Abstract
Objectives:
To determine the prevalence of refractive errors and visual impairment in Down syndrome (DS) patients compared to normal controls.
Materials and Methods:
Cycloplegic refraction was tested in 213 DS patients and 184 normal age- and gender-matched controls using autorefraction followed by retinoscopy. Data from the worse eye of each case were used in the analyses.
Results:
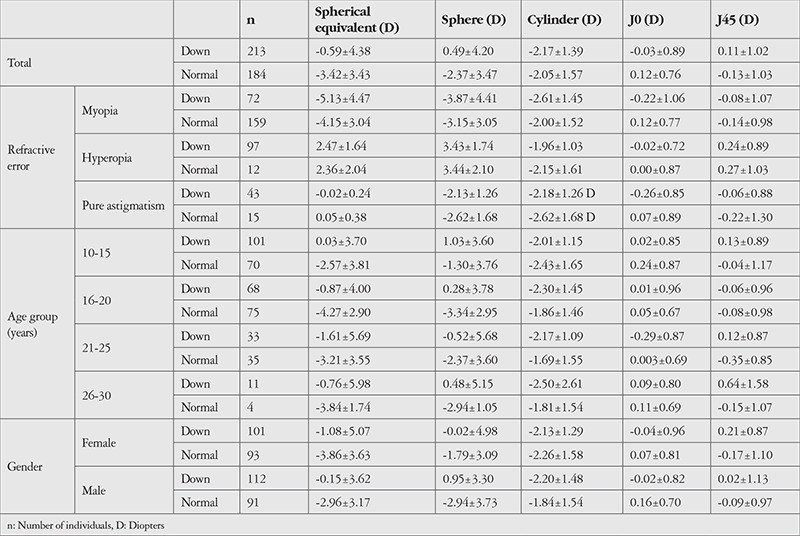
In the DS and control groups, respectively, mean age was 17.2±4.8 and 17.2±4.4 years (p=0.993) and 53.0% and 49.5% were male (p=0.473). In the DS and control groups, respectively, mean spherical equivalent (SE) was -5.13±4.47 and -4.15±3.04 diopters (D) in myopics (p=0.050) and 2.47±1.64 and 2.36±2.04 D in hyperopics (p=0.482), mean cylinder error was -2.17±1.39 and -2.05±1.57 D (p=0.451), mean J0 was -0.03±0.89 and 0.12±0.76 D (p=0.086), and mean J45 was 0.11±1.02 and -0.13±1.03 D (p=0.024). The prevalence of oblique astigmatism was higher in the DS group (20.4% vs. 6.1%) while against-the-rule astigmatism was more prevalent in the control group (84.0% vs. 71.6%) (p<0.001). The prevalence of anisometropia was not significantly different between the groups (19.4% vs. 13.8%). Visual impairment was detected in 11.7% of the DS and 0.5% of the control group (p<0.001). The prevalence of amblyopia was 36.3% and 3.8% in the DS and control groups, respectively (p<0.001). Based on the multiple model, only absolute SE inversely correlated with age and differed between males and females (all p<0.05).
Conclusion:
In DS patients, the prevalence rates of refractive errors, amblyopia, and visual impairment are higher than those in non-DS individuals, and emmetropization appears to be either defective or slow. Cylinder error is stable in this age range, but the rotation of astigmatism axis is different from normal samples.
Keywords: Refractive errors, visual impairment, amblyopia, emmetropization, Down syndrome, comparative study
Introduction
Refractive errors are one of the main items and the fifth priority of the 2020 Vision: Right to Sight Initiative.1 In 2012, a systematic review of surveys in 39 countries showed that uncorrected refractive errors were the leading cause of visual impairment (43%).2 A systematic review in 2018 reported the pooled prevalence of myopia, hyperopia, and astigmatism in children worldwide to be 11.7%, 4.6%, and 14.9% respectively, which are considerably high prevalence rates.3 Studies have shown a significant correlation between refractive errors, socioeconomic status, and lifestyle.4 In patients with Down syndrome (DS), quality of life is reduced due to medical conditions,5 and declines further as they age.6 Therefore, the identification and correction of refractive errors in this population deserves even higher priority than in normal populations.
