole person care: reaching national and international consensus. J Palliat Med. 2014;17(6):642–656. doi: 10.1089/jpm.2014.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newsom C., Stroebe M.S., Schut H. Community-based counseling reaches and helps bereaved people living in low-income households. Psychother Res. 2019;29(4):479–491. doi: 10.1080/10503307.2017.1377359. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen M.K., Neergaard M.A., Jensen A.B. Do we need to change our understanding of anticipatory grief in caregivers? A systematic review of caregiver studies during end-of-life caregiving and bereavement. Clin Psychol Rev. 2016;44:75–93. doi: 10.1016/j.cpr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Hanna J.R., McCaughan E., Semple C.J. Challenges and support needs of parents and children when a parent is at end of life: a systematic review. Palliat Med. 2019;33(8):1017–1044. doi: 10.1177/0269216319857622. [DOI] [PubMed] [Google Scholar]
- 15.Fearnley R., Boland J.W. Communication and support from health-care professionals to families, with dependent children, following the diagnosis of parental life-limiting illness: a systematic review. Palliat Med. 2017;31(3):212–222. doi: 10.1177/0269216316655736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haine R.A., Wolchik S.A., Sandler I.N. Positive parenting as a protective resource for parentally bereaved children. Death Stud. 2006;30(1):1–28. doi: 10.1080/07481180500348639. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Brufau S., Gaines K., Nicolas C.T. The fifth vital sign? Nurse worry predicts inpatient deterioration within 24 hours. JAMIA Open. 2019;2(4):465–470. doi: 10.1093/jamiaopen/ooz033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekse R.J.T., Hunskår I., Ellingsen S. The nurse’s role in palliative care: a qualitative meta-synthesis. J Clin Nurs. 2018;27(1-2):e21–e38. doi: 10.1111/jocn.13912. [DOI] [PubMed] [Google Scholar]
- 19.Parola V., Coelho A., Sandgren A. Caring in palliative care. J Hosp Palliat Nurs. 2018;20(2):180–186. doi: 10.1097/NJH.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 20.Li J., Smothers A., Fang W. Undergraduate nursing students’ perception of end-of-life care education placement in the nursing curriculum. J Hosp Palliat Nurs. 2019;21(5):E12–E18. doi: 10.1097/NJH.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillman L., Adams J., Kovac R. Strategies to promote coping and resilience in oncology and palliative care nurses caring for adult patients with malignancy: a comprehensive systematic review. JBI Database Sys Rev Implement Rep. 2015;13(5):131–204. doi: 10.11124/jbisrir-2015-1898. [DOI] [PubMed] [Google Scholar]
- 22.Sawatzky R., Porterfield P., Roberts D. Embedding a palliative approach in nursing care delivery. Adv Nurs Sci. 2017;40(3):263–279. doi: 10.1097/ANS.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui D., Hess K., Dos Santos R. A diagnostic model for impending death in cancer patients: preliminary report. Cancer. 2015;121(21):3914–3921. doi: 10.1002/cncr.29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolan R.D., McSorley S.T., Horgan P.G. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134–146. doi: 10.1016/j.critrevonc.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Simmons C.P.L., McMillan D.C., McWilliams K. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53(5):962–970.e10. doi: 10.1016/j.jpainsymman.2016.12.330. [DOI] [PubMed] [Google Scholar]
- 26.Hui D., Dos Santos R., Chisholm G.B. Symptom expression in the last seven days of life among cancer patients admitted to acute palliative care units. J Pain Symptom Manage. 2015;50(4):488–494. doi: 10.1016/j.jpainsymman.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baena-Cañada J.M., Campini Bermejo A., Gámez Casado S. Experiences with prescribing large quantities of systemic anticancer therapy near death. J Palliat Med. 2019;22(12):1515–1521. doi: 10.1089/jpm.2019.0017. [DOI] [PubMed] [Google Scholar]
