Highlights
-
•
The semiological hallmark of anti-LGI-1 antibody encephalitis is 'FBDS'.
-
•
These seizures are not always dystonic in nature and sometimes of a mixed phenotype.
-
•
The case of a patient with faciobrachial tonic-myoclonic seizures is presented.
-
•
The phenotypes include tonic, clonic, dystonic and myoclonic +/- plus features.
-
•
‘Faciobrachial motor seizures’ is a more apt description for these seizures.
Keywords: Anti-LGI1 encephalitis, Faciobrachial seizures, Electroencephalography
Abstract
Autoimmune encephalitis associated with antibodies against leucine-rich glioma inactivated protein (LGI1) is classically associated with brief, recurrent, contractions of facial and upper limb muscles, typically on the same side. Commonly described as ‘faciobrachial dystonic seizures’ (FBDS), these seizures have become the semiological hallmark of anti-LGI1 encephalitis. However, the facial and upper limb contractions observed in patients with anti-LGI1 encephalitis associated seizures are not always dystonic in nature. Here, we briefly highlight the case of a patient who was admitted to our institution with faciobrachial tonic-myoclonic seizures to emphasize the fact that faciobrachial seizures in anti-LGI1 encephalitis are not always dystonic. We also review the literature on the semiology of these seizures in patients diagnosed with anti-LGI1 encephalitis and propose a more apt description for this phenomenon. Our case as well as the literature highlights that in anti-LGI1 encephalitis the typical seizure semiology of faciobrachial distribution includes tonic, clonic, dystonic, and myoclonic activity in isolation or combination with or without plus features. Given that accurate labelling of clinical phenomenology enables a better understanding of the underlying epileptic networks and precise diagnosis, we would suggest a more inclusive term ‘faciobrachial motor seizures’ instead of ‘faciobrachial dystonic seizures’ to describe the typical seizure semiology of anti-LGI1 encephalitis. Based on the presence or absence of specific clinical features, these seizures can be further sub-classified as focal aware faciobrachial motor seizures, focal impaired awareness faciobrachial motor seizures or focal faciobrachial motor plus seizures (aware or impaired awareness).
Introduction
Autoimmune encephalitis associated with antibodies against leucine-rich glioma inactivated protein (LGI1) is classically associated with brief, recurrent, contractions of facial and upper limb muscles typically on the same side. Initially described as seizure-like episodes [1], early debate focussed on whether the manifestation was a seizure or a movement disorder [2]. These events were first described as faciobrachial dystonic seizures (FBDS) by Irani et al in 2011 in their seminal publication and it remains the most commonly used terminology to describe typical seizures in anti-LGI1 encephalitis [3].
Since the original description, FBDS has become the semiological hallmark of anti-LGI1 encephalitis. However, the facial and upper limb contractions observed in patients with anti-LGI1 encephalitis associated seizures are not always dystonic in nature. The semiologic descriptions of these seizures have been variably described as tonic, clonic, myoclonic, dystonic and motor spasms in the literature (Table 1). Prior to the key publication by Irani et al. [3], another group of researchers described the semiology of tonic seizures with faciobrachial and lower limb distribution in anti-LGI1 encephalitis [4]. Despite this variability, this seizure type is almost always referred to as dystonic in the literature.
Table 1.
Summary of findings of the literature review.
