Abstract
Background
Oncological care was considerably impacted by the COVID-19 pandemic. Worrisome declines in diagnostic procedures and cancer diagnoses in 2020 have been reported; however, nationwide, population-based evidence is limited. Quantification of the magnitude and distribution of the remaining outstanding diagnoses is likewise lacking.
Methods
Using accelerated delivery of data from pathology laboratories to the Belgian Cancer Registry, we compared the nationwide rates of new diagnoses of invasive cancers in 2020 to 2019.
Results
We observed a 44% reduction in total diagnoses of invasive cancers in April 2020 compared with April 2019, coinciding with the first wave of the COVID-19 pandemic. The reduction was largest in older patients and for skin cancers (melanoma and nonmelanoma). Reductions in diagnosis were less pronounced among children and adolescents (0-19 years). A smaller decline was observed for most cancers with typically poorer prognosis or obvious symptoms, including some hematological malignancies, lung, and pancreatic cancer. Suspension of organized population screening programs was reflected in a strong decline in diagnosis in the screening age groups for female breast cancer (56%) and for colorectal cancer in both men (49%) and women (60%). The number of diagnoses began to increase from the end of April and stabilized at the beginning of June at or just above 2019 levels. There has yet to be a complete recovery in cancer diagnoses, with an estimated 6%, or ∼4000 diagnoses, still outstanding for all of 2020. Among solid tumors, head and neck cancers have the largest remaining year-over-year decrease in diagnoses at 14%.
Conclusion
These results add to the evidence of a profound impact of the COVID-19 pandemic on oncological care and identify groups at risk for continuing diagnostic delays. These data should stimulate health care providers worldwide to facilitate targeted, accessible, and efficient procedures for detection of cancers affected by this delay.
Key words: COVID-19 pandemic, diagnostic delay, neoplasm, hematological malignancy, head and neck cancer, population-based cancer registry
Highlights
-
•
A 44% reduction in diagnosis of invasive tumors during first wave of the COVID-19 pandemic, in April 2020 versus April 2019.
-
•
Persistent 6% decline in diagnosis of all invasive cancers in 2020, with largest decline for head and neck cancer (14%).
-
•
Decline in diagnosis smaller for tumors with typically poorer prognosis (lung, pancreatic cancers).
-
•
More profound and persistent decline in diagnoses in older population (aged 80+ years).
-
•
Children and adolescents (0-19 years) less impacted in first wave (12% decline); 4% of diagnoses outstanding for all of 2020.
Introduction
Following the necessary measures taken globally to counteract the spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), concerns were raised about the impact of these measures on timely cancer diagnosis. Studies looking at the use of specific diagnostic modalities or first referrals for oncology services alerted to a potential influence of the COVID-19 pandemic on cancer detection.1, 2, 3, 4, 5, 6, 7, 8, 9 Likewise, single-center and multicenter studies, and population-level studies focused on specific tumor types have reported declines in numbers of cancer diagnoses.1,2,7,9, 10, 11, 12, 13, 14, 15 Evidence from large-scale studies quantifying these declines on a population level is limited. Two nationwide studies, from the Netherlands16 and Denmark,17 reported declines in the overall number of cancer diagnoses in March 2020 and March to May 2020, respectively. Little is known about the magnitude of the diagnostic decline in younger age groups and pediatric populations, or the distribution of the declines over subgroups of heterogenous cancer types, especially between the different categories of hematological malignancies.
The first confirmed case of COVID-19 in Belgium, a country with a population of 11.5 million inhabitants, occurred on 4 February 2020.18 On 14 March 2020, all consultations, medical tests, and interventions deemed ‘non-essential’ were temporarily halted. Additionally, organized population screening programs for female breast cancer, cervical cancer, and colorectal cancer were suspended in the week of 16 March 2020 and only resumed from mid-May 2020. In early July 2020, pathologists reported that markedly fewer samples were submitted to pathology laboratories, particularly noting a decrease in samples related to cancer screening.19
Mandatory reporting of all cancer diagnoses in Belgium20 by oncological care programs and pathology laboratories, in combination with compulsory health insurance for all residents, virtually eliminates selection bias in the data collected by the Belgian Cancer Registry. The Belgian Cancer Registry is estimated to be 95% complete for diagnoses made from 2004 onwards,21 and these data have been used in global studies of cancer epidemiology.22, 23, 24 In this study, we tracked the number of new cancer diagnoses from January through December 2020, relative to 2019. Our results cover the first wave of the COVID-19 pandemic in Belgium, marked by strict confinement measures; the subsequent months following the softening of restrictions, with numerous campaigns to encourage patients to consult a physician if they had symptoms, and the restoration of ‘non-essential’ health care; and finally, the second wave of the pandemic. We provide a population-level quantification of the reduction in diagnosis in 2020 by type of tumor. We also examine the distribution of the decline over various age groups, examining COVID-19 high-risk age groups, younger adults, and pediatric and adolescent populations, and we make a specific investigation for screening age groups for female breast cancer and colorectal cancer.
