Abstract
With the US Food and Drug Administration (FDA) approval of four CD19- and one BCMA-targeted chimeric antigen receptor (CAR) therapy for B cell malignancies, CAR T cell therapy has finally reached the status of a medicinal product. The successful manufacturing of autologous CAR T cell products is a key requirement for this promising treatment modality. By analyzing the composition of 214 apheresis products from 210 subjects across eight disease indications, we found that high CD14+ cell content poses a challenge for manufacturing CAR T cells, especially in patients with non-Hodgkin’s lymphoma and multiple myeloma caused by the non-specific phagocytosis of the magnetic beads used to activate CD3+ T cells. We demonstrated that monocyte depletion via rapid plastic surface adhesion significantly reduces the CD14+ monocyte content in the apheresis products and simultaneously boosts the CD3+ content. We established a 40% CD14+ threshold for the stratification of apheresis products across nine clinical trials and demonstrated the effectiveness of this procedure by comparing manufacturing runs in two phase 1 clinical trials. Our study suggests that CD14+ content should be monitored in apheresis products, and that the manufacturing of CAR T cells should incorporate a step that lessens the CD14+ cell content in apheresis products containing more than 40% to maximize the production success.
Keywords: CAR T cell, cGMP, clinical grade, large-scale manufacturing, monocyte depletion
Graphical abstract
By analyzing 214 apheresis products and CAR T cell manufacturing runs across multiple disease indications, IR and colleagues have established a threshold of 40% CD14+ cell content as the trigger for the introduction of a rapid plastic adherence monocyte depletion step to enable a 97% manufacturing success rate.
Introduction
The approval by the US Food and Drug Administration (FDA) of four CD19-targeted chimeric antigen receptor (CAR) T cell therapies for the treatment of pediatric acute lymphoblastic leukemia (ALL) and relapsed or refractory large B cell and mantle cell lymphoma, as well as one B cell maturation antigen (BCMA)-targeted CAR T cell therapy for the treatment of multiple myeloma (MM), marked a new era for the treatment of cancer.1,2 The integral component for the success of this promising therapy relies on reproducible CAR T cell manufacturing. The manufacturing process for autologous CAR T cells is complex and includes T cell isolation, activation, transduction, expansion, formulation, and cryopreservation.3 More than 10 years ago, we established a robust modular CAR T cell production platform starting from patient leukapheresis.4 Our manufacturing process is initiated by the selection and activation of CD3+ T cells from the apheresis product, followed by transduction using retroviral vector stocks and expansion of transduced cells on the WAVE/Xuri bioreactor. By using this platform, we not only supported multiple CAR T cell clinical trials targeting CD195, 6, 7, 8 but also obtained the FDA breakthrough designation for the treatment of adult patients with ALL in 20145,8 and also expanded our spectrum to other cancer types and tumor antigens, such as prostate-specific membrane antigen (PSMA),9 Muc 16,10 mesothelin,11 and BCMA.12,13
Among the CD19-targeted CAR T clinical trials for patients with adult ALL,8 pediatric ALL (pALL),14 chronic lymphocytic leukemia (CLL),7,15 and non-Hodgkin’s lymphoma (NHL),16 we encountered more challenges in patients with NHL. Prior to the initiation of the trial in patients with NHL, we manufactured more than a hundred CAR T cells products with a success rate of 93.2% for patients with ALL and CLL. However, for patients with NHL, the manufacturing success rate was only 75%, suggesting that the higher failure rate could be related to patients’ disease and/or specific disease pre-treatments (Figure S1). By characterizing the profiles of 214 collected apheresis products from 2015 to 2019, including those from patients with NHL, we found that apheresis products from subjects with various diseases exhibited different levels of CD14+ monocytes.
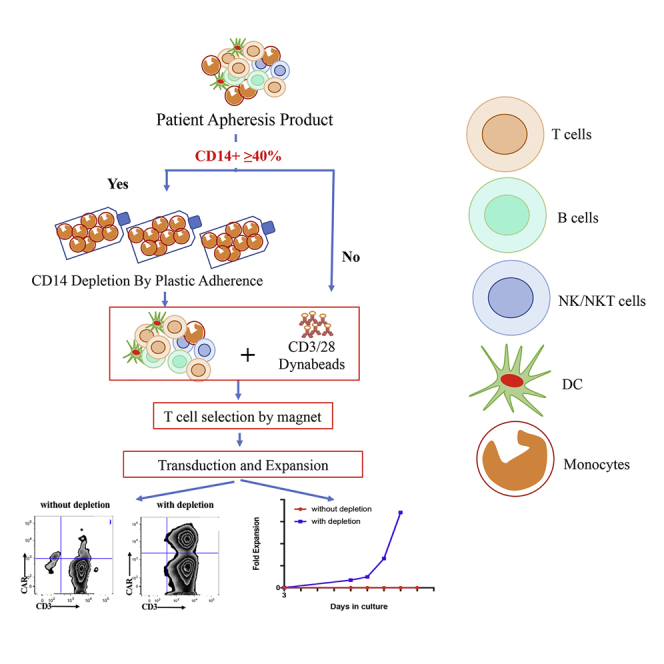
Monocytes are characterized by their phenotypic expression of CD14. They play an important role in host defense as circulating monocytes and differentiate into tissue macrophages and dendritic cells that display potent antigen-presenting capability. Monocytes are professional phagocytes that represent one of the innate defense mechanisms of the host immune system.17 As we observed at the initiation of the manufacturing process that some cells adhered to the cell culture bags and that they were engulfing magnetic beads, we hypothesized that monocytes present in the apheresis products could pose a challenge to our cGMP large-scale CAR T cell manufacturing platform starting from unmanipulated apheresis products.4 We indeed established that these cells were CD14+, and there was a threshold in CD14+ cell content that should not be exceeded in order to ensure manufacturing success.
By stratifying apheresis products based on their monocyte content, we established a 40% cutoff in CD14+ monocytes content as a threshold for the introduction of a plastic adhesion monocyte depletion prior to selection and activation with magnetic beads. By using this modified procedure, we successfully manufactured 42 of 43 CAR T cell products using apheresis products containing ≥40% monocytes. To validate our threshold of 40% monocyte content as the stratification criteria, we compared CAR T cell manufacturing runs of all subjects enrolled in both our CD19 CAR clinical protocol for CLL patients (ClinicalTrials.gov: NCT03085173) and BCMA CAR clinical protocol for MM patients (ClinicalTrials.gov: NCT03070327). We found that the short 2-h plastic adhesion step is sufficient to deplete adequate subsets and numbers of CD14+ cells, and allows successful manufacturing of CAR T cells for subjects with high CD14+ content. Since we have implemented this procedure, we have manufactured a total of 201 CAR T products with a success rate of 99.0%, among which 43 of the manufacturing runs required the CD14 depletion step (Figure S1).