| Study and Year | Number of patients | FBS topography | FBS seizures as described in the publication by the authors | Plus features | Interpretation of FBS subtype | Ictal EEG Change | Ictal EEG Pattern |
|---|---|---|---|---|---|---|---|
| Ahn, 2014 | 1 | Face, limbs | Tonic/dystonic seizure involving face and limbs | NA | Mixed tonic-dystonic | Yes | Spike-and-wave burst |
| Beimer, 2017 | 1 | Face | Right-hand manual automatisms, an unusual facial expression or dystonic facial contraction, tachycardia and outbursts of nonsensical ictal speech | Manual automatisms, tachycardia, ictal speech | Dystonic | Yes | Rhythmic delta activity |
| Casault, 2015 | 1 | Face, upper limb, lower limb | History of spasms affecting face and bilateral limbs with predominance for the right side, occurring nearly hourly, associated with postictal confusion. Observed to have spontaneous jerks, occurring numerous times per hour, characterised by contraction of right face with flexion of ipsilateral shoulder, arm, wrist and hip with extension of the right knee and plantar flexion right foot lasting 0.5–2 seconds | NA | Clonic | No | NA |
| Celliers, 2016 | 1 | Face, arm | Brief dystonic jerks of left arm and face | NA | Dystonic | No | NA |
| Chen, 2017 | 4 FBS only 8 FBS plus |
NR | In patients with FBS only, patients reportedly showed “typical symptoms” with short episodes, frequent attacks and in one case postural change was a trigger. In the group with FBS plus, vast majority had automatisms (such as hand groping, soliloquizing and oral-automatisms). One patient had dystonic posturing plus subsequent hand and arm elevation. Other types of epileptic seizures occurred before or after FBS in two patients. |
Automatisms or other types of epileptic seizures | Dystonic | Yes (in group with plus features only) | NA |
| d’Orsi, 2018 | 1 | Face, upper limb | Sudden, short, frequent contractions of the upper limb and the left hemiface. The EEG/EMG pattern resembling an asymmetric tonic spasm was always followed by oral and gestural automatisms with dystonic posturing of the upper limbs. | Automatisms | Mixed tonic-dystonic | Yes | Focal contralateral EEG wave (fronto-central) preceding muscle activity |
| Duncan, 2014 | 1 | Face, upper limb | History of violent muscle jerks involving the right side of his body and face that impaired his gait and balance, occurring many times throughout the day. Observed to have myoclonus involving the right side of his face and right upper extremity which were associated with loss of awareness and dystonic posturing of the right arm. | NA | Mixed myoclonic-dystonic | No | NA |
| Gravier, 2019 | 1 | Upper limb, face, sometimes leg | Right side dystonic posturing involving the upper limb, the face and sometimes the leg followed by speech arrest but without loss of consciousness. The events lasted less than 10 seconds. | NA | Dystonic | Yes | Left temporal seizure concomitant with FBS |
| Irani, 2011 | 29 | Arm, face, leg, trunk | Images show ipsilateral facial grimacing and dystonic arm posturing. The authors report that the events are very brief (<3 seconds), very frequent, involve the arm, commonly the ipsilateral face, leg and sometimes trunk. Ictal vocalisation was present in 24%. Ictal loss of awareness was present in a minority of seizures in 66% of patients. Auditory stimuli and high emotions were reported to be triggers, in 28% of patients. | Vocalisation | Dystonic | Yes (in some) | 2–4 Hz left fronto-temporal spike-wave activity (in one patient) |
| Irani, 2013 | 10 | Arm, face, leg | The motor component was always dystonic and involved the arm both proximally and distally. Ipsilateral face involvement was commonly present and leg involvement was seen in almost half cases. Events were frequent and typically brief, but lasted between 10–30 seconds in four patients. Triggers included heightened emotion, movement and noise. An aura comprising of sensory symptoms and auditory hallucination was seen in some patients. Automatisms were present in three cases. Speech arrest, agitation, fear and tearfulness were seen following the motor event in some patients. | Automatisms Aura |
Predominantly dystonic; with clonic components in 2 patients | Yes (in 3 patients) | Focal slowing during seizure in two patients; the third patient had rhythmic 4–5 Hz left-hemispheric discharges associated with disorientation and automatisms |
| Li, 2016 | 7 | Face, upper limb | Described in four patients as sudden, short, and predominantly tonic contractions of the upper limbs. The end of the movement was accompanied by a dystonic posturing and ipsilateral facial grimacing, lasting 1–2 seconds. One case was preceded by an autonomic aura with palpitations lasting 5 seconds before the onset of FBS. | Autonomic aura | Mixed tonic-dystonic | Yes (in 1 patient) | Diffuse voltage depression with ‘palmodic aura’ |