Methods
In 2020, registration of new cancer diagnoses by pathology laboratories was expedited, to allow for rapid analysis of the impact of COVID-19. These laboratories generate data on the localization, behavior, and histological diagnosis of a tumor, based on the results of all types of samples analyzed. Only diagnoses of invasive tumors were included in this analysis. Data from oncological care programs are delivered to the Belgian Cancer Registry at a later time (6 months after year of incidence) and were, therefore, not available for 2020 at the time of this study. Without clinical data from oncological care programs, stage at diagnosis could not be assessed.
Patients with a history of invasive cancer on 31 December of the preceding year were excluded from analysis. Per invasive tumor diagnosis, the date of the first sample was taken as the incidence date. If a patient had multiple tumors within the same organ group, only the first invasive tumor was included in the analysis. Patient age was determined on the date of the first sample. For 2019, the analysis was likewise limited to data from pathology laboratories and the same methods were applied. To avoid any artifacts that might be generated by limiting the analysis to data delivered by the pathology laboratories, as well as to smooth fluctuations in diagnosis due to annual holidays, all results are reported as a ratio of the number of diagnoses reported by pathology laboratories in 2020 compared with 2019. For estimates of absolute numbers of missing tumors, the percentage decline estimated based on reports from pathology laboratories in 2020 versus 2019 is multiplied by the true incidence in 2018, based on registrations from both pathology laboratories and oncological care programs. A 14-day moving average was calculated using the 7th day of the period as the date reported. January and February 2020, before the COVID-19 crisis in Belgium, serve as baseline months, when the ratio of diagnoses in 2020 compared with 2019 oscillated around baseline (0% change). This is the result that would be expected, indicating that the 2020 database can be regarded as complete. Analyses of all cancers together exclude nonmelanoma skin cancer. In addition, analyses were carried out separately for a selection of tumor types, including nonmelanoma skin cancer. Larger time frames are used for analyzing types of hematological malignancies due to smaller absolute numbers.
Results
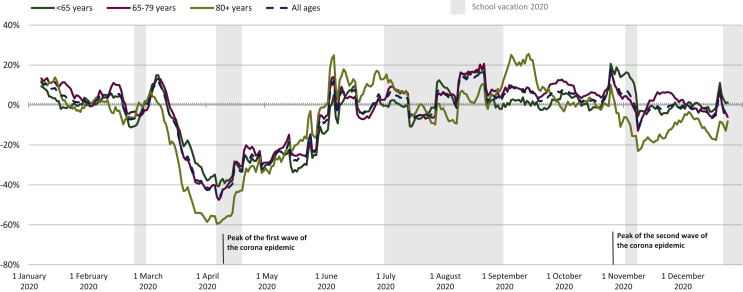
Before the COVID-19 pandemic in Belgium, in January and February 2020, the overall number of diagnoses for all invasive tumors (excluding nonmelanoma skin cancer) was comparable to 2019 (Figure 1). From mid-March, a steep decline in cancer diagnoses was observed. This decline was largest in April 2020, during which diagnosis of invasive tumors (excluding nonmelanoma skin cancer) decreased by 44% compared with April 2019. This coincides with the first wave of the COVID-19 pandemic in Belgium (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100197). Cancer diagnoses then began to recover from the second half of April 2020, reaching 2019 levels by the beginning of June, and remained stable throughout the rest of the summer and early autumn. Some excursions above 2019 levels were observed, indicating a beginning rebound in the number of diagnoses. During the second wave of the pandemic, in November 2020, a small 2% dip in diagnoses was observed overall. Over the whole of 2020 (January-December), 6% of diagnoses were still not made.
Figure 1.
Two-week moving average of the change in new invasive cancer diagnoses in Belgium from January to December 2020 relative to January to December 2019. Nonmelanoma skin cancer is excluded from this analysis.
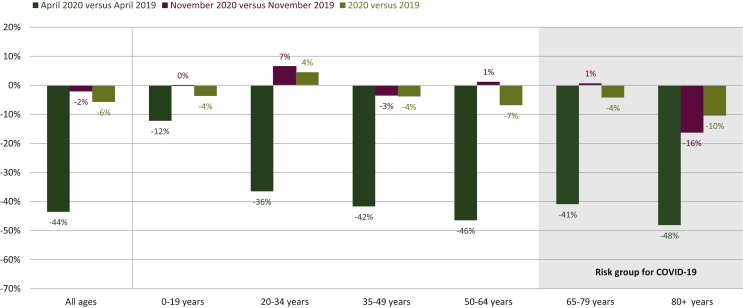
The decline in cancer diagnoses in April 2020 was more pronounced in the oldest patient populations (48% decline for patients aged ≥80 years), whereas for children and adolescents (0-19 years of age), the decline was limited to 12% (Figure 2). The second wave in November 2020 primarily impacted the group aged ≥80 years, where the decline in diagnosis was 16%. The largest remaining declines were seen in the older populations; particularly in the group aged ≥80 years, a decline of 10% persisted. The number of diagnoses in children and adolescents in all of 2020 was 4% lower compared with 2019.