- 28.Nieder C., Angelo K., Dalhaug A. Palliative radiotherapy during the last month of life: predictability for referring physicians and radiation oncologists. Oncol Lett. 2015;10(5):3043–3049. doi: 10.3892/ol.2015.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yerramilli D., Parker G., Lebaron V. Ethical issues in patients referred for palliative radiation therapy. Ann Palliat Med. 2019;8:231–239. doi: 10.21037/apm.2019.06.02. [DOI] [PubMed] [Google Scholar]
- 30.Lutz S.T., Chow E.L., Hartsell W.F., Konski A.A. A review of hypofractionated palliative radiotherapy. Cancer. 2007;109(8):1462–1470. doi: 10.1002/cncr.22555. [DOI] [PubMed] [Google Scholar]
- 31.Glisch C., Hagiwara Y., Gilbertson-White S. Immune checkpoint inhibitor use near the end of life is associated with poor performance status, lower hospice enrollment, and dying in the hospital. Am J Hosp Palliat Med. 2020;37(3):179–184. doi: 10.1177/1049909119862785. [DOI] [PubMed] [Google Scholar]
- 32.Davis M.P., Panikkar R. Checkpoint inhibitors, palliative care, or hospice. Curr Oncol Rep. 2018;20(1):2. doi: 10.1007/s11912-018-0659-0. [DOI] [PubMed] [Google Scholar]
- 33.Cherny N.I. ESMO Clinical Practice Guidelines for the management of refractory symptoms at the end of life and the use of palliative sedation. Ann Oncol. 2014;25:iii143–iii152. doi: 10.1093/annonc/mdu238. [DOI] [PubMed] [Google Scholar]
- 34.Fallon M., Giusti R., Aielli F. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(suppl 4):iv166–iv191. doi: 10.1093/annonc/mdy152. [DOI] [PubMed] [Google Scholar]
- 35.Bouleuc C., Anota A., Cornet C. Impact on health-related quality of life of parenteral nutrition for patients with advanced cancer cachexia: results from a randomized controlled trial. Oncologist. 2020;25:5. doi: 10.1634/theoncologist.2019-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arends J., Strasser F., Gonella S. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open. 2021;6(3):100092. doi: 10.1016/j.esmoop.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roeland E.J., Bohlke K., Baracos V.E. Management of cancer cachexia: ASCO Guideline. J Clin Oncol. 2020;38(21):2438–2453. doi: 10.1200/JCO.20.00611. [DOI] [PubMed] [Google Scholar]
- 38.Druml C., Ballmer P.E., Druml W. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr. 2016;35(3):545–556. doi: 10.1016/j.clnu.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Lokker M.E., van der Heide A., Oldenmenger W.H. Hydration and symptoms in the last days of life. BMJ Support Palliat Care. 2019 doi: 10.1136/bmjspcare-2018-001729. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coelho T.A., Wainstein A.J.A., Drummond-Lage A.P. Hypodermoclysis as a strategy for patients with end-of-life cancer in home care settings. Am J Hosp Palliat Med. 2020;37(9):675–682. doi: 10.1177/1049909119897401. [DOI] [PubMed] [Google Scholar]
- 41.Todd A., Husband A., Andrew I. Inappropriate prescribing of preventative medication in patients with life-limiting illness: a systematic review. BMJ Support Palliat Care. 2017;7(2):113–121. doi: 10.1136/bmjspcare-2015-000941. [DOI] [PubMed] [Google Scholar]
- 42.Lindsay J., Dooley M., Martin J. The development and evaluation of an oncological palliative care deprescribing guideline: the ‘OncPal deprescribing guideline’. Support Care Cancer. 2015;23(1):71–78. doi: 10.1007/s00520-014-2322-0. [DOI] [PubMed] [Google Scholar]
- 43.Coleman R., Hadji P., Body J.-J. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2020;31(12):1650–1663. doi: 10.1016/j.annonc.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Bush S.H., Lawlor P.G., Ryan K. Delirium in adult cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(suppl 4):iv143–iv165. doi: 10.1093/annonc/mdy147. [DOI] [PubMed] [Google Scholar]
