Abstract
Transcriptional silencing in Saccharomyces cerevisiae occurs at several genetic loci, including the ribosomal DNA (rDNA). Silencing at telomeres (telomere position effect [TPE]) and the cryptic mating-type loci (HML and HMR) depends on the silent information regulator genes, SIR1, SIR2, SIR3, and SIR4. However, silencing of polymerase II-transcribed reporter genes integrated within the rDNA locus (rDNA silencing) requires only SIR2. The mechanism of rDNA silencing is therefore distinct from TPE and HM silencing. Few genes other than SIR2 have so far been linked to the rDNA silencing process. To identify additional non-Sir factors that affect rDNA silencing, we performed a genetic screen designed to isolate mutations which alter the expression of reporter genes integrated within the rDNA. We isolated two classes of mutants: those with a loss of rDNA silencing (lrs) phenotype and those with an increased rDNA silencing (irs) phenotype. Using transposon mutagenesis, lrs mutants were found in 11 different genes, and irs mutants were found in 22 different genes. Surprisingly, we did not isolate any genes involved in rRNA transcription. Instead, multiple genes associated with DNA replication and modulation of chromatin structure were isolated. We describe these two gene classes, and two previously uncharacterized genes, LRS4 and IRS4. Further characterization of the lrs and irs mutants revealed that many had alterations in rDNA chromatin structure. Several lrs mutants, including those in the cdc17 and rfc1 genes, caused lengthened telomeres, consistent with the hypothesis that telomere length modulates rDNA silencing. Mutations in the HDB (RPD3) histone deacetylase complex paradoxically increased rDNA silencing by a SIR2-dependent, SIR3-independent mechanism. Mutations in rpd3 also restored mating competence selectively to sir3Δ MATα strains, suggesting restoration of silencing at HMR in a sir3 mutant background.
Heterochromatin in eukaryotic chromosomes is usually associated with transcriptional silencing of nearby genes and also the suppression of recombination. In Saccharomyces cerevisiae, silencing occurs at several different genetic loci, including the cryptic mating-type loci (HML and HMR) (for a review see reference 50), and telomeres (34), both of which are generally recognized as the yeast heterochromatin equivalent. Remarkably, silencing of several RNA polymerase II (Pol II)-transcribed reporter genes also occurs within the rDNA locus (11, 70), even though this region of the yeast genome is very actively transcribed by Pol I and III. The ribosomal DNA (rDNA) of S. cerevisiae consists of 100 to 200 copies of a 9.1-kb unit organized into a perinuclear tandem array (59, 60), an arrangement reminiscent of the heterochromatin of higher eukaryotes.
Silencing in yeast is mediated by a specialized heterochromatin-like structure that is dependent on a series of trans-acting factors, including the proteins encoded by the four silent information regulator (SIR) genes. Efficient silencing at the HM loci requires all four SIR genes, while telomere position effect (TPE) requires SIR2, SIR3, and SIR4 (2). SIR1 contributes to the efficient establishment of silencing only at the HM loci (61). TPE and HM silencing also share requirements for Rap1, histones H3 and H4, and several other factors (50). As a result of this overlap in required silencing factors, the underlying mechanism of repression is thought to be similar between the HM loci and telomeres, although the mechanism is not yet well understood. Current models indicate that Sir2p, Sir3p, and Sir4p form a multimeric complex which interacts with the hypoacetylated N-terminal tails of histones H3 and H4 within nucleosomes, leading to the formation of a silenced chromatin domain at telomeres and the HM loci (35). The Sir proteins may therefore be structural components of yeast heterochromatin, although their exact functions have not yet been identified.
rDNA silencing is distinct from TPE and HM silencing in that SIR2 is the only absolutely required SIR gene (11, 70), implying that there are underlying differences in the mechanism of repression. Furthermore, unlike the HM and telomere loci, the rDNA is possibly the most transcriptionally active region of the entire genome, making rDNA silencing paradoxical. It is currently unknown whether the Pol I or Pol III transcription of rDNA plays any role in silencing. rDNA silencing is also exquisitely sensitive to alterations in SIR2 dosage (31, 71), suggesting that Sir2p is a structural component of rDNA chromatin; indeed, Sir2p specifically associates with rDNA by chromatin immunoprecipitation analysis (32). Although SIR4 function is not directly required for efficient rDNA silencing, it plays a regulatory role, mediating competition between telomeres and the rDNA for limiting amounts of Sir2 protein (71). This is consistent with the cellular localization of Sir2p, which is mostly nucleolar; smaller amounts of Sir2p also localize to perinuclear telomeric foci (32).
There are several potential functions of rDNA silencing in the yeast cell. The first is suppression of mitotic and meiotic recombination within the tandemly repeated rDNA. Deletion of SIR2 not only causes a loss of silencing in the rDNA (11, 70) but also increases the rate of rDNA recombination (33). The second is suppression of a cryptic Pol II promoter in the rDNA that overlaps with the well-characterized Pol I promoter (20). The third is modulation of rRNA transcription by Pol I. Deletion of SIR2 increases the percentage of rDNA repeats that are actively transcribed by Pol I (70). Fourth, silencing has been linked to the regulation of life span in yeast cells (47, 69). Certain mutations of SIR4 which promote longevity (47) also strengthen rDNA silencing (71), suggesting that there may be a link between the counteraction of aging and rDNA silencing. However, this link is complex, as aging is associated with redistribution of Sir3p and Sir4p to the nucleolus (46), yet neither of these proteins participates directly in rDNA silencing, as operationally defined by the silencing of Pol II reporter genes placed in the rDNA (70).
Thus far, a few genes other than SIR2 and SIR4 have been implicated as rDNA silencing factors. These genes encode topoisomerase I (TOP1), the ubiquitin-conjugating enzyme (UBC2/RAD6), histones H2A and H2B (11), and most recently Sas10p (42). Using multiple rDNA silencing reporter genes, we have performed a genetic screen that identified numerous non-SIR genes with rDNA silencing functions. Interestingly, multiple genes with known roles in DNA replication and/or chromatin modulation were identified. Several of the rDNA silencing genes identified in this screen have similar functions in TPE and HM silencing, implying that the rDNA silencing mechanism is distinct yet has some features in common with the other forms of silencing in yeast. We propose a model for rDNA silencing in which multiple cellular processes collaboratively lead to an rDNA chromatin structure that is repressive to Pol II reporter gene expression and recombination.
MATERIALS AND METHODS
Media, plasmids, and yeast strains.
Unless stated otherwise, media used were as previously described (63, 70). Pb2+-containing medium (MLA) consisted of 0.3% peptone, 0.5% yeast extract, 4% glucose, 0.02% (wt/vol) ammonium acetate, 0.1% Pb(NO3)2, and 2% agar. Glucose was the sole carbon source in all media. All yeast strains used (Table 1) were congenic to GRF167 (6, 70). All liquid and plate incubations of yeast strains were performed at 30°C. pJSS70-9 (2μm TRP1 SIR2) was constructed by ligating a XhoI-NotI SIR2 fragment from pCAR237 (70) into the XhoI-NotI sites of pRS424 (17).
TABLE 1.
Yeast strains used
| Strain | Genotypea |
|---|---|
| JB721 | MATa his3Δ200 ura3-167 |
| JS237b | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 |
| JS260 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 |
| JS262 | MATa/MATα his3Δ200/his3Δ200 LEU2/leu2Δ1 MET15/met15Δ0 TRP1/trp1 Δ63 ura3-167/ura3-167 RDN1/RDN1::Ty1-MET15 mURA3/HIS3 |
| JS306 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 |
| JS311 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 |
| JS314 | MATα his3Δ200 leu2Δ1 met15Δ0 ura3-167 |
| JS315 | MATa his3Δ200 leu2Δ1 met15Δ0 ura3-167 |
| JS343c | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 sir2Δ::kanMX4 |
| JS400 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 cac1Δ::kanMX4 |
| JS401 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 cac1Δ::kanMX4 |
| JS418 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 rif1Δ::kanMX4 |
| JS420 | MATa/MATα his3Δ200/his3Δ200 leu2Δ1/leu2Δ1 met15Δ0/met15Δ0 trp1Δ63/trp1 Δ63 ura3-167/ura3-167 rif1::mTn3/RIF1 SIR3/sir3Δ::kanMX4 RDN1/RDN1::Ty1-MET15 |
| JS421 | MATa/MATα his3Δ200/his3Δ200 leu2Δ1/leu2Δ1 met15Δ0/met15Δ0 trp1Δ63/trp1Δ63 ura3-167/ura3-167 rif1::mTn3/RIF1 SIR4/sir4Δ::HIS3 RDN1/RDN1::Ty1-MET15 |
| JS333c | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 |
| JS337c | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 sir4Δ::HIS3 |
| JS335c | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 sir3Δ::kanMX4 |
| JS422 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 rif1::mTn3 |
| JS424 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 rif1::mTn3 sir3Δ::kanMX4 |
| JS426 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 rif1::mTn3 sir4Δ::HIS3 |
| JS430 | MATa/MATα his3Δ200/his3Δ200 leu2Δ1/leu2Δ1 met15Δ0/met15Δ0 trp1Δ63/trp1Δ63 ura3-167/ura3-167 CDC17/cdc17::mTn3 sir3Δ::kanMX4/SIR3 RDN1::Ty1-MET15/RDN1 |
| JS431 | MATa/MATα his3Δ200/his3Δ200 leu2Δ1/leu2Δ1 met15Δ0/met15Δ0 trp1Δ63/trp1Δ63 ura3-167/ura3-167 CDC17/cdc17::mTn3 sir4Δ::HIS3/SIR4 RDN1::Ty1-MET15/RDN1 |
| JS432 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 cdc17::mTn3 |
| JS434 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 cdc17::mTn3 sir3Δ::kanMX4 |
| JS436 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 cdc17::mTn3 sir4Δ::HIS3 |
| JS442 | MATa/MATα his3Δ200/his3Δ200 leu2Δ1/leu2Δ1 met15Δ0/met15Δ0 trp1Δ63/trp1Δ63 ura3-167/ura3-167 rfc1::mTn3/RFC1 SIR4/sir4Δ::HIS3 RDN1/RDN1::Ty1-MET15 |
| JS443 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 rfc1::mTn3 |
| JS445 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15 rfc1::mTn3 sir4Δ::HIS3 |
| JS490 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 rpd3Δ::kanMX4 |
| JS493 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 sin3Δ::kanMX4 |
| JS495 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-mURA3 rif2Δ::kanMX4 |
| JS497 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-mURA3 rif2Δ::kanMX4 rif1::mTn3 |
| JS523 | JS306 pRS424 |
| JS524 | JS306 pJSS70-9 |
