Abstract
Background
Prevalence, respiratory symptoms, and quality of life (QoL) in post-tuberculosis (TB) subjects with bronchiectasis are not well elucidated.
Methods
Subjects who participated in the Korea National Health and Nutritional Examination Survey 2007–2009 were enrolled in this cross-sectional study. We evaluated the prevalence of physician-diagnosed bronchiectasis among post-TB subjects. We compared respiratory symptoms, physical activity limitations, and QoL between post-TB subjects with and without bronchiectasis.
Results
The prevalence of bronchiectasis was 3.3% among 963 post-TB subjects. Post-TB subjects with bronchiectasis showed a higher rate of asthma (29.6% vs. 4.9%, P<0.001) than those without bronchiectasis. Post-TB subjects with bronchiectasis showed more cough (23.9% vs. 6.7%, P=0.033) and physical activity limitations due to respiratory diseases (35.0% vs. 8.9%, P=0.033) than those without bronchiectasis. Furthermore, compared with post-TB subjects without bronchiectasis, those with bronchiectasis had lower QoL measured by the EuroQoL five-dimension (EQ-5D) index (0.84 vs. 0.93, P=0.048). Linear regression analysis found that the EQ-5D index in post-TB subjects with bronchiectasis was significantly lower than in those without bronchiectasis (difference estimate =–0.089, P=0.030), especially in the anxiety/depression component.
Conclusions
Post-TB subjects with bronchiectasis had more cough and physical activity limitations and lower health-related QoL than those without bronchiectasis.
Keywords: Tuberculosis (TB), bronchiectasis, quality of life (QoL), Korea NHANES
Introduction
Tuberculosis (TB) remains a major global health burden (1). The treatment outcome of TB is highly effective, with a treatment success rate of approximately 90% (2). In contrast to the microbiologic cure, up to half of TB survivors suffer from lung function decline and lower quality of life (QoL) after TB treatment (3-6). Pulmonary impairment includes airflow obstruction as well as a restrictive ventilatory defect, which can be explained by TB-associated lung pathologies such as pulmonary cavitation, bronchiectasis, and fibrosis (4,7-10).
Bronchiectasis is a well-known TB-induced lung damage (4,11). In post-mortem studies of TB patients conducted in the 1950s, post-TB bronchiectasis was found in 19–65% of patients (12,13). However, those studies were performed in referral centers, and possibly, they evaluated patients with severe forms of TB compared to those which were performed in community settings. Thus, despite the sufficiently probable association between bronchiectasis and post-TB subjects, there is limited information on the prevalence of post-TB bronchiectasis.
Patients with active TB have impaired QoL and its impact continues until the end of treatment (14-16). Although it is suggested that post-TB subjects may have persistent respiratory symptoms and lower QoL, especially in those who develop chronic lung disease such as bronchiectasis, there have been few studies regarding this issue.
Therefore, the aim of the present study was to investigate the prevalence of bronchiectasis among post-TB subjects and evaluate symptomatic burden in terms of respiratory symptoms and health-related QoL in these patients.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-20-3028).
Methods
Study population
This study was performed on data obtained from the Korea National Health and Nutrition Examination Survey (NHANES) from 2007 to 2009, a nationally representative health survey conducted by the Ministry of Health and Welfare. We used health-related questionnaires, health examinations, and spirometry results from the database. The presence of previous pulmonary TB was defined based on a history of physician diagnosis. Bronchiectasis was defined using the NHANES questionnaire on whether subjects had been previously diagnosed with bronchiectasis by physicians (17).
We restricted our analysis to participants over 20 years old (n=24,871). Post-TB subjects were identified using a questionnaire on a previous physician TB diagnosis (n=963). The post-TB subjects were classified into two groups based on the presence or absence of bronchiectasis: those without bronchiectasis (n=931) and those with bronchiectasis (n=32).
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital (application No. 2020-04-035). Informed consent was not required because this study was based on the NHANES database, which includes fully anonymized and de-identified data.