Figure 2.
Decline in the number of new cancer diagnoses in Belgium by age group in 2020 relative to 2019. Nonmelanoma skin cancer is excluded from this analysis.
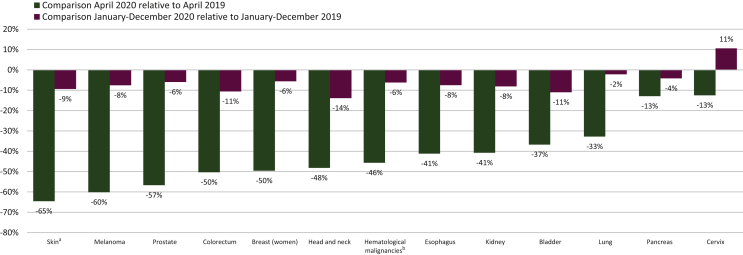
We found a more pronounced decline in diagnoses of cancers with typically a good prognosis25 (Figure 3). Overall, the largest declines observed in April 2020 were seen for nonmelanoma skin cancer (65%), melanoma (60%), and prostate cancer (57%), whereas the smallest initial declines were observed for pancreatic cancer and cervical cancer (13%). By the end of 2020, <10% of diagnoses were outstanding for most tumor types. A decline of only 2% persisted for lung cancer and 4% for pancreatic cancer. However, for head and neck cancers, a 14% decrease persisted. For cervical cancer, we found an 11% increase in overall diagnoses in 2020 relative to 2019.
Figure 3.
Decline in number of new cancer diagnoses by tumor type in Belgium in 2020 compared with 2019. a Skin indicates nonmelanoma skin cancers. b Hematological malignancies are further divided in Figure 5.
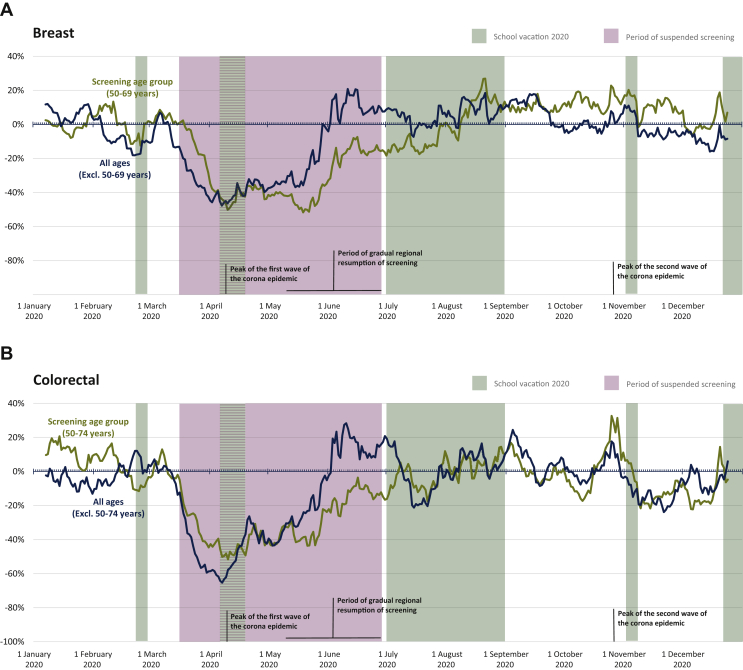
A large decline in diagnoses was observed for female breast cancer and colorectal cancer, particularly in the age groups targeted by organized population screening programs. In April 2020, female breast cancer diagnoses in the screening population (age 50-69 years) declined by 56% (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100197). Compared with the population not targeted by screening, the decline in diagnoses persisted longer for the screening population, with recovery only beginning from the end of May and reaching baseline levels around August (Figure 4A). This correlates with the resumption of screening activities. Breast cancer diagnoses rebounded, with only 6% of diagnoses missing by the end of 2020 (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100197) in the screening population, which was equivalent to the general population (Figure 1). A similar trend was observed for colorectal cancer (Figure 4B), with an initial decline in April 2020 of 54% (49% male, 60% female) in the screening population (age 50-74 years; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100197), and a rebound lagging behind the trend for the population not targeted by screening until July. This rebound resulted in 12% (14% male, 9% female) outstanding diagnoses in the screening population at the end of 2020 (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2021.100197) compared with 11% in the general population (Figure 1).
Figure 4.
Two-week moving average of the change in new breast (female) and colorectal cancer diagnoses in Belgium from January to December 2020 relative to January to December 2019 in screening and non-screening populations. (A) Breast cancer diagnoses. (B) Colorectal cancer diagnoses.