| JS527 | M179 pRS424 |
| JS528 | M179 pJSS70-9 |
| JS533 | M154 pRS424 |
| JS534 | M154 pJSS70-9 |
| JS537 | JS311 pRS424 |
| JS538 | JS311 pJSS70-9 |
| JS541 | JS490 pRS424 |
| JS542 | JS490 pJSS70-9 |
| JS549 | M489 pRS424 |
| JS550 | M489 pJSS70-9 |
| JS555 | MATa/MATα his3Δ200/his3Δ200 leu2Δ1/leu2Δ1 met15Δ0/met15Δ0 trp1Δ63/trp1Δ63 ura3-167/ura3-167 RDN1/RDN1::Ty1-MET15, mURA3/HIS3 cac1Δ::kanMX4/CAC1 RPD3/rpd3::mTn3 |
| JS556 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 |
| JS557 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 cac1Δ::kanMX4 |
| JS558 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 cac1Δ::kanMX4 rpd3::mTn3 |
| JS560 | MATa/MATα his3Δ200/his3Δ200 leu2Δ1/leu2Δ1 met15Δ0/met15Δ0 trp1Δ63/trp1Δ63 ura3-167/ura3-167 RDN1/RDN1::Ty1-MET15, mURA3/HIS3 rif1::mTn3/RIF1 RPD3/rpd3Δ::kanMX4 |
| JS561 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 |
| JS562 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 rif1::mTn3 |
| JS563 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 rpd3Δ::kanMX4 |
| JS564 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 rif1::mTn3 rpd3Δ::kanMX4 |
| JS566 | JS311 made sir2Δ::kanMX4 |
| JS568 | M480 made sir2Δ::kanMX4 |
| JS574 | JS306 made lrs4Δ::kanMX4 |
| JS576 | JS306 made sir2Δ::kanMX4 |
| JS625 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 sir3Δ::kanMX4 |
| JS626 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 sir3Δ::kanMX4 |
| JS627 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 rpd3::mTn3 sir3Δ::kanMX4 |
| JS628 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 rpd3::mTn3 sir3Δ::kanMX4 |
| JS629 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 rpd3::mTn3 |
| JS633 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 sir4Δ::HIS3 |
| JS634 | MATα his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 rpd3::mTn3 sir4Δ::HIS3 |
| JS635 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 sir4Δ::HIS3 |
| JS636 | MATa his3Δ200 leu2Δ1 met15Δ0 trp1Δ63 ura3-167 rpd3::mTn3 sir4Δ::HIS3 |
| JS639 | MATa his3Δ200 leu2Δ1 met15Δ0 ura3-167 RDN1::Ty1-MET15, mURA3/HIS3 rpd3Δ::kanMX4 sir2Δ::HIS3 |
JS306, JS311, JS314, and JS315 were congenic haploid spores dissected from the diploid JS262 (Table 1). JS262 was constructed as follows. The mURA3/HIS3 expression cassette (“m” indicates the minimal TRP1 promoter) was integrated into the 18S rRNA-coding region of yeast strain JS237, using a PCR product generated from pJSS51-9 as a template (70) and oligonucleotides JB1271 (5′ ACATGGTATAACCGTGGTAATTCTAGAGCTAATACATGCTATACGACTCACTATAGGGCG 3′), and JB1272 (5′ TATCTAATAAATTCATCTCTTCCAAAGGGTCGAGATTTTAAAGGGAACAAAAGCTGGAGC 3′). The underlined sequences are complementary to regions flanking the multiple cloning site of pRS vectors. Using this PCR product, the expression cassette was inserted between nucleotides 3333 and 3334 of the rDNA repeat (68, 70). The resulting strain was called JS260 (Table 1). JS260 was then mated to JB721 to produce the JS262 diploid. JS262 was sporulated and tetrads dissected to produce haploid spores which were used in this study. Throughout this report, transposon insertion mutations are written in lowercase and deletions are designated by the suffix “Δ.” JS401 was created by elimination of the mURA3/HIS3 and Ty1-MET15 markers from the rDNA of strain JS400. JS422, JS424, and JS426 were haploids derived from the parental diploids JS420 and JS421 (Table 1). Haploids JS432, JS434, and JS436 were derived from the diploids JS430 and JS431. Haploids JS443 and JS445 were derived from the diploid JS442. Haploids JS556, JS557, and JS558 were derived from the diploid JS555. The haploids JS561, JS562, JS563, and JS564 were derived from the diploid JS560.
Mutagenesis and identification of affected genes.
Haploid strains JS306 and JS311 were mutagenized using transposon Tn3::lacZ::LEU2 as previously described by Burns et al. (13). A yeast genomic DNA library was obtained from Mike Snyder’s lab (via Susan Michaelis). This library had been mutagenized in Escherichia coli by random Tn3::lacZ::LEU2 integration events. The mutated genomic DNA inserts were removed from the vector backbone by digestion with NotI and transformed into JS306 and JS311 by using a high-efficiency lithium acetate-polyethylene glycol-dithiothreitol procedure. Cells were plated onto leucine-deficient synthetic complete (SC−Leu) medium (approximately 200 to 250 transformants/plate) to select for Leu+ mutant colonies in which a transposon-disrupted DNA fragment had integrated into the genome by homologous recombination.
Leu+ colonies grown for 3 days were replica plated to SC−Leu, SC−Ura, SC−His, and MLA media to detect changes in rDNA silencing phenotypes. Over a period of 3 days, the replica plates were monitored daily for colonies which significantly differed from other colonies on the same plate. After 3 days, colonies were selected for further study only if they had altered silencing phenotypes on both SC−Ura and MLA plates. Two major classes of mutants, loss of rDNA silencing (lrs) and increased rDNA silencing (irs), were obtained. The HIS3 reporter was useful for the increased rDNA silencing screen because irs mutant colonies were often phenotypically Ura− but still His+, allowing them to be differentiated from colonies which simply lost the mURA3/HIS3 reporter cassette from the rDNA. Mutant colonies were picked and restreaked for single colonies on SC−Leu medium, grown 3 days, and replica plated to SC−Ura, SC−His, and MLA media to retest the mutant phenotypes (2° screen).
The lrs mutants were classified into (i) those which have an rDNA recombination phenotype measured by hypersectoring of MLA-grown colonies and (ii) those which do not sector. The transposon-disrupted gene in each hypersectoring mutant was identified by plasmid rescue and DNA sequencing. For most lrs mutants, the mURA3/HIS3 reporter gene was first removed from the rDNA by simply plating cells onto YPD, and through replica-plating of the resulting colonies, Ura− His− colonies were easily identified. Ura− versions of each lrs mutant were inoculated into 10-ml YPD cultures and grown approximately 16 h. Each mutant was then transformed with approximately 0.5 μg of PvuI-linearized Yip5 vector. Ura+ colonies were selected, and genomic DNA was isolated by using a Teeny prep spheroplasting method (6). Recovered DNA was digested with NsiI and circularized by self-ligation overnight at 4°C with T4 DNA ligase. The ligation mixture was transformed into E. coli DH5α and selected on LB supplemented with carbenicillin (50 μg/ml). Plasmid DNA was recovered, and transposon recovery was verified by restriction mapping. The genomic DNA flanking the recovered transposon was identified by DNA sequencing.
For the irs mutants, the procedure used was similar except that the mURA3/HIS3 cassette was not removed from the rDNA. Instead, ScaI linearized pRS404 (TRP1) was used in the plasmid rescue. Recovered yeast genomic DNA was cut with SpeI before circularization and transformation into E. coli.
Dominance tests.
Each mutant was mated to a strain of the opposite mating type which did not contain reporter genes in the rDNA and was Trp+. Briefly, mutant strains were streaked out for single colonies on SC−Leu, grown for 3 days, and replica plated onto a YPD plate, along with a lawn of either JS314 (MATα) or JS315 (MATa). The two strains were allowed to mate for 5 h at 30°C and the YPD mating plate was then replica plated to SC−Leu−Trp and grown overnight to select for diploid formation. The resulting diploids were then replica plated to the silencing indicator medium SC−Leu−Trp−Ura or to MLA to test for dominance. The heterozygous lrs diploids were also restreaked for single colonies onto MLA to test for dominance by the rDNA sectoring assay. Backcross analysis was used to confirm cosegregation of the mutant phenotype with the Tn3::lacZ::LEU2 transposon insertion for mutants that were represented by a single isolate in the screen.
PCR-mediated gene disruption.
Complete open reading frame (ORF) deletions were made for several genes identified in the screen to confirm the Lrs− or Irs− silencing phenotype of that particular mutant. PCR-mediated gene disruption was performed as described elsewhere (5, 51). The dominant drug resistance marker, kanMX4 (79), was PCR amplified from pRS400 (8, 71), using oligonucleotide primers containing 39 nucleotides complementary to the 5′ and 3′ ends of the targeted ORF. The resulting PCR fragments were transformed into JS306 or JS311, and gene replacement with kanMX4 was selected for by growth on YPD medium containing G418 (200 μg/ml).
Silencing growth assays.
Strains to be tested were patched onto YPD, or selective medium if they contained a plasmid, and grown overnight. Cells were scraped from the plates and resuspended in 1 ml of sterile water. The cell suspension was normalized to an A600 reading of 0.5 and then serially diluted in fivefold increments; 5 μl of each dilution was spotted onto either nonselective or selective SC agar plates, using an eight-channel pipette. Plates were incubated for 2 to 5 days. Polaroid photographs were taken of all plates except SC−Ura after 2 days. Photographs of SC−Ura plates were taken after 4 days unless specified otherwise in the figure legends.
Colony color silencing assays.
Strains to be tested were patched onto YPD and grown overnight. Cells were then streaked onto MLA plates with four or six sectors per plate. The plates were wrapped with Parafilm to prevent dehydration and then allowed to grow 5 days. Photographs were taken at day 5, using a Leica stereoscopic microscope equipped with a 35-mm color camera.
Telomere length analysis.
Two independent colonies for each mutant were lightly inoculated into 10 ml of YPD cultures and incubated for approximately 16 h. Cells were pelleted, and genomic DNA was isolated as previously described, using a Teeny prep spheroplasting method (6). Nucleic acid pellets were resuspended in 100 μl of Tris-EDTA (TE), and 15 μl (2 to 4 μg of DNA) was digested in a 30-μl reaction with XhoI and separated on a 0.7% agarose gel. The DNA was transferred to Genescreen Plus (NEN-Dupont), UV cross-linked, and hybridized with a telomere specific probe in Church and Gilbert hybridization solution (1 mM EDTA, 0.5 M Na2HPO4 [pH 7.2], 7% sodium dodecyl sulfate, and 1% bovine serum albumin) at 60°C overnight. The blot was washed once at room temperature and three times (10 min) at 60°C with washing solution (2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% sodium dodecyl sulfate). The probe was a 350-bp EcoRI fragment from plasmid pYLPV, which contained a 280-bp TG1–3 telomeric repeat (81).
Psoralen cross-linking analysis.