Measurements
Data on age, sex, body mass index (BMI), smoking history, physical activity, occupation, the EuroQoL five-dimension questionnaire (EQ-5D) index values, and spirometry results were obtained from the Korea NHANES database. Physical activity limitations due to respiratory diseases (hereafter physical activity limitations) were defined using the questionnaire on whether subjects had experienced physical activity limitations due to respiratory diseases before. The EQ-5D index values range between 0 (worst imaginable health state) and 1 (best imaginable health state). Spirometry was performed according to the recommendations of the American Thoracic Society and European Respiratory Society (18). Absolute values of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were obtained, and the percentages of predicted values (% predicted) for FEV1 and FVC were calculated using the reference equation obtained on analysis of a representative Korean sample (19). We categorized occupation type according to the major groups outlined in the 6th Korean Standard Classification of Occupation into five groups: manager, professional, office worker, service or sales worker, agriculture or fishery worker, skilled labor or machine operator and manual laborer (20). Comorbidities of asthma, chronic obstructive pulmonary disease (COPD), diabetes mellitus, systemic hypertension, dyslipidemia, cardiovascular disease, osteoporosis, osteoarthritis or rheumatoid arthritis, and depression were self-reported based on previous physician diagnoses.
Outcomes
Firstly, we compared respiratory symptoms, physical activity limitations, spirometry results, and QoL (as measured by EQ-5D index) between post-TB subjects with bronchiectasis and those without bronchiectasis. Secondly, we analyzed the impact of bronchiectasis on respiratory symptoms and perceived health status among post-TB subjects. Thirdly, we analyzed the impact of bronchiectasis on the EQ-5D index value and its components among post-TB subjects.
Statistical analysis
Analysis was performed using survey commands in STATA 15.1 version (StataCorp LP, College Station, TX, USA) to account for the complex sampling design and survey weights. Values were presented as weighted mean [95% confidence interval (CI)] or as weighted proportions (95% CI) (21). All tests were two-tailed, and P values <0.05 were considered statistically significant differences.
The association between bronchiectasis and respiratory symptoms was analyzed using logistic regression models and presented as an odds ratio (OR) and 95% CI. The first model was a crude model (Model 1); the second model was adjusted for age (categorized as ≥65 years old or not), sex, and BMI (Model 2); the third model was additionally adjusted for education level (categorized as high school or less vs. college or above) and family income (categorized as low or high) (Model 3). The association between bronchiectasis and perceived health status was also analyzed using logistic regression models. A linear regression model was used to assess the association between bronchiectasis, the EQ-5D index values, and other covariates as mentioned above, and presented as difference estimates and 95% CI.
Results
Baseline characteristics
The baseline characteristics of the subjects are summarized in Table 1. The prevalence of bronchiectasis was 3.3% (n=32) among 963 post-TB subjects. There were no significant differences in age (P=0.769), sex (P=0.761), smoking history (P=0.226), family income (P=0.205), education level (P=0.591), type of occupation (P=0.488), comorbidities including diabetes mellitus (P=0.468), cardiovascular disease (P=0.996), osteoporosis (P=0.193), arthritis (P=0.114), or depression (P=0.106) between groups. There were no significant differences in spirometry results including FVC, FEV1, and FEV1/FVC ratio between the groups. Compared with post-TB subjects without bronchiectasis, those with bronchiectasis had lower BMI (22.2 vs. 23.5 kg/m2, P=0.009), higher asthma rate (29.6% vs. 4.9%, P<0.001), lower systemic hypertension rate (13.6% vs. 34.3%, P=0.023) and dyslipidemia (15.5% vs. 44.1%, P=0.003).
Table 1. Baseline characteristics.