- 45.Mandalà M., Falanga A., Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(suppl 6):vi85–vi92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 46.Tardy B., Picard S., Guirimand F. Bleeding risk of terminally ill patients hospitalized in palliative care units: the RHESO study. J Thromb Haemost. 2017;15(3):420–428. doi: 10.1111/jth.13606. [DOI] [PubMed] [Google Scholar]
- 47.Baghban A., Juthani-Mehta M. Antimicrobial use at the end of life. Infect Dis Clin North Am. 2017;31(4):639–647. doi: 10.1016/j.idc.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Preston N.J., Hurlow A., Brine J., Bennett M.I. Blood transfusions for anaemia in patients with advanced cancer. Cochrane Database Syst Rev. 2012;2012(2):CD009007. doi: 10.1002/14651858.CD009007.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres M.E.U., Rodríguez J.N.R., Ramos J.L.S. Transfusion in palliative cancer patients: a review of the literature. J Palliat Med. 2014;17(1):88–104. doi: 10.1089/jpm.2013.0387. [DOI] [PubMed] [Google Scholar]
- 50.Aapro M., Beguin Y., Bokemeyer C. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(suppl 4):iv96–iv110. doi: 10.1093/annonc/mdx758. [DOI] [PubMed] [Google Scholar]
- 51.Fabi A., Bhargava R., Fatigoni S. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31:713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Teunissen S.C.C.M., Wesker W., Kruitwagen C. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34(1):94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 53.Toscani F., Di Giulio P., Brunelli C. How people die in hospital general wards: a descriptive study. J Pain Symptom Manage. 2005;30(1):33–40. doi: 10.1016/j.jpainsymman.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Seow H., Barbera L., Sutradhar R. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29(9):1151–1158. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 55.Kanji S., MacPhee H., Singh A. Validation of the critical care pain observation tool in critically ill patients with delirium. Crit Care Med. 2016;44(5):943–947. doi: 10.1097/CCM.0000000000001522. [DOI] [PubMed] [Google Scholar]
- 56.Thorns A., Sykes N. Opioid use in last week of life and implications for end-of-life decision-making. Lancet. 2000;356(9227):398–399. doi: 10.1016/S0140-6736(00)02534-4. [DOI] [PubMed] [Google Scholar]
- 57.Zheng J., He J., Wang W. The impact of pain and opioids use on survival in cancer patients: results from a population-based cohort study and a meta-analysis. Medicine (Baltimore) 2020;99(9):e19306. doi: 10.1097/MD.0000000000019306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novy D.M., Nelson D.V., Koyyalagunta D. Pain, opioid therapy, and survival: a needed discussion. Pain. 2020;161(3):496–501. doi: 10.1097/j.pain.0000000000001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derry S., Wiffen P.J., Moore R.A. Oral nonsteroidal anti-inflammatory drugs (NSAIDs) for cancer pain in adults. Cochrane Database Syst Rev. 2017;2017(7):CD012637. doi: 10.1002/14651858.CD012638.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh D., Davis M., Ripamonti C. 2016 Updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25(1):333–340. doi: 10.1007/s00520-016-3371-3. [DOI] [PubMed] [Google Scholar]
- 61.Sande T.A., Laird B.J.A., Fallon M.T. The management of opioid-induced nausea and vomiting in patients with cancer: a systematic review. J Palliat Med. 2019;22(1):90–97. doi: 10.1089/jpm.2018.0260. [DOI] [PubMed] [Google Scholar]
- 62.Dzierżanowski T. Prospects for the use of cannabinoids in oncology and palliative care practice: a review of the evidence. Cancers (Basel) 2019;11(2):129. doi: 10.3390/cancers11020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hui D., Maddocks M., Johnson M.J. Management of breathlessness in patients with cancer: ESMO Clinical Practice Guidelines. ESMO Open. 2020;5(6):e001038. doi: 10.1136/esmoopen-2020-001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hui D., Bohlke K., Bao T. Management of dyspnea in advanced cancer: ASCO Guideline. J Clin Oncol. 2021;39:1389–1411. doi: 10.1200/JCO.20.03465. [DOI] [PubMed] [Google Scholar]