Results
High CD14+ monocyte content in apheresis product poses a manufacturing challenge for CAR T cells
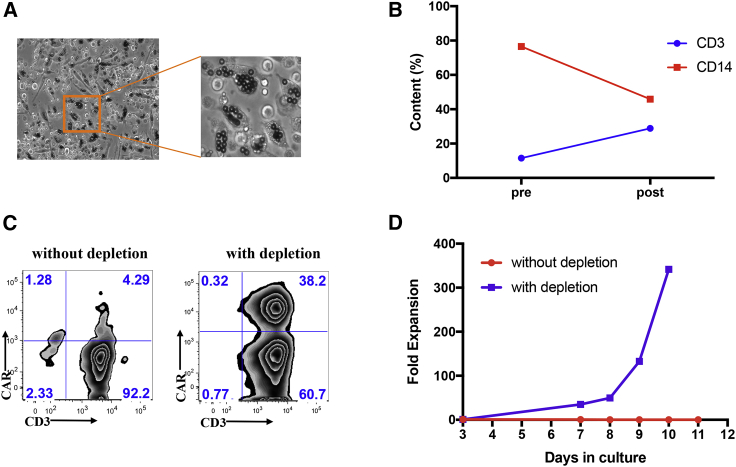
We recently reported the long-term follow-up of adult patients with relapsed ALL who received an infusion of autologous CD19 CAR T cell therapy (ClinicalTrials.gov: NCT01044069) at our center.8 In this particular phase I clinical trial for ALL, we manufactured CD19 CAR T cell products with a success rate of over 97%. We encountered our first manufacturing challenge with an apheresis product containing 76.5% CD14+ monocytes, whereas monocytes in the circulating peripheral blood of healthy individuals typically make up 10%–30% of the total mononuclear cell population.18, 19, 20 During the manufacturing run for this ALL patient, we observed that a large number of cells adhered to the cell bag, and we observed a pronounced engulfment of magnetic beads by these adherent cells after the selection and activation steps (Figure 1A). The purity of the CD3+ cells in the CAR T cell product was 96.5%, while the transduction efficiency was merely 4.3% (Figure 1C), and the T cells did not expand (Figure 1D). Although the manufacturing process was initiated with 345 × 106 CD3+ T cells, by the end of production on day 11, the total number of viable cells was 12.3 × 106, with only 14.8% CD3+ T cells (Figure 1D). We hypothesized that the unspecific sequestration of magnetic beads by monocytes resulted in inadequate selection of CD3+ cells leading to the low purity of CD3+ T cells, as well as insufficient activation of the T cells and subsequent suboptimal transduction and expansion.21
Figure 1.
Removal of CD14+ cells by plastic adhesion rescues large-scale clinical CAR T manufacturing for an ALL patient (ClinicalTrials.gov: NCT01044069)
(A) Microscopic observation of Dynabeads uptaken by cells adhering to PermaLife bag 3 days after selection and activation during CAR T cell manufacturing run (without CD14+ cell removal by plastic adhesion). (B) Percentage of CD3+ (blue) and CD14+ (red) contents prior to and after two-step, 1.5 h and overnight, plastic adhesion monocyte depletion procedure. (C) Transduction efficiency of CAR T cells for manufacturing runs without (left) or with (right) monocyte depletion procedure. (D) Cumulative fold expansion of total viable cells for the large-scale manufacturing runs with (blue) or without (red) monocyte depletion step since the day of transduction.
Based on these observations, we reasoned that the removal of CD14+ monocytes by plastic surface adhesion before T cell selection and activation could restore the suitability of the apheresis product for our manufacturing procedure. To this end, using the same apheresis product from the ALL patient containing 76.5% CD14+ cells, we conducted a manufacturing run whereby 350 × 106 CD3+ T cells from the thawed and washed apheresis product were incubated in 10 T175 flasks for 1.5 h, then selected and activated by magnetic beads with a cell-to-bead ratio of 1:1, followed by a second overnight plastic surface adhesion in a new set of T175 flasks, and reactivation of the cells in suspension at a bead-to-cell ratio of 1:10 the following day. We found that the plastic adhesion steps led to a significant reduction of CD14+ cells from 76.5% to 45.9% and a corresponding increase of CD3+ T cells from 11.5% to 28.9% (Figure 1B). On day 3, the activated cells were transduced with the same CD19-CAR retroviral vector stocks in Retronectin-coated cell bags using the same vector concentration. We achieved a transduction efficiency of 38.2%, a drastic increase from 4.3% in the previous production run conducted without monocyte depletion (Figure 1C). Moreover, the cells expanded 350-fold (Figure 1D) with 98.9% CD3+ purity (Figure 1C) at the end of the production by day 10. These results strongly supported our hypothesis that high level of CD14+ monocytes in the apheresis products posed a significant manufacturing challenge.
CD14+ cell content in apheresis products from patients with various diseases
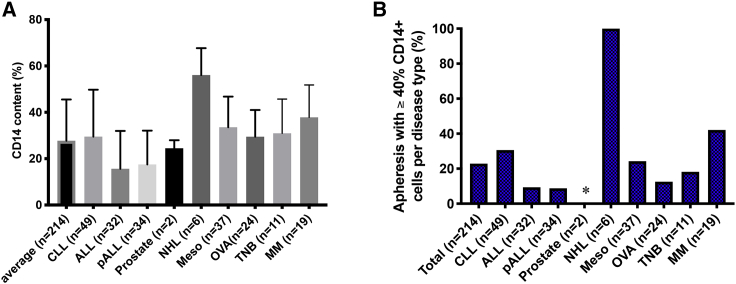
Based on the stark difference of outcome between production runs conducted with or without CD14 depletion using the same apheresis product, we started monitoring the CD14+ monocyte content in all of the following incoming patients. Interestingly, analysis of 214 apheresis products with various disease indications, including CLL (ClinicalTrials.gov: NCT00466531, NCT01416974, and NCT03085173), adult ALL (ClinicalTrials.gov: NCT01044069), pALL gov:NCT01860937), prostate cancer (ClinicalTrials.gov: NCT01140373), NHL (ClinicalTrials.gov: NCT01840566), mesothelioma (Meso) (ClinicalTrials.gov: NCT02414269), ovarian cancer (OVA) (ClinicalTrials.gov: NCT02498912), triple-negative breast (TNB) cancer (ClinicalTrials.gov: NCT02792114), and MM (ClinicalTrials.gov: NCT03070327), revealed that disease indication and potentially also prior treatments have a notable impact on the CD14+ monocyte apheresis content. The average CD14+ monocyte content in the above patient pool (n = 214) was 27.7% (range 0%–76.7%) (Figure 2A). The average percentages of CD14+ monocytes in the apheresis products collected from adult ALL (n = 32) and pALL patients (n = 34) were 15.7% and 17.5%, respectively, which is within the physiological level of 10%–30%,18,20 but significantly lower than the average in our patient pool. Only two subjects with prostate cancer were enrolled since we started monitoring CD14+ cell content. Although the CD14+ cell contents were 22% and 27%, similar to the general population average, we would need to accumulate data on more patients with this disease to determine whether this observation would hold. The average CD14+ content in apheresis products from patients with CLL (n = 49), Meso (n = 37), OVA (n = 24), TNB (n = 11), and MM (n = 19) were 29.5% (range 0%–76.7%), 33.6% (range 1.6%–67.4%), 37.9% (range 10%–56.4%), 31% (range 14%–66.8%), and 37.9% (range 18.9%–68.5%), respectively, and did not differ significantly from our monitored patient pool. In contrast, the average CD14+ content in apheresis products from patients with NHL was 56.1% (n = 6, range 43.5%–71.7%), which was statistically higher than in the patient pool average (Figure 2A; Table S1). These observations suggest that the content in CD14+ monocytes could be influenced by the disease indication itself and/or by the various treatments used in the frontline setting in the different disease indications.