| Variable | Total (N=963) | Post-TB subjects without bronchiectasis (n=931) | Post-TB subjects with bronchiectasis (n=32) | P value |
|---|---|---|---|---|
| Age, years | 51.4 (49.6–53.0) | 51.4 (49.5–53.3) | 52.5 (45.3–59.7) | 0.769 |
| Sex, male | 58.4 (53.4–63.3) | 58.6 (53.3–63.8) | 54.8 (31.8–75.9) | 0.761 |
| BMI, kg/m2 | 23.5 (23.1–23.8) | 23.5 (23.2–23.9) | 22.2 (21.3–23.1) | 0.009 |
| Smoking history | 0.226 | |||
| Never-smoker | 45.7 (40.4–51.0) | 46.5 (41.0–52.0) | 31.1 (13.8–56.1) | |
| Current- or ex-smoker | 54.3 (49.0–59.6) | 53.5 (48.0–59.0) | 68.9 (43.9–86.2) | |
| Family income | 0.205 | |||
| Low | 44.4 (38.0–51.0) | 43.6 (37.1–50.3) | 59.5 (35.1–80.0) | |
| High | 55.6 (49.0–62.0) | 56.4 (49.7–62.9) | 40.5 (20.0–64.9) | |
| Education | 0.591 | |||
| High school or less | 74.4 (68.7–79.3) | 74.1 (68.3–79.2) | 79.9 (55.0–92.8) | |
| College or above | 25.6 (20.7–31.3) | 25.9 (20.8–31.7) | 20.1 (7.2–45.0) | |
| Type of occupation | 0.488 | |||
| Manager/professional/office worker | 34.8 (28.2–42.0) | 35.6 (28.8–42.9) | 16.7 (3.5–52.6) | |
| Service/sales worker | 20.5 (15.0–27.3) | 20.5 (14.9–27.5) | 19.9 (4.4–57.4) | |
| Agriculture/fishery worker | 10.9 (7.6–15.4) | 10.1 (6.8–14.7) | 28.7 (9.7–60.1) | |
| Skilled labor/machine operation | 18.1 (12.6–25.3) | 18.0 (12.4–25.4) | 19.4 (2.9–65.8) | |
| Manual laborer | 15.8 (11.4–21.4) | 15.8 (11.3–21.6) | 15.3 (3.1–50.2) | |
| Comorbidities | ||||
| Pulmonary comorbidity | ||||
| Asthma | 6.1 (4.0–9.1) | 4.9 (3.0–7.9) | 29.6 (14.0–52.1) | <0.001 |
| COPD | 30.2 (24.6–36.4) | 29.0 (23.3–35.6) | 50.5 (26.6–74.2) | 0.096 |
| Extrapulmonary comorbidity | ||||
| Diabetes mellitus | 10.6 (7.4–15.1) | 10.9 (7.5–15.5) | 6.5 (1.5–24.4) | 0.468 |
| Hypertension | 33.2 (28.1–38.8) | 34.3 (28.9–40.0) | 13.6 (5.1–31.5) | 0.023 |
| Dyslipidemia | 42.6 (37.1–48.3) | 44.1 (38.3–50.0) | 15.5 (6.4–33.2) | 0.003 |
| Cardiovascular disease | 4.4 (2.4–8.1) | 4.4 (2.3–8.3) | 4.4 (0.6–25.9) | 0.996 |
| Osteoporosis | 5.8 (3.8–8.8) | 5.5 (3.6–8.6) | 12.3 (3.9–32.5) | 0.193 |
| Osteoarthritis or rheumatoid arthritis | 15.3 (11.8–19.6) | 14.7 (11.2–19.0) | 27.6 (12.5–50.5) | 0.114 |
| Depression | 5.0 (3.0–8.0) | 4.5 (2.6–7.7) | 13.1 (3.8–36.6) | 0.106 |
| Spirometry | ||||
| FVC, L (n=752) | 3.62 (3.50–3.74) | 3.63 (3.50–3.76) | 3.48 (3.05–3.92) | 0.519 |
| FVC, % predicted (n=749) | 88.9 (87.4–90.4) | 89.0 (87.4–90.6) | 87.1 (79.8–94.3) | 0.606 |
| FEV1, L (n=752) | 2.73 (2.62–2.84) | 2.75 (2.63–2.86) | 2.44 (1.97–2.91) | 0.207 |
| FEV1, % predicted (n=751) | 84.4 (82.6–86.2) | 84.9 (83.0–86.7) | 75.4 (64.0–86.7) | 0.109 |
| FEV1/FVC ratio (n=752) | 0.75 (0.74–0.76) | 0.75 (0.74–0.76) | 0.69 (0.61–0.77) | 0.123 |
Data are presented as the weighted mean (95% confidence interval) or weighted percentage (95% confidence interval). TB, tuberculosis; BMI, body mass index; COPD, chronic obstructive pulmonary disease; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second.