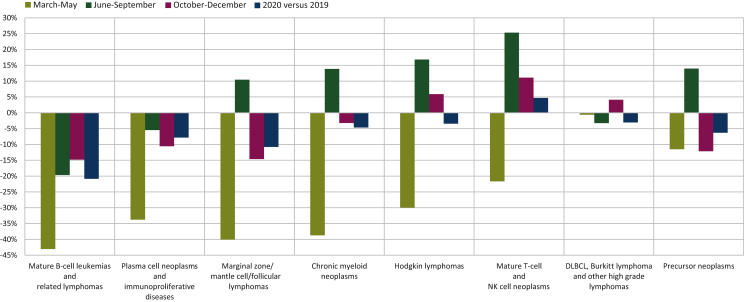
All hematological malignancies together had an initial decline of 46% in April 2020 recovering to 6% by the end of 2020. However, hematological malignancies are heterogenous and the initial decline and recovery in diagnoses differed strongly by type of hematological malignancy (Figure 5). The more indolent mature lymphoid B-cell neoplasms (i.e. mature B-cell leukemias, plasma cell neoplasms, and low-grade B-cell lymphomas) showed the largest decreases, with no or very limited rebound during June-September 2020, between the two waves of the pandemic. On the contrary, no obvious impact (1%) was observed for the most aggressive mature B-cell lymphomas [i.e. diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma, and other high-grade lymphomas] in March-May 2020, with a 3% decline over the whole year, mostly observed in the older population (7% decline over the whole year in patients aged ≥65 years with a profound initial decline in diagnosis of 33% in March-May 2020 observed in the patients aged ≥80 years—data not shown). For the other lymphoid neoplasms [Hodgkin and mature T-cell/natural killer cell (T/NK cell) lymphomas], the initial declines observed during the first wave (March-May) were compensated by rapid recoveries during June-December 2020. The decline of chronic myeloid neoplasms (mostly myelodysplasia and myeloproliferative neoplasms) and of precursor neoplasms (mainly acute lymphoid and myeloid leukemias) was limited to 5% and 6%, respectively, for the whole year 2020. However, the results for the latter two categories, the diagnoses of which usually require clinical biology analyses, should be interpreted with caution due to the indirect estimation based on pathology data (Figure 5).
Figure 5.
Decline in number of new diagnoses of hematological malignancies in Belgium in 2020 compared with 2019. DLBCL, diffuse large B-cell lymphoma; NK, natural killer.
Discussion
Cancer diagnoses dropped by almost half in April 2020, coinciding with the first wave of the COVID-19 pandemic in Belgium. While Belgium registered high numbers of deaths attributed to COVID-19 (with or without PCR confirmation), excess mortality during the first wave of the COVID-19 pandemic was comparable to average levels for Europe.26 Despite the varied impact of the pandemic and the diversity of confinement measures to slow the spread of COVID-19 around the world, single-center and multicenter studies, from Italy,5,15 Germany,13 Austria,12 Slovenia,9 Poland,2 Spain,11 the UK,1 and the USA,3,4,6,14 as well as nationwide, population-level studies from the Netherlands16 and Denmark,17 all support a global decline in cancer diagnosis during the early stage of the pandemic. The scarcity of large, population-based studies on cancer diagnoses during the pandemic, to date, is presumably linked to delays in data collection. Population-based cancer registries play a critical role in cancer control and surveillance27,28 and are at the forefront of monitoring cancer care on a large scale with minimal selection bias. In times of crisis, such as the COVID-19 pandemic, when enormous pressure is placed on health care systems, the need for prompt data collection is emphasized.29 For this study, the Belgian Cancer Registry was able to obtain data with only a delay of 2-3 months, due to expedited registration from pathology laboratories in Belgium. This has been critical in encapsulating the impact of this health care crisis. Rapid registration will also be necessary for informing the implementation of strategies to efficiently capture missing cancer diagnoses and monitor the success of these efforts.