In vivo psoralen cross-linking assays were performed as previously described (14, 22, 70), with several minor modifications. Fresh 50-ml YPD cultures were inoculated from saturated YPD cultures to an A600 of 0.3 and grown for 6.5 h into log phase. Approximately 2.5 × 108 cells were washed with ice-cold H2O and resuspended in 0.7 ml of cold TE in a 24-well tissue culture plate; 40 μl of a 200-μg/ml solution of 4,5′,8-trimethylpsoralen (Sigma) in 100% ethanol was added to each well, and the plate was UV irradiated for 5 min on ice at a distance of 6 cm five times. The light source was a long-wave UV lamp (model B-100A; Ultraviolet Products, Inc.). Cells were washed, spheroplasted with zymolyase at 37°C, lysed, proteinase K treated, phenol-chloroform extracted, and ethanol precipitated. Total nucleic acid was resuspended in 40 μl of TE and normalized to an A260 of 0.04. DNA (4 μl) was digested for 5 h at 37°C with EcoRI in a 30-μl reaction containing RNase A (80 ng/μl). DNA was separated on 1.3% agarose gel (14.5 by 24 cm) at 80 V for 17 h. Cross-linking was reversed in a Stratagene Stratalinker at 0.6 J/cm2. DNA was transferred to Genescreen Plus in 10× SSC and hybridized with probe A, which is a 2.2-kb EcoRI-SmaI fragment of the rDNA nontranscribed spacer (NTS).
RESULTS
Identification of rDNA silencing mutants.
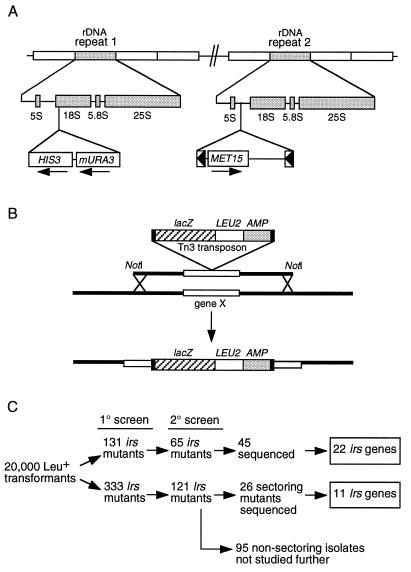
As a first step toward understanding the molecular mechanism of rDNA silencing, we carried out a genetic screen designed to identify mutations in genes which contribute either positively or negatively to silencing. Strains JS306 (MATa) and JS311 (MATα) were constructed to contain three different Pol II-transcribed reporter genes in the rDNA, namely, a single MET15 reporter gene (embedded in a Ty1 element) located within NTS2 of one rDNA repeat and a mURA3/HIS3 expression cassette within the 18S rRNA-coding region of a second repeat (Fig. 1A). Met+ strains produce white colonies on Pb2+-containing (MLA) medium, whereas Met− strains produce dark brown colonies (21, 58). rDNA silencing of MET15 results in a characteristic intermediate tan colony color (70). Mutants which weaken rDNA silencing were predicted to produce a lighter colony color than wild type (WT), and mutants which strengthen rDNA silencing were predicted to produce darker colonies. The mURA3 reporter was also repressed by the rDNA and resulted in a very weak Ura+ phenotype (70). HIS3 was not observed as silenced in previous studies in a replica plating test (11, 70), and so it was used as a marker for the presence of the tightly linked mURA3 reporter. The relevant WT phenotypes of these reporter strains were therefore a tan colony color on MLA plates, weakly Ura+, and fully His+. During this study we found that HIS3 is in fact partially silenced when assayed by a more quantitative colony spotting assay.
FIG. 1.
Genetic screen for rDNA silencing mutants. (A) Schematic representation of the rDNA structure of strains JS306 and JS311, showing the positions of each reporter gene. (B) Schematic drawing showing the transposon mutagenesis strategy used in the screen. NotI yeast genomic DNA fragments disrupted with the transposon mTn3::lacZ::LEU2 (13), were transformed into strains JS306 and JS311. Homologous recombination resulted in the replacement of a specific segment of yeast chromosome (gene X), with an identical DNA fragment which was disrupted with the transposon. Transposon-disruption mutants are selected for the presence of LEU2 by growth on SC−Leu medium. (C) Flow chart describing the screening procedure and number of isolates at each stage. Sequences for 20 of the 65 of the 2° irs isolates were not recovered.
Mutations which weakened rDNA silencing, such as sir2Δ, had the potential to also increase the amount of mitotic recombination between rDNA repeats (33), making the silencing reporter genes unstable and more difficult to work with. To facilitate cloning the affected genes, we used a transposon-mediated gene disruption strategy developed by Burns et al. (13). The transposon mutagenesis method allows for direct recovery and sequencing of the mutated gene. Complementation cloning using potentially unstable silencing reporters is thus avoided. On the other hand, use of transposon mutagenesis biases toward the recovery of nonessential genes.
Approximately 20,000 Leu+ transformants of JS306 and JS311 were generated by transformation with a collection of yeast genomic library inserts previously mutagenized in E. coli by transposon Tn3::lacZ::LEU2 insertions (13) (Fig. 1B). These Leu+ transformants were then replica plated to MLA to observe changes in colony color, SC−Ura to observe changes in mURA3 reporter expression, SC−His to track the presence of the mURA3/HIS3 cassette, and to SC−Leu as a nonselective growth control.
Several different classes of mutants were generated. Colonies which were lighter in color than WT on MLA plates, more Ura+, and still His+ were classified as lrs mutants; those which were darker than WT on MLA and less Ura+ (and sometimes less His+) were classified as irs mutants. The screening process is summarized in Fig. 1C.
We identified the disrupted gene in a large subset of the lrs isolates, specifically those with phenotypes similar to sir2 mutants, which had, in addition to the loss of rDNA silencing (Lrs−) phenotypes, a hypersectoring phenotype by the MET15 color assay, indicative of increased MET15 marker loss through mitotic rDNA recombination (70). The flanking genomic DNAs were recovered into E. coli, and the disrupted genes were identified by DNA sequencing. We identified 11 different LRS genes (LRS1 through LRS11) and 22 different IRS genes (IRS1 through IRS22). We chose not to analyze the nonsectoring class of lrs mutants because most of them were dominant and likely to reflect simple rDNA amplification events that increased reporter gene copy number. However, informative mutants may exist in this collection; this possibility will be addressed in the future. Even though a total of 33 different genes were identified, the screen was not saturated; two known rDNA silencing genes, SIR2 and RAD6, were not recovered.
Description of lrs mutants and their silencing phenotypes.
The LRS class of genes were predicted to encode proteins which contributed structurally or were positive regulators of rDNA silencing. In this paper we describe LRS1 (RFC1), LRS4 (YDR439W), LRS5 (TOP1), LRS7 (RIF1), LRS8 (CAC1), and LRS9 (CDC17) (Table 2). The remaining LRS genes will be described elsewhere. Figure 2 shows the effects of a subset of these mutants on mURA3, HIS3, and MET15 silencing in the rDNA as measured by quantitative growth assays, which were previously shown to correlate with reporter gene expression levels (71). Figure 3 shows the effect of the mutations on MET15 expression as measured by a qualitative colony color assay. Each lrs mutant was more Ura+ and His+ than WT (Fig. 2A) and produced white colonies with a hyperrecombination sectoring phenotype on Pb2+ medium (Fig. 3). The lrs5 insertions were in the topoisomerase I gene (TOP1), a known rDNA silencing factor (11, 18), and therefore validated the specificity of the lrs screen.
TABLE 2.
Mutants isolated from the genetic screen
| Mutation | Gene/ORF name | Isolate no. | Transposon insertion sitea | Transposon orientationb | Gene function | Additional phenotypes |
|---|---|---|---|---|---|---|
| lrs1 | RFC1/CDC44 | M65 | Promoter | Plus | DNA replication/processivity factor | Slow growth, long telomeres |
| lrs4 | YDR439W | M114 | 78/347 | Plus | Unknown | Slow growth |
| M169 | 78/347 | Plus | Slow growth | |||
| M345 | 78/347 | Plus | Slow growth | |||
| lrs5 | TOP1 | M122 | 10/769 | Plus | Topoisomerase I | |
| M154 | 267/769 | Minus | ||||
| M160 | 55/769 | Plus | ||||
| lrs6 | DPB3 | M155 | 72/201 | Plus | DNA polymerase ɛ/subunits C and C′ | Increased spontaneous mutation rate |
| M172 | 34/201 | Minus | ||||
| M182 | 72/201 | Plus | ||||
| M286 | 72/201 | Plus | ||||
| lrs7 | RIF1 | M98 | 444/1916 | Minus | Telomere length control | Long telomeres |
| M158 | 183/1916 | Plus | ||||
| lrs8 | CAC1 | M179 | 272/606 | Plus | Nucleosome assembly during replication | UV sensitive |
| lrs9 | CDC17/POL1 | M326 | 237/1468 | Minus | DNA polymerase α | Long telomeres |
| irs1 | SIR4 | M87 | 20/1358 | Minus | Silencing factor | Mating defective |
| irs2 | RPD3 | M480 | 135/433 | Minus | Histone deacetylase | Pleiotropicc |
| irs4 | YKR019C | M469 | 69/615 | Plus | Unknown | Slow growth on Pb2+ |
| irs8 | YMR263W/SAP30 | M475 | 107/201 | Plus | Sin3-associated protein | Pleiotropicc |
| irs10 | HIR3 | M390 | 150/1648 | Plus | Regulates histone expression | |
| M411 | 702/1648 | Plus | ||||
| M487 | 702/1648 | Plus | ||||
| M489 | 1496/1648 | Minus | ||||
| irs18 | TUP1 | M419 | Promoter | Minus | Transcriptional repressor | Flocculent |
| M420 | Promoter | Minus | Flocculent | |||
| M432 | 478/713 | Minus | Flocculent |
Position of transposon insertion relative to protein sequence in amino acids (insertion site/total amino acids). Exceptions are M65, M419, and M420, which had insertions within the promoter sequences.
Plus orientation means the lacZ gene of the transposon is in the same transcriptional orientation as the target ORF.
Cycloheximide sensitive, derepression of PHO5, ethidium bromide sensitivity (78), temperature sensitive at 37°C.
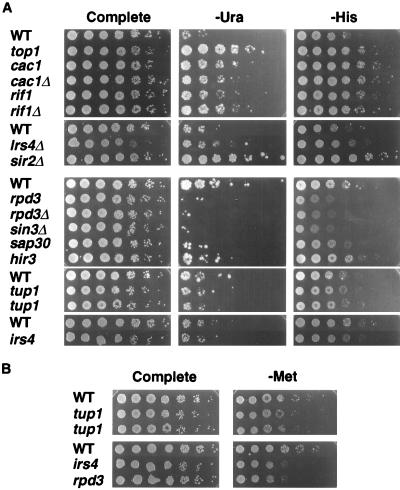
FIG. 2.
rDNA silencing phenotypes of selected mutants. (A) Quantitative growth assays measuring the silencing of mURA3 and HIS3 within the rDNA. Fivefold serial dilutions of freshly grown cells were plated onto either SC (Complete), SC−Ura (−Ura), or SC−His (−His) medium. Strains shown are WT (JS306 or JS311), top1 (M122), cac1 (M179), cac1Δ (JS400), rif1 (M158), rif1Δ (JS418), lrs4Δ (JS574), sir2Δ (JS576), rpd3 (M480), rpd3Δ (JS490), sin3Δ (JS493), sap30 (M475), hir3 (M489), tup1 (M419 and M432), and irs4 (M469). The photographs were taken for the SC−Ura plates at day 3 for the lrs mutant series (top of panel) and day 4 for the irs series (bottom of panel). (B) Quantitative growth assay measuring silencing of MET15 in NTS2. Fivefold serial dilutions of cells were plated onto SC and SC−Met −Cys medium. Strains were the same as for panel A.