Figure 2.
CD14 content in apheresis products from multiple disease indications
(A) Total number of apheresis collections was 214, including n = 49 from patients with chronic lympholytic leukemia (CLL), n = 32 from adult patients with acute lymphoblastic leukemia (ALL), n = 34 from pediatric patients with ALL (pALL), n = 2 from patients with prostate cancer, n = 6 from patients with non-Hodgkin’s lymphoma (NHL), n = 37 from patients with mesothelioma (Meso), n = 24 from patients with ovarian cancer (OVA), n = 11 from patients with triple-negative breast (TNB) cancer, and n = 19 from patients with multiple myeloma (MM). Average CD14 content + SEM in apheresis products from different disease backgrounds are shown and compared with the average of the total patient pool using unpaired Student’s t test. The p values of the different disease types versus patient pool are as follows: CLL versus average, p = 0.54; ALL versus average, p = 0.0004; pALL versus average, p = 0.0016; prostate cancer versus average, p = 0.80; NHL versus average, p = 0.0001; Meso versus average, p = 0.058; OVA versus average, p = 0.64; TNB cancer versus average, p = 0.56; and MM versus average, p = 0.017. The CD14 content in apheresis products collected from patients with different diseases are also compared with each other, and the p values are listed in Table S1. (B) Frequency of apheresis products containing ≥40% CD14+ monocytes according to disease indication.
Based on the successful manufacturing run yielded after reducing the content in CD14+ cells in the apheresis product from 76.5% to 45.9% following plastic adhesion (Figure 1B), we hypothesized that we could stratify the apheresis products into two categories by setting the threshold of CD14+ cells around 40% to trigger the implementation of the monocyte depletion procedure. Using 40% CD14+ monocytes as the threshold, we further analyzed the frequency of apheresis products with ≥40% of CD14+ monocytes based on disease backgrounds. 22.9% of our monitored apheresis product pool (49/214 products) had ≥40% CD14+ monocytes. Interestingly, only 9.4% adult ALL (3/32), 8.8% pALL (3/34), and 12.5% OVA (3/21) apheresis products had ≥40% of CD14+ monocytes, significantly lower than that of the monitored patient pool average. On the contrary, 100% of NHL (6/6) apheresis products collected since we started monitoring the percentage of CD14 contained ≥40% of CD14+ monocytes (Figure 2B). The second highest frequency (42.1%; 8/19) of apheresis products with ≥40% of CD14 monocytes was from patients with MM; the third highest (30.6%; 15/49) was in patients with CLL. As for apheresis products from patients with other disease indications, 24.3% (9/37) from Meso patients and 18.2% (2/11) from TNB cancer patients had ≥40% of CD14+ monocytes (Figure 2B). If we lowered the threshold to ≥30% of CD14+ cells, 62.2% of apheresis products from Meso patients (23/37), 63.2% of apheresis products from MM patients (12/19), and 57% of apheresis products from CLL patients (28/49) would have met the criteria. This analysis also indirectly informed our decision in setting the threshold at 40% CD14+ cells, which required fewer procedure deviations and limited further manipulation from our established manufacturing procedure while maintaining our success manufacturing rate above 97%.
A short-time plastic adhesion step is sufficient to remove CD14 monocytes
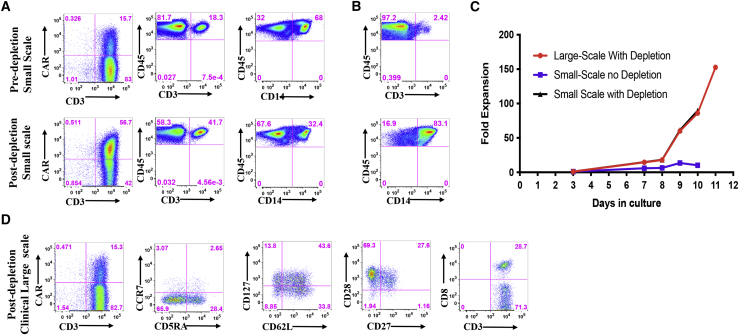
We next set out to test whether we can simplify the burdensome two-step plastic surface adhesion and next day re-stimulation procedure to a simpler one-step 2-h plastic adhesion. Apheresis products with ≥40% CD14+ monocytes were first incubated in a set of 10 T175 flasks in CO2 incubator for 2 h post wash. Subsequently, cells in suspension were selected and activated with magnetic beads and then manufactured per our validated procedure.4
The first clinical CAR T manufacturing run using this simple adhesion procedure was from an apheresis product with 68% CD14+ monocytes. We set up in parallel a small-scale manufacturing comparator arm with the same apheresis product. In the experiment at small scale in six-well plates, the CD14+ cell content dropped from 68% to 32.4%, and the CD3+ cell content increased from 18.3% to 41.7% as measured in the cells that remained in suspension post-adhesion (Figure 3A). Among the cells that adhered to the plates, only 2.4% were CD3+ and 83.1% were CD14+ high (Figure 3B). At small scale in six-well plate, the transduction efficiency went from 15.7% without depletion to 56.7% post-depletion (Figure 3A), and the low expansion of 10.2-fold without depletion went to 89.7-fold with depletion in 10 days (Figure 3C). For the clinical run at large scale, the transduction in bags post-monocyte depletion was only 15.3% (Figure 3D); however, the fold expansion was 86-fold on day 10 and went up to 153-fold on day 11 (Figure 3C). The end-of-process cells from the clinical arm contained 28.7% CD8+ T cells and an adequate level of effector memory and central memory T cells based on the frequency of CD45RA−, CD62L+, CD27+, CD28+, and CD127+ cells (Figure 3D).
Figure 3.
Comparison of CAR T cell expansion with or without a 2-h plastic adhesion monocyte depletion for an NHL patient
(A) Transduction efficiency and percentage of CD3+ and CD14+ cells in the apheresis product without (top row) and with 2-h plastic adhesion monocyte depletion procedure (bottom row). (B) Percentage of CD3+ (top row) and CD14+ (bottom row) cells that adhered to the plastic surface. (C) Cumulative fold expansion of total viable cells from the day of transduction (day 3) until the end of the production run (day 10 or 11). Small-scale manufacturing runs with (black) or without (blue) monocyte depletion (10-day run). Clinical large-scale manufacturing runs with (red) monocyte depletion (11-day run). (D) Expression of effector memory and central memory markers, CD45RA, CCR7, CD62L, CD127, CD27, and CD28, and distribution of CD8+ cells in CAR+ T cells were determined at the end of the production on day 11.