Our data demonstrate that the rate of diagnosis of invasive tumors returned to baseline levels (i.e. comparable to 2019) by early June 2020, and remained relatively stable thereafter. The second wave of the COVID-19 pandemic in Belgium in November 2020 had a limited impact on diagnosis, with only a 2% decline. As in Belgium, the Netherlands and Northern Ireland saw marked declines in diagnoses in the first wave of the COVID-19 pandemic.16 Compared with Belgium, the Netherlands report a smaller percentage decline in diagnoses by the end of 2020, which is largely restricted to colon and breast cancer,30 while reports from Northern Ireland show relatively higher rates of outstanding diagnoses.31 In the whole of Belgium, there were 6% fewer invasive tumors diagnosed for 2020 compared with 2019—equivalent to ∼4000 invasive tumors. However, absolute incidence of all invasive cancers (excluding nonmelanoma skin cancer) was projected to increase in Belgium by 1.6% in 2020 relative to 2019.32,33 As such, the relative decline of 6% for 2020 compared with 2019 likely underestimates the true number of missing expected diagnoses in 2020. The 8% decline in diagnosis of melanoma in 2020 relative to 2019 (equivalent to ∼265 tumors) is particularly concerning, since melanoma was projected to have one of the largest increases in diagnoses (4.2%) in 2020 relative to 2019.32,33
While the first wave of the COVID-19 crisis had a profound impact on the rate of diagnosis of invasive tumors, the effect of the second wave was more limited (Figure 2 and Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100197). During the first wave, all non-essential health care services were halted nearly a month before the peak of the wave to prevent spread of SARS-CoV-2 and to divert resources to the care of COVID-19 patients. As more became known about the transmission of SARS-CoV-2, structural changes were made in hospitals and private practices to prevent the spread of SARS-CoV-2. This allowed for restoration of services shortly after the first wave and continuity of care during the second wave of the pandemic. Although a small number of hospitals in Belgium briefly had to limit non-essential procedures that required in-patient hospital care in order to have enough resources to treat COVID-19 patients during the second wave, efforts were made to maintain diagnostic services at normal levels.
Following initial indications of declines in cancer diagnosis in the first half of 2020, patient groups, government, and professional organizations of physicians, as well as the Belgian Cancer Registry, alerted the public to the necessity of timely consultation for symptoms. This likely contributed to the recovery of diagnostic rates, particularly for cancers that have more conspicuous symptoms. Declines in cancer diagnosis were generally less pronounced for tumors with a typically poorer prognosis25 or with more conspicuous symptoms, and strong recoveries in diagnostic trends were observed for several of these tumors including pancreatic and lung cancers and mature T/NK cell lymphomas. Decline in pancreatic cancer diagnosis in April 2020 was 13%, but this recovered to 4%, or ∼85 missing diagnoses, for all of 2020. Lung cancer initially showed a 33% decline in diagnosis in April 2020, but recovered to just 2% under 2019 levels for all of 2020, or an estimated 190 diagnoses outstanding. This strong recovery may partly be explained by incidental findings of lung cancer in the course of a COVID-19 diagnostic work-up.34 The persistent decline for all hematological malignancies in 2020 remains within the range of invasive tumors (6% decline in 2020 versus 2019, or ∼470 outstanding diagnoses), yet there is high heterogeneity between entities. The largest persistent declines are observed for the more indolent mature lymphoid neoplasms (∼375 missing diagnoses at the end of 2020), while the effect was very limited for the high-grade B-cell lymphoid neoplasms, with the exception of the older population.
Head and neck tumors failed to show an encouraging recovery, with 14% of diagnoses, equivalent to ∼380 tumors, still outstanding at the end of 2020. Analyses indicate that the specific decline in oral cavity cancers was more than twice that of all other head and neck cancers (data not shown). Since dental practices play an important role in the initial discovery of suspicious oral lesions, this decline may be partially explained by the initial closure and continued adapted services in dental practices. The necessity of close contact with the patient’s face mask removed could augment patients’ reluctance to attend regular dental check-ups, for fear of contracting or spreading SARS-CoV-2. Desire to reduce the use of aerosol-generating procedures, such as upper gastrointestinal (GI) endoscopy, could also contribute to the overall decline in head and neck cancer diagnoses. In the UK, upper GI endoscopy was limited to the most concerning cases, indicated by the increased percent of upper GI endoscopy with positive findings, though the absolute number of procedures was profoundly decreased.7 Head and neck cancers are also linked to socio-economic status,35 and despite the availability of socialized health care, the increased financial burden of the COVID-19 crisis might have disproportionately impacted groups with an increased risk for these types of tumors.
Total diagnoses of solid tumors and hematological malignancies in children and adolescents were only mildly affected by the COVID-19 crisis. Given the small number of cancer diagnoses in children and adolescents, the absolute number of missing diagnoses over the whole year is expected to be <20 tumors in the whole of Belgium for 2020. Since COVID-19 is currently understood to be less prevalent, cause milder disease, and have a better prognosis in children and young adolescents,36,37 barriers to accessing care may have been smaller for these age groups. Symptoms of cancers in children and adolescents may also be less likely to be overlooked. However, a global survey of pediatric cancer care noted reductions in suspected and diagnosed new cancer cases in several countries with low, middle, and high income.38 This survey also received reports of disruptions in surgical care, radiotherapy, and availability of chemotherapy agents from a sample of countries at all income levels,38 indicating that, globally, children and adolescents are certainly not invulnerable to the impacts of COVID-19 on cancer care.