FIG. 3.
Qualitative colony color assay showing the lrs and irs phenotypes of selected mutants. Freshly grown cells were scraped from YPD medium and streaked onto MLA medium for single colonies. Cells were grown 5 days before photographs were taken. lrs mutants produce white colonies and often display a hypersectoring phenotype; irs mutants produce a darker colony color, indicative of reduced MET15 expression compared to WT. lrs strains shown are WT (JS306), top1 (M122), rif1 (M158), cac1 (M179), sir2Δ (JS576), and lrs4Δ (JS574); irs strains shown are WT (JS311), hir3 (M487), rpd3 (M480), sin3Δ (JS493), sap30, M475, irs4 (M469), and tup1 (M419).
LRS1 was identified as a single transposon insertion into the promoter region of the gene encoding the large subunit of replication factor C (RFC1), which acts as a processivity factor for DNA polymerases δ and ɛ. RFC1 is an essential gene, and this mutation likely alters its expression level. The LRS9 gene was identified as encoding the essential catalytic subunit of DNA polymerase α (CDC17/POL1). The single lrs9 mutation consisted of a transposon integrated within the N-terminal portion of the ORF. We confirmed that no WT copy of CDC17 was present in the strain by PCR and backcross analysis (data not shown), indicating that this mutation of cdc17 is indeed viable. This suggests that there may be a cryptic yeast promoter in the 5′ end of the promoterless lacZ gene of the transposon, which can transcribe a 5′ truncated version of the CDC17 ORF.
LRS7 was identified as the RIF1 gene. RIF1 (Rap1-interacting factor 1) was originally isolated from a two-hybrid screen for proteins which interact with the C terminus of the essential silencing factor Rap1p (38). Deletion of RIF1 increases telomere length, strengthens TPE and weakens silencing at HMR, probably due to a shift in the balance of Sir3 and Sir4 proteins between the HM locus and telomeric chromatin compartments (12, 38).
LRS8 was identified as CAC1 (chromatin assembly complex), which encodes the large subunit of yeast chromatin assembly factor I (yCAF-I). yCAF-I is composed of three protein subunits (Cac1p [p90]; Cac2p [p60], and Cac3p [p50]) and preferentially assembles newly synthesized histones H3 and H4 with a deposition-competent acetylation pattern into nucleosomes on newly replicated DNA (45), similar to the activity of human CAF-I (hCAF-I) (72). The CAC genes are not essential, but their deletion causes modest UV sensitivity and weakens TPE and HM silencing (28, 45). While the exact function of yCAF-I in silencing is not known, it was recently shown to be required for the stable maintenance of repressed chromatin at telomeres and HM loci (27, 54). Both the original lrs8 (cac1) mutant and a cac1 deletion mutant derepressed all three rDNA reporter genes (Fig. 2A and 3). Furthermore, deletion of CAC2 or CAC3 resulted in an Lrs− phenotype (data not shown), indicating that the yCAF-I complex as a whole contributes to rDNA silencing.
LRS4 was identified as the previously uncharacterized ORF YDR439W. This gene is not highly homologous to any genes of known function and encodes a positively charged protein (pI = 10.34). It does appear to encode a coiled-coil protein with limited homology to myosin and other coiled-coil proteins. Its function in rDNA silencing is not known.
Description of irs mutants and their phenotypes.
The IRS class of genes was predicted to include negative regulators of rDNA silencing. IRS1 was identified as SIR4, deletion of which was previously shown to increase rDNA silencing (70). Isolation of a sir4 mutant therefore validated the specificity of this screen for irs mutants. Other IRS genes described in this study are IRS2 (RPD3), IRS4 (YKR019C), IRS8 (SAP30), IRS10 (HIR3), and IRS18 (TUP1) (Table 2). Each of these genes (except IRS4) has previously been shown to have a chromatin-related function (26, 39, 44, 64, 73, 84). Other IRS genes will be described elsewhere.
IRS2 was identified as the histone deacetylase gene RPD3. Rpd3p is part of a larger multiprotein complex called histone deacetylase B (HDB) (64), which also contains the transcriptional corepressor Sin3p (41, 43). Physical interactions between Sin3p and specific DNA binding proteins target HDB to various promoters which causes local histone deacetylation and transcriptional repression (41, 65). It was therefore surprising to isolate a mutation in rpd3 that caused a strong increased rDNA silencing (Irs−) phenotype (Fig. 2 and 3). Identical Irs− phenotypes were observed when RPD3 or SIN3 were deleted (Fig. 2A and 3), suggesting that the defect of the irs2 mutant was a loss of activity by the HDB complex. While unexpected, this phenotype was fully consistent with previous work showing that rpd3 or sin3 mutations also strengthen TPE and HM silencing (23, 64, 77).
Another recently identified member of HDB, called SAP30 (84), was isolated from our screen as IRS8. Similar to rpd3 and sin3Δ mutants, the irs8 (sap30) mutant had an Irs− phenotype (Fig. 2A and 3). However, its increase in silencing was not as strong as the rpd3 or sin3Δ mutants as measured by each rDNA silencing assay (Fig. 2A and 3). Since the loss of HDB activity alters the expression of multiple genes, the effects of HDB mutants on silencing could potentially be indirect.
IRS10 was identified as the histone regulator gene HIR3, which, like HIR1 and HIR2, encodes a transcriptional corepressor required for a feedback control system that regulates the expression of the HTA1-HTB1 genes in response to cellular histone H2A and H2B levels (73). hir mutations derepress histone transcription during the entire cell cycle, rather than normally in late G1 or early S phase (67). Interestingly, hir mutations exacerbate the telomeric silencing defects of cac mutants but have little or no TPE phenotype on their own (44, 62). Compared to rpd3 mutants, hir3 mutants were relatively mild in their strengthened rDNA silencing (Fig. 2A and 3).
IRS18 was identified as the global transcriptional repressor gene, TUP1. Tup1p and its partner, Ssn6p, are recruited to many yeast genes by interactions with specific DNA binding proteins to repress transcription through a histone H3- and H4-dependent mechanism (39). Direct interactions of Tup1p with the N-terminal tails of histones H3 and H4 contribute to repression (26). Even though irs18 isolates M419 and M420 each contained mTn3 insertions within the TUP1 promoter, they had silencing phenotypes similar to M432, in which mTn3 disrupted the ORF. The tup1 mutants appeared to have only modest effects on silencing of mURA3 and HIS3 (Fig. 2A) but had a more pronounced Irs− phenotype for the MET15 reporter (Fig. 2B and 3). This is the first report of mutations in tup1 having effects on SIR-mediated silencing. However, it is likely that this effect is indirect, because Tup1p represses the expression of many genes, yet the mutants increase the strength of silencing.
IRS4 was identified as ORF YKR019C. Its major distinguishing characteristic was a C-terminal Eps15 homology (EH) domain, a recently discovered protein-protein interaction domain (24). The prototypical yeast EH protein is Pan1p, which is involved in actin organization and endocytosis (75). Interestingly, Irs4p also contains a DNA polymerase B signature motif (YDDTDS) at amino acid positions 390 to 396. Similar to the tup1 mutants, the irs4 mutant had only modest effects on the mURA3 and HIS3 reporters (Fig. 2A). A more dramatic Irs− phenotype (approximately 25-fold increase in silencing) was observed for the MET15 reporter positioned in NTS2 (Fig. 2B).
Importantly, the expression of mURA3 or MET15 reporters located outside the rDNA was not significantly altered by the lrs or irs mutations that we tested (data not shown). These mutations included cac1Δ, rpd3Δ, rif1Δ, lrs4Δ, and sin3Δ. These results suggested that most of the effect of these mutations on mURA3 and MET15 expression in the rDNA is due to the general influence of rDNA chromatin and not to specific effects on the individual promoters used.
rDNA silencing in lrs and irs mutants is controlled by Sir2p levels.
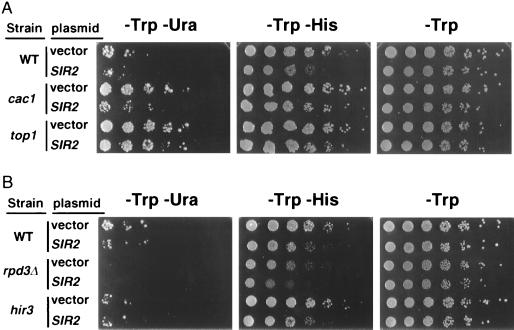
rDNA silencing is exquisitely sensitive to SIR2 dosage (31, 71). We were therefore interested in determining whether the lrs and irs mutants remained susceptible to SIR2 dosage effects. If any of these genes were downstream of SIR2 in a silencing pathway, then their Lrs− or Irs− phenotypes should become resistant to changes in SIR2 dosage. To test this hypothesis, we overexpressed SIR2 in several mutant backgrounds and tested rDNA silencing strength in Ura+ and His+ growth assays. SIR2 overexpression increased the silencing strength of the cac1 (lrs8) mutant, as measured by less Ura+ growth, compared to a strain containing an empty vector (Fig. 4A). However, less of an effect was observed in the His+ assay, consistent with the HIS3 reporter being more resistant to rDNA silencing in this mutant. The effect of SIR2 overexpression was less pronounced for the top1 mutant in the Ura+ and His+ assays, suggesting that top1 mutants become partially resistant to increased SIR2 dosage. rif1 and lrs4 mutants were fully sensitive to SIR2 dosage (data not shown). SIR2 overexpression also further strengthened rDNA silencing in all irs mutants tested, including tup1, sin3Δ, sap30 (not shown), rpd3Δ, and hir3 (Fig. 4B). These results indicated that most lrs and irs mutants are fully responsive to elevations in SIR2 dosage, and they localized these genes either upstream of SIR2 or in different genetic pathways to rDNA silencing.
FIG. 4.
Effect of SIR2 overexpression on rDNA silencing mutants. (A) The high-copy-number empty vector pRS424 or the SIR2 vector pJSS70-9 was transformed into the WT strain JS306, several lrs mutants including cac1 (M179), and the top1 mutant (M154). Fivefold serial dilutions were spotted onto SC−Trp, which selects for the plasmid, SC−Trp−Ura to measure silencing of mURA3, and SC−Trp −His to measure silencing of HIS3. (B) Fivefold serial dilutions of irs mutants containing a high-copy-number empty vector or a high-copy-number SIR2 vector.
Several mutants with long telomeres also weaken rDNA silencing.