To date, several studies have been done on the prevalence and degree of refractive errors in patients with DS.7,8,9,10,11 These studies were mostly carried out in age groups 10 years of age and younger, and one study reported non-cycloplegic refraction in a group with a mean age of 15 years (range, 4 to 60 years).8,9,10,11
The goal of this study was to determine the prevalence and distribution of refractive errors, type of astigmatism, visual impairment, and amblyopia in order to provide a comprehensive report on the refractive status in this particular population. We used cyclopentolate, as evidence showed that it is the gold standard for epidemiological studies of refraction and increases the reliability of findings.12 The distribution of refraction in DS patients was compared to a group of age- and gender-matched normal controls.
Materials and Methods
Study Subjects
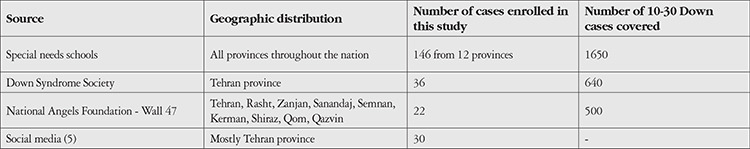
The sampling details have been described elsewhere.13 This report is part of a larger comparative study in which 10- to 30-year-old DS patients recruited from the nation’s special needs schools, the DS Society, and relevant non-governmental organizations were consecutively screened for eligibility and enrolled in the study (Table 1). Inclusion criteria were a diagnosis of DS and minimum age of 10 years. Exclusion criteria were any concomitant mental illnesses such as autism or Klinefelter syndrome. Of the 250 respondents, 16 were not eligible due to mental disabilities, Klinefelter syndrome, or autism. The remaining 234 underwent clinical and paraclinical examinations at Noor Eye Hospital. For a comparison group, 200 non-DS participants were consecutively selected from candidates for refractive surgery presenting for their first work-up session (87 cases) and individuals presenting for a vision check-up (113 cases) in Noor Eye Hospital. This group had no personal or family history of DS or other intellectual disabilities.
Table 1. Summary of sources from which Down syndrome patients were recruited.
Ethical Consideration
Prior to enrollment, the goals and methods of the study were explained and written consent was obtained. For all cases in the DS group and those under 18 years of age in the control group, informed consent was obtained from their parents/guardians, and participants were asked for verbal assent before any procedure. This project was approved by the Ethics Committee of Tehran University of Medical Sciences (ID: 1397-091) and adhered to the Declaration of Helsinki at all stages.
Examinations
Visual acuity was evaluated with Snellen chart (SC-2000; Nidek Co., Tokyo, Japan) without correction (uncorrected distance visual acuity; UDVA) and with correction (corrected distance visual acuity [CDVA]). Manifest refraction was first evaluated using autorefraction (ARK-510A, NIDEK, Gamagory, Japan), followed by retinoscopy (ParaStop HEINE BETA 200; HEINE Optotechnik, Herrsching, Germany). Cycloplegic refraction was done in participants who, as determined by the physician, had no contraindication for cycloplegia and whose parents consented to the procedure. This was done 20 minutes after instilling 2 drops of cyclopentolate 10 mg/ml eye drops (Novartis, Barcelona, Spain) 10 minutes apart.
Definitions
Spherical equivalent (SE) was calculated as the spherical error plus half of the cylinder error. Myopia and hyperopia were defined as an SE ≤-0.5 diopter (D) and ≥0.5 D in the worse eye, respectively, and the prevalence of these conditions was determined. Myopia was categorized into 4 groups: mild (-0.51 to -3.0 D), moderate (-3.01 to -6.0 D), high (-6.01 to -9.0 D), and extreme (<-9.0 D), and hyperopia was categorized into 3 groups: mild (0.51 to 2.0 D), moderate (2.01 to 4.0 D), and high (>4.0 D). The worse eye was the one with higher absolute SE value, and if refractive error data were available for only one eye, it was considered the worse eye.