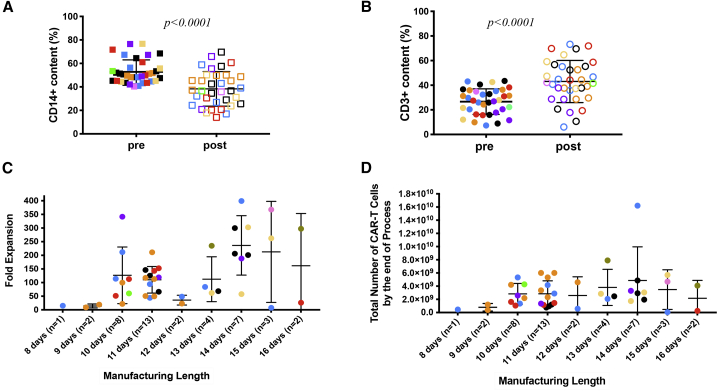
Since we started the 2-h plastic adhesion monocyte removal procedure for apheresis products with ≥40% CD14+ cell contents, we have encountered 43 such products with a CD14+ content ranging from 40.3% to 76.7%. We monitored the CD14+ and CD3+ cell contents before and after the adhesion step in the majority of the manufacturing runs (n = 34). Before the adhesion step, the average CD14+ cell content in these apheresis products was 54% (range 40.3%–76.7%), and the average CD14+ cell content dropped to 39% (range 16.4%–73.3%) after the monocyte depletion step (Figure 4A), while the average CD3+ cell content increased from 30.0% (range 7.3%–43.4%) to 44.0% (range 6%–73.2%) correspondingly (Figure 4B). The relative depletion in CD14+ and gain in CD3+ T cells post-adhesion step varied from one apheresis product to the other, for some of the products rather drastically and for others less seemingly (Figures 4A and 4B). The statistical analysis of these changes indicated that this simple adhesion step led to a significant change for both CD14+ and CD3+ cell contents of the apheresis products (Figures 4A and 4B). Among the 43 products, 42 CAR T cell manufacturing runs successfully yielded the required cell dose and met all release criteria with an average manufacturing length of 11.9 days (range 8–16 days) (Figure 4C), an average fold expansion from time of transduction to end of production of 134-fold (range 7.5–399-fold) (Figure 4C), and an average of 10.3 billion (range, 147 million to 56.2 billion) total viable cells (data not shown) and 3.1 billion end of process (EOP) CAR T cells (range, 55.7 million to 16.2 billion) (Figure 4D). All end-of-process products met our release criteria, with CD3+ content in the EOP CAR T cell products ranging from 95.9% to 100% (median 99.6%) and the transduction efficiency ranging from 10.3% to 62.2% (median 33.9%).
Figure 4.
Pre- and post-monocyte depletion percentage of CD14+ and CD3+ cells, fold expansion and total number of CAR T cells by the end of production runs following CD14+ cell removal
(A) A 2-h plastic adhesion monocyte depletion step significantly altered the percentage of CD14+ and CD3+ cells in apheresis products with ≥40% CD14+ monocytes. A total of 35 apheresis products with originally ≥40% CD14+ monocytes from different disease indications (CLL: ivory [ClinicalTrials.gov: NCT00466531] and orange [ClinicalTrials.gov: NCT03085173]; ALL: dark purple; pALL: green; NHL: red; meso: blue; OVA: tan; TNB: light purple; and MM: black) were monitored. Means ± SEM of the percentage of CD14+ cell content pre- and post-plastic adhesion procedure are shown and compared using Student’s t test, p < 0.0001. Solid squares represent % CD14 cells in suspension pre-attachment, and empty squares represent remaining % CD14 cells in suspension post-attachment. (B) Means ± SEM of the percentage of CD3+ cells pre- and post-plastic adhesion procedure in the same apheresis products as in (A) are shown and compared using Student’s t test, p < 0.0001. The solid circles represent the % CD3+ cells in suspension pre-attachment, and the empty circles represent the % CD3+ cells in suspension post-attachment. (C and D) Fold expansion (C) and total number of CAR T cells obtained at the end of each manufacturing process (D), plotted according to length of manufacturing for 42 manufacturing runs derived from apheresis products with initial CD14+ monocyte content ≥40% and that underwent 2-h plastic adherence step. Different colors represent different disease indications as specified in (A).
For the single failed run for a patient with ALL disease, we unexpectedly found that the CD14+ monocyte content insignificantly dropped from 58.2% to 56.7%, and the CD3+ cells decreased from 23.9% to 18.3% post-depletion. This observation was in contrast with the other products and may be patient specific. However, the outcome was in line with our predicated finding that high CD14+ monocyte content poses a manufacturing challenge. The 97.7% success rate (42/43) for manufacturing runs initiated from apheresis products containing ≥40% CD14+ monocytes supports our strategy to use 40% CD14+ monocyte content as the threshold to stratify apheresis products.
Case study of CAR T cell manufacturing runs for patients with same disease indication
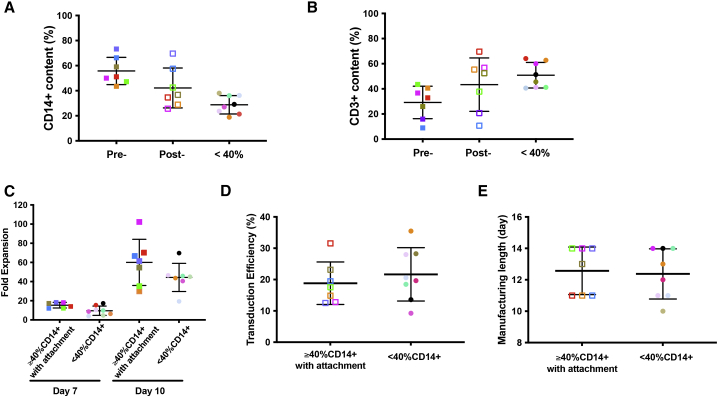
To further demonstrate that CD14+ monocyte removal from apheresis products with ≥40% CD14+ cell content can render the product as manageable as the apheresis product with an initial content ≤40% CD14+ cells, we analyzed productions run for all the patients enrolled in our MM phase 1 clinical trial (ClinicalTrials.gov: NCT03070327). A total of 15 patients were enrolled in this protocol, among which seven apheresis products contain ≥40% CD14+ cells and eight apheresis products contain <40% CD14+ cells. The average CD14+ cell contents of the seven apheresis products with ≥40% CD14+ prior to CD14+ cell depletion was 51.6% ± 8.3% versus 28.8% ± 7.3% for the eight apheresis products with <40% CD14+ cells. After the depletion, the average CD14+ content dropped from 51.6% to 42.2%, which was closer to the 28.8% average for those apheresis products with <40% CD14+ monocytes (Figure 5A). Correspondingly, the average CD3+ cell contents increased from 29.1% pre-depletion to 43.3% post-depletion, which was not significantly different from the 50.8% average CD3+ contents of those apheresis products with ≤40% CD14+ monocyte content (Figure 5B). At day 7 during production runs, the expansion of products initiated from apheresis products with ≥40% CD14+ cells and post-depletion appeared to be slightly higher than those started from apheresis products with <40% CD14+ cells. However, no significant difference was found by day 10 (Figure 5C). In addition, there was no statistically significant difference in either transduction efficiency (18.8% ± 6.8% for apheresis products with ≥40% CD14+ followed by monocyte depletion versus 21.7% ± 8.5% for apheresis products with initially <40% CD14+ monocyte) (Figure 5D), effector memory or central memory phenotype (Figure S2), or manufacturing length between these two groups (12.6 ± 0.57 days for apheresis products with ≥40% CD14+ followed by monocyte depletion step versus 12.4 ± 0.57 days for apheresis products with initially <40% CD14+ monocyte) (Figure 5E).