- 65.Likar R., Michenthaler M.C., Traar R. Clinical factors influencing death rattle breathing in palliative care cancer patients. Z Gerontol Geriatr. 2017;50(4):332–338. doi: 10.1007/s00391-016-1042-0. [DOI] [PubMed] [Google Scholar]
- 66.Kolb H., Snowden A., Stevens E. Systematic review and narrative summary: treatments for and risk factors associated with respiratory tract secretions (death rattle) in the dying adult. J Adv Nurs. 2018;74(7):1446–1462. doi: 10.1111/jan.13557. [DOI] [PubMed] [Google Scholar]
- 67.Lokker M.E., van Zuylen L., van der Rijt C.C.D. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage. 2014;47(1):105–122. doi: 10.1016/j.jpainsymman.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Kloke M., Cherny N. Treatment of dyspnoea in advanced cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2015;26(suppl 5):v169–v173. doi: 10.1093/annonc/mdv306. [DOI] [PubMed] [Google Scholar]
- 69.Care of dying adults in the last days of life. NICE guideline [NG31] https://www.nice.org.uk/guidance/ng31 Available at. [PubMed]
- 70.Kintzel P.E., Chase S.L., Thomas W. Anticholinergic medications for managing noisy respirations in adult hospice patients. Am J Heal Pharm. 2009;66(5):458–464. doi: 10.2146/ajhp080194. [DOI] [PubMed] [Google Scholar]
- 71.American Psychiatric Association . 5th ed. American Psychiatric Association; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 72.Abarshi E., Rietjens J., Robijn L. International variations in clinical practice guidelines for palliative sedation: a systematic review. BMJ Support Palliat Care. 2017;7(3):223–229. doi: 10.1136/bmjspcare-2016-001159. [DOI] [PubMed] [Google Scholar]
- 73.Maltoni M., Scarpi E., Rosati M. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30(12):1378–1383. doi: 10.1200/JCO.2011.37.3795. [DOI] [PubMed] [Google Scholar]
- 74.Miccinesi G., Caraceni A., Maltoni M. Palliative sedation: ethical aspects. Minerva Anestesiol. 2017;83(12):1317–1323. doi: 10.23736/S0375-9393.17.12091-2. [DOI] [PubMed] [Google Scholar]
- 75.Hui D., De La Rosa A., Wilson A. Neuroleptic strategies for terminal agitation in patients with cancer and delirium at an acute palliative care unit: a single-centre, double-blind, parallel-group, randomised trial. Lancet Oncol. 2020;21(7):989–998. doi: 10.1016/S1470-2045(20)30307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tursunov O., Cherny N.I., De Keyser Ganz F. Experiences of family members of dying patients receiving palliative sedation. Oncol Nurs Forum. 2016;43(6):E226–E232. doi: 10.1188/16.ONF.E226-E232. [DOI] [PubMed] [Google Scholar]
- 77.Butow P., Price M.A., Shaw J.M. Clinical pathway for the screening, assessment and management of anxiety and depression in adult cancer patients: Australian guidelines. Psychooncology. 2015;24(9):987–1001. doi: 10.1002/pon.3920. [DOI] [PubMed] [Google Scholar]
- 78.Grassi L., Caruso R., Sabato S. Psychosocial screening and assessment in oncology and palliative care settings. Front Psychol. 2015;5:1475. doi: 10.3389/fpsyg.2014.01485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patkar A.A., Pae C.-U. Atypical antipsychotic augmentation strategies in the context of guideline-based care for the treatment of major depressive disorder. CNS Drugs. 2013;27(suppl 1):S29–S37. doi: 10.1007/s40263-012-0031-0. [DOI] [PubMed] [Google Scholar]
- 80.WHO, World Health Assembly 67 . 2014. Strengthening of Palliative Care as a Component of Comprehensive Care Throughout the Life Course; pp. 1–5. Sixty-seventh World Health Assembly. [Google Scholar]