| Rizzi, 2019 | 1 | Arm, leg, face | History of brief episodes lasting a few seconds of involuntary movement and tingling of the right hand followed by contraction of the right arm, leg, mouth deviation and occasionally LOC and falling. Observed to have frequent “jerks” (facial grimace with winking and mouth deviation to one side and rapid abduction of right shoulder) | Sensory symptoms | Tonic | Yes (in some) | Spike-wave bursts |
| Schmerler, 2016 | 1 | Face, arm | Brief (5–10 s) jaw-pulling and arm flexion episodes associated with altered awareness. | NA | Tonic | No | NA |
| Simabukuro, 2016 | 2 | Face, arm | Short, unilateral jerking movements affecting the right arm and face in one patient. These events lasted a few seconds in duration and were frequent. Focal seizures characterised by brisk and sudden shock-like jerks of the left upper limb in another patient. The image demonstrates dystonic arm posturing and ipsilateral face grimacing in both patients |
NA | Mixed myoclonic-dystonic | Yes (in 1 patient) | Electrodecremental pattern |
| Steriade, 2016 | 5 | Face, arm, leg | The most frequent electroclinical event comprised of sudden tonic spasm of the face, arm and/or leg. | NA | Tonic | Yes (in a minority of seizures, and typically during longer duration events) | Electrodecremental pattern |
| Sweeney, 2017 | 1 | Face, arm | History of involuntary, recurrent, episodic contractions of the left face and right hand. Observed to have episodic left face tightening and grimacing along with bilateral arm flexing and hand tightening. | NA | Tonic | No | NA |
| Wennberg, 2018 | 4 | Arm, face, leg | Patient 2 reported to have tonic flexion spasms, involving sudden involuntary flexion at the hips and knees often with abduction of the shoulders. She occasionally experienced an aura of bilateral feet parasthesiae lasting one second. A majority of her events consisted of just the bilateral flexion spasms, whereas a minority (~30%) started as flexion spasms and ended as lateralised ‘FBDS’ or, least frequently (~10%), consisted of ‘typical FBDS’ | Sensory symptoms | Predominantly tonic | Yes | Frontal infraslow activity Electrodecremental pattern |
| Yu, 2016 | 2 | Face, arm | Case 1 described as initially having a numb and itchy feeling in chest, left shoulder and left face, which typically lasted for several seconds and occurred several times daily. The symptoms progressed to include muscle tension in the left arm, which lasted for several seconds. The patient also experienced occasional muscle contortion and the tongue lolled out. | Sensory symptoms | Tonic | No | NA |
| Yoo, 2014 | 1 | Face, hand | History of confusion and twitching of the arm and face. Family reported facial contortions and simultaneous hand dystonic movements lasting under 2 seconds and happening many times a day. Observed to have frequent, though very brief (0.5–2 seconds) facial grimaces, (right > left), sometimes with subtle right-hand movements (mostly wrist flexion). | NA | Mixed tonic-dystonic | No | NA |
| Navarro, 2016 | 22 | Upper limb, face, lower limb | Tonic-dystonic seizures consisted of sudden, short and predominant distal contraction of the upper limb of approximately one second duration. In some patients, the hemiface and lower limb were involved. The end of the movement was accompanied by a dystonic posture, with the fingers of the hand moving apart and a few brief automatisms | Automatisms | Mixed tonic-dystonic | Yes (in patients with isolated tonic-dystonic seizures) | Focal, frontal contralateral EEG wave. Bilateral changes seen in patients with bilateral asynchronous tonic-dystonic seizures. |
| Andrade, 2011 | 3 | Face, arm, leg | Strong, massive movements (of the face, shoulders, arms, or legs) which were slightly slower than typical myoclonus. Consciousness was preserved during the movements. | NA | Tonic | Yes (longer events) | Electrodecremental pattern |
| Boesebeck, 2013 | 1 | Face, upper limb | Right shoulder abduction and elbow flexion with dystonic posture of right fingers, followed by dystonic movement of right cheek and tongue abduction to the right | NA | Dystonic | No | NA |
Plus features - refers to presence of additional clinical findings in association with faciobrachial seizures such as automatisms, dysphasia, vocalization etc. NR – not reported. NA – not applicable. LOC – loss of consciousness. FBS – faciobrachial seizures. FBDS – Faciobrachial dystonic seizures. Full list of references for studies summarised in Table 1 can be found in Supplementary References List (Supplementary data 2).