The definition and prevalence of astigmatism was based on a cylinder error <-0.5 D in the worse eye (higher absolute astigmatism). Astigmatism types were with-the-rule (WTR, steep axis 90°±30°), against-the-rule (ATR, steep axis 180°±30°), and oblique (other axes). If cylinder error data were available for only one eye, it was considered the worse eye. Pure astigmatism was defined as a spherical error of -0.5 to 0.5 D and a cylinder error higher than 0.5 D.
Thibos astigmatism vector analysis14 was used to decompose cylinder error to J0 and J45. As such, J0 = -C/2cos2ɑ and J45 = -C/2sin2α, where C is the cylinder value and α is the cylinder axis. A positive value for J0 indicates WTR astigmatism and a negative value indicates ATR. J45 represents oblique astigmatism at 45° and 135°, and a positive value indicates + cylinder axis >90°.
Anisometropia was reported in terms of an interocular SE difference more than 1.0 D and visual impairment was based on a CDVA <20/60 in the worse eye. Amblyopia was defined as 2 lines or greater interocular difference in CDVA in the absence of correctable pathology.15
Statistical Analysis
Prevalence was calculated as the ratio of cases with a given condition in at least one eye to the total number of people who were examined for the condition. Data of the worse eye were used in the analysis. Four age groups of 10-15, 16-20, 21-25, and 26-30 years of age were defined, and prevalence rates were determined for all age and gender subgroups. Multiple linear regression was used to examine the correlation of absolute refractive error with age (continuous variable), gender (female: 0 and male: 1), and group (normal: 0 and DS: 1). In addition, multinomial regression model (baseline: emmetropia) was used to test the correlation of the prevalence of refractive errors with age, gender, and group. The prevalence of anisometropia, visual impairment, and amblyopia was compared between the two groups using the chi-square test.
Results
After applying the inclusion criteria for this report (having cycloplegic refraction results, no ectasia, and no history of corneal surgery), of the 234 patients with DS and 200 normal controls enrolled into the study, data from 213 DS cases and 184 controls were used in the analyses.
In the DS and control groups, respectively, the mean age was 17.2±4.8 and 17.2±4.4 years (p=0.993), and 53.0% and 49.5% were male (p=0.473). In the DS and control groups, mean UDVA was 0.36±0.34 and 0.86±0.62 logMAR (p<0.001) and mean CDVA was 0.20±0.11 and 0.02±0.06 logMAR (p<0.001), respectively.
Distribution of Refractive Errors
Table 2 summarizes refractive indices in the two studied groups and in refractive error, age, and gender subgroups. Based on multiple analysis, absolute SE was significantly correlated with age (b=0.11, p=0.002), gender (b=0.853, p=0.007), and group (b=-0.81, p=0.010). Mean cylinder error, J0, and pure astigmatism were not correlated with these parameters (all p>0.05). J45 was higher in the DS group (b=0.24, p=0.022).
Table 2. Distribution of refractive components by age and gender in 10- to 30-year-old Down syndrome patients and normal control group.
Prevalence of Refractive Errors, Visual Impairment, and Amblyopia
Table 3 presents the prevalence of emmetropia, myopia, hyperopia, and pure astigmatism in the DS and control groups and their age and gender subgroups. Figure 1 shows the subtypes of refractive errors in each group. Anisometropia >1.0 D was detected in 19.4% of the DS and 13.8% of the control group (p=0.136). Visual impairment was observed in 11.7% of the DS and 0.5% of the control group (p<0.001). The prevalence of amblyopia was 36.3% and 3.8% in the DS and control groups (p<0.001), respectively.
Table 3. Frequency (%) of refractive components by age and gender in 10- to 30-year-old Down syndrome patients and normal control group.
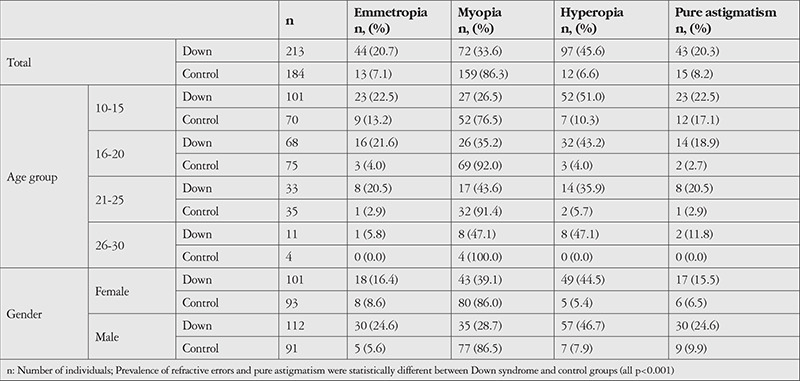
Figure 1.
