Abstract
Osteoarthritis (OA) is a debilitating disease generally of old age manifested as degeneration of articular cartilage. With no definitive treatment available, ongoing research aims at early detection and use specific noninvasive imaging markers to monitor therapeutic efficacy of disease modifying osteoarthritic drug (DMOAD) to reverse or/and arrest the disease process. Articular cartilage degradation and loss, as well as bone remodelling, are typical biomarkers of OA. As a result, an ideal imaging technique for early detection of OA is required, which must be sensitive to both soft tissue and bone health. PET/MRI is emerging as an imaging tool which can be used to study the underlying pathogenesis of OA as it enables us to assess molecular activity with PET markers while also linking them to qualitative and quantitative MRI indices of OA. In this regard recent work was exploring the role of 18F–Na Fluoride which is a marker of bone remodelling together with MRI in early detection of OA on simultaneous PET/MRI. In this article we intend to present different patterns of OA (mild to severe stages of OA) that we had observed on 18F-Sodium Fluoride (18F–NaF) PET/MRI.
Keywords: Osteoarthritis (OA), PET/MRI, 18F–NaF, Standard uptake value (SUV)
1. Introduction
Osteoarthritis (OA) is a debilitating disease generally of old age manifested as degeneration of articular cartilage.1 Magnetic resonance imaging (MRI) has evolved as a valuable diagnostic tool for OA research because it can evaluate molecular level pathology of whole joint thematically that are not visible on radiography by using traditional MRI as well as advanced methods, i.e., compositional MRI but functional bone imaging poses a hurdle.2 Positron emission tomography (PET) has an idiosyncratic ability to provide quantitative data on physiological as well as molecular activity, which can often harbinger structural and biochemical alterations but it requires the assistance of higher-resolution morphological information in order to locate these physiologic events.3 As a result, it's plausible to conceptualise that the integrated PET/MRI system allows comprehensive imaging of the entire joint, has a lot of potential for studying complex disease processes in OA4 and to harness its potential to study efficacy of disease modifying osteoarthritic drug (DMOAD). In this regard recent work was exploring the role of 18F–Na Fluoride which is a marker of bone remodelling5 together with MRI in early detection of OA on simultaneous PET/MRI. The images were acquired on simultaneous PET/MRI Biograph mMR (Siemens, Erlangen, Germany) by using mMR body matrix coil. Acquisition was done after 45 minutes of intravenous injection of 18F–NaF 185–370 MBq (5–10 mCi) for 40 minutes while several MRI sequences were carried out over continuous PET acquisition. The images of MRI were analysed by using MOAKS criteria6 and modified outerbridge criteria specifically for cartilage grading.7