To place these semiologic variations into clinical context, it would be useful to study the characteristic phenotypes. Tonic activity is defined as sustained increase in muscle contraction that may last a few seconds to minutes. It differs from myoclonic seizures characterized by sudden, brief (typically < 100 msec) involuntary, single or multiple contractions of muscle(s) or muscle group(s) and clonic (regular, repetitive jerking involving the same muscle groups) activity. Both clonic and myoclonic activity can arise from the primary motor cortex or the premotor cortex. Furthermore, clonic activity may also arise from the supplementary sensorimotor area whereas myoclonic activity may arise from the primary somatosensory area [5]. Tonic activity can result in sustained posturing [5], [6]. Dystonic activity is characterized by sustained contractions of agonist–antagonist muscle pairs producing athetoid or twisting movements which may result in abnormal posturing [6]. Tonic activity can be difficult to distinguish from dystonic activity but the presence of a rotational component can be a helpful distinguishing feature [7]. Whereas focal tonic seizures are thought to reflect the involvement of the frontal lobe, particularly peri-rolandic and mesial frontal regions [8], metabolic studies indicate that dystonic posturing in seizures may reflect the involvement of the putamen, as well as, extratemporal cortical areas such as the insula, inferior and superior frontal gyri, anterior cingulate gyri and parietal areas [9]. Functional neuroimaging studies of patients with FBDS have also demonstrated altered glucose metabolism in the basal ganglia in some cases [3].
Here, we briefly highlight the case of a patient who was admitted to our institution with faciobrachial tonic-myoclonic seizures to emphasize the fact that faciobrachial seizures in anti-LGI1 encephalitis are not always dystonic. We also review the literature on the semiology of these seizures in patients diagnosed with anti-LGI1 encephalitis and propose a more apt description for this phenomenon.
Materials and methods
We obtained approval from the Human Research Ethics Committee at Monash Health for the publication of clinical data.
To investigate the semiologic variability of FBDS, we conducted a review of the literature on the semiology and ictal EEG features of anti-LGI1 encephalitis associated seizures using Pubmed (1966 to October 2020), Medline (1964 to October 2020) and PsycInfo (1967 to October 2020). Using Boolean operators and MESH terms where appropriate, we combined the following search terms: LGI1 antibody encephalitis, faciobrachial, seizure, clinical, electroclinical, semiology and electroencephalography. We only included publications that described the semiology of anti-LGI1 encephalitis associated seizures with a faciobrachial distribution. Review articles, non-English language publications and unclear literature were excluded.