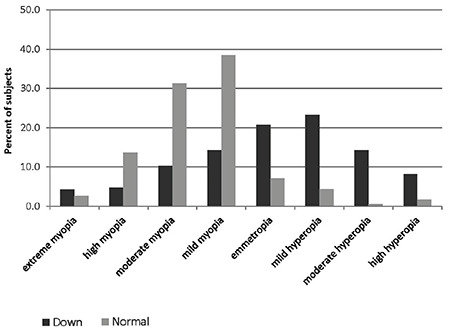
The subtypes of refractive errors in patients with Down syndrome patients and normal control group
Based on multinomial analysis, the prevalence of myopia increased with age (odds ratio [OR]=1.11, p=0.004) and was higher in the control group (OR=7.95, p<0.001). The prevalence of hyperopia was age-independent and higher in the DS group (OR=2.36, p=0.049). The prevalence of pure astigmatism was age-independent and higher in the DS group (b=2.83, p<0.001). The prevalence of refractive errors and pure astigmatism did not correlate with gender.
The prevalence of oblique astigmatism was higher in DS patients (20.4% vs. 6.1%), while WTR (84.0% vs. 71.6%) and ATR (9.9% vs. 8.1%) astigmatism was more common in the control group (p<0.001). The multinomial regression model showed that the prevalence of WTR astigmatism decreased with age (OR=0.893, p=0.003) and was not significantly different between the two groups (p=0.940). Oblique astigmatism was age-independent and the prevalence was higher in the DS group (OR=4.24, p=0.003) (Figure 2). The prevalence rates of the three types of astigmatism orientation were not significantly different between genders (all p>0.05).
Figure 2.
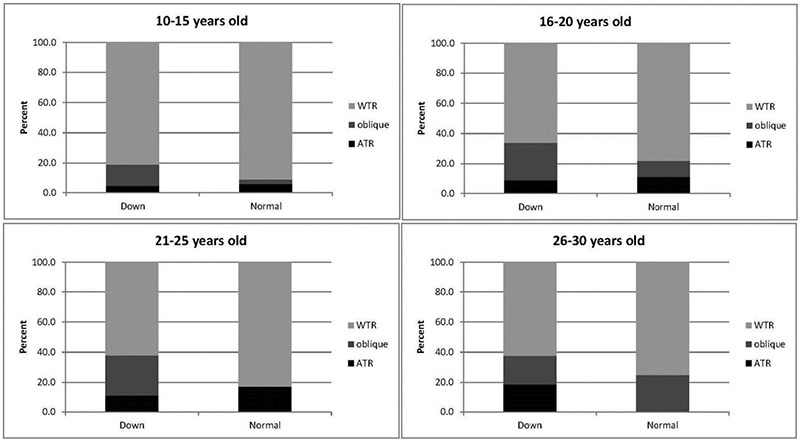
The types of astigmatism in patients with Down syndrome patients and normal control group based on age groups
WTR: With-the-rule astigmatism, ATR: Against-the-rule astigmatism
Age and gender were not significantly correlated with anisometropia (p=0.764 and p=0.136), visual impairment (p=0.133 and p=0.220), or amblyopia (p=0.482 and p=0.118, respectively).
Discussion
In this large comparative study, we showed the distribution and prevalence of myopia, hyperopia, astigmatism, amblyopia, and visual impairment in a sample of DS patients aged 10-30 years (when the incidence of refractive errors is highest) and compared the results to a group of age- and gender-matched controls. The strength of this study was selecting DS patients from different sources and creating a sample with diverse cases. Although several studies have been done on refractive errors in DS patients, they have often been studied in the 10-year-old age group7,9,10,11 or a broad age range (3 months to 60 years) without cycloplegia.8 As emmetropization has been suggested to be incomplete in DS patients even up to 17 years of age16 and the prevalence of myopia tends to increase in non-DS individuals after the age of 9 years17 and continue up to 30 years of age18, in this study we considered the age range of 10 to 30 years.