2. 18F–NaF PET/MRI findings observed in:-
2.1. Mild osteoarthritic knee
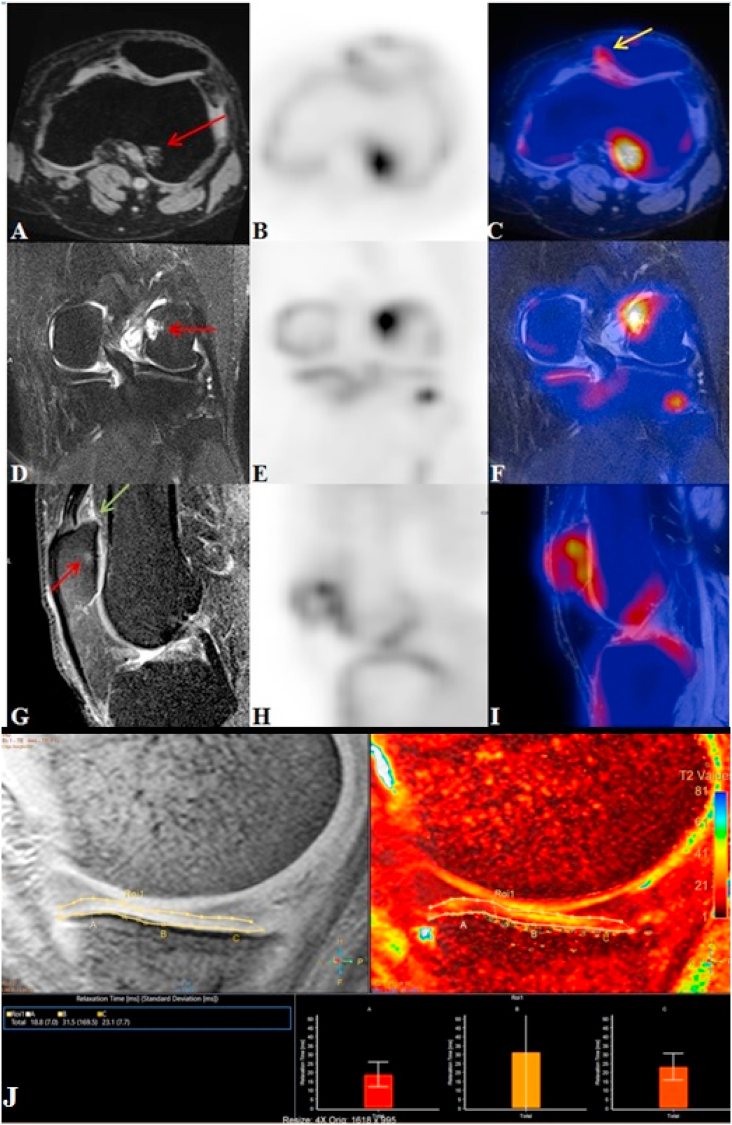
A 36 year old man came for screening standing knee radiograph. He had no pain in the knees and KL grade was 0 for bilateral knee. But on the PET/MRI, we found that in the right knee all the regions had grade 1 articular changes with corresponding subchondral uptake: 2.63(1.80–3.35) SUVmean, 3.29 (2.32–4.42) SUVmax and T2∗ values 30.25 (21.9–40.0). In whole knee joint, there was only one bone marrow lesion (BML) with grade 1 on MRI and NaF uptake values were 1.32 SUVmean and 1.41 SUVmax. There was grade 1 osteophytes in 3 regions with 1.43 (1.08–2.07) SUVmean and 1.62 (1.62–2.21) SUVmax and 2 regions had high uptake of NaF i.e. 2.95 (2.62–3.37) SUVmean and 3.97 (3.71–4.23) SUVmax without any structural changes. Also MRI showed mucoid degeneration with intact fibres seen along the ACL and grade 1 changes in posterior horn of medial meniscus (Fig. 1).
Fig. 1.
PET/MRI of right knee of 36 year male having KL grade 0 showing high tracer uptake in medial central femur region (B) without any morphological changes in corresponding sagittal T2 SPIR image (A) also spatially correlated on fused PET/MRI image (C). Grade 1 osteophyte in lateral posterior tibial region in sagittal T1 TSE image (D) showing increased tracer uptake in PET (E) better appreciated on fused PET/MRI image (F). The T2 ∗ relaxometry cartigram overlayed on sagittal T2 SPIR image (G) showed mild changes in the cartilage of medial aspect of tibia (Green arrow for osteophytes and yellow arrow for region having intense tracer uptake without morphological changes). Physiological uptake was noted at tendon insertion sites on patella & tibial tuberosity.
2.2. Moderate osteoarthritic knee
PET/MRI of 42 year male who had KL grade 2 for left knee showed grade 2 cartilage changes in 8 regions with corresponding subchondral uptake of 1.68 (1.06–3.23) SUVmean, 2.21 (1.13–5.04) SUVmax and 30.9 (29.4–44.7) T2∗ values, grade 1 cartilage changes in 3 regions with 1.65 (1.05–2.14) SUVmean, 3.23 (2.29–4.02) SUVmax and 24.4 (18.8–31.5) T2∗ values; and 3 regions had grade 0 cartilage changes with 1.38 (1.34–1.47) SUVmean and 2.09 (1.7–2.57) SUVmax and 26.8 (25.7–28.8) T2∗ values. One region had grade 1 BML with 1.21 SUVmean and 1.57 SUVmax, grade 3 BML in 1 region with 3.78 SUVmean and 8.24 SUVmax and two grade 2 BMLs with 4.26 (3.40–5.12) SUVmean and 6.88 (5.17–8.59) SUVmax. Eight regions had grade 1 osteophytes with 1.53 (1.05–1.82) SUVmean and 1.96 (1.16–2.74) SUVmax. In one region there was intense NaF uptake i.e. 2.38 SUVmean and 3.93 SUVmax without any morphological changes on MRI. The reduced joint space, subchondral marrow edema in the intercondylar notch and mucoid degeneration with ganglion cyst formation along the ACL was seen (Fig. 2).
Fig. 2.