Comparison of symptoms, physical activity, perceived health and QoL
As shown in Table 2, there were no significant intergroup differences in symptoms of sputum (P=0.380) and dyspnea (P=0.674), and perceived health (P=0.691). However, post-TB subjects with bronchiectasis had more cough (23.9% vs. 6.7%, P=0.033) and physical activity limitations (35.0% vs. 8.9%, P=0.033). Furthermore, the EQ-5D index values were significantly lower in post-TB subjects with bronchiectasis than those without bronchiectasis (weighted mean 0.84 vs. 0.93, P=0.048). Among the EQ-5D components, post-TB subjects with bronchiectasis reported significantly more anxiety or a depressive mood (43.8% vs. 11.9%, P<0.001) than those without bronchiectasis. There were no intergroup differences between the other EQ-5D components.
Table 2. Comparison of symptoms, physical activity, perceived health, and quality of life.
| Variable | Total (N=963) | Post-TB subjects without bronchiectasis (n=931) | Post-TB subjects with bronchiectasis (n=32) | P value |
|---|---|---|---|---|
| Symptoms | ||||
| Cough | 7.5 (4.7–11.7) | 6.7 (4.1–11.0) | 23.9 (7.4–55.0) | 0.033 |
| Sputum | 14.4 (9.7–20.8) | 14.0 (9.2–20.7) | 23.9 (7.4–55.0) | 0.380 |
| Dyspnea | 1.3 (0.5–3.5) | 1.4 (0.5–3.7) | 0 | 0.674 |
| Physical activity limitations due to respiratory diseases | 11.1 (5.9–19.8) | 8.9 (4.3–17.4) | 35.0 (10.3–71.6) | 0.033 |
| Perceived health | 0.691 | |||
| Good/very good/not good/not bad | 73.6 (68.5–78.1) | 73.8 (68.5–78.4) | 69.8 (47.9–85.3) | |
| Bad/very bad | 26.4 (21.9–31.5) | 26.2 (21.6–31.5) | 30.2 (14.7–52.1) | |
| EQ-5D component | ||||
| Mobility | 15.6 (12.2–19.8) | 15.1 (11.7–19.3) | 25.3 (11.2–47.8) | 0.205 |
| Self-care | 4.3 (2.6–7.0) | 4.6 (2.8–7.4) | 0 | 0.355 |
| Usual activity | 10.8 (8.0–14.4) | 10.4 (7.6–14.1) | 18.6 (7.5–38.9) | 0.216 |
| Pain/discomfort | 30.4 (25.3–36.0) | 29.9 (24.7–35.7) | 39.6 (20.5–62.6) | 0.377 |
| Anxiety/depression | 13.5 (10.0–18.1) | 11.9 (8.5–16.4) | 43.8 (22.5–67.8) | <0.001 |
| EQ-5D index | 0.93 (0.91–0.94) | 0.93 (0.92–0.94) | 0.84 (0.76–0.93) | 0.048 |
Data are presented as the weighted mean (95% confidence interval) or weighted percentage (95% confidence interval). TB, tuberculosis; EQ-5D, EuroQoL five-dimension questionnaire.
The impact of bronchiectasis on respiratory symptoms, physical activity and QoL among post-TB subjects
Bronchiectasis was associated with cough among post-TB subjects in the crude model (OR =4.33; 95% CI =1.01, 18.63; P=0.049); however, the association was not significant in the adjusted models. Additionally, based on logistic regression analyses, bronchiectasis was not significantly associated with sputum, physical activity limitations or a bad/very bad state of perceived health (Table 3).
Table 3. The impact of bronchiectasis on respiratory symptoms and perceived health status among post-TB subjects.
| Respiratory symptom | Model | Post-TB subjects without bronchiectasis (n=931) | Post-TB subjects with bronchiectasis (n=32) | P value |
|---|---|---|---|---|
| Cough | Model 1 | Reference | 4.33 (1.01, 18.63) | 0.049 |
| Model 2 | Reference | 4.46 (0.80, 24.86) | 0.088 | |
| Model 3 | Reference | 2.08 (0.50, 8.58) | 0.311 | |
| Sputum | Model 1 | Reference | 1.93 (0.43, 8.56) | 0.387 |
| Model 2 | Reference | 1.72 (0.31, 9.49) | 0.534 | |
| Model 3 | Reference | 1.07 (0.24, 4.78) | 0.926 | |
| Physical activity limitations | Model 1 | Reference | 5.51 (0.99, 30.81) | 0.052 |
| Model 2 | Reference | 4.84 (0.91, 25.68) | 0.064 | |
| Model 3 | Reference | 0.54 (0.06, 4.75) | 0.576 | |
| Bad/very bad state of perceived health | Model 1 | Reference | 1.22 (0.46, 3.23) | 0.692 |
| Model 2 | Reference | 1.17 (0.44, 3.10) | 0.749 | |
| Model 3 | Reference | 0.70 (0.28, 1.76) | 0.443 |
Data are presented as an odds ratio (95% confidence interval). Model 1, crude model; Model 2, adjusted for age (categorized as ≥65 years old or not), sex, and BMI (kg/m2); Model 3, further adjusted for education (categorized as high school or less vs. college or above) and family income (categorized as low or high). TB, tuberculosis.