Distribution of Refractive Errors
Non-cycloplegic SE values were reported as 1.43±2.86 D in the age group of 15-22 years (mean 17 years) in a study by Doyle et al.16 and as -1.86±5.28 D in a sample aged 3 months to 60 years (mean 15 years) in a study by Alio et al.8 In our DS group, with a mean age of 17 years, cycloplegic SE (-0.59±4.38 D) was lower than that in the mentioned studies. However, the cylinder error in our sample was 0.5 to 1.0 D higher than in these two studies. In other words, the lower SE in the present study was due to the lower spherical error. The effect of cyclopentolate on refractive measurements should be considered, especially in the ≤20 age group. The use of cyclopentolate in our study can explain the more positive SE19 than in the study by Alio et al.8 In the study by Doyle et al.16, SE was positive (hyperopia) in 80% of cases, which differs considerably from the results of the present study (46% hyperopia). Given that the difference between cycloplegic and non-cycloplegic refraction is significant in hyperopics up to 30 years of age12, the difference between the two studies is expected, albeit ethnicity and age may be influential factors as well.20
Prevalence of Refractive Errors and Visual Impairment
The prevalence rates of myopia, hyperopia, emmetropia (defined as SE >-0.5 and <0.5 D), and pure astigmatism in our DS group were 33.6%, 45.7%, 20.7%, and 20.3%, respectively. Of the myopic cases, 12.8% were extreme, and among hyperopic cases, 17.9% were high hyperopic. In some studies, cycloplegic evaluation of DS patients under 1 year to 18 years of age9,10,11 showed higher rates of hyperopia (SE ≥0.75 D) than myopia (SE ≤-0.75 D) in these populations (59.0% vs. 9.0%9, 28.0% vs. 25.0%10, and 36.9% vs. 24.6%11). In a study by Adio and Wajuihian7, myopia (SE ≤-0.5 D) was predominant compared to hyperopia in patients up to 28 years of age (38.1% vs. 9.5%). Another study in the 15- to 22-year age range reported a hyperopia prevalence of 80% in DS patients.16 In the present study (10-30 years), the prevalence of hyperopia was approximately 46% (i.e., 1.4 times of the rate of myopia), and 17.9% had hyperopia greater than 4.0 D. Except for the Adio and Wajuihian7 study, other studies with different age groups have shown hyperopia to be the most common refractive error in DS individuals. The difference in the frequency of refractive errors in these studies is due to differences in sample age, threshold for the definition of SE, and inducing cycloplegia.
Comparing the prevalence of refractive errors in the DS group of this study (33.6% for myopia and 45.6% for hyperopia) with the rates in the age- and gender-matched control group (86.3% for myopia and 6.6% for hyperopia) suggested that emmetropization in DS patients is defective or slow. Unlike normal populations, where the prevalence of hyperopia is higher in men and the prevalence of myopia is higher in women,21,22 there was no significant inter-gender difference in our DS group in terms of the prevalence of refractive errors. Similarly, there was no inter-gender difference in terms of astigmatism type as in healthy samples.23 The ratio of oblique/ATR astigmatism in DS patients in comparison with the control group (2.06 vs. 0.75) points to a defective emmetropization process in DS patients.
In the current study, the prevalence of visual impairment was 11.7% in DS patients, while other studies reported rates of 46% (in a 50- to 59-year-old sample) up to 85% (in those ≥60 years of age), which might indicate a higher prevalence with age.24,25,26 The prevalence of amblyopia in our sample was 36.3%. Others have reported rates of 8.5% for a sample age between 1 and 31 years and 13% in those between 6 months and 14 years of age.27,28 Ugurlu ve Altinkurt29 reported a prevalence of 36.4% for amblyopia in DS patients with a mean age of 13 years in Turkey, which is very close to our study. These variations are perhaps due to ethnic differences, sample age, or the prevalence and severity of refractive errors.