The population aged ≥80 years consistently had the largest decline in diagnosis, both for solid tumors and hematological malignancies, with a 10% decline (∼1430 expected tumors) persisting over the whole of 2020. This age group also showed a stronger impact of the second wave of the COVID-19 crisis in November 2020, while other age groups were nearly unaffected. Given that this population is also at high risk of complications from COVID-19, it is logical that this group would be most reluctant to seek medical attention. In Belgium in 2020, there were an estimated 19 441 deaths from COVID-19,39 >80% of which were in patients aged ≥75 years. As such, it is possible that a limited portion of the missing diagnoses in 2020 could be attributed to patients who died from COVID-19 before a diagnosis of invasive cancer was made. Hospitalized patients with solid cancer have a higher risk of dying from COVID-19 compared with patients without cancer,40 so COVID-19 patients with underlying, undiagnosed cancer may also be at increased risk of dying.
In Belgium, organized screening programs for the general population for breast, cervical, and colorectal cancer were suspended during the first wave of the COVID-19 pandemic. For breast and colorectal cancer, we observed strong initial drops in diagnoses, with a tendency towards a delayed recovery in the screening age groups compared with age groups not targeted by screening. Outstanding diagnoses in the screening age groups alone are estimated at 285 invasive breast tumors and 500 invasive colorectal tumors in 2020. In situ tumors and precursor lesions, which would normally also be detected by organized population screening programs, are not included in this estimate. The criteria to define target groups for organized population screening did not change from 2019 to 2020 and were not influenced by the COVID-19 pandemic. For cancers in organized population screening programs, a model of diagnostic delays during the COVID-19 pandemic predicted a 7.9%-9.6% increase in 5-year mortality for breast cancer and a 15.3%-16.6% increase in 5-year mortality for colorectal cancer in a UK study.41 Likewise, models based on delays in start of treatment at a single hospital in France predict a 2.25% increase in 5-year mortality, with most patients only having a delay to treatment of <7 days.42 As such, further suspensions of cancer screening should be avoided if possible. Interpretation of data pertaining to cervical cancer is difficult because of the small number of invasive tumors; the observed overall 11% increase in diagnosis corresponds to an estimated 65 additional diagnoses in 2020 relative to 2019. It is possible that this comparatively young age group responded particularly well to campaigns encouraging people with symptoms to consult a medical professional and attend screening.
While the observed decline in diagnosis of invasive tumors appears to have been a temporary phenomenon, largely restricted to the first wave of the COVID-19 pandemic in Belgium, the recovery of these outstanding diagnoses is ongoing, and some barriers to diagnosis persist. In the beginning of the COVID-19 pandemic, an abrupt shift was made in patient-care strategies, both in general practice and in specialized centers,43 which hindered diagnosis. Telemedicine was introduced, and in-person visits were limited to urgent, non-respiratory symptoms.43,44 Among general practitioners in the Flemish region of Belgium, there is worry that telemedicine, and even the necessary additional personal protective equipment at in-person visits, hinders communication, particularly non-verbal communication, which could lead to missed diagnoses if patients are unable to adequately express their concerns.44 Furthermore, some patients with alarming symptoms refused in-person visits for fear of contracting COVID-19.44
A new and important challenge to the health care system is timely treatment and quality support for patients whose diagnosis was delayed during the COVID-19 pandemic. Delayed diagnosis is expected to have a measurable effect on patient morbidity and mortality and is particularly concerning for cancers that may (rapidly) progress to a higher stage before being diagnosed. Models based on overall cancer diagnoses in the UK predicted that, for patients diagnosed with stage I-III cancer, an average 2-month delay for 50% of referrals for diagnosis could result in a 6% increase in deaths within 10 years.45 A shift towards higher stage at diagnosis increases the burden on both patients and oncological care programs, as these high-stage cancers may require more extensive treatment. If capacities for oncological care are exceeded, excess burden could additionally exacerbate treatment delays and further impact prognosis for the patient.
Conclusions
Our results demonstrate a profound decline in diagnosis of invasive cancers and hematological malignancies in Belgium in the beginning of the COVID-19 pandemic, which is only partially and variably recovered by the end of 2020. These data should stimulate targeted searches for outstanding diagnoses in at-risk populations, particularly older patients and those at higher risk of head and neck cancers, as well as those patients overdue for participation in organized population screening programs. Time between diagnosis and start of treatment should, where possible, be minimized to avoid compounding the effects of delayed diagnosis. Trends in stage at diagnosis, including in situ and precursor lesions, as well as outcomes for patients diagnosed during the COVID-19 pandemic in Belgium will be monitored by the Belgian Cancer Registry over the coming months and years. Quantification of the impact of these delays in diagnosis on patient outcomes can be used to inform decision making by authorities during the continuation of this pandemic and potential future crises.
Acknowledgements
We wish to express special appreciation to all the pathologists who participated in the expedited data deliveries for this study. We thank all the data managers at the Belgian Cancer Registry who contributed to preparation of the data. We are especially grateful to the efforts of Petra Denolf, Anne-Dominique Petit, Geert Silversmit, and Cindy De Gendt.