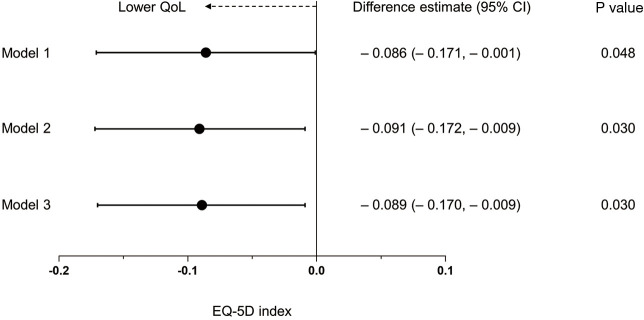
Linear regression analysis revealed that post-TB subjects with bronchiectasis had a significantly lower EQ-5D index value than those without bronchiectasis in all the models: difference estimate =–0.086, 95% CI =–0.171 to –0.001, P=0.048 in Model 1; difference estimate =–0.091, 95% CI =–0.172 to –0.009, P=0.030 in Model 2; difference estimate =–0.089, 95% CI =–0.170 to –0.009, P=0.030 in Model 3 (fully adjusted model) (Figure 1).
Figure 1.
Forest plot of difference estimate for EQ-5D index in post-TB subjects with bronchiectasis relative to those without bronchiectasis. Data are presented as a difference estimate (95% confidence interval). Model 1, crude model; Model 2, adjusted for age (categorized as ≥65 years old or not), sex, and BMI (kg/m2); Model 3, further adjusted for education (categorized as high school or less vs. college or above) and family income (categorized as low or high). EQ-5D, EuroQoL five-dimension questionnaire; TB, tuberculosis; CI, confidence interval; QoL, quality of life; BMI, body mass index.
Discussion
Using nationally representative data in Korea, the present study showed that the prevalence of physician-diagnosed bronchiectasis was 3.3% in post-TB subjects. Post-TB subjects with bronchiectasis had significantly lower BMI and a higher rate of comorbid asthma than those without bronchiectasis. They also experienced significantly more cough and physical activity limitations than those without bronchiectasis. Furthermore, the post-TB subjects with bronchiectasis showed a significantly lower QoL compared to those without bronchiectasis, especially in the anxiety/depression component.
One of the noteworthy results of this study is the prevalence of bronchiectasis in post-TB subjects. Despite the well-known relationship between bronchiectasis and past pulmonary TB history (22,23), there was limited information on its prevalence. The post-mortem studies of TB patients in the 1950s found bronchiectasis in 19–65% of those examined; however, the numbers should be cautiously interpreted since they were old studies investigating very severe forms of pulmonary TB (12,13,24). A systematic review and a recent study also reported that 35–86% of TB patients have bronchiectasis based on computed tomography (CT) scans after TB treatment (25,26). The reason why the prevalence of bronchiectasis in this study is relatively lower than that of previous studies is not clear. Nonetheless, we postulate that previous studies might have included more severe forms of TB compared to those treated in the community, as previous studies were performed in referral centers. All of the post-TB subjects underwent CT scans in previous studies, even though it is not routinely performed in real-world practice, which might overestimate the prevalence of post-TB bronchiectasis. In contrast, our study may underestimate the prevalence due to the lack of data on chest CT scans in the NHANES database. Taken together, future research is warranted to identify the exact prevalence of bronchiectasis in post-TB subjects.