Figure 5.
Case study of CAR T cell manufacturing runs for patients with MM (ClinicalTrials.gov: NCT03070327; n = 15)
(A) Percentages of CD14+ cells in apheresis products with initially ≥40% CD14+ monocytes pre- (Pre-) and post- (Post-) 2-h plastic adhesion procedure (n = 7), in comparison with the CD14+ monocyte content in apheresis products with initially <40% CD14+ monocytes (<40%) (n = 8). Average CD14+ content by FACS ± SEM is shown for each group, and the groups were compared using Student’s t test. For pre-adhesion versus post-adhesion, p = 0.042; for post-adhesion versus product with initially <40% CD14+ monocytes, p = 0.052. (B) Percentages of CD3+ cells in apheresis products with initially ≥40% CD14+ monocytes before (Pre-) and after (Post-) 2-h plastic adhesion procedure (n = 7), in comparison with the CD3+ cell content of apheresis products with initially <40% CD14+ monocytes (<40%) (n = 8). Average CD3+ content by FACS ± SEM is shown for each group, and the groups were compared using Student’s t test. For pre-adhesion versus post-adhesion, p = 0.046; for post-adhesion versus product with <40% CD14+ monocytes, p = 0.39. (C) Comparison of cumulative fold expansion of total viable cells in CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+ monocytes, which underwent a 2-h plastic adhesion depletion step (n = 7, left group on days 7 and 10), and the manufacturing runs started with apheresis products containing <40% CD14+ monocytes (n = 8, right group on days 7 and 10). Average ± SEM is shown for each group at days 7 and 10 and compared using Student’s t test. On day 7, p = 0.02; at day 10, p = 0.17. (D) Comparison of transduction efficiency between CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+ monocytes, which underwent a 2-h plastic adhesion monocyte depletion step (n = 7) and the manufacturing runs started with apheresis products containing <40% CD14+ monocytes (n = 8). Average transduction efficiency by FACS ± SEM is shown for each group, and the groups were compared using Student’s t test, p = 0.49. (E) Comparison of manufacturing length between CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+, which underwent a 2-h plastic adhesion monocyte depletion step (n = 7) and the manufacturing runs started with apheresis products containing <40% CD14+ monocytes (n = 8). Average manufacturing length ± SEM is shown for each group, and the groups were compared using Student’s t test, p = 0.81. The average length of the runs started from apheresis products with ≥40% CD14+ and monocyte depletion step (12.6 ± 0.57 days) was comparable with that of runs started from apheresis products with <40% CD14+ monocytes (12.4 ± 0.57 days). Each solid-colored square represents an individual apheresis product with initially ≥40% CD14+ monocytes pre-depletion, and the same-colored empty square corresponds to the same product post-depletion. Each solid-colored circle represents an individual apheresis product with initially <40% CD14+ monocytes.
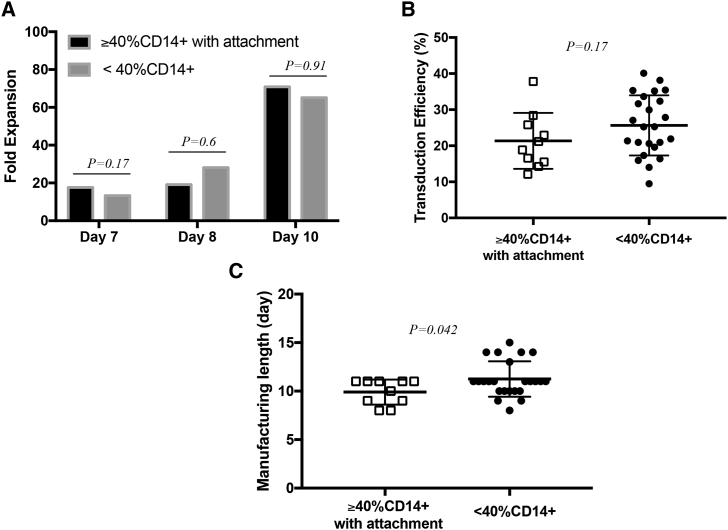
We further compared the manufacturing runs for all the patients with relapsed or refractory CLL enrolled in our phase 1 clinical trial of anti-CD19 “armored” CAR T cells (ClinicalTrials.gov: NCT03085173). A total of 34 CAR T cell manufacturing runs were performed for this protocol, of which 10 were initiated from apheresis products with ≥40% CD14+ monocytes and included the depletion step and 24 started from apheresis products with <40% CD14+ cells and went through our established manufacturing procedure.4 We found comparable levels of expansion (day 7: 17.6 ± 8.9-fold versus 13.3 ± 5.4-fold, p = 0.17; day 8: 19.2 ± 17.1-fold versus 28.2 ± 39-fold, p = 0.60; day 10: 70.9 ± 52.5-fold versus 65.1 ± 65.1-fold, p = 0.91 for CAR T cells derived from apheresis products with ≥40% CD14+ followed by monocyte depletion versus CAR T cells derived from apheresis products with initially <40% CD14+ monocyte, respectively) (Figure 6A) and transduction efficiency (21.4% ± 7.8% versus 25.6% ± 8.3%) for both groups (Figure 6B), similar to our findings in production runs derived from MM patients (Figure 5). In the case of patients with CLL, the average length of the runs started from apheresis products containing ≥40% CD14+ and that underwent monocyte depletion was significantly shorter (9.9 ± 0.41 days) than that of the runs started from apheresis products with <40% CD14+ monocytes (11.3 ± 0.37 days) (p = 0.042) (Figure 6C).
Figure 6.
Case study of CAR T cell manufacturing runs for patients with relapsed/refractory CLL (ClinicalTrials.gov: NCT03085173; n = 34)
(A) Comparison of cumulative fold expansion of total viable cells between CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+ monocytes that underwent a 2-h plastic adhesion monocyte depletion step (n = 10), and the manufacturing runs started with apheresis products containing initially <40% CD14+ monocytes (n = 24). Average fold expansion is shown and compared using Student’s t test. At day 7, p = 0.17; at day 8, p = 0.6; and at day 10, p = 0.91. (B) Comparison of transduction efficiency between CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+ monocytes that underwent a 2-h plastic adhesion monocyte depletion step (n = 10, open squares) and shown for each group, and the groups were compared using Student’s t test, p = 0.17. (C) Comparison of manufacturing length between CAR T cell manufacturing runs started with apheresis products containing initially ≥40% CD14+ monocytes that underwent a 2-h plastic adhesion monocyte depletion step (n = 10, open squares), and the manufacturing runs started with apheresis products containing initially <40% CD14+ monocytes (n=24, solid circles). Average manufacturing length ± SEM is shown for each group, and the groups were compared using Student’s t-test, p = 0.042.
These findings based on CAR T cell manufacturing runs of patients from the same disease indication further strengthened the validity of our hypothesis that apheresis products could be stratified based on a threshold of ≥40% CD14+ cell content. We also demonstrated that removal of CD14+ cells by a simple 2-h plastic surface adhesion from apheresis product with ≥40% CD14+ monocytes rendered these products suitable for large-scale cGMP CAR T cell manufacturing.