- 81.Puchalski C.M., Sbrana A., Ferrell B. Interprofessional spiritual care in oncology: a literature review. ESMO Open. 2019;4(1):e000465. doi: 10.1136/esmoopen-2018-000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borneman T., Ferrell B., Puchalski C.M. Evaluation of the FICA tool for spiritual assessment. J Pain Symptom Manage. 2010;40(2):163–173. doi: 10.1016/j.jpainsymman.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 83.Meaningful Ageing Australia. Meaningful Ageing Australia; Parkville: 2016. National Guidelines for Spiritual Care in Aged Care. [Google Scholar]
- 84.Swift C. NHS; England: 2015. NHS Chaplaincy Guidelines 2015: Promoting Excellence in Pastoral, Spiritual and Religious Care. [Google Scholar]
- 85.Balboni T.A., Paulk M.E., Balboni M.J. Provision of spiritual care to patients with advanced cancer: associations with medical care and quality of life near death. J Clin Oncol. 2010;28(3):445–452. doi: 10.1200/JCO.2009.24.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCabe R., Murray R., Austin P. Spiritual and existential factors predict pain relief in a pain management program with a meaning-based component. J Pain Manag. 2018;11(2):163–170. [Google Scholar]
- 87.Lucchetti G., Lucchetti A.L.G., Koenig H.G. Impact of spirituality/religiosity on mortality: comparison with other health interventions. Explore. 2011;7(4):234–238. doi: 10.1016/j.explore.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 88.Delgado-Guay M.O., Chisholm G., Williams J. Frequency, intensity, and correlates of spiritual pain in advanced cancer patients assessed in a supportive/palliative care clinic. Palliat Support Care. 2016;14(4):341–348. doi: 10.1017/S147895151500108X. [DOI] [PubMed] [Google Scholar]
- 89.NCCN Clinical Practice Guidelines in Oncology. Distress management. Version 2.2021 – January 5, 2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf. Accessed March 30, 2021.
- 90.Cannon A.J., Darrington D.L., Reed E.C. Spirituality, patients’ worry, and follow-up health-care utilization among cancer survivors. J Support Oncol. 2011;9(4):141–148. doi: 10.1016/j.suponc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 91.The Joint Commission and Spiritual Care. Spiritual Directors International. https://www.sdicompanions.org/the-joint-commission-and-spiritual-care/ Available at. Accessed July 30, 2021.
- 92.Puchalski C., Ferrell B., Virani R. Improving the quality of spiritual care as a dimension of palliative care: the report of the Consensus Conference. J Palliat Med. 2009;12(10):885–904. doi: 10.1089/jpm.2009.0142. [DOI] [PubMed] [Google Scholar]
- 93.Ferrell B.R., Twaddle M.L., Melnick A. National consensus project Clinical Practice Guidelines for Quality Palliative Care Guidelines, 4th Edition. J Palliat Med. 2018;21(12):1684–1689. doi: 10.1089/jpm.2018.0431. [DOI] [PubMed] [Google Scholar]
- 94.Rodríguez Vega B., Palao A., Torres G. Combined therapy versus usual care for the treatment of depression in oncologic patients: a randomized controlled trial. Psychooncology. 2010;20(9):943–952. doi: 10.1002/pon.1800. [DOI] [PubMed] [Google Scholar]
- 95.Lichtenthal W.G., Catarozoli C., Masterson M. An open trial of meaning-centered grief therapy: rationale and preliminary evaluation. Palliat Support Care. 2019;17(1):2–12. doi: 10.1017/S1478951518000925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chochinov H.M., Hack T., Hassard T. Dignity and psychotherapeutic considerations in end-of-life care. J Palliat Care. 2004;20(3):134–142. [PubMed] [Google Scholar]
- 97.Bennett K.M., Morselli D., Spahni S. Trajectories of resilience among widows: a latent transition model. Aging Ment Health. 2020;24(12):2014–2021. doi: 10.1080/13607863.2019.1647129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Improving supportive and palliative care for adults with cancer. http://www.nice.org.uk/guidance/csgsp/resources/supportive-and-palliative-care-the-manual-2 Available at. Accessed July 28, 2021. [PubMed]