Many subjects with chronic respiratory diseases may have physical activity limitations due to their respiratory diseases. Longitudinal studies assessing patients with COPD have consistently revealed the association between low levels of physical activity and a higher risk of both mortality and hospitalization (27-29). In addition, low levels of physical activity were also associated with a risk of hospitalization due to bronchiectasis exacerbation (30). This study revealed that bronchiectasis significantly worsened physical activity limitations in post-TB subjects. Though Korea NHANES data do not provide information on healthcare use, physical activity limitations may lead post-TB subjects with bronchiectasis to require more healthcare use.
Compared to post-TB subjects without bronchiectasis, those with bronchiectasis had lower QoL despite similar respiratory symptoms. As previous studies mostly focused on lung function impairment after TB (6,31-33), there had been only a few studies investigating the QoL in patients with TB sequelae (16,34). Furthermore, no studies have focused on the QoL in subjects with post-TB bronchiectasis. From this perspective, our study is the first, which investigated the association of bronchiectasis and health-related QoL in post-TB subjects using national representative samples. In this study, the difference of EQ-5D index values between post-TB subjects with bronchiectasis and those without bronchiectasis was 0.089. Considering the minimum important difference of the EQ-5D index was 0.051 in COPD (35), and it was suggested as approximately 0.07 in asthma studies (36,37), the difference of the EQ-5D between post-TB subjects with and without bronchiectasis in this study also seems to be significant. Accordingly, clinicians should be keen to assess the presence of bronchiectasis in post-TB subjects for adequate management in terms of health-related QoL. Interestingly, of the EQ-5D components, post-TB subjects with bronchiectasis had more problems with anxiety/depression. In line with our study results, previous studies also revealed that anxiety and depression were common in patients with bronchiectasis and might negatively affect health related QoL in those with bronchiectasis (38-40). Thus, our study results suggest that more attention is needed for anxiety and depression in post-TB subjects with bronchiectasis.
When it comes to comorbidities, asthma was more frequently observed in post-TB subjects with bronchiectasis than those without bronchiectasis. There is a complexity in the relationship between TB, asthma, and bronchiectasis. Many previous studies showed corticosteroid use in asthma is associated with pulmonary TB (41), which in turn, may be related to post-TB bronchiectasis. It is also well established that bronchiectasis is a common comorbidity in subjects with severe asthma (42-44). As our study is cross-sectional, we could not provide the causal inferences of those conditions. However, our study results suggest that asthma might be a triggering factor for bronchiectasis, as well as a common etiology/comorbid condition of bronchiectasis, and TB is linked to both conditions. Well-designed prospective studies are needed to determine the complex interaction of those conditions.
The major strength of this study is that it is the first to investigate the impact of bronchiectasis on QoL in post-TB subjects using nationally representative data. However, there are also several limitations to this study. Firstly, this study was performed in a representative sample of the Korean population. Thus, our data might not be generalizable to other ethnic groups or populations. Secondly, the Korea NHANES database did not provide chest CT data; therefore, we defined bronchiectasis based on physician diagnosis. Thus, we may have underestimated the prevalence of bronchiectasis. Thirdly, the number of post-TB subjects with bronchiectasis was relatively small, which may have led to a lack of statistical significance in respiratory symptoms and spirometry results.
In conclusion, the estimated prevalence of bronchiectasis was 3.3% among post-TB subjects in Korea. There was a significant gap between the prevalence of physician-diagnosed bronchiectasis in our study and CT-based bronchiectasis in other cohort studies. Post-TB subjects with bronchiectasis had lower health-related QoL than those without bronchiectasis. To attenuate the symptomatic burden of post-TB bronchiectasis, strategies for early diagnosis and proper management of post-TB bronchiectasis is urgently needed.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This research was supported by the Hallym University Research Fund 2019 (HURF-2019-39); Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communications Technologies (NRF-2019R1G1A1008692). The funder had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Ethical Statement: The authors are accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the institutional review board of Hallym University Kangnam Sacred Heart Hospital (application No. 2020-04-035). Informed consent was not required because this study was based on the NHANES database, which includes fully anonymized and de-identified data.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-20-3028
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-20-3028
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-20-3028). The authors declare no conflicts of interest.