Case summary
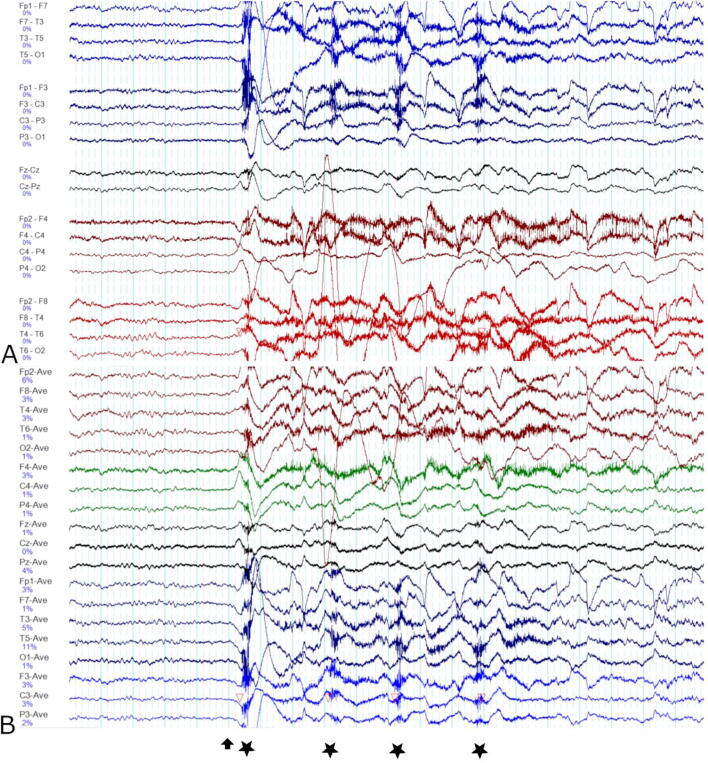
A 73-year-old woman, with no apparent risk factors for epilepsy, was admitted to our institution in the setting of recurrent ‘twitching’, confusion and agitation. Investigations were notable for the finding of mild hyponatremia (Na 133 mmol/L), normal cerebrospinal fluid (CSF) cell count and biochemistry and normal brain MRI. Continuous video EEG monitoring captured brief, recurrent, and frequent episodes of predominantly myoclonic contraction of the left upper limb (sudden, brief, involuntary contractions), with ipsilateral tonic contraction (upper and lower facial tonic spasm) of the face (Video 1). These events were associated with preserved awareness and could last between 0.5 to 10 seconds in duration. These events were observed in sleep as well as wakefulness. Additional neurologic symptoms such as automatisms or sensory symptoms were not present. The pattern of involvement was always unilateral (almost exclusively left-sided). Dystonic seizures in the faciobrachial distribution were also seen occasionally in the same patient (Video 2). The interictal EEG demonstrated intermittent right frontotemporal slowing. The ictal EEG demonstrated an electrodecremental pattern (Fig. 1). Serum and CSF autoimmune encephalitis screen returned positive for anti-LGI1 antibodies. She made a good recovery with immunotherapy (intravenous immunoglobulin and rituximab) and antiseizure medications (phenytoin and lacosamide).
Fig. 1.
Ictal EEG pattern of faciobrachial motor seizures in our patient. The ‘double banana’ (bipolar) montage is shown in Image A. The common average reference montage is shown in Image B. Electrodecremental pattern is noted immediately before the initial myoclonic contraction of the left upper limb (marked with an arrow). Tonic contraction of the left hemiface occurs concurrently. The tonic hemiface contraction and ipsilateral upper limb myoclonus (marked with a star) are brief and recur four times in quick succession before the faciobrachial motor seizure terminates. During the muscular contractions, the background EEG is obscured by EMG and lead movement artefact. A 20 second window is shown. The sensitivity and filter settings are as follows: sensitivity 10 uV/mm, notch filter at 50 Hz, low pass filter at 70 Hz and high pass filter at 0.5 Hz.
Results
After removing duplicate entries, screening the abstract, reviewing the full text of identified citations and screening the references list of included publications, we identified 35 relevant publications (Supplementary Fig. 1 and Supplementary References List Supplementary data 2). Table 1 summarises the key findings from our literature review and only includes publications that describe the semiology of these seizures.
Discussion
This case highlights several key points regarding anti-LGI1 encephalitis associated seizures with faciobrachial distribution. These seizures are typically brief (lasting between 0.5 and 10 seconds in duration in our patient) very frequent, sometimes precede other clinical symptoms such as cognitive impairment and are highly responsive to immunotherapy (more so than antiseizure medication) [10]. Hyponatremia and falls are commonly associated with seizures in this patient group [10]. Other seizure types (e.g. focal onset non-motor seizures with impaired awareness and tonic-clonic seizures) can be concurrently present [11]. An ictal EEG correlate for faciobrachial seizures is only seen in approximately one-third of cases [12]. Reported ictal EEG patterns include: bursts of spike-and-wave activity, electrodecremental pattern, contralateral fronto-central slow wave and evolving rhythmic activity [3], [13], [14], [15].