Conclusion
Overall, in our sample of 10- to 30-year-old DS patients, the prevalence of refractive errors, astigmatism, visual impairment, and amblyopia was higher than that of their age- and gender-matched controls, and emmetropization appeared to be either defective or slow. The prevalence of refractive and visual complications was similar between males and females. Cylinder error appears to be stable in this age range in DS patients, but the rotation of its axis was different from the controls. These findings are useful for refractive errors correction services for DS patients.
Footnotes
Ethics
Ethics Committee Approval: This project was approved by the Ethics Committee of Tehran University of Medical Sciences (ID: 1397-091) and adhered to the Declaration of Helsinki at all stages.
Informed Consent: Obtained.
Peer-review: Externally peer reviewed.
Authorship Contributions
Surgical and Medical Practices: H.H., Concept: S.A., Design: S.A., Data Collection or Processing: H.H., S.M., Analysis or Interpretation: S.A., S.M., Literature Search: S.A., F.D.N., Writing: S.A., S.M., H.H., F.D.N.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.World Health Organization (WHO). Blindness and vision impairment prevention. Priority eye diseases; 2018 [accessed December 17, 2018]. [Internet] http://www.who.int/blindness/causes/priority/en .
- 2.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 3.Hashemi H, Fotouhi A, Yekta A, Pakzad R, Ostadimoghaddam H, Khabazkhoob M. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J Curr Ophthalmol. 2018;30:3–22. doi: 10.1016/j.joco.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster PJ, Jiang Y. Epidemiology of myopia. Eye (Lond) 2014;28:202–208. doi: 10.1038/eye.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haddad F, Bourke J, Wong K, Leonard H. An investigation of the determinants of quality of life in adolescents and young adults with Down syndrome. PloS One. 2018;13:e0197394. doi: 10.1371/journal.pone.0197394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields N, Leonard H, Munteanu S, Bourke J, Lim P, Taylor NF, Downs J. Parent-reported health-related quality of life of children with Down syndrome: a descriptive study. Dev Med Child Neurol. 2018;60:402–408. doi: 10.1111/dmcn.13670. [DOI] [PubMed] [Google Scholar]
- 7.Adio AO, Wajuihian SO. Ophthalmic manifestations of children with Down syndrome in Port Harcourt, Nigeria. Clin Ophthalmol. 2012;6:1859–1864. doi: 10.2147/OPTH.S36685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alio JL, Vega-Estrada A, Sanz P, Osman AA, Kamal AM, Mamoon A, Soliman H. Corneal Morphologic Characteristics in Patients With Down Syndrome. JAMA Ophthalmol. 2018;136:971–978. doi: 10.1001/jamaophthalmol.2018.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fimiani F, Iovine A, Carelli R, Pansini M, Sebastio G, Magli A. Incidence of ocular pathologies in Italian children with Down syndrome. Eur J Ophthalmol. 2007;17:817–822. doi: 10.1177/112067210701700521. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Hwang JM, Kim HJ, Yu YS. Characteristic ocular findings in Asian children with Down syndrome. Eye (Lond) 2002;16:710–714. doi: 10.1038/sj.eye.6700208. [DOI] [PubMed] [Google Scholar]
- 11.Stirn Kranjc B. Ocular abnormalities and systemic disease in Down syndrome. Strabismus. 2012;20:74–77. doi: 10.3109/09273972.2012.680234. [DOI] [PubMed] [Google Scholar]
- 12.Morgan IG, Iribarren R, Fotouhi A, Grzybowski A. Cycloplegic refraction is the gold standard for epidemiological studies. Acta Ophthalmol. 2015;93:581–585. doi: 10.1111/aos.12642. [DOI] [PubMed] [Google Scholar]
- 13.Makateb A, Hashemi H, Farahi A, Mehravaran S, Khabazkhoob M, Asgari S. Ocular alignment, media, and eyelid disorders in Down syndrome. Strabismus. 2020;28:42–48. doi: 10.1080/09273972.2019.1699582. [DOI] [PubMed] [Google Scholar]