- 99.Aoun S.M., Breen L.J., Howting D.A. Who needs bereavement support? A population based survey of bereavement risk and support need. PLoS One. 2015;10(3):e0121101. doi: 10.1371/journal.pone.0121101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waller A., Turon H., Mansfield E. Assisting the bereaved: a systematic review of the evidence for grief counselling. Palliat Med. 2016;30(2):132–148. doi: 10.1177/0269216315588728. [DOI] [PubMed] [Google Scholar]
- 101.Aoun S.M., Breen L.J., O’Connor M. A public health approach to bereavement support services in palliative care. Aust N Z J Public Health. 2012;36(1):14–16. doi: 10.1111/j.1753-6405.2012.00825.x. [DOI] [PubMed] [Google Scholar]
- 102.Johannsen M., Damholdt M.F., Zachariae R. Psychological interventions for grief in adults: a systematic review and meta-analysis of randomized controlled trials. J Affect Disord. 2019;253:69–86. doi: 10.1016/j.jad.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 103.Sealey M., Breen L.J., O’Connor M., Aoun S.M. A scoping review of bereavement risk assessment measures: implications for palliative care. Palliat Med. 2015;29(7):577–589. doi: 10.1177/0269216315576262. [DOI] [PubMed] [Google Scholar]
- 104.Nielsen M.K., Neergaard M.A., Jensen A.B. Predictors of complicated grief and depression in bereaved caregivers: a nationwide prospective cohort study. J Pain Symptom Manage. 2017;53(3):540–550. doi: 10.1016/j.jpainsymman.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 105.Kissane D.W., Zaider T.I., Li Y. Randomized controlled trial of family therapy in advanced cancer continued into bereavement. J Clin Oncol. 2016;34(16):1921–1927. doi: 10.1200/JCO.2015.63.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roulston A., Campbell A., Cairnduff V. Bereavement outcomes: a quantitative survey identifying risk factors in informal carers bereaved through cancer. Palliat Med. 2017;31(2):162–170. doi: 10.1177/0269216316649127. [DOI] [PubMed] [Google Scholar]
- 107.Neimeyer R.A., Burke L.A. In: Counseling Clients Near End Life A Practical Guide for Mental Health Professionals. Werth J.L.J., editor. New York, NY: Springer Publishing Co.; 2013. Complicated grief and the end-of-life: risk factors and treatment considerations. [Google Scholar]
- 108.Stroebe M.S., Folkman S., Hansson R.O. The prediction of bereavement outcome: development of an integrative risk factor framework. Soc Sci Med. 2006;63(9):2440–2451. doi: 10.1016/j.socscimed.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 109.Vegsund H.K., Reinfjell T., Moksnes U.K. Resilience as a predictive factor towards a healthy adjustment to grief after the loss of a child to cancer. PLoS One. 2019;14(3):e0214138. doi: 10.1371/journal.pone.0214138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boerner K., Mancini A.D., Bonanno G.A. In: Complicated Grief Scientific Foundations for Health Care Professionals. 1st ed. Stroebe M., Schut H., van den Bout J., editors. Routledge; London and New York: 2013. On the nature and prevalence of uncomplicated and complicated patterns of grief. [Google Scholar]
- 111.Caruso R., Nanni M.G., Riba M.B. The burden of psychosocial morbidity related to cancer: patient and family issues. Int Rev Psychiatry. 2017;29(5):389–402. doi: 10.1080/09540261.2017.1288090. [DOI] [PubMed] [Google Scholar]
- 112.McCabe C.J., Thomas K.J., Brazier J.E. Measuring the mental health status of a population: a comparison of the GHQ-12 and the SF-36 (MHI-5) Br J Psychiatry. 1996;169(4):517–521. doi: 10.1192/bjp.169.4.516. [DOI] [PubMed] [Google Scholar]
- 113.Dykewicz C.A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33(2):139–144. doi: 10.1086/321805. (Adapted from: Gross PA, Barrett TL, Dellinger EP, et al. Purpose of quality standards for infectious diseases. Clin Infect Dis. 1994;18:421). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.