Of particular importance, the episodes of facial and upper limb contraction are not always dystonic in nature, as alluded to by the term FBDS. In our patient, the movements were predominantly myoclonic (accompanied by ipsilateral tonic contraction of the face) rather than dystonic and the ictal EEG demonstrated an electrodecremental pattern which can be seen in tonic seizures and epileptic spasms (Video 1 and Fig. 1) [16]. The EEG finding of electrodecrement pattern has also been described in seizures of faciobrachial distribution in anti-LGI1 encephalitis (Table 1) [4]. It must be noted that a ‘mixed’ clinical phenotype, comprising of two different types of muscular movement (e.g. tonic-dystonic or myoclonic-dystonic) is not unique to our patient and is reported in more than one-third of all the cases published in the literature (Table 1). Of these mixed phenotypes, tonic-dystonic was the commonest phenotype [13], [14], [17], [18], [19], followed by myoclonic-dystonic subtype [20], [21].
As revealed by our literature search and summarized in the Table 1, patients with this condition usually experience brief (ranging from 0.5 to 10 seconds in duration), recurrent and frequent episodes of tonic, dystonic, clonic or myoclonic contractions, mostly affecting the face and upper limb, but sometimes also the lower limb and trunk [3], [22]. Although these contractions typically occur on the same side, bilateral and/or alternating patterns of involvement (side-to-side involvement) have been reported in the literature [3]. Loss of awareness may occur but is not always present [1]. These seizures can occur in sleep and wakefulness. Although faciobrachial motor seizures are usually spontaneous, they can also be triggered by movement, postural change, high emotion and noise in some patients [3], [22]. The end of the movement can be associated with dystonic posturing but this was not reported in all cases [13], [14]. Furthermore, patients with seizures of faciobrachial distribution sometimes experience additional, concurrent clinical symptoms or signs that have been labelled as "plus features". Plus features were reported in 10 out of 22 (45.5%) publications summarized in Table 1. Examples of plus features include automatisms, sensory symptoms, autonomic features, dysphasia and vocalization [14], [15], [22].
Although seizures in the faciobrachial distribution are characterized by dystonic contraction of the face and upper limb in some patients, it must be emphasized that other movements in the faciobrachial distribution are also reported in association with anti-LGI1 encephalitis. In the 22 studies summarized in Table 1, the tonic subtype is reported in seven publications (31.8%), the dystonic subtype in six publications (27.3%), the clonic subtype in one publication (4.5%) and the mixed subtype (comprising of a mixture of myoclonic, clonic, tonic and/or dystonic subtypes) in eight publications (36.4%). Plus features were reported in almost half the publications summaried in Table 1.
Despite the variability in semiology, all but three of these publications (86.4%) described these seizures as FBDS [4], [13], [17]. This term does not take into account the different seizure subtypes that are observed in patients with anti-LGI1 encephalitis associated seizures of faciobrachial distribution. Patients who do not present with the ‘typical’ dystonic subtype may not be immediately considered to have anti-LGI1 encephalitis potentially resulting in delayed diagnosis. This is of particular relevance given that delayed diagnosis and treatment is associated with poor prognostic outcomes in these patients [23].