We previously proposed that rDNA silencing strength could be modulated by telomere length due to a competition between the rDNA and telomeres for a limited pool of Sir2p (71). Similarly, extra telomeres weaken silencing of a telomeric reporter gene by titrating an unidentified silencing factor (82). Certain mutations in the DNA replication genes cdc17 (pol1) and rfc1, and null mutations of rif1, cause telomeres to lengthen compared to WT strains (1, 38). Lrs− insertion alleles were isolated for each of these genes (Table 2; Fig. 5A). The Lrs− phenotype of these mutants might therefore be due to long telomeres sequestering Sir2p and depleting it from the rDNA. As predicted by this model, the viable rfc1 (lrs1) and cdc17 (lrs9) mutants indeed had significantly longer telomeres than the parent strains (Fig. 5B). The rif1 (lrs7) mutant had longer telomeres which produced a qualitatively different banding pattern than the other mutants (Fig. 5B). This unusual banding pattern was specific to the rif1 mutation because it cosegregated in backcrosses.
FIG. 5.
A class of rDNA silencing mutants with abnormally long telomeres. (A) The DNA replication mutants cdc17 and rfc1, and the telomere regulation mutant rif1, were each isolated as lrs mutants. Each mutant was crossed to a sir4Δ::HIS3 mutant of the opposite mating type to produce a heterozygous diploid which was sporulated and dissected for tetrads. The resulting haploid strains were grown on MLA medium and assayed for loss of silencing of MET15 as measured by colony color. Strains shown are WT (JS333), sir4Δ (JS337), cdc17 (JS432), cdc17 sir4Δ (JS436), rfc1 (JS443), rfc1 sir4Δ (JS445), rif1 (JS422), and rif1 sir4Δ (JS426). (B) Steady-state telomere length of lrs mutants in combination with sir3Δ and sir4Δ mutations. Genomic DNA was isolated from two isolates of each mutant listed, digested with XhoI, and separated on a 0.7% agarose gel. The transferred DNA was detected with a C1–3A-specific DNA probe, which on this blot will detect typical Y′ telomeres (shown schematically at bottom). The variable-length telomeres are visualized as a smear (brackets). In addition to the mutants described in panel A, there are congenic sir3Δ (JS335), cdc17 sir3Δ (JS434), rif1 sir3Δ (JS424), rif2Δ (JS495), and rif2Δ rif1 (JS497) strains.
Each rDNA repeat contains an origin of replication (74). It was therefore possible that in addition to their effect on telomere length, Cdc17p and Rfc1p may have a more direct role in rDNA silencing related to their DNA replication functions. Furthermore, Top1p and Dpb3p, two other DNA replication proteins for which we isolated lrs mutant alleles, did not have lengthened telomeres (data not shown). To differentiate this replication model from the telomere competition model, we combined the rfc1, cdc17, and rif1 mutations with sir3Δ and sir4Δ mutations and tested whether the Lrs− phenotype was reversed in the double mutants. Deletion of SIR3 or SIR4 in the lrs mutants was predicted to prevent telomeric titration of Sir2p to telomeres and away from the rDNA. Sir2p exclusively localizes in the nucleolus of sir4Δ mutant strains (32). For rfc1 and cdc17, double-mutation combinations with sir4Δ indeed reversed the Lrs− phenotype to WT or slightly stronger than WT levels (Fig. 5A, bottom row). However, the full Irs− phenotype expected of a sir4Δ mutant was not observed. These results suggest that much, but perhaps not all, of the effect on rDNA silencing caused by rfc1 and cdc17 mutations was due to telomere competition. Similar results were observed when rfc1 and cdc17 were combined with a sir3Δ mutation (data not shown). Telomere length for these double-mutant strains was also intermediate between WT and single-mutant lengths (Fig. 5B). Furthermore, telomere length did not change during prolonged strain passage (data not shown).
In contrast, the rif1 sir4Δ double mutant had weakened rDNA silencing compared to WT, similar to the rif1 single mutant (Fig. 5A). This result suggested that Rif1p may have a direct role in rDNA silencing that is independent of telomere competition. Rif1p physically interacts with another Rap1-interacting factor called Rif2p (83), which also functions in telomere length regulation. Unexpectedly, deletion of RIF2 had no effect on the strength of rDNA silencing (data not shown), even though these strains had long telomeres (Fig. 5B). Furthermore, a rif1 rif2Δ mutant had extremely long telomeres (Fig. 5B) but did not weaken rDNA silencing more than a rif1 single mutant (data not shown). These results suggest that the telomere lengthening effects of rif1 mutants may be unrelated to their Lrs− phenotype.
lrs and irs mutants alter the structure of rDNA chromatin.
To determine whether mutations which weaken rDNA silencing (lrs) also disrupt rDNA chromatin structure, we tested each lrs mutant in an in vivo psoralen cross-linking assay (22). This assay measures the intracellular accessibility of the rDNA to psoralen cross-linking. The Lrs− phenotype of sir2 mutants was previously shown to be associated with an increase in psoralen cross-linking at the rDNA, reflecting a more open chromatin structure (70). Increased psoralen cross-linking was detected by the slower mobility of isolated rDNA fragments on a native agarose gel. The accessibility of the NTS in a subset of the lrs mutants is shown in Fig. 6A. The 2.5-kb NTS fragment released by EcoRI digestion displayed a slower gel mobility for each lrs mutant, similar to the sir2Δ control. This result indicated that the NTS had a more open chromatin structure in most lrs mutants, not just those carrying sir2Δ.
FIG. 6.
rDNA chromatin accessibility of mutants as measured by in vivo psoralen cross-linking. (A) Log-phase cultures of lrs mutants were UV cross-linked with psoralen in vivo. Isolated DNA was digested with EcoRI and separated on a 1.3% agarose gel, and the transferred DNA was detected with a probe specific for the NTS of the rDNA (C). The sir2Δ strain (JS343) acted as a positive control for increased accessibility to psoralen cross-linking. Other strains shown are WT (JS306), top1 (M122), rif1 (M158), and cac1Δ (JS400). (B) An identical cross-linking procedure using the same NTS-specific probe was carried out on a subset of the irs mutants. The strains tested are WT (JS311), rpd3Δ (JS490), sin3Δ (JS493), sap30 (M475), hir3 (M411), and tup1 (M419). The control lanes show the 2.5-kb EcoRI fragment which is observed when the strains are not cross-linked. (C) Schematic drawing of the EcoRI rDNA fragment detected from this assay. ARS, autonomously replicating sequence.
Since the lrs mutants had an open rDNA chromatin structure, we anticipated that the irs mutants might have a more closed chromatin structure which would be consistent with stronger silencing. To test this hypothesis, the psoralen cross-linking assay was repeated for a subset of irs mutants (Fig. 6B). The only mutant tested which produced an NTS fragment with moderately faster gel mobility than WT (less cross-linking) was irs18 (tup1). The sir4Δ (data not shown) and hir3 mutants had no detectable effect on the rDNA chromatin in this assay. Since this assay measures the average state of the rDNA chromatin, we cannot rule out that the chromatin associated with the marker genes has not been altered in these cases. Unexpectedly, the rpd3Δ, sin3Δ, and sap30 (HDB) mutants each produced NTS fragments with slower gel mobility, indicative of a more open chromatin structure (Fig. 6B). Histone acetylation is thought to increase the access of transcription-associated factors to DNA. The more open chromatin structure of the rpd3Δ, sin3Δ, and sap30 mutants was therefore fully consistent with histone hyperacetylation in the rDNA caused by a lack of histone deacetylation, and it suggested that HDB may directly deacetylate rDNA histones. However, this result was again inconsistent with the paradoxical Irs− phenotype of the HDB mutants.
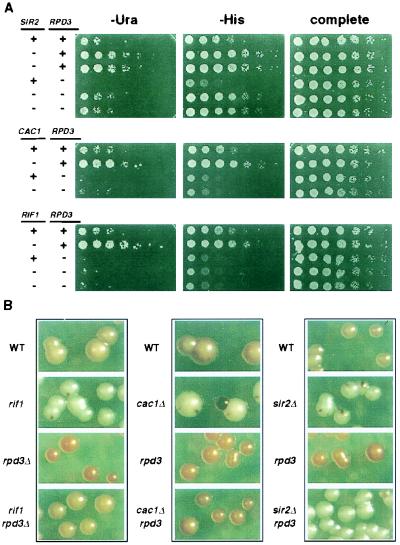
The Irs− phenotype of an rpd3 mutant is partially reversed by cac1, rif1, and sir2 mutations.
The opposing silencing and chromatin accessibility phenotypes of rpd3, sin3, and sap30 mutants prompted a more in-depth examination of the genetic interactions between RPD3 and the LRS genes. We asked whether the Irs− phenotype caused by rpd3 mutations depends on any of the LRS genes, specifically SIR2, CAC1, or RIF1. Double-mutant combinations were generated and tested in an epistasis analysis for rDNA silencing strength using the Ura+ and His+ growth assays and the colony color assay (Fig. 7). We obtained different epistasis results that correlated with the location of the silencing reporter gene in the rDNA repeat. For example, with the mURA3/HIS3 cassette readouts, rpd3 mutations fully overrode the effects of the cac1Δ and rif1 mutations (Fig. 7A). However, for the MET15 reporter in the same strains, the double mutants produced Met+ (data not shown) and colony color phenotypes that were intermediate between those of the two single mutants (Fig. 7B). Thus, rpd3Δ partially overrode the cac1Δ and rif1 Lrs− phenotypes. Therefore, mURA3 and HIS3, both located within the 18S coding region, appeared to be influenced by the rpd3 mutant effect more than MET15, which was located within NTS2. Alternatively, these differences could be caused by simple promoter differences.
FIG. 7.
Epistasis analysis between rpd3 and lrs mutations for rDNA silencing phenotypes. (A) Silencing reporter strains were generated with combinations of rpd3 mutations with either sir2Δ, cac1Δ, or rif1 mutations. The resulting strains were assayed for rDNA silencing strength in Ura+ and His+ growth assays. Fivefold serial dilutions of each haploid strain were spotted on indicator plates. A plus sign indicates the strain is WT for a particular gene, and a minus sign indicates a mutation of a particular gene. (B) The same strains were plated onto MLA medium and tested for silencing strength in the colony color assay. Each column represents strains grown on the same plate.
The rpd3 sir2Δ combination of mutations produced a significantly different result. The double mutant again resulted in an intermediate rDNA silencing phenotype but in this case had an Lrs− phenotype relative to WT in each assay (Fig. 7). This result indicated that SIR2 was required for the Irs− phenotype of the rpd3 mutant but that sir2Δ was not completely epistatic to rpd3. For the rpd3 sir2Δ combination, there was also no differential effect on silencing based on the reporter gene position in the rDNA repeat sequence. To determine whether the other SIR genes contributed to the Irs− phenotype of an rpd3 mutant, we generated rpd3 sir3Δ double mutants through backcrossing. Deletion of sir3 in an rpd3 mutant did not reverse the Irs− phenotype (data not shown), indicating that the Irs− phenotype of an rpd3 mutant occurs through a SIR3-independent, SIR2-dependent mechanism.
rpd3 mutations partially restore mating competence to sir3Δ mutants.