- 14.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–375. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Ohlsson J. Defining amblyopia: the need for a joint classification. Strabismus. 2005;13:15–20. doi: 10.1080/09273970590890949. [DOI] [PubMed] [Google Scholar]
- 16.Doyle SJ, Bullock J, Gray C, Spencer A, Cunningham C. Emmetropisation, axial length, and corneal topography in teenagers with Down’s syndrome. Br J Ophthalmol. 1998;82:793–796. doi: 10.1136/bjo.82.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumura H, Hirai H. Prevalence of myopia and refractive changes in students from 3 to 17 years of age. Surv Ophthalmol. 1999;44(Suppl 1):109–115. doi: 10.1016/s0039-6257(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 18.Hashemi H, Nabovati P, Yekta A, Shokrollahzadeh F, Khabazkhoob M. The prevalence of refractive errors among adult rural populations in Iran. Clin Exp Optom. 2018;101:84–89. doi: 10.1111/cxo.12565. [DOI] [PubMed] [Google Scholar]
- 19.Sankaridurg P, He X, Naduvilath T, Lv M, Ho A, Smith E 3rd, Erickson P, Zhu J, Zou H, Xu X. Comparison of noncycloplegic and cycloplegic autorefraction in categorizing refractive error data in children. Acta Ophthalmol. 2017;95:633–640. doi: 10.1111/aos.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen G, Tarczy-Hornoch K, McKean-Cowdin R, Cotter SA, Borchert M, Lin J, Kim J, Varma R; Multi-Ethnic Pediatric Eye Disease Study Group. Prevalence of myopia, hyperopia, and astigmatism in non-Hispanic white and Asian children: multi-ethnic pediatric eye disease study. Ophthalmology. 2013;120:2109–2116. doi: 10.1016/j.ophtha.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czepita D, Mojsa A, Ustianowska M, Czepita M, Lachowicz E. Role of gender in the occurrence of refractive errors. Ann Acad Med Stetin. 2007;53:5–7. [PubMed] [Google Scholar]
- 22.Hashemi H, Fotouhi A, Mohammad K. The age- and gender-specific prevalences of refractive errors in Tehran: the Tehran Eye Study. Ophthalmic Epidemiol. 2004;11:213–225. doi: 10.1080/09286580490514513. [DOI] [PubMed] [Google Scholar]
- 23.Fotouhi A, Hashemi H, Yekta AA, Mohammad K, Khoob MK. Characteristics of astigmatism in a population of schoolchildren, Dezful, Iran. Optom Vis Sci. 2011;88:1054–1059. doi: 10.1097/OPX.0b013e318221727d. [DOI] [PubMed] [Google Scholar]
- 24.Evenhuis HM, Theunissen M, Denkers I, Verschuure H, Kemme H. Prevalence of visual and hearing impairment in a Dutch institutionalized population with intellectual disability. J Intellect Disabil Res. 2001;45:457–464. doi: 10.1046/j.1365-2788.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 25.van Splunder J, Stilma JS, Bernsen RM, Evenhuis HM. Prevalence of ocular diagnoses found on screening 1539 adults with intellectual disabilities. Ophthalmology. 2004;111:1457–1463. doi: 10.1016/j.ophtha.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 26.Van Buggenhout GJ, Trommelen JC, Schoenmaker A, De Bal C, Verbeek JJ, Smeets DF, Ropers HH, Devriendt K, Hamel BC, Fryns JP. Down syndrome in a population of elderly mentally retarded patients: genetic-diagnostic survey and implications for medical care. Am J Med Genet. 1999;85:376–384. doi: 10.1002/(sici)1096-8628(19990806)85:4<376::aid-ajmg14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Hiles DA, Hoyme SH, McFarlane F. Down’s syndrome and strabismus. Am Orthop J. 1974;24:63–68. [PubMed] [Google Scholar]
- 28.Jaeger EA. Ocular findings in Down’s syndrome. Trans Am Ophthalmol Soc. 1980;158:808–845. [PMC free article] [PubMed] [Google Scholar]
- 29.Ugurlu A, Altinkurt E. Ophthalmologic Manifestations and Retinal Findings in Children with Down Syndrome. J Ophthalmol. 2020;2020:9726261. doi: 10.1155/2020/9726261. [DOI] [PMC free article] [PubMed] [Google Scholar]