Conclusion
Our case as well as the literature highlights that in anti-LGI1 encephalitis the typical seizure semiology of faciobrachial distribution includes tonic, clonic, dystonic, and myoclonic activity in isolation or combination with or without "plus" features (referring to the presence of additional neurologic symptoms or signs such as automatisms or sensory symptoms). Given that accurate labelling of clinical phenomenology enables a better understanding of the underlying epileptic networks and precise diagnosis, we suggest that the more accurate and inclusive term ‘faciobrachial motor seizures’ be used instead of ‘faciobrachial dystonic seizures’ to describe the typical seizure semiology of anti-LGI1 encephalitis. Based on the presence or absence of specific clinical features, these seizures can be further sub-classified as focal aware faciobrachial motor seizures, focal impaired awareness faciobrachial motor seizures or focal faciobrachial motor plus seizures (aware or impaired awareness).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebr.2021.100476.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Tonic-myoclonic seizures in the faciobrachial distribution. Brief myoclonic contraction of the left upper limb and tonic contraction of the ipsilateral hemiface occurring in drowsiness. The upper limb contraction results in flexion of the forearm and to a lesser extent the upper arm. The movements recur four times over a 10 second period. There is no dystonic posturing, plus features or loss of awareness associated with the episode.
Dystonic seizure in the faciobrachial distribution. Contraction of the left face and ipsilateral upper limb with dystonic posturing of the left hand at the end of the movement is noted in the same patient. The seizure occurs in wakefulness and there is no associated loss of awareness or plus features associated with the episode.
References
- 1.Irani S.R., Buckley C., Vincent A., Cockerell O.C., Rudge P., Johnson M.R., et al. Immunotherapy-responsive seizure-like episodes with potassium channel antibodies. Neurology. 2008;71(20):1647–1648. doi: 10.1212/01.wnl.0000326572.93762.51. [DOI] [PubMed] [Google Scholar]
- 2.Striano P. Faciobrachial dystonic attacks: seizures or movement disorder? Ann Neurol. 2011;70(1):179–180. doi: 10.1002/ana.22470. author reply 180. [DOI] [PubMed] [Google Scholar]
- 3.Irani S.R., Michell A.W., Lang B., Pettingill P., Waters P., Johnson M.R., et al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69(5):892–900. doi: 10.1002/ana.22307. [DOI] [PubMed] [Google Scholar]
- 4.Andrade D.M., Tai P., Dalmau J., Wennberg R. Tonic seizures: a diagnostic clue of anti-LGI1 encephalitis? Neurology. 2011;76(15):1355–1357. doi: 10.1212/WNL.0b013e3182152808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foldvary-Schaefer N., Unnwongse K. Localizing and lateralizing features of auras and seizures. Epilepsy Behav. 2011;20(2):160–166. doi: 10.1016/j.yebeh.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Fisher R.S., Cross J.H., D'Souza C., et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58(4):531–542. doi: 10.1111/epi.13671. [DOI] [PubMed] [Google Scholar]
- 7.Bleasel A., Kotagal P., Kankirawatana P., Rybicki L. Lateralizing value and semiology of ictal limb posturing and version in temporal lobe and extratemporal epilepsy. Epilepsia. 1997;38(2):168–174. doi: 10.1111/j.1528-1157.1997.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 8.Werhahn K.J., Noachtar S., Arnold S., Pfander M., Henkel A., Winkler P.A., et al. Tonic seizures: their significance for lateralization and frequency in different focal epileptic syndromes. Epilepsia. 2000;41(9):1153–1161. doi: 10.1111/j.1528-1157.2000.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 9.Rusu V., Chassoux F., Landre E., Bouilleret V., Nataf F., Devaux B.C., et al. Dystonic posturing in seizures of mesial temporal origin: electroclinical and metabolic patterns. Neurology. 2005;65(10):1612–1619. doi: 10.1212/01.wnl.0000184510.44808.50. [DOI] [PubMed] [Google Scholar]