In the process of analyzing the interactions of rpd3 and sir3Δ mutations, we made some surprising observations about effects on HM locus silencing. Previous studies have noted enhancement of silencing effects by rpd3 mutations on a weakened HMRΔA silencer (77). We observed behavior suggesting that rpd3 mutations can restore silencing to the native HMR silencer in sir3Δ mutants. All of the 17 MATα rpd3 sir3Δ spores resulting from a cross of rpd3 and sir3Δ strains efficiently mated to a MATa tester strain, indicating that the rpd3 mutation partially reversed the silencing defect caused by the sir3Δ mutation. This effect was specific for MATα cells, suggesting that HMRa was affected but HMLα was not affected (Fig. 8). In contrast, the rpd3 mutation did not rescue the mating defect of MATα sir2Δ or MATα sir4Δ strains (Fig. 8).
FIG. 8.
Mating phenotypes of rpd3 sir double-mutation combinations. The rpd3::mTn3 mutation was combined with either sir2Δ, sir3Δ, or sir4Δ mutation to determine the effect on mating ability. Strains were patched onto YPD, grown for 24 h, and then mated for 6 h with a lawn of MATa or MATα tester strains. Diploids were selected on SD minimal medium by growth for 18 h. The original YPD master plate was replica plated to SC medium as a nonselective growth control. The double-mutation combinations were tested in both MATα and MATa genetic backgrounds. Mating is measured by the efficiency of diploid formation on SD medium.
DISCUSSION
Multiple genetic pathways to rDNA silencing.
An unanticipated outcome from this screen was the absence of genes involved in transcription by RNA Pol I or III, especially since this specialized transcription is intimately associated with the rDNA. It was possible that rDNA silencing of Pol II reporter genes was caused by promoter interference from Pol I or III. Instead, we recovered multiple genes involved with DNA replication or chromatin modulation. The DNA replication genes included POL1, RFC1, and DPB3. The chromatin modulating class included RPD3, SIN3, SAP30, TUP1, HIR3, SIR4, and RIF1. The TOP1 and CAC1 genes could be placed in either DNA replication or chromatin modulating classes, since they participate in both processes. These results, coupled with the intermediate phenotypes of several double-mutation combinations, are strong evidence for several independent pathways which converge on rDNA silencing (Fig. 9). The SIR2 pathway is extremely important, but it appears that all the pathways must be functional to achieve full silencing strength.
FIG. 9.
Model of multiple pathways to rDNA silencing. Sir2p is a central factor in rDNA silencing. Cdc17p and Rfc1p inhibit formation of extended telomeres, limiting the impact of telomere-rDNA competition for Sir2p, which is mediated by Sir4p. Cdc17p and Rfc1p may also positively act on rDNA silencing through their DNA replication functions. Silencing in most lrs and irs mutants can be increased by overexpression of Sir2p. CAF-I and Rif1p could be upstream of Sir2p, act in cooperation with Sir2p, or be completely separate inputs. Rpd3p counteracts rDNA silencing mostly through a Sir2p-dependent mechanism but also through a SIR-independent mechanism. Topoisomerase I may influence rDNA silencing by a mechanism that is partially independent of Sir2p.
There are several reasons why a role for Pol I or Pol III transcription in rDNA silencing cannot be ruled out by this study. First, the screen was not carried out to saturation, since we did not recover transposon insertions into SIR2 or RAD6, two genes already known to be required for rDNA silencing (11, 70). Second, many genes involved in Pol I and Pol III transcription are essential and thus are more difficult to recover with this mutagenesis method than nonessential genes. Third, a bias was placed on the lrs mutant selection when we chose to study the hypersectoring class. Pol I or Pol III mutants might lack rDNA recombination phenotypes. Future work will address whether there is any role of specialized rRNA transcription in rDNA silencing.
Telomere length effects on rDNA silencing.
Sir4p mediates competition between the rDNA and telomeres for a limiting amount of Sir2 protein (71). Variations in telomere length are therefore predicted to influence the strength of rDNA silencing by changing the balance of Sir2p between the rDNA and telomere compartments. Indeed, three different lrs mutants with significantly longer than WT telomeres were isolated. Also consistent with this competition model, introducing a sir4Δ mutation into rfc1 or cdc17 mutant strain backgrounds partially reversed their Lrs− phenotypes back to at least WT silencing strength and also significantly reduced telomere length. This was an important result because in the absence of Sir4p, all cellular Sir2p is localized in the nucleolus and not sequestered at telomeres (32). Taken together, these two findings suggest that rfc1 and cdc17 mutations weaken the Irs− phenotype of a sir4Δ mutant through a telomere length-independent mechanism, perhaps related to their replication functions.
This model was complicated by our finding that rif2Δ mutants had long telomeres but did not weaken rDNA silencing. Furthermore, unlike the case for cdc17 and rfc1 mutants the Lrs− phenotype of rif1 mutants was not dramatically reversed by introduction of sir4Δ or sir3Δ mutations. This result suggested that rif1 plays a direct role in rDNA silencing, which partially uncouples telomere length control from rDNA silencing. Rif1p and Rif2p interact with each other and with Rap1p (83). They also have recently been shown to interact with telomeric DNA sequences in a one-hybrid assay (7). Deletion of RIF2 causes telomeres longer than those in WT strains and improves TPE but has little or no effect on HMR silencing (83). Since deletion of RIF2 also has little or no effect on rDNA silencing, rif2Δ mutant telomeres probably contain normal amounts of Sir2p, even though they are longer.
In addition to causing loss of rDNA silencing (this study), rif1 mutations shorten the life span of yeast (4). The short life span of rif1Δ mutants was proposed to result from sequestration of the Sir2p-Sir3p-Sir4p silencing complex by longer telomeres (4). However, our data suggest that a rif1 mutation has negative effects on rDNA silencing that are telomere length independent. Introduction of sir4 mutations which lengthen life span (47), and increase rDNA silencing (71), into a rif1Δ mutant background only partially reverses the short life span phenotype (4). The remaining short life span effect observed in those double mutants is therefore also consistent with a direct role for Rif1p in rDNA silencing. A final piece of evidence for a direct Rif1p role in rDNA silencing is that Rif1p directly interacts with Sir2p in 2-hybrid and biochemical assays (67a).
DNA replication and rDNA silencing.
Connections between DNA replication and silencing are not unprecedented. First, establishment of silencing at the HM loci requires progression through S phase (30, 53). Second, the six-subunit origin recognition complex (ORC) is required for silencing at HML and HMR (29, 52). One role of ORC in silencing at the HM loci appears to be recruitment of the Sir-silencing complex through interactions with Sir1p (16, 76). However, ORC is also required for silencing at telomeres in a SIR1-independent manner (30). Interestingly, the silencing function of one ORC subunit (Orc5p) at the HMR-E silencer can be genetically separated from its replication initiation function, implying that replication initiation is not required for silencing (25). Silencing at the rDNA is qualitatively different from HM and telomere silencing, and so it is currently unknown whether ORC functions in rDNA silencing.
Each rDNA repeat contains an origin of DNA replication, which is located upstream of the 5S rRNA in NTS2 (68). It was therefore possible that DNA replication could play a role in rDNA silencing. Indeed, we have shown that relatively mild mutant alleles of rfc1 (lrs1) and cdc17 (lrs9) weakened rDNA silencing. Part of this effect was likely due to lengthened telomeres (see above), but not all of this effect could be explained by this model. The remainder of the Lrs− phenotype could be caused by replication defects. Consistent with this hypothesis, another lrs mutant gene (lrs6) suffered a Tn3 insertion into a nonessential subunit of DNA polymerase ɛ (DPB3). Unlike the cdc17 and rfc1 mutants, the dpb3 mutants had normal-length telomeres, indicating that replication mutants can affect rDNA silencing independent of telomere length control.
Finding a DNA polymerase ɛ (Pol ɛ) subunit in the lrs screen is intriguing because DNA Pol ɛ is required not only for chromosomal replication but also for base excision repair (80). RFC-1 is also involved in base excision repair. Furthermore, the large subunit of Pol ɛ (Pol2) is required for replication and DNA damage checkpoints in S phase (57). dpb3Δ strains have an elevated spontaneous mutation rate, suggesting it is involved in maintaining replication fidelity (3). Taken together, an alternative model to explain the role of the DNA replication genes in rDNA silencing could be a contribution from the DNA repair machinery.
Histone balancing act.
Nucleosomes are formed from two molecules of each of the core histones H2A, H2B, H3, and H4 which tightly associate with 146 bp of DNA. Histones H3 and H4 have been shown to contribute directly to silencing at the HM and telomere loci (35). Furthermore, histones H2A and H2B are important for rDNA silencing. Deletion of HTA1-HTB1, one of two gene pairs producing H2A and H2B, results in a loss of rDNA silencing (11), suggesting that histone stoichiometry within the nucleosome may be critical. We recovered four independent mutations in the HIR3 gene from our screen as irs10 mutants. Mutations in HIR1, HIR2, or HIR3 cause deregulation of a transcriptional feedback mechanism which controls HTA1-HTB1 expression (73). The Irs− phenotype of irs10 (hir3) mutants may therefore be due to increased H2A H2B expression levels. Consistent with this model, telomeric silencing is also slightly increased in a hir1 mutant strain (44).
The Lrs− phenotype of cac1, cac2, and cac3 mutants may also result from altered histone stoichiometry. hCAF-I and yCAF-I (chromatin assembly complexes) assemble newly synthesized histones H3 and H4 onto newly replicating DNA (45, 72). The CAC genes are not essential in yeast, but mutations of these genes weaken silencing at all silenced loci (28, 45). It is therefore possible that CAF-I is important for assembly of a silencing-competent nucleosome structure. Indeed, the HM and telomere silencing defects of cac mutants are caused by poor maintenance of the silent chromatin states (27, 54). In the absence of CAF-I activity, an alternative nucleosome assembly activity may take over and deposit silencing-incompetent nucleosomes that lack the proper histone acetylation pattern or are assembled improperly for efficient silencing. Another straightforward hypothesis for the effect of cac mutants on rDNA silencing is that a certain minimal nucleosome density is required to build silencing competent chromatin. The cac mutants may fail to deposit nucleosomes at a sufficient density to maintain silencing, even if histone levels are normal. Epigenetic switching between silent and active chromatin states has not been demonstrated for the rDNA, suggesting that the Lrs− phenotype of cac mutants is due to a direct structural defect of the rDNA chromatin. Indeed, the structure of rDNA chromatin is altered in a cac1Δ mutant as measured by an in vivo psoralen accessibility assay (Fig. 6), consistent with a silencing maintenance defect.
Histone deacetylation and rDNA silencing.
Transcriptionally silenced regions of eukaryotic genomes are generally hypoacetylated. In S. cerevisiae, the HM loci and telomeres are hypoacetylated on histones H3 and H4, with an acetylation pattern similar to that in higher eukaryotes (10). The yeast RPD3 gene product is the proposed catalytic component of a large multiprotein histone deacetylase complex (HDB) which acts in targeted transcriptional repression (40, 64). Sin3p, another subunit of HDB, acts to target the complex to specific Pol II promoters through association with specific DNA binding proteins (41). Sap30p is also a member of this complex (84). Paradoxically, rpd3 and sin3 mutations increase silencing at HM loci (77), telomeres (23, 64), and the rDNA (this study). Similarly, Drosophila TPE is enhanced by null mutations of its RPD3 homolog (23). In another study, sap30 mutants strengthened all types of silencing in yeast, including the rDNA (37). These findings were completely consistent with our results for the irs8 (sap30) mutation using a different strain background and different rDNA silencing reporters. In both studies, these results are paradoxical because loss of histone deacetylase function is expected to result in hyperacetylation of histone N-terminal tails, resulting in derepression of transcription.