Discussion
Monocytes are professional phagocytes that play an important role in adaptive and innate immunity.17 Under conditions such as stress, inflammation,18,19 and cancer, myeloid-derived suppressor cells (MDSCs), which represent a pathologic state of activation of monocytes, develop the ability to inhibit T cell function and thus contribute to the pathogenesis of these diseases. They play a pro-tumorigenic role in solid tumors. Higher levels of MDSCs in lymphomas, MM, and leukemias have also been reported in multiple clinical studies.22 MDSCs are characterized by the ability to suppress both innate and adaptive immune responses mostly through the direct inhibition of T cell activation and expansion, including high level of arginase, inducible nitric oxidase, or reactive oxygen species production, as well as indoleamine 2,3-dioxygenase activity and prostaglandin E2.22
In our study, by analyzing 214 apheresis products collected from patients enrolled in clinical trials with different disease indications, we found that apheresis products collected from patients with NHL appear to have the highest frequency of CD14+ monocyte content ≥40%, and that the probability to get such an apheresis product is higher in patients with NHL and MM. On the other end, apheresis products collected from adult ALL and pALL patients appeared to have lower CD14+ content, and the probability of getting an apheresis product with a high CD14+ content is lower for these patients.
We showed in two small-scale experiments using apheresis products with ≥40% CD14+ cells from one patient with ALL and one patient with NHL that the cultures expanded poorly without CD14+ cell depletion (<10-fold), while upon CD14+ cell depletion the cultures achieved superior expansion (350- and 153-fold, respectively) comparable with that of CAR T cell products derived from aphereses initially containing <40% CD14+ cells. In both cases, CAR T cells could not be expanded in response to Dynabeads stimulation prior to the adherence depletion, which suggest that their activation function was impaired by the presence of CD14+ cells, likely because of the immunosuppressive functions of the CD14+ cells22 in addition to the unspecific sequestration of Dynabeads by the monocytes.
Other groups, such as Künkele et al.,23 have shown that most granulocyte colony stimulating factor (G-CSF) mobilized peripheral blood stem cell units from patients with neuroblastoma that contained 80%–90% monocytes (n = 6/8) can serve as starting material for CAR T cell manufacturing pending magnetic CD14 depletion to eliminate the growth-inhibiting monocytes before T cell activation. Stroncek et al.24,25 also reported similar challenges and remedies in the context of their CD19 and GD2 CAR T cell manufacturing runs.
More recently, it was also elegantly demonstrated by Noaks et al.26 that removal of monocytes from healthy donor leukapheresis products improved T cell activation, increased transduction efficiency, and promoted a more resting and naive phenotype in end-of-process CAR T cells. In addition, Boucher et al.27 have observed that gene transfer was lower in CAR T cells co-cultured with MDSCs. They also observed a reduction in total T cell numbers, in T cell activation, cytotoxic killing, and interferon γ (IFNγ) secretion,27 similarly to Braun et al.,28 who investigated the function of tumor-infiltrating T cells upon “panning” of monocytes. Another potential mechanism of inhibition could take place through the secretion of interleukin-10 by monocytes as described by Mielcarek et al.29 in G-CSF-treated donors. In our experience, we observed that the transduction efficiency in the case of the ALL patient at small scale went from 15.7% pre-depletion to 56.7% post-depletion; however, the transduction efficiency was only 15.3% with CD14+ depletion at large scale despite the fact that the depletion of the CD14+ cells was similar to the small scale (CD14+ cell content was reduced to 30.6% at large scale versus 32.4% at small scale). Because we did not perform the transduction at large scale without depletion because of the cost of the large-scale experiments, we do not know what the transduction would have been. In general, with apheresis products from healthy donors originally containing <40% CD14+ cells, we observe a 20%–50% reduction in transduction efficiency from small scale in tissue culture plates to large scale in bags (data not shown). Therefore, we would have anticipated an even lower transduction efficiency at large scale without monocyte depletion.
We also observed in a cohort of patients with MM (n = 15) that, upon removal of CD14+ cells from apheresis products ≥40% (n = 7), the CAR T cell products retained effector memory and central memory phenotype that are similar to the CAR T cell products generated from <40% of CD14+ cells (n = 8).
To date, 42 of the 43 manufacturing runs (97.7%) that were initiated with apheresis products containing ≥40% CD14+, and in which we included the monocyte adhesion step, successfully reached the required cell dose (range 1 × 106 to 60 × 106 CAR T cells/kg) and met all release criteria for infusion.5, 6, 7, 8, 9,14,16 The analysis of 35 of our 42 large-scale CAR T manufacturing runs initiated with apheresis products with high CD14+ contents showed that the monocyte depletion step could significantly bring down the level of CD14+ cells and restore the ability of the products to expand and reach the clinical dose.
The comparable characteristics of the manufacturing runs initiated with apheresis products containing ≥40% CD14+ monocytes followed by monocyte depletion to that of the runs initiated with apheresis products with <40% CD14+ monocytes in two clinical studies of patients with either MM (ClinicalTrials.gov: NCT03070327; n = 15) or CLL (ClinicalTrials.gov: NCT03085173; n = 34) further strengthen the threshold of 40% CD14+ that we set to stratify the incoming apheresis products into those that require monocyte depletion versus those that do not. Interestingly, we noted that the depletion step did not always bring down the CD14+ monocyte content lower than the threshold of 40%. However, this simple procedure was successful in 42 apheresis products, allowing an overall success manufacturing rate of 97%. We can either hypothesize that the monocytes that are more suppressive are more adherent and therefore better removed by the adherence depletion step and/or that the procedure sufficiently reduces the total number of CD14+ cells to a threshold that restores the ability of the Dynabeads to activate these products. Interestingly, in the recently published study by Noaks et al.,26 the authors demonstrate that a “stronger” T cell activation with, for example, TransAct beads instead of Dynabeads, can overcome the inhibitory effect of monocytes on transduction efficiency and expansion, at least in apheresis from healthy donors. This observation suggests that the strength of activation plays a role in overcoming monocyte inhibition and can be affected by the ratio of beads to monocytes. In addition, the seeding density for the plastic adherence depletion is based on 300 × 106 CD3+ cells seeded in 10 T175 flasks. Because there was no further adjustment of seeding density based on total cell number, it is possible that the variable level of CD14+ reduction is due to different total cell concentrations per square centimeter, thus affecting the surface available for optimal monocytes removal. There was only one product that did not expand to meet the cell dose requirement, for which we found a drop in CD3+ content and an insignificant removal of CD14+ monocytes, which could relate to other factors prior to collection, such as prior chemotherapy and disease stage. It is possible that elutriation24 or a more deliberate upfront CD4+ and CD8+ cell selection using CliniMACS device30 would be more effective for such apheresis products. Additionally, adherence depletion in flasks is not a closed system, and the manipulation of multiple flasks increases the risk for contamination and human error. It also does not allow the removal of all the CD14+ monocytes. Consequently, CD4+/CD8+ positive cell selection or CD14+ negative cell selection with microbeads is more suitable to overcome these challenges instead of adherence depletion. Indeed, Shah et al.30 also reported recently that monocyte frequencies negatively affected CAR T cell manufacturing by inhibiting transduction and expansion of anti-CD22 CAR T cells, and that upfront incorporation of CD4/8-T cell selection effectively salvaged apheresis material unable to be used for CAR T cell manufacturing using their previous selection method. One potential complication with upfront CD4/CD8 selection, though, is that monocytes also express CD4 on their surface;31 therefore, it still may be worthwhile to monitor the monocyte content in incoming apheresis and post-selected products to ensure the CD14 content is within the acceptable manufacturing threshold of ≤40% as established here.