- 10.Irani S.R., Bien C.G., Lang B. Autoimmune epilepsies. Curr Opin Neurol. 2011;24(2):146–153. doi: 10.1097/WCO.0b013e3283446f05. [DOI] [PubMed] [Google Scholar]
- 11.van Sonderen A., Thijs R.D., Coenders E.C., Jiskoot L.C., Sanchez E., de Bruijn M.A.A.M., et al. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology. 2016;87(14):1449–1456. doi: 10.1212/WNL.0000000000003173. [DOI] [PubMed] [Google Scholar]
- 12.Thompson J, Bi M, Murchison AG, et al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain 2018;141(2):348–356. [DOI] [PMC free article] [PubMed]
- 13.Navarro V., Kas A., Apartis E., Chami L., Rogemond V., Levy P., et al. Motor cortex and hippocampus are the two main cortical targets in LGI1-antibody encephalitis. Brain. 2016;139(4):1079–1093. doi: 10.1093/brain/aww012. [DOI] [PubMed] [Google Scholar]
- 14.Li Z., Cui T., Shi W., Wang Q. Clinical analysis of leucine-rich glioma inactivated-1 protein antibody associated with limbic encephalitis onset with seizures. Medicine. 2016;95(28) doi: 10.1097/MD.0000000000004244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beimer N.J., Selwa L.M. Seizure semiology of anti-LGI1 antibody encephalitis. Epileptic Disorders. 2017;19(4):461–464. doi: 10.1684/epd.2017.0936. [DOI] [PubMed] [Google Scholar]
- 16.Sazgar M., Young M.G. Absolute Epilepsy and EEG Rotation Review: Essentials for Trainees. Springer International Publishing; Cham: 2019. pp. 163–182. [DOI] [Google Scholar]
- 17.Ahn S.-W., Kim J.-M., Kim J.-E., Lee S.-T., Ahn D.-W., Sung J.-J. Development of LGI1 antibody encephalitis after treatment of lung cancer. Can J Neurol Sci. 2014;41(5):669–671. doi: 10.1017/cjn.2014.17. [DOI] [PubMed] [Google Scholar]
- 18.d'Orsi G., Martino T., Lalla A., Claudio M.T.D., Carapelle E., Avolio C. Faciobrachial dystonic seizures expressed as epileptic spasms, followed by focal seizures in anti-LGI1 encephalitis: a video-polygraphic study. Epileptic Disorders. 2018;20(6):525–529. doi: 10.1684/epd.2018.1010. [DOI] [PubMed] [Google Scholar]
- 19.Yoo J.Y., Hirsch L.J. Limbic encephalitis associated with anti-voltage-gated potassium channel complex antibodies mimicking Creutzfeldt-Jakob disease. JAMA Neurol. 2014;71(1):79–82. doi: 10.1001/jamaneurol.2013.5179. [DOI] [PubMed] [Google Scholar]
- 20.Duncan M., Cholfin J., Restrepo L. Clinical reasoning: A 72-year-old man with rapid cognitive decline and unilateral muscle jerks. Neurology. 2014;82(22):e194–e197. doi: 10.1212/WNL.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 21.Simabukuro M.M., Nóbrega P.R., Pitombeira M., Cavalcante W.C.P., Grativvol R.S., Pinto L.F., et al. The importance of recognizing faciobrachial dystonic seizures in rapidly progressive dementias. Dementia Neuropsychol. 2016;10(4):351–357. doi: 10.1590/s1980-5764-2016dn1004016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irani SR, Stagg CJ, Schott JM, et al. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013;136(10):3151–3162. [DOI] [PubMed]
- 23.Broadley J., Seneviratne U., Beech P., Buzzard K., Butzkueven H., O'Brien T., et al. Prognosticating autoimmune encephalitis: a systematic review. J Autoimmun. 2019;96:24–34. doi: 10.1016/j.jaut.2018.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tonic-myoclonic seizures in the faciobrachial distribution. Brief myoclonic contraction of the left upper limb and tonic contraction of the ipsilateral hemiface occurring in drowsiness. The upper limb contraction results in flexion of the forearm and to a lesser extent the upper arm. The movements recur four times over a 10 second period. There is no dystonic posturing, plus features or loss of awareness associated with the episode.
Dystonic seizure in the faciobrachial distribution. Contraction of the left face and ipsilateral upper limb with dystonic posturing of the left hand at the end of the movement is noted in the same patient. The seizure occurs in wakefulness and there is no associated loss of awareness or plus features associated with the episode.