Two separate hypotheses have been proposed to explain the rpd3Δ effect on silencing (23, 64, 77). The first is that the effect is indirect due to the increased expression of a critical, dosage-dependent silencing factor. This is unlikely to be SIR2 itself because increased SIR2 expression does not increase silencing at the HM loci but actually slightly derepresses it (19). The second hypothesis is that loss of rpd3 function causes an increase in the acetylation of an N-terminal lysine residue on one of the core histones which correlates with increased silencing. Lysine 12 of histone H4 has been proposed to be a critical lysine, because it is specifically acetylated in the chromatin of the silent HM loci compared to the expressed MAT locus (10). This modification was suggested to enhance the interaction between H4 and the Sir3 and Sir4 proteins, thus strengthening silencing (10). If this were true, then it was possible that Sir3p and/or Sir4p were being recruited to the rDNA by increased histone H4 Lys12 acetylation to increase silencing in an rpd3 mutant. However, this does not appear to be the case for rDNA silencing, because deletion of SIR3 had no effect on the Irs− phenotype of an rpd3 mutant. Furthermore, SIR4 is also not required for the Irs− phenotype of an rpd3 mutant (37). Whatever the mechanism, it is clear that SIR2 is required for the Irs− phenotype of the HDB mutants.
A third possible explanation for the increased silencing effect caused by an rpd3 mutation is that the activity of an unidentified silencing factor is modulated by acetylation on internal lysine residues. Perhaps the HDB activity is required to regulate this potential silencing factor through deacetylation. Loss of deacetylase activity would then hyperactivate the silencing factor, leading to stronger silencing. Several nonhistone proteins including p53 and HMG-I(Y) are known to be acetylated in mammalian cells by the histone acetyltransferases CBP/p300 and P/CAF (36, 56, 66). However, acetylation of a nonhistone protein by a yeast histone acetyltransferase has not been demonstrated.
During the course of these experiments, we made the unexpected observation that rpd3 mutations efficiently suppress the mating defect of sir3Δ strains, but only in the MATα background. No such mating was seen in rpd3 sir2Δ or rpd3 sir4Δ strains. Thus, this effect appears to be SIR3 specific. We interpret this restoration of mating in sir3Δ mutants as an HMR-specific restoration of silencing, although other more complex interpretations are possible (e.g., RPD3 could be required for a1/α2 mediated repression of α-specific genes). The latter type of interpretation seems unlikely because it does not explain the sir3 specificity of the rpd3 mutation. A direct effect of the rpd3 mutation on HMR silencing could be explained by a direct effect of the histone deacetylase at HMR. Increased acetylation of histone H4 Lys12 in an rpd3 mutant might render HMR silenceable in the absence of SIR3. Alternatively, an RPD3-regulated gene encoding a redundant function with SIR3 could be activated in the rpd3 mutant and substitute for SIR3 at HMR. In either case, there is clearly a differential effect of the rpd3 mutation on the α mating type, presumably indicating differential silenceability of HMR versus HML.
A similar pattern of HMR-specific suppression was seen in a sir2Δ mutant when the related gene HST1 was overexpressed (9). Furthermore, the SUM1-1 mutation suppresses all sir mutations, also in an HMR-preferential manner (15, 48, 49). However, the situation with an rpd3 mutation is distinct, in that it suppresses only the HMR silencing defects of sir1 (77) and sir3 mutations (this study). The basis for specific suppression of the HMR silencing defect is not known, but it could be related to the observation that HMR silencing is more resistant than HML silencing to mutations in non-SIR silencing genes such as nat1 and ard1 (55). Silencing at HMR therefore appears to be more robust than silencing at HML, which could be due to the redundancy of the HMR-E silencer (15). In the absence of Sir3p, there may be a residual form of silencing at HMR which becomes detectable in an rpd3Δ genetic background through mating assays.
Although rDNA silencing is different from TPE and HM silencing in that SIR1, SIR3, and SIR4 are not required, this and other recent studies have drawn some interesting correlations. There are now several shared factors required for all types of silencing, including Sir2p, Cac1p, and Rad6p. In addition, mutation of rif1 weakens silencing at both HMR and the rDNA. On the other hand, the RPD3, SIN3, and SAP30 genes counteract all forms of silencing. All of these correlations further solidify the notion that rDNA silencing is mediated by a specialized repressive chromatin structure that is distinct from yet related to the heterochromatin of telomeres and the HM loci.
ACKNOWLEDGMENTS
We thank Michael Hampsey, Danny Reinberg, David Shore, and Susan Gasser for communicating results prior to publication, Paul Kaufman, Jasper Rine, and David Shore for helpful discussions, and Greg Cost and Nurjana Bachman for comments on the manuscript. We also thank Forrest Spencer for providing access to a photography-equipped stereoscopic microscope, Virginia Zakian and Lorraine Pillus for the telomere probe plasmid, and Mike Snyder and Susan Michaelis for the transposon insertion library.
J.S.S. was supported by a postdoctoral fellowship from the Leukemia Society of America and an NIH postdoctoral training grant. This work was supported in part by National Institutes of Health grants CA16519 and GM36481 to J.D.B.
REFERENCES
- 1.Adams A K, Holm C. Specific DNA replication mutations affect telomere length in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4614–4620. doi: 10.1128/mcb.16.9.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aparicio O M, Billington B L, Gottschling D E. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 3.Araki H, Hamatake R K, Morrison A, Johnson A L, Johnston L H, Sugino A. Cloning DPB3, the gene encoding the third subunit of DNA polymerase II of Saccharomyces cerevisiae. Nucleic Acids Res. 1991;19:4867–4872. doi: 10.1093/nar/19.18.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austriaco N R, Jr, Guarente L P. Changes in telomere length cause reciprocal changes in the lifespan of mother cells in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1997;94:9768–9772. doi: 10.1073/pnas.94.18.9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boeke J D, Garfinkel D J, Styles C A, Fink G R. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- 7.Bourns B D, Alexander M K, Smith A M, Zakian V A. Sir proteins, Rif proteins, and Cdc13p bind Saccharomyces telomeres in vivo. Mol Cell Biol. 1998;18:5600–5608. doi: 10.1128/mcb.18.9.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brachmann C B, Davies A, Cost G J, Caputo E, Li J, Hieter P, Boeke J D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Brachmann C B, Sherman J M, Devine S E, Cameron E E, Pillus L, Boeke J D. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- 10.Braunstein M, Sobel R E, Allis C D, Turner B M, Broach J R. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol Cell Biol. 1996;16:4349–4356. doi: 10.1128/mcb.16.8.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryk M, Banerjee M, Murphy M, Knudsen K E, Garfinkel D J, Curcio M J. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 1997;11:255–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- 12.Buck S W, Shore D. Action of a RAP1 carboxy-terminal silencing domain reveals an underlying competition between HMR and telomeres in yeast. Genes Dev. 1995;9:370–384. doi: 10.1101/gad.9.3.370. [DOI] [PubMed] [Google Scholar]
- 13.Burns N, Grimwade B, Ross-Macdonald P B, Choi E-Y, Finberg K, Roeder G S, Snyder M. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- 14.Cavalli G, Bachmann D, Thoma F. Inactivation of topoisomerases affects transcription-dependent chromatin transitions in rDNA but not in a gene transcribed by RNA polymerase II. EMBO J. 1996;15:590–597. [PMC free article] [PubMed] [Google Scholar]
- 15.Chi M-H, Shore D. SUM1-1, a dominant suppressor of SIR mutations in Saccharomyces cerevisiae, increases transcriptional silencing at telomeres and HM mating-type loci and decreases chromosome stability. Mol Cell Biol. 1996;16:4281–4294. doi: 10.1128/mcb.16.8.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chien C T, Buck S, Sternglanz R, Shore D. Targeting of SIR1 protein establishes transcriptional silencing at HM loci and telomeres in yeast. Cell. 1993;75:531–541. doi: 10.1016/0092-8674(93)90387-6. [DOI] [PubMed] [Google Scholar]
- 17.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Multifunctional yeast high-copy number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 18.Christman M F, Dietrich F S, Fink G R. Mitotic recombination in the rDNA of S. cerevisiae is suppressed by the combined action of DNA topoisomerases I and II. Cell. 1988;55:413–425. doi: 10.1016/0092-8674(88)90027-x. [DOI] [PubMed] [Google Scholar]
- 19.Cockell, M., M. Gotta, F. Palladino, S. G. Martin, and S. M. Gasser. Targeting limiting pools of Sir proteins to sites of action: a general mechanism for regulated repression. Cold Spring Harbor Symp. Quant. Biol., in press. [DOI] [PubMed]
- 20.Conrad-Webb H, Butow R. A polymerase switch in the synthesis of rRNA in Saccharomyces cerevisiae. Mol Cell Biol. 1995;15:2420–2428. doi: 10.1128/mcb.15.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cost G J, Boeke J D. A useful colony colour phenotype associated with the yeast selectable/counterselectable marker MET15. Yeast. 1996;12:939–941. doi: 10.1002/(SICI)1097-0061(199608)12:10%3C939::AID-YEA988%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Dammann R, Lucchini R, Koller T, Sogo J M. Chromatin structures and transcription of rDNA in yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:2331–2338. doi: 10.1093/nar/21.10.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Rubertis F, Kadosh D, Henchoz S, Pauli D, Reuter G, Struhl K, Spierer P. The histone deacetylase RPD3 counteracts genomic silencing in Drosophila and yeast. Nature. 1996;384:589–591. doi: 10.1038/384589a0. [DOI] [PubMed] [Google Scholar]
- 24.Di Fiorre P P, Pelicci P G, Sorkin A. EH: a novel protein-protein interaction domain potentially involved in intracellular sorting. Trends Biochem Sci. 1997;22:411–413. doi: 10.1016/s0968-0004(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 25.Dillin A, Rine J. Separable functions of ORC5 in replication initiation and silencing in Saccharomyces cerevisiae. Genetics. 1997;147:1053–1062. doi: 10.1093/genetics/147.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmondson D G, Smith M M, Roth S Y. Repression domain of the yeast global repressor Tup1 interacts directly with histones H3 and H4. Genes Dev. 1996;10:1247–1259. doi: 10.1101/gad.10.10.1247. [DOI] [PubMed] [Google Scholar]
- 27.Enomoto S, Berman J. Chromatin assembly factor I contributes to the maintenance, but not the re-establishment, of silencing at the yeast silent mating loci. Genes Dev. 1998;12:219–232. doi: 10.1101/gad.12.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enomoto S, McCune-Zierath P D, Gerami-Nejad M, Sanders M A, Berman J. RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev. 1997;11:358–370. doi: 10.1101/gad.11.3.358. [DOI] [PubMed] [Google Scholar]
- 29.Foss M, McNally F J, Laurenson P, Rine J. Origin recognition complex (ORC) in transcriptional silencing and DNA replication in S. cerevisiae. Science. 1993;262:1838–1844. doi: 10.1126/science.8266071. [DOI] [PubMed] [Google Scholar]
- 30.Fox C A, Ehrenhofer-Murray A E, Loo S, Rine J. The origin recognition complex, SIR1, and the S phase requirement for silencing. Science. 1997;276:1547–1551. doi: 10.1126/science.276.5318.1547. [DOI] [PubMed] [Google Scholar]
- 31.Fritze C E, Verschueren K, Strich R, Esposito R E. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotta M, Strahl-Bolsinger S, Renauld H, Laroche T, Kennedy B K, Grunstein M, Gasser S M. Localization of Sir2p: the nucleolus as a compartment for silent information regulators. EMBO J. 1997;16:3243–3255. doi: 10.1093/emboj/16.11.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gottlieb S, Esposito R E. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- 34.Gottschling D E, Aparicio O M, Billington B L, Zakian V A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 35.Grunstein M. Molecular model for telomeric heterochromatin in yeast. Curr Opin Cell Biol. 1997;9:383–387. doi: 10.1016/s0955-0674(97)80011-7. [DOI] [PubMed] [Google Scholar]
- 36.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 37.Hampsey, M. Personal communication.