References
- 1.World Health Organization. Global tuberculosis report 2019. Geneva: World Health Organization, 2019. [Google Scholar]
- 2.Zumla A, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med 2013;368:745-55. 10.1056/NEJMra1200894 [DOI] [PubMed] [Google Scholar]
- 3.Pasipanodya JG, Miller TL, Vecino M, et al. Pulmonary impairment after tuberculosis. Chest 2007;131:1817-24. 10.1378/chest.06-2949 [DOI] [PubMed] [Google Scholar]
- 4.Ravimohan S, Kornfeld H, Weissman D, et al. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev 2018;27:170077. 10.1183/16000617.0077-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000;55:32-8. 10.1136/thorax.55.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross J, Ehrlich RI, Hnizdo E, et al. Excess lung function decline in gold miners following pulmonary tuberculosis. Thorax 2010;65:1010-5. 10.1136/thx.2009.129999 [DOI] [PubMed] [Google Scholar]
- 7.Menezes AM, Hallal PC, Perez-Padilla R, et al. Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J 2007;30:1180-5. 10.1183/09031936.00083507 [DOI] [PubMed] [Google Scholar]
- 8.Byrne AL, Marais BJ, Mitnick CD, et al. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015;32:138-46. 10.1016/j.ijid.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 9.Amaral AF, Coton S, Kato B, et al. Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J 2015;46:1104-12. 10.1183/13993003.02325-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Mora IL, Martínez-Oceguera D, Laniado-Laborín R. Chronic airway obstruction after successful treatment of tuberculosis and its impact on quality of life. Int J Tuberc Lung Dis 2015;19:808-10. 10.5588/ijtld.14.0983 [DOI] [PubMed] [Google Scholar]
- 11.Ko JM, Kim KJ, Park SH, et al. Bronchiectasis in active tuberculosis. Acta Radiol 2013;54:412-7. 10.1177/0284185113475796 [DOI] [PubMed] [Google Scholar]
- 12.Jones EM, Peck WM. Relationships between tuberculosis and bronchiectasis; a study of clinical and of post-mortem material. Am Rev Tuberc 1950;61:387-98. [DOI] [PubMed] [Google Scholar]
- 13.Salkin D. Tuberculosis as a cause of upper lobe bronchiectasis. Calif Med 1950;73:577-80. [PMC free article] [PubMed] [Google Scholar]
- 14.Bauer M, Leavens A, Schwartzman K. A systematic review and meta-analysis of the impact of tuberculosis on health-related quality of life. Qual Life Res 2013;22:2213-35. 10.1007/s11136-012-0329-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal AN. Quality of life with tuberculosis. J Clin Tuberc Other Mycobact Dis 2019;17:100121. 10.1016/j.jctube.2019.100121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maguire GP, Anstey NM, Ardian M, et al. Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int J Tuberc Lung Dis 2009;13:1500-6. [PubMed] [Google Scholar]
- 17.Yang B, Choi H, Lim JH, et al. The disease burden of bronchiectasis in comparison with chronic obstructive pulmonary disease: a national database study in Korea. Ann Transl Med 2019;7:770. 10.21037/atm.2019.11.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005;26:319-38. 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 19.Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberculosis and Respiratory Diseases 2005;58:230-42. 10.4046/trd.2005.58.3.230 [DOI] [Google Scholar]
- 20.Statistics Korea (KOSTAT). Korean Standard Statistical Classification. Seoul: Statistics Korea.
- 21.Centers for Disease Control and Prevention (CDC). National Health and Nutritional Examination Survey Module 3: Weighting.
