Results from the Surveillance, Epidemiology and End Results (SEER) Program and National Cancer Data Base (NCDB) indicate that among men with localized, high-grade prostate cancer, those with low prostate-specific antigen (PSA) levels at diagnosis have worse prognosis compared to men with intermediate PSA levels [1], This prior analysis was based on data with rather short median follow-up of only 2–4 yr, which is limited given the long natural history of prostate cancer, and only reported on a limited number of prostate cancer deaths. The aim of our analysis was to validate these findings among men with Gleason score 8–10 prostate cancer within the US Health Professionals Follow-up Study (HPFS). Gleason scores were re-reviewed by experienced genitourinary pathologists according to the most recent grading recommendations [2]. The primary endpoint was lethal prostate cancer, defined as death from prostate cancer or the development of metastases during follow-up. The association between PSA and lethal prostate cancer was modeled using restricted cubic splines with four knots and Cox regression.
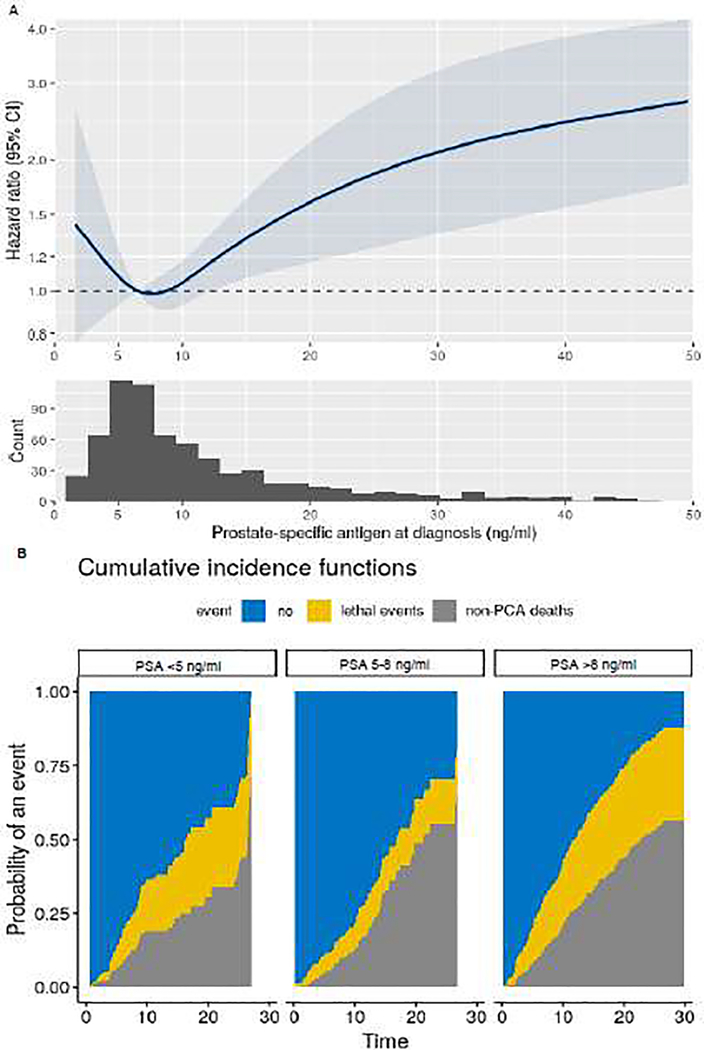
Of 4908 men with localized prostate cancer diagnosed between 1988 and 2015 and data on PSA available at diagnosis and follow-up, 716 had Gleason score 8–10 tumors. The median age at diagnosis was 70 yr (interquartile range 66–77). Primary treatment included radical prostatectomy (43%), radiotherapy (33%), brachytherapy (8%), hormonal therapy (9%), and watchful waiting or other therapies (9%; Supplementary Table 1). Over median follow-up of 13 yr (interquartile range 8–19), 156 men (22%) experienced progression to lethal prostate cancer and 259 (36%) noncancer deaths occurred. The association between diagnostic PSA levels and lethal disease is presented in Fig. 1A. After adjustment for clinical stage, Gleason score, and treatment received, men with low PSA (<5 ng/ml) at diagnosis were at higher risk of lethal progression compared to men with intermediate PSA levels (5–8 ng/ml; hazard ratio [HR] 1.83, 95% confidence interval [CI] 1.05–3.20). Men with high PSA (>8 ng/ml) also had an excess risk of lethal prostate cancer (HR 2.14, 95% CI 1.35–3.40) compared to those with intermediate PSA. Competing-risk analyses revealed that all-cause mortality exceeded lethal cancer events among the whole cohort and that lethal prostate cancer was more common for men with low or high PSA at diagnosis (Fig. 1B).
Fig. 1 –
Lethal prostate cancer (PCA) according to prostate-specific antigen (PSA) levels at cancer diagnosis among men diagnosed with localized, Gleason score 8–10 PCA between 1986 and 2015 within the Health Professional Follow-up Study. (A) Multivariable hazard ratios from Cox proportional hazards regression, with PSA modeled as a restricted cubic spline, adjusted for clinical stage, Gleason score, and treatment received. The solid curve represents point estimates and the highlighted blue area the 95% confidence interval (CI). (B) Cumulative incidence of lethal PCA (yellow) and all-cause mortality (gray) among men with high or low PSA levels compared to men with intermediate PSA levels.
These results suggest a J-shaped rather than a linear association between PSA and lethal disease among men with localized high-grade prostate cancer. Data from this prospective cohort with long-term follow-up confirm prior findings of worse oncological outcomes for men with high-grade prostate cancer and low PSA at diagnosis. Previous research suggested that altered neuroendocrine/small-cell histology and androgen receptor signaling might be a reason for the inferior oncological outcomes [1]. This may explain why men with high-grade prostate cancer and low PSA often respond differently to primary or salvage therapies [3]. Our data support the need for an amended clinical definition of “highest-risk” localized prostate cancer expanded to include men in the low PSA group. Further research is needed to explore genomic and/or genetic tests, lifestyle modification, and different treatment regimens for this group.
Supplementary Material
Acknowledgments
This study was supported by funding for C.D.F. from the University Hospital of Zurich and medAlumni Zurich, Stiftung für urologische Forschung, Marlis Geiser-Lemken Stiftung, Fonds zur Förderung des akademischen Nachwuchses, Ernst Göhner Stiftung, a SAKK/Dr. Paul Janssen Fellowship, and Arnold U. und Susanne Huggenberger-Bischoff Stiftung zur Krebsforschung; and for L.A.M. from the National Institutes of Health (U01 CA167552), the US Army Prostate Cancer Research Program (Idea Development Award PC060389, an Early-Investigator Research Award (W81XWH-18-1-0330), and Dana-Farber/Harvard Cancer Center Prostate SPORE Career Development Award (NIH/NCI P50 CA090381). L.A.M., K.L.P., K.H.S., and M.A.P. are Young Investigators of the Prostate Cancer Foundation. The funding bodies had no influence on the design or conduct of the study, analysis and interpretation of the data, or preparation of the article. We are grateful to the participants and staff of the Health Professionals Follow-up Study for their valuable contributions. We also thank the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nevada, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Footnotes
Conflicts of interest: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mahal BA, Yang DD, Wang NQ, et al. Clinical and genomic characterization of low–prostate-specific antigen, high-grade prostate cancer. Eur Urol 2018;74:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stark JR, Perner S, Stampfer MJ, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol 2009;27:3459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rickman DS, Beltran H, Demichelis F, Rubin MA. Biology and evolution of poorly differentiated neuroendocrine tumors. Nat Med 2017;23:664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.