Funding
This work was supported by the Foundation against Cancer (Stichting tegen Kanker/Fondation contre le Cancer), Brussels, Belgium [grant number 2019-108].
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Earnshaw C.H., Hunter H.J.A., McMullen E., Griffiths C.E.M., Warren R.B. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183(4):792–794. doi: 10.1111/bjd.19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maluchnik M., Podwojcic K., Wieckowska B. Decreasing access to cancer diagnosis and treatment during the COVID-19 pandemic in Poland. Acta Oncol. 2021;60(1):28–31. doi: 10.1080/0284186X.2020.1837392. [DOI] [PubMed] [Google Scholar]
- 3.Litchman G.H., Rigel D.S. The immediate impact of COVID-19 on US dermatology practices. J Am Acad Dermatol. 2020;83(2):685–686. doi: 10.1016/j.jaad.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maganty A., Yu M., Anyaeche V.I. Referral pattern for urologic malignancies before and during the COVID-19 pandemic. Urol Oncol. 2021;39:268–276. doi: 10.1016/j.urolonc.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toss A., Isca C., Venturelli M. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open. 2021;6(2):100055. doi: 10.1016/j.esmoop.2021.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh K.D., Ramaiya N.H., Kikano E.G. COVID-19 pandemic impact on decreased imaging utilization: a single institutional experience. Acad Radiol. 2020;27(9):1204–1213. doi: 10.1016/j.acra.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutter M.D., Brookes M., Lee T.J., Rogers P., Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis. Gut. 2021;70(3):537–543. doi: 10.1136/gutjnl-2020-322179. [DOI] [PubMed] [Google Scholar]
- 8.Langabeer S.E. Reduction in molecular diagnostics of myeloproliferative neoplasms during the COVID-19 pandemic. Ir J Med Sci. 2021;190(1):27–28. doi: 10.1007/s11845-020-02303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zadnik V., Mihor A., Tomsic S. Impact of COVID-19 on cancer diagnosis and management in Slovenia - preliminary results. Radiol Oncol. 2020;54(3):329–334. doi: 10.2478/raon-2020-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turkington R.C., Lavery A., Donnelly D., Cairnduff V., McManus D.T., Coleman H.G. The impact of the COVID-19 pandemic on Barrett's esophagus and esophagogastric cancer. Gastroenterology. 2021;160:2169–2171.e1. doi: 10.1053/j.gastro.2021.01.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez J., Mata E., Guerra A. Impact of the COVID-19 pandemic during Spain's state of emergency on the diagnosis of colorectal cancer. J Surg Oncol. 2021;123(1):32–36. doi: 10.1002/jso.26263. [DOI] [PubMed] [Google Scholar]
- 12.Tsibulak I., Reiser E., Bogner G. Decrease in gynecological cancer diagnoses during the COVID-19 pandemic: an Austrian perspective. Int J Gynecol Cancer. 2020;30(11):1667–1671. doi: 10.1136/ijgc-2020-001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob L., Loosen S.H., Kalder M., Luedde T., Roderburg C., Kostev K. Impact of the COVID-19 pandemic on cancer diagnoses in general and specialized practices in Germany. Cancers (Basel) 2021;13(3):408. doi: 10.3390/cancers13030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3(8):e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vincentiis L., Carr R.A., Mariani M.P., Ferrara G. Cancer diagnostic rates during the 2020 ‘lockdown', due to COVID-19 pandemic, compared with the 2018-2019: an audit study from cellular pathology. J Clin Pathol. 2021;74(3):187–189. doi: 10.1136/jclinpath-2020-206833. [DOI] [PubMed] [Google Scholar]
- 16.Dinmohamed A.G., Visser O., Verhoeven R.H.A. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21(6):750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skovlund C.W., Friis S., Dehlendorff C., Nilbert M.C., Morch L.S. Hidden morbidities: drop in cancer diagnoses during the COVID-19 pandemic in Denmark. Acta Oncol. 2021;60(1):20–23. doi: 10.1080/0284186X.2020.1858235. [DOI] [PubMed] [Google Scholar]
- 18.Eén gerepatrieerde landgenoot testte positief op het nieuwe coronavirus. 2020. Available at https://www.info-coronavirus.be/nl/news/gerepatrieerde-landgenoot-testte-positief-op-het-nieuwe-coronavirus/. Accessed October 7, 2020
- 19.de Pelsemaeker M.C., Guiot Y., Vanderveken J., Galant C., Van Bockstal M.R. The impact of the COVID-19 pandemic and the associated Belgian governmental measures on cancer screening, surgical pathology and cytopathology. Pathobiology. 2021;88(1):46–55. doi: 10.1159/000509546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wet Houdende Diverse Bepalingen Betreffende Gezondheid van 13 December 2006 Hoofdstuk VI Artikel 39. B.S. 2006. [Google Scholar]
- 21.Henau K., Van Eycken E., Silversmit G., Pukkala E. Regional variation in incidence for smoking and alcohol related cancers in Belgium. Cancer Epidemiol. 2015;39(1):55–65. doi: 10.1016/j.canep.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Bray F., Colombet M., Mery L. Cancer Incidence in Five Continents, Vol. XI (electronic version) 2017. https://ci5.iarc.fr Available at. Accessed March, 2021.