- 38.Hardy C F, Sussel L, Shore D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 39.Huang L, Zhang W, Roth S Y. Amino termini of histones H3 and H4 are required for a1-α2 repression in yeast. Mol Cell Biol. 1997;17:6555–6562. doi: 10.1128/mcb.17.11.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadosh D, Struhl K. Histone deacetylase activity of Rpd3 is important for transcriptional repression in vivo. Genes Dev. 1998;12:797–805. doi: 10.1101/gad.12.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadosh D, Struhl K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell. 1997;89:365–371. doi: 10.1016/s0092-8674(00)80217-2. [DOI] [PubMed] [Google Scholar]
- 42.Kamakaka R T, Rine J. Sir- and silencer independent disruption of silencing in Saccharomyces by Sas10p. Genetics. 1998;149:903–914. doi: 10.1093/genetics/149.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasten M M, Dorland S, Stillman D J. A large protein complex containing the yeast Sin3p and Rpd3 transcriptional regulators. Mol Cell Biol. 1997;17:4852–4858. doi: 10.1128/mcb.17.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaufman P D, Cohen J L, Osley M A. Hir proteins are required for position-dependent gene silencing in Saccharomyces cerevisiae in the absence of chromatin assembly factor I. Mol Cell Biol. 1998;18:4793–4806. doi: 10.1128/mcb.18.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaufman P D, Kobayashi R, Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11:345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy B K, Gotta M, Sinclair D A, Mills K, McNabb D S, Murthy M, Pak S M, Laroche T, Gasser S M, Guarente L. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997;89:381–391. doi: 10.1016/s0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy B K, Nicanor J, Austriaco R, Zhang J, Guarente L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell. 1995;80:485–496. doi: 10.1016/0092-8674(95)90499-9. [DOI] [PubMed] [Google Scholar]
- 48.Klar A J, Kakar S N, Ivy J M, Hicks J B, Livi G P, Miglio L M. SUM1, an apparent positive regulator of the cryptic mating-type loci in Saccharomyces cerevisiae. Genetics. 1985;111:745–758. doi: 10.1093/genetics/111.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laurenson P, Rine J. SUM1-1: a suppressor of silencing defects in Saccharomyces cerevisiae. Genetics. 1991;129:685–696. doi: 10.1093/genetics/129.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loo S, Rine J. Silencing and heritable domains of gene expression. Annu Rev Biol Dev. 1995;11:519–548. doi: 10.1146/annurev.cb.11.110195.002511. [DOI] [PubMed] [Google Scholar]
- 51.Lorenz M C, Muir R S, Lim E, McElver J, Weber S C, Heitman J. Gene disruption with PCR products in Saccharomyces cerevisiae. Gene. 1995;158:113–117. doi: 10.1016/0378-1119(95)00144-u. [DOI] [PubMed] [Google Scholar]
- 52.Micklem G, Rowley A, Harwood J, Nasmyth K, Diffley J F. Yeast origin recognition complex is involved in DNA replication and transcriptional silencing. Nature. 1993;366:87–89. doi: 10.1038/366087a0. [DOI] [PubMed] [Google Scholar]
- 53.Miller A M, Nasmyth K A. Role of DNA replication in the repression of silent mating type loci in yeast. Nature. 1984;312:247–251. doi: 10.1038/312247a0. [DOI] [PubMed] [Google Scholar]
- 54.Monson E K, deBruin D, Zakian V A. The yeast Cac1 protein is required for the stable inheritance of transcriptionally repressed chromatin at telomeres. Proc Natl Acad Sci USA. 1997;94:13081–13086. doi: 10.1073/pnas.94.24.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mullen J R, Kayne P S, Moerschell R P, Tsunasawa S, Gribskov M, Colavito-Shepanski M, Grunstein M, Sherman F, Sternglanz R. Identification and characterization of genes and mutants for an N-terminal acetyltransferase from yeast. EMBO J. 1989;8:2067–2075. doi: 10.1002/j.1460-2075.1989.tb03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMG I(Y) by CBP turns off IFNB expression by disrupting the enhanceosome. Mol Cell. 1998;2:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- 57.Navas T A, Zhou Z, Elledge S J. DNA polymerase epsilon links the DNA replication machinery to the S phase checkpoint. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 58.Ono B, Ishii N, Fujino S, Aoyama I. Role of hydrosulfide ions in methylmercury resistance in Saccharomyces cerevisiae. Appl Environ Microbiol. 1991;57:3183–3186. doi: 10.1128/aem.57.11.3183-3186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petes T D, Botstein D. Simple Mendelian inheritance of the reiterated ribosomal DNA of yeast. Proc Natl Acad Sci USA. 1977;74:5091–5095. doi: 10.1073/pnas.74.11.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Philippsen P, Thomas M, Kramer R A, Davis R W. Unique arrangement of coding sequences for 5S, 5.8S, 18S, and 25S ribosomal RNA in Saccharomyces cerevisiae as determined by R-loop and hybridization analysis. J Mol Biol. 1978;123:387–404. doi: 10.1016/0022-2836(78)90086-4. [DOI] [PubMed] [Google Scholar]
- 61.Pillus L, Rine J. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell. 1989;59:637–647. doi: 10.1016/0092-8674(89)90009-3. [DOI] [PubMed] [Google Scholar]
- 62.Qian Z, Huang H, Hong J Y, Burck C L, Johnston S D, Berman J, Carol A, Liebman S W. Yeast Ty1 retrotransposition is stimulated by a synergistic interaction between mutations in chromatin assembly factor I and histone regulatory proteins. Mol Cell Biol. 1998;18:4783–4792. doi: 10.1128/mcb.18.8.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rose M D, Winston F, Heiter P. Methods in yeast genetics. A laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 64.Rundlett S E, Carmen A A, Kobayashi R, Bavykin S, Turner B M, Grunstein M. HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc Natl Acad Sci USA. 1996;93:14503–14508. doi: 10.1073/pnas.93.25.14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rundlett S E, Carmen A A, Suka N, Turner B M, Grunstein M. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature. 1998;392:831–835. doi: 10.1038/33952. [DOI] [PubMed] [Google Scholar]
- 66.Sakaguchi K, Herrera J E, Saito S I, Miki T, Bustin M, Vassilev A, Anderson C W, Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sherwood P W, Tsang S V, Osley M A. Characterization of HIR1 and HIR2, two genes required for regulation of histone gene transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:28–38. doi: 10.1128/mcb.13.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67a.Shore, D. Personal communication.
- 68.Skryabin K G, Eldarov M A, Larionov V L, Bayev A A, Klootwijk J, de Regt V C H F, Veldman G M, Planta R J, Georgiev O I, Hadjiolov A A. Structure and function of the nontranscribed spacer regions of yeast rDNA. Nucleic Acids Res. 1984;12:2955–2968. doi: 10.1093/nar/12.6.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smeal T, Claus J, Kennedy B, Cole F, Guarente L. Loss of transcriptional silencing causes sterility in old mother cells of S. cerevisiae. Cell. 1996;84:633–642. doi: 10.1016/s0092-8674(00)81038-7. [DOI] [PubMed] [Google Scholar]
- 70.Smith J S, Boeke J D. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- 71.Smith J S, Brachmann C B, Pillus L, Boeke J D. Distribution of a limited Sir2 protein pool regulates the strength of yeast rDNA silencing and is modulated by Sir4p. Genetics. 1998;149:1205–1219. doi: 10.1093/genetics/149.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication. Cell. 1989;58:15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- 73.Spector M S, Raff A, DeSilva H, Lee K, Osley M A. Hir1p and Hir2p function as transcriptional corepressors to regulate histone gene transcription in the Saccharomyces cerevisiae cell cycle. Mol Cell Biol. 1997;17:545–552. doi: 10.1128/mcb.17.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Szostak J W, Wu R. Insertion of a genetic marker into the ribosomal DNA of yeast. Plasmid. 1979;2:536–554. doi: 10.1016/0147-619x(79)90053-2. [DOI] [PubMed] [Google Scholar]
- 75.Tang H Y, Cai M. The EH-domain-containing protein Pan1 is required for normal organization of the actin cytoskeleton in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4897–4914. doi: 10.1128/mcb.16.9.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Triolo T, Sternglanz R. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature. 1996;381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- 77.Vannier D, Balderes D, Shore D. Evidence that the transcriptional regulators SIN3 and RPD3, and a novel gene (SDS3) with similar functions, are involved in transcriptional silencing in S. cerevisiae. Genetics. 1996;144:1343–1353. doi: 10.1093/genetics/144.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vidal M, Gaber R F. RPD3 encodes a second factor required to achieve maximum positive and negative transcriptional states in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:6317–6327. doi: 10.1128/mcb.11.12.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z, Wu X, Friedberg E C. DNA repair synthesis during base excision repair in vitro is catalyzed by DNA polymerase epsilon and is influenced by DNA polymerases alpha and delta in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:1051–1058. doi: 10.1128/mcb.13.2.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wellinger R J, Wolf A J, Zakian V A. Saccharomyces telomeres acquire single-strand TG1–3 tails late in S phase. Cell. 1993;72:51–60. doi: 10.1016/0092-8674(93)90049-v. [DOI] [PubMed] [Google Scholar]
- 82.Wiley E A, Zakian V A. Extra telomeres, but not internal tracts of telomeric DNA, reduce transcriptional repression at Saccharomyces telomeres. Genetics. 1995;139:67–79. doi: 10.1093/genetics/139.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 1997;11:748–760. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Sun Z W, Iratni R, Erdjument-Bromage H, Tempst P, Hampsey M, Reinberg D. SAP30, a novel protein conserved between human and yeast, is a component of a histone deacetylase complex. Mol Cell. 1998;1:1021–1031. doi: 10.1016/s1097-2765(00)80102-1. [DOI] [PubMed] [Google Scholar]