PET/MRI of left knee of 42 year male having KL grade 2 showed high tracer uptake in medial patellar region (B) without any structural abnormality in axial reconstructed 3D MEDIC image (A) which was spatially localized on fused PET/MRI image (C). Lateral posterior femur region had grade 3 BML in axial image (A) and coronal T2 SPIR image (D) with intense tracer uptake on PET (B & E) which was spatially correlated on fused PET/MRI image (C & F). The medial region of patella also had grade 1 BML and osteophyte in T1 sagittal T2 SPIR image (G) with high tracer uptake (H) which was spatially localized on fused PET/MRI image (I). The T2 ∗ relaxometry cartigram overlayed on sagittal T1 TSE image (J) showed moderate changes in the cartilage of in medial tibial region (Green arrow for osteophytes, red arrow for BML and yellow arrow for region having intense tracer uptake without morphological changes).
2.3. Severe osteoarthritic knee
The PET/MRI of left knee of 63 year old female having KL grade 3 showed grade 4 cartilage changes in 2 regions with 3.33 (2.88–3.79) SUVmean, 5.86 (5.18–6.55) SUVmax and 47.3 T2∗ value; 1 region had grade 3 changes with 3.1 SUVmean, 4.89 SUVmax and 34.4 T2∗ value; 5 regions had grade 2 cartilage changes with 1.21(1.09–1.96) SUVmean, 1.6 (1.2–2.04) SUVmax and 31.78 (24.3–36.8) T2∗ values; six regions had grade 1 cartilage changes with 1.14 (0.96–1.61) SUVmean, 1.69 (1.44–2.18) SUVmax and 26.98 (31.4–23.3) T2∗ values. Four regions had grade 1 BML with 2.57 (1.69–3.68) SUVmean and 3.25 (1.9–4.68) SUVmax. Four regions had grade 3 osteophytes with 3.37 (2.38–5.11) SUVmean and 5.17 (3.33–7.87) SUVmax, four regions had grade 2 osteophytes with 1.14 (0.98–1.44) SUVmean and 1.34 (1.15–1.59) SUVmax, two regions had grade 1 osteophytes with 1.5 (1.1–2.01) SUVmean and 1.9 (1.2–2.65) SUVmax. The knee joint also had grade 1 synovitis. There were degenerative changes in both menisci (Fig. 3).
Fig. 3.
PET/MRI of left knee of 63 year old female with KL grade 3 revealed grade 3 ostephytes in medial patellar and medial trochlear region in reconstructed 3D MEDIC sagittal (A) & axial (D) images showing high tracer uptake (B & E) better localized on fused PET/MRI images (C & F). Grade 2 osteophyte in medial posterior tibial region (A) shows high uptake on PET (B) spatially correlated on fused PET/MRI (C). Grade 1 BML in medial patellar region (D) also shows increased tracer uptake on PET (E) better visualised on fused PET/MRI (F). The lateral posterior femur had grade 3 BML in sagittal econstructed 3D MEDIC image (G) having increased tracer uptake (H) which was better localized on PET/MRI fused images (I). The T2 ∗ relaxometry cartigram overlayed on sagittal T2 SPIR image (J) showed increased relaxation times in the cartilage of lateral central and posterior femur (Green arrow for osteophytes and red arrow for BML).
3. Discussion-
Recently PET/MRI is emerging as an imaging tool which can be used to study the underlying pathogenesis of OA as it enables us to assess spatially correlated OA's molecular activity with markers of PET and linking them to qualitative and quantitative MRI indices of OA bone diseases with lesser radiation dose.8 There are drastic alterations in subchondral bone composition as well as structural organisation in case of OA joints, causing harmful effects to the overlaying articular cartilage. BMLs, microdamage and bone cysts are histopathological attributes of osteoarthritic subchondral bone. In a study, it was observed that the phenotype of subchondral trabecular bone beneath healthy cartilage was identical to that of severely injured cartilage, implying that subchondral bone changes in early OA could precede cartilage degradation.9 Uptake of [18F]NaF indicates remodelling as well as bone metabolism, and is correlated with mineral apposition rate measured by bone histomorphometry. Early OA has been linked to alterations in remodelling of subchondral bone as well as vascularisation; as subchondral bone is metabolically active. [18F] NaF uptake is predominantly concentrated at areas of newly mineralizing bone, where hydroxyl ions is interchanged with fluoride ions in newly formed hydroxyapaptite crystals on the surface of bone.10
In our cases we found intense uptake in some regions without any structural abnormalities which was also observed by Kogan et al.11 This symbolizes that metabolic abnormalities that looked to be normal on MRI can be detected with the help of PET/MRI. The pattern of tracer uptake in BML was grade 3-2 > grade 1. In case of osteophytes the uptake pattern was grade 3 > grade 2-1 which is in line with results of Kogan et al., who also observed higher uptake in the subchondral lesions, however 49% grade 1 osteophytes didn't show intense uptake in their study.11 The pattern of NaF uptake in articular cartilage was: grade 4- 3 > grade 2-1 > grade 0 and pattern of relaxometry values: grade 4 > grade 3 > grade 2 > grade 1 > grade 0. Higher relaxation time in cartilage implying deterioration in combination with increased bone turnover was discovered by researchers.12 In this study we found that simultaneous evaluation of morphological MRI, compositional MRI and PET is feasible leading to detection of metabolic abnormalities with or without subchondral bone changes on MRI that can help in early detection of diseases and in research of OA pathophysiology and clinical trials of DMOAD.