- 23.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 24.ECIS - European Cancer Information System Breast cancer burden in EU-27; 2020. https://ecis.jrc.ec.europa.eu/ Available at.
- 25.Belgian Cancer Registry . 2020. Cancer Burden in Belgium 2004-2017. Brussels. [Google Scholar]
- 26.Eurostat Excess mortality in the European Union between January 2020 and January 2021. 2021. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Excess_mortality-_statistics&oldid=518814#Excess_mortality_in_the_European_Union_between_January_202 0_and_January_2021 Available at.
- 27.Armstrong B.K. The role of the cancer registry in cancer control. Cancer Causes Control. 1992;3(6):569–579. doi: 10.1007/BF00052754. [DOI] [PubMed] [Google Scholar]
- 28.Parkin D.M. The role of cancer registries in cancer control. Int J Clin Oncol. 2008;13(2):102–111. doi: 10.1007/s10147-008-0762-6. [DOI] [PubMed] [Google Scholar]
- 29.Zanetti R., Schmidtmann I., Sacchetto L. Completeness and timeliness: cancer registries could/should improve their performance. Eur J Cancer. 2015;51(9):1091–1098. doi: 10.1016/j.ejca.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 30.Integraal kankercentrum Nederland COVID-19 en kanker. 2021. https://iknl.nl/covid-19 Available at.
- 31.Northern Ireland Cancer Registry Impact of COVID-19 on cancer diagnosis. 2021. https://www.qub.ac.uk/research-centres/nicr/Publications/ImpactofCOVID-19onCancerDiagnosis/ Available at.
- 32.Silversmit G., Vaes E., Van Eycken L. 2017. Cancer incidence in Belgium projected to 2025. Paper presented at: GRELL, Brussels. [DOI] [PubMed] [Google Scholar]
- 33.Belgian Cancer Registry . 2017. Cancer Incidence Projections in Belgium 2015 to 2025. Brussels; 2017. [Google Scholar]
- 34.Iadevaia C., Perrotta F., Mazzeo G. Incidental diagnosis of lung adenocarcinoma following coronavirus OC 43 severe pneumonia. Monaldi Arch Chest Dis. 2020;90(3) doi: 10.4081/monaldi.2020.1313. [DOI] [PubMed] [Google Scholar]
- 35.Olsen M.H., Boje C.R., Kjaer T.K. Socioeconomic position and stage at diagnosis of head and neck cancer – a nationwide study from DAHANCA. Acta Oncol. 2015;54(5):759–766. doi: 10.3109/0284186X.2014.998279. [DOI] [PubMed] [Google Scholar]
- 36.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta N.S., Mytton O.T., Mullins E.W.S. SARS-CoV-2 (COVID-19): what do we know about children? A systematic review. Clin Infect Dis. 2020;71(9):2469–2479. doi: 10.1093/cid/ciaa556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graetz D., Agulnik A., Ranadive R. Global effect of the COVID-19 pandemic on paediatric cancer care: a cross-sectional study. Lancet Child Adolesc Health. 2021;5(5):332–340. doi: 10.1016/S2352-4642(21)00031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sciensano . 2021. COVID-19 Wekelijks epidemiologisch bulletin (31 December 2020); [Google Scholar]
- 40.de Azambuja E., Brandao M., Wildiers H. Impact of solid cancer on in-hospital mortality overall and among different subgroups of patients with COVID-19: a nationwide, population-based analysis. ESMO Open. 2020;5(5):e000947. doi: 10.1136/esmoopen-2020-000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maringe C., Spicer J., Morris M. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Mol P., Franken A., Dooms C. LBA78 A microsimulation model to assess the impact of SARS-CoV-2 on cancer outcomes, healthcare organization and economic burden. Ann Oncol. 2020;31:S1207. [Google Scholar]
- 43.Onesti C.E., Rugo H.S., Generali D. Oncological care organisation during COVID-19 outbreak. ESMO Open. 2020;5(4):e000853. doi: 10.1136/esmoopen-2020-000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verhoeven V., Tsakitzidis G., Philips H., Van Royen P. Impact of the COVID-19 pandemic on the core functions of primary care: will the cure be worse than the disease? A qualitative interview study in Flemish GPs. BMJ Open. 2020;10(6):e039674. doi: 10.1136/bmjopen-2020-039674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sud A., Torr B., Jones M.E. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol. 2020;21(8):1035–1044. doi: 10.1016/S1470-2045(20)30392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.