- 22.Zhou YM, Wang C, Yao WZ, et al. The prevalence and risk factors of bronchiectasis in residents aged 40 years old and above in seven cities in China. Zhonghua Nei Ke Za Zhi 2013;52:379-82. [PubMed] [Google Scholar]
- 23.Kwak HJ, Moon JY, Choi YW, et al. High prevalence of bronchiectasis in adults: analysis of CT findings in a health screening program. Tohoku J Exp Med 2010;222:237-42. 10.1620/tjem.222.237 [DOI] [PubMed] [Google Scholar]
- 24.Jordan TS, Spencer EM, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology 2010;15:623-8. 10.1111/j.1440-1843.2010.01749.x [DOI] [PubMed] [Google Scholar]
- 25.Meghji J, Simpson H, Squire SB, et al. A Systematic Review of the Prevalence and Pattern of Imaging Defined Post-TB Lung Disease. PLoS One 2016;11:e0161176. 10.1371/journal.pone.0161176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meghji J, Lesosky M, Joekes E, et al. Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax 2020;75:269-78. 10.1136/thoraxjnl-2019-213808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demeyer H, Donaire-Gonzalez D, Gimeno-Santos E, et al. Physical Activity Is Associated with Attenuated Disease Progression in Chronic Obstructive Pulmonary Disease. Med Sci Sports Exerc 2019;51:833-40. 10.1249/MSS.0000000000001859 [DOI] [PubMed] [Google Scholar]
- 28.Furlanetto KC, Donária L, Schneider LP, et al. Sedentary Behavior Is an Independent Predictor of Mortality in Subjects With COPD. Respir Care 2017;62:579-87. 10.4187/respcare.05306 [DOI] [PubMed] [Google Scholar]
- 29.Gimeno-Santos E, Frei A, Steurer-Stey C, et al. Determinants and outcomes of physical activity in patients with COPD: a systematic review. Thorax 2014;69:731-9. 10.1136/thoraxjnl-2013-204763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcaraz-Serrano V, Gimeno-Santos E, Scioscia G, et al. Association between physical activity and risk of hospitalisation in bronchiectasis. Eur Respir J 2020;55:1902138. 10.1183/13993003.02138-2019 [DOI] [PubMed] [Google Scholar]
- 31.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med 1989;83:195-8. 10.1016/S0954-6111(89)80031-9 [DOI] [PubMed] [Google Scholar]
- 32.Rhee CK, Yoo KH, Lee JH, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis 2013;17:67-75. 10.5588/ijtld.12.0351 [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich RI, Adams S, Baatjies R, et al. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tuberc Lung Dis 2011;15:886-91. 10.5588/ijtld.10.0526 [DOI] [PubMed] [Google Scholar]
- 34.Pasipanodya JG, McNabb SJ, Hilsenrath P, et al. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health 2010;10:259. 10.1186/1471-2458-10-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolan CM, Longworth L, Lord J, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax 2016;71:493-500. 10.1136/thoraxjnl-2015-207782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClure NS, Sayah FA, Xie F, et al. Instrument-Defined Estimates of the Minimally Important Difference for EQ-5D-5L Index Scores. Value Health 2017;20:644-50. 10.1016/j.jval.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 37.Hernandez G, Dima AL, Pont À, et al. Impact of asthma on women and men: Comparison with the general population using the EQ-5D-5L questionnaire. PLoS One 2018;13:e0202624. 10.1371/journal.pone.0202624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao YH, Guan WJ, Zhu YN, et al. Anxiety and depression in adult outpatients with bronchiectasis: Associations with disease severity and health-related quality of life. Clin Respir J 2018;12:1485-94. 10.1111/crj.12695 [DOI] [PubMed] [Google Scholar]
- 39.Girón Moreno RM, Fernandes Vasconcelos G, Cisneros C, et al. Presence of anxiety and depression in patients with bronchiectasis unrelated to cystic fibrosis. Arch Bronconeumol 2013;49:415-20. 10.1016/j.arbr.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 40.Olveira C, Olveira G, Gaspar I, et al. Depression and anxiety symptoms in bronchiectasis: associations with health-related quality of life. Qual Life Res 2013;22:597-605. 10.1007/s11136-012-0188-5 [DOI] [PubMed] [Google Scholar]
- 41.Lee CH, Kim K, Hyun MK, et al. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax 2013;68:1105-13. 10.1136/thoraxjnl-2012-203175 [DOI] [PubMed] [Google Scholar]
- 42.Coman I, Pola-Bibián B, Barranco P, et al. Bronchiectasis in severe asthma: Clinical features and outcomes. Ann Allergy Asthma Immunol 2018;120:409-13. 10.1016/j.anai.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 43.Crimi C, Ferri S, Crimi N. Bronchiectasis and asthma: a dangerous liaison? Curr Opin Allergy Clin Immunol 2019;19:46-52. 10.1097/ACI.0000000000000492 [DOI] [PubMed] [Google Scholar]
- 44.Choi H, Lee H, Ryu J, et al. Bronchiectasis and increased mortality in patients with corticosteroid-dependent severe asthma: a nationwide population study. Ther Adv Respir Dis 2020;14:1753466620963030. 10.1177/1753466620963030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as