Declaration of competing interest
No Conflict of Interest.
Acknowledgement
The authors acknowledge contribution of Prerana Rana, MSc (Nuclear Medicine), Apollo Hospitals Education & Research Foundation, Indraprastha Apollo Hospitals, New Delhi for support in preparing the manuscript and data evaluation.
The authors also acknowledge President AHERF (Apollo Hospitals Educational and Research Foundation) for funding to carry out the work under Faculty Development Program (FDP).
Contributor Information
Amarnath Jena, Email: drjena2002@gmail.com.
Nidhi Goyal, Email: nidhi.i.goyal@gmail.com.
Raju Vaishya, Email: raju.vaishya@gmail.com.
References
- 1.Karsdal M.A., Ladel C., Siebuhr A.S. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage. 2016 Dec;24(12):2013–2021. doi: 10.1016/j.joca.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Binks D.A., Hodgson R.J., Ries M.E. Quantitative parametric MRI of articular cartilage: a review of progress and open challenges. Br J Radiol. 2013;86(1023):20120163. doi: 10.1259/bjr.20120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogan F., Broski S.M., Yoon D., Gold G.E. Applications of PETMRI in musculoskeletal disease. J Magn Reson Imag. 2018;48(1):27–47. doi: 10.1002/jmri.26183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torigian D.A., Zaidi H., Kwee T.C. PET/MR imaging: technical aspects and potential clinical applications. Radiology. 2013;267(1):26–44. doi: 10.1148/radiol.13121038. [DOI] [PubMed] [Google Scholar]
- 5.Blau M., Nagler W., Bender M.A. Fluorine-18: a new isotope for bone scanning. J Nucl Med. 1962;3:332–334. [PubMed] [Google Scholar]
- 6.Hunter D.J., Guermazi A., Lo G.H. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score) Osteoarthritis Cartilage. 2011 Aug;19(8):990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paunipagar B.K., Rasalkar D.D. Imaging of articular cartilage. Indian J Radiol Imag. 2014 Jul;24(3):237–248. doi: 10.4103/0971-3026.137028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menendez M.I., Wei L., Knopp M. Correlation between 18F-FDG and F-18 sodium fluoride (NaF) PET/MRI and gross morphology of the knee in a canine model of osteoarthritis. Osteoarthritis Cartilage. 2016;24(1):S63–S534. doi: 10.1016/j.joca.2016.01.548. [DOI] [Google Scholar]
- 9.Zhu X., Chan Y.T., Yung P.S.H., Tuan R.S., Jiang Y. Subchondral Bone Remodeling: a therapeutic target for osteoarthritis. Front Cell Dev Biol. 2021;8 doi: 10.3389/fcell.2020.607764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins L., MacKay J., Haddock B. Assessment of quantitative [18F]Sodium fluoride PET measures of knee subchondral bone perfusion and mineralization in osteoarthritic and healthy subjects. Osteoarthritis Cartilage. 2021;29:849–858. doi: 10.1016/j.joca.2021.02.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kogan F., Fan A., McWalter E., Oei E.H.G., Quon A., Gold G.E. PET/MR imaging of metabolic activity in osteoarthritis: a feasibility study. J Magn Reson Imag. 2017;45(6):1736–1745. doi: 10.1002/jmri.25529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savic D., Pedoia V., Seo Y. Imaging bone–cartilage interactions in osteoarthritis using [18F]-NaF PET-MRI. Mol Imag. 2016;15:1–12. doi: 10.1177/1536012116683597. [DOI] [PMC free article] [PubMed] [Google Scholar]