In conclusion, high monocyte content poses a serious challenge for CAR T cell manufacturing starting from unmanipulated apheresis products. Our study suggests that manufacturing of CAR T cells should incorporate a step that lessens the content of CD14+ cells in apheresis products containing more than 40% CD14+ cells to maximize the rate of successful CAR T cell productions.
Materials and methods
Patient apheresis products
All apheresis products evaluated were collected from consenting patients enrolled into the clinical trials approved by the human studies institutional review board (IRB) at Memorial Sloan Kettering Cancer Center (MSKCC). The collection was conducted in the MSKCC blood donor room using a blood cell separator (COBE Spectra, COBE Optia). Approximately 7 L of blood was processed for each collection.
CAR T cell manufacturing
CAR T cells were manufactured from cryopreserved apheresis product as previously described.4 In brief, on day 0, cryopreserved apheresis product was washed, and CD3+ cells were selected and activated by paramagnetic Dynabeads CD3/28 (Dynabeads ClinEx vivo CD3/CD28; Invitrogen) at a ratio of either 3:1 or 1:1 (bead to cell). On day 3, the activated cells were transduced with gamma retroviral vector in Retronectin-coated PermaLife cell bags (OriGen Biomedical) with 1-h spinoculation at room temperature. Transduced cells were subsequently inoculated in WAVE/Xuri bioreactor (GE Healthcare). The medium used for the culture was X-VIVO 15 (Lonza) supplemented with 5% heat-inactivated human AB Serum (Gemini), 1% GlutaMAX, and 100–200 IU/mL IL-2. The culture was kept at 37°C with 5% CO2. At the end of process, the paramagnetic Dynabeads CD3/28 were removed using Dynal ClinExVIVO MPC magnet (Invitrogen), washed, and formulated. All products must meet all releasing criteria before patient use. For small-scale CAR T cell production in Figure 3, washed apheresis product containing 5 × 106 CD3+ cells was selected and activated with paramagnetic Dynabeads CD3/28 (Dynabeads ClinEx vivo CD3/CD28; Invitrogen) at a ratio of 3:1 on day 0. On day 3, the activated cells were transduced with gamma retroviral vector in Retronectin-coated PL7 cell bags (OriGen Biomedical) with 1-h spinoculation at room temperature. Transduced cells were subsequently cultured at 0.6–0.8 million/mL in tissue culture flask (Corning) at 37°C with 5% CO2. At the end of the process, the paramagnetic Dynabeads CD3/28 were removed using DynaMag-50 (Invitrogen).
Plastic adhesion
For apheresis products with ≥40% CD14+ contents, on day 0, the washed apheresis product containing approximately 300 × 106 CD3+ cells was plated in 10 T175 flasks (Corning) and kept in 37°C and 5% CO2 incubator for 2 h. The cells in suspension were used as the starting cell population for selection and activation as described above. For small-scale CAR T cell production in Figure 3, washed apheresis products containing 5 × 106 CD3+ cells were plated in one T175 flask (Corning) and kept in 37°C and 5% CO2 incubator for 2 h. The cells in suspension were used as the starting cell population for selection and activation.
Flow cytometry
Immunophenotyping of the apheresis was conducted with the following fluorophore-conjugated antibodies: CD3-phycoerythrin (PE) (Beckman Coulter), CD8-PE-Cy7 (Invitrogen), CD14-allophycocyanin (APC) (eBioscience), and CD45-fluorescein isothiocyanate (FITC) (Beckman Coulter). In-process transduction efficiency of the CD19-targeted CAR T cells was evaluated with the CD3-APC (Invitrogen) and biotinylated goat-anti-mouse Fab (Jackson Immunoresearch Lab) followed by PE-conjugated streptavidin (MP Biomedicals). In-process transduction efficiency of the BCMA-targeted CAR T cells was evaluated with the CD3-APC (Invitrogen) and BCMA-Fc-APC.12 The effector memory and central memory immunophenotyping was conducted using the following monoclonal antibodies: CD27-APC, CD28-FITC, CD62L-FITC, CCR7-FITC, CD45RA-APC (Invitrogen), and CD127-eFlour450 (eBioscience). Dead cells were excluded from analysis using either 7AAD or DAPI staining. Flow data acquisition was performed on an LSRII (BD Biosciences), and data analysis was performed using FlowJo Software (Tree Star).
Statistical analysis
Statistical analysis was conducted using Prism (GraphPad Software). Data were presented as mean ± standard deviation. p values were determined using Student’s two-tailed paired or unpaired t test.
Acknowledgments
We thank Dr. Michel Sadelain for his critical reading and input on the manuscript. We thank the members of the Cellular Immunology Laboratory at MSK for the fluorescence-activated cell sorting (FACS) analysis of the cryopreserved apheresis products. All MSK investigators acknowledge MSK Cancer Center Support Core Grant P30 CA008748. This work is supported by the NCI (P30 CA08748 and PO1 CA008748-T Cell Therapies), NIH (R01 CA236615-01, R01 CA235667, and K08 CA241400), Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Center for Experimental Therapeutics at Memorial Sloan Kettering Cancer Center, the William Lawrence and Blanche Hughes Foundation, Alliance for Cancer Gene Therapy Foundation, Major Family, Memorial Sloan Kettering Society, Cycle for Survival, Stand Up To Cancer/AACR, DOD (BC132124, LC160212, CA170630, and CA180889), the Miner Fund for Mesothelioma Research, American Society of Blood and Marrow Transplantation New Investigator Award, Juno Therapeutics, and ATARA Biotherapeutics.
Author contributions
X.W. and I.R. conceived the experiments, analyzed the data, and wrote the manuscript. O.B.O., J.S., F.D., J.Q., J.C., K.T., M.Z., L.S., M.H., and P.G. manufactured the products and contributed to data analysis. Y.W., B.S., and D.S. coordinated the QC release tests and released the apheresis for processing and CAR T manufacturing. P.S.A., R.J.B., K.C., M.B.G., S.M., R.O.C., J.H.P., C.S., S.S., and E.L.S. recruited and obtained consent from all the patients, reviewed the manuscript, and provided feedback.
Declaration of interests
P.S.A. has received research funding from ATARA Biotherapeutics; has served on the Scientific Advisory Board or as consultant to ATARA Biotherapeutics, Bayer, Carisma Therapeutics, Imugene, and Takeda Therapeutics; and has patents, royalties, and intellectual property on mesothelin-targeted CARs and other T cell therapies, method for detection of cancer cells using virus, and pending patent applications on T cell therapies. E.L.S. has patents, royalties, and intellectual property on BCMA-targeted CARs and serves as consultant for BMS. I.R. has intellectual property rights from Juno Therapeutics.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2021.06.014.
Supplemental information
References
- 1.Sadelain M. CD19 CAR T Cells. Cell. 2017;171:1471. doi: 10.1016/j.cell.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 2.June C.H., Sadelain M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hollyman D., Stefanski J., Przybylowski M., Bartido S., Borquez-Ojeda O., Taylor C., Yeh R., Capacio V., Olszewska M., Hosey J. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J. Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geyer M.B., Manjunath S.H., Evans A.G., Park J.H., Davila M.L., Cutler C.S., Wang X., Wang Y., Senechal B., Rivière I. Concurrent therapy of chronic lymphocytic leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia utilizing CD19-targeted CAR T-cells. Leuk. Lymphoma. 2018;59:1717–1721. doi: 10.1080/10428194.2017.1390237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geyer M.B., Rivière I., Sénéchal B., Wang X., Wang Y., Purdon T.J., Hsu M., Devlin S.M., Halton E., Lamanna N. Autologous CD19-Targeted CAR T Cells in Patients with Residual CLL following Initial Purine Analog-Based Therapy. Mol. Ther. 2018;26:1896–1905. doi: 10.1016/j.ymthe.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J.H., Rivière I., Gonen M., Wang X., Sénéchal B., Curran K.J., Sauter C., Wang Y., Santomasso B., Mead E. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slovin S., Wang X., Borquez-Ojeda O., Stefanski J., Olszewska M., Taylor C., Waisielewska T., Bartido S., Poon S., Scher H.I. Targeting Castration Resistant Prostate Cancer (CRPC) with Autologous PSMA-Directed Chimeric Antigen Receptor T Cells. Mol. Ther. 2012;20(Suppl 1):S33. [Google Scholar]
- 10.Koneru M., Purdon T.J., Spriggs D., Koneru S., Brentjens R.J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. OncoImmunology. 2015;4:e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adusumilli P.S., Cherkassky L., Villena-Vargas J., Colovos C., Servais E., Plotkin J., Jones D.R., Sadelain M. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith E.L., Staehr M., Masakayan R., Tatake I.J., Purdon T.J., Wang X., Wang P., Liu H., Xu Y., Garrett-Thomson S.C. Development and Evaluation of an Optimal Human Single-Chain Variable Fragment-Derived BCMA-Targeted CAR T Cell Vector. Mol. Ther. 2018;26:1447–1456. doi: 10.1016/j.ymthe.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh A., Mailankody S., Giralt S.A., Landgren C.O., Smith E.L., Brentjens R.J. CAR T cell therapy for multiple myeloma: where are we now and where are we headed? Leuk. Lymphoma. 2018;59:2056–2067. doi: 10.1080/10428194.2017.1393668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curran K.J., Margossian S.P., Kernan N.A., Silverman L.B., Williams D.A., Shukla N., Kobos R., Forlenza C.J., Steinherz P., Prockop S. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134:2361–2368. doi: 10.1182/blood.2019001641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geyer M.B., Rivière I., Sénéchal B., Wang X., Wang Y., Purdon T.J., Hsu M., Devlin S.M., Palomba M.L., Halton E. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight. 2019;5:e122627. doi: 10.1172/jci.insight.122627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauter C.S., Senechal B., Rivière I., Ni A., Bernal Y., Wang X., Purdon T., Hall M., Singh A.N., Szenes V.Z. CD19 CAR T cells following autologous transplantation in poor-risk relapsed and refractory B-cell non-Hodgkin lymphoma. Blood. 2019;134:626–635. doi: 10.1182/blood.2018883421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 18.Passlick B., Flieger D., Ziegler-Heitbrock H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–2534. [PubMed] [Google Scholar]
- 19.Ziegler-Heitbrock L. Blood Monocytes and Their Subsets: Established Features and Open Questions. Front. Immunol. 2015;6:423. doi: 10.3389/fimmu.2015.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiveland C.R. In: The Impact of Food Bioactives on Health: in vitro and ex vivo models [Internet] Chapter 15. Verhoeckx K., Cotter P., López-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. Springer; Cham (CH): 2015. Peripheral Blood Mononuclear Cells; pp. 161–167. [PubMed] [Google Scholar]
- 21.Wang X., Qu J., Stefanski J., Du F., Borquez-Ojeda O., Hack A., Riviere I. Depletion of High-Content CD14+ Cells from Apheresis Products is Critical for the Successful Transduction and Expansion of CAR T Cells During Large-Scale cGMP Manufacturing. Mol. Ther. 2015;23(Suppl 1):S35. doi: 10.1016/j.omtm.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv M., Wang K., Huang X.J. Myeloid-derived suppressor cells in hematological malignancies: friends or foes. J. Hematol. Oncol. 2019;12:105. doi: 10.1186/s13045-019-0797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Künkele A., Brown C., Beebe A., Mgebroff S., Johnson A.J., Taraseviciute A., Rolczynski L.S., Chang C.A., Finney O.C., Park J.R., Jensen M.C. Manufacture of Chimeric Antigen Receptor T Cells from Mobilized Cyropreserved Peripheral Blood Stem Cell Units Depends on Monocyte Depletion. Biol. Blood Marrow Transplant. 2019;25:223–232. doi: 10.1016/j.bbmt.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Stroncek D.F., Lee D.W., Ren J., Sabatino M., Highfill S., Khuu H., Shah N.N., Kaplan R.N., Fry T.J., Mackall C.L. Elutriated lymphocytes for manufacturing chimeric antigen receptor T cells. J. Transl. Med. 2017;15:59. doi: 10.1186/s12967-017-1160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroncek D.F., Ren J., Lee D.W., Tran M., Frodigh S.E., Sabatino M., Khuu H., Merchant M.S., Mackall C.L. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy. 2016;18:893–901. doi: 10.1016/j.jcyt.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noaks E., Peticone C., Kotsopoulou E., Bracewell D.G. Enriching leukapheresis improves T cell activation and transduction efficiency during CAR T processing. Mol. Ther. Methods Clin. Dev. 2021;20:675–687. doi: 10.1016/j.omtm.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boucher J., Cervantes E.V., Lee S.B., Spitler K., Reid K., Davila M.L. Optimization of CAR T cell co-stimulation reduces to MDSC suppression. J. Immunol. 2020;204(Suppl 1):170.3. [Google Scholar]
- 28.Braun M.W., Abdelhakim H., Li M., Hyter S., Pessetto Z., Koestler D.C., Pathak H.B., Dunavin N., Godwin A.K. Adherent cell depletion promotes the expansion of renal cell carcinoma infiltrating T cells with optimal characteristics for adoptive transfer. J. Immunother. Cancer. 2020;8:e000706. doi: 10.1136/jitc-2020-000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mielcarek M., Graf L., Johnson G., Torok-Storb B. Production of interleukin-10 by granulocyte colony-stimulating factor-mobilized blood products: a mechanism for monocyte-mediated suppression of T-cell proliferation. Blood. 1998;92:215–222. [PubMed] [Google Scholar]
- 30.Shah N.N., Highfill S.L., Shalabi H., Yates B., Jin J., Wolters P.L., Ombrello A., Steinberg S.M., Martin S., Delbrook C. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J. Clin. Oncol. 2020;38:1938–1950. doi: 10.1200/JCO.19.03279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filion L.G., Izaguirre C.A., Garber G.E., Huebsh L., Aye M.T. Detection of surface and cytoplasmic CD4 on blood monocytes from normal and HIV-1 infected individuals. J. Immunol. Methods. 1990;135:59–69. doi: 10.1016/0022-1759(90)90256-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.