Abstract
Pregabalin is a drug that can cause psychiatric symptoms via pregabalin withdrawal. Prior reports on pregabalin withdrawal have mainly focused on cases with pregabalin dependence or abuse, and little attention has been paid to patients who are prescribed regular doses of pregabalin. Herein, we report three cases of pregabalin withdrawal in patients without psychiatric disorders, taking regular doses of pregabalin, who developed psychiatric symptoms such as insomnia and anxiety after abrupt discontinuation of pregabalin. In addition, we conducted a systematic review of six case reports (previous studies) of pregabalin withdrawal under regular doses of pregabalin. Among the six cases, three patients had no comorbid mental or substance use disorders, the dose of pregabalin ranged from 150 to 600 mg/d, and the duration of pregabalin use ranged from a few weeks to many years. Of these six cases of pregabalin withdrawal, five had psychopathological symptoms, three had vegetative symptoms, and three had neurologic and physical complications. We concluded that since pregabalin withdrawal can occur even with regular doses and short‐term use, clinicians must carefully reduce pregabalin doses when reducing or discontinuing treatment, paying close attention to withdrawal symptoms. Our case series sheds light on the scant evidence from previous research on physical dependence in patients who are taking regular doses of pregabalin. Furthermore, our cases were also valuable in demonstrating that pregabalin withdrawal can occur even after a relatively short period of 2 months.
Keywords: anxiety, dependence, insomnia, pregabalin, withdrawal
This case series shows pregabalin withdrawal in patients without psychiatric disorders taking a regular dose of pregabalin. Our cases demonstrated that pregabalin withdrawal can occur even after a relatively short period of 2 months following regular doses of pregabalin.

1. INTRODUCTION
Pregabalin is a drug prescribed for neuropathic pain, anxiety disorders, and partial epilepsy.1, 2, 3 In Japan, pregabalin is approved for neuropathic pain (150‐600 mg/d) and pain associated with fibromyalgia (150‐450 mg/d). Pregabalin is presumed to exert its effect by binding to the α2δ subunit of voltage‐gated Ca2+ channels, thereby inhibiting Ca2+ influx into the nerves and decreasing neurotransmitter release.4
Pregabalin can cause psychiatric symptoms during withdrawal. Prior reports on pregabalin withdrawal have mainly focused on cases with pregabalin dependence or abuse; previous studies have not focused on patients who were prescribed regular doses of pregabalin.5, 6 Herein, we report three cases of pregabalin withdrawal in patients without psychiatric disorders taking regular doses of pregabalin, who developed psychiatric symptoms such as insomnia and anxiety after the dose of pregabalin was reduced. Written informed consent was obtained from each patient for the publication of this case series. Furthermore, we conducted a systematic review of previous case reports that reported pregabalin withdrawal under regular doses of pregabalin.
2. CASE PRESENTATIONS
2.1. Case 1
A 39‐year‐old man visited the psychiatric department of our hospital. The patient's medical history included cervical spondylosis and right upper extremity neuropathic pain. The patient had no history of mental disorders. Two months before the visit, the patient was started on pregabalin for right upper extremity neuropathic pain and was administered 600 mg/d of pregabalin and 200 mg/d of celecoxib at the time of the visit. The patient experienced symptoms of cold 9 days before the visit; therefore, all prescription drugs were discontinued at his discretion. Seven days before the visit, the patient experienced dyspnea, loss of appetite, insomnia, and anxiety. With these symptoms as the chief complaint, the patient visited our emergency department for 3 consecutive days (4‐6 days before the visit). The patient's blood test results were normal; however, due to prolonged respiratory and psychological symptoms, 4 days before the visit, the patient was admitted to the otolaryngology department of the hospital. The patient's appetite and cold symptoms improved during hospitalization, and he was discharged on the fifth day of hospitalization. However, the patient was referred to a psychiatrist on the day of discharge due to worsening of insomnia and anxiety.
The patient had severe insomnia. Palpitations, restlessness, and anxiety were the main complaints. No psychotic symptoms, such as hallucinatory delusions or mood episodes, were observed. As the patient was unaware that the discontinuation of pregabalin could potentially have caused these symptoms, he did not mention pregabalin discontinuation. As an initial treatment, zolpidem 10 mg/d was prescribed for insomnia.
Two days later, his insomnia and psychiatric symptoms persisted. A detailed review of the patient's medical history during this visit suggested the possibility of pregabalin withdrawal; therefore, 300 mg/d of pregabalin was resumed from the same day. His respiratory distress, palpitations, and insomnia rapidly improved within the next few days. The dose of pregabalin was returned to 600 mg/d. After 16 days, the patient's anxiety symptoms disappeared, no sleeping pills were required, and the follow‐up was completed.
2.2. Case 2
A 74‐year‐old woman was referred to the psychiatry department of our hospital. She had a history of shingles. She had no history of mental disorders. She was being treated with 150 mg/d of pregabalin for postherpetic neuralgia for 10 years and brotizolam of 0.25 mg/d for insomnia for 11 years. A year before the referral, the patient was instructed by her orthopedic surgeon to reduce the intake of pregabalin. However, she consistently failed to reduce the dose of the medication owing to physical and psychological discomfort. Sixteen days before her referral, pregabalin was switched to tramadol hydrochloride acetaminophen combination tablets due to pain. Nine days before the referral, the patient experienced cold sweats, headaches, and finger tremors. In addition, 6 days before the referral, the patient started to experience insomnia, loss of appetite, dyspnea, lacrimation, epigastric discomfort, insomnia, and depression. Two days before the referral, the patient felt restless and suicidal, and she attempted suicide by strangling herself with a towel, after which the patient was brought to our emergency department by her family. Examinations conducted in the emergency room, including head computed tomography (CT) scans, were normal. Subsequently, she was referred to the psychiatric department for continued suicidal ideation and restlessness (16 days after pregabalin discontinuation).
The patient awoke several times every 1‐2 hours and consumed very little food. The patient experienced depression, restlessness, anxiety, and suicidal ideation. No manic or psychotic episodes were observed. Following this, pregabalin withdrawal was suspected, and pregabalin 150 mg/d was resumed.
Two days later, her psychiatric symptoms, except for insomnia, had disappeared. Her insomnia had also improved rapidly after resuming pregabalin. Therefore, patient follow‐up was terminated.
2.3. Case 3
A 69‐year‐old man was referred to the psychiatry department of our hospital. The patient's medical history included hypertension and sciatica, with no history of mental illness. The patient was being treated with 40 mg/d of telmisartan for hypertension. The patient was administered 600 mg/d of pregabalin for sciatica for 19 years. The patient had been instructed by his doctor to reduce pregabalin intake according to his pain. However, the patient found this challenging since he previously experienced chest tightness during the dose reduction period. The patient suddenly stopped pregabalin intake 2 days before the referral as he had lost the prescribed pregabalin. The patient started to experience chest tightness, suffocation, chills, and insomnia from the same evening. He visited our emergency department for 2 consecutive days after pregabalin discontinuation, complaining of the above symptoms. Blood tests and chest CT scans were normal. An emergency physician considered that the patient had psychiatric issues and immediately referred him to the psychiatric department.
The patient started to experience insomnia and was experiencing depression, chest anguish, chills, and loss of appetite, with no psychotic episodes or symptoms. Pregabalin withdrawal was then suspected, and pregabalin was immediately resumed at 600 mg/day.
After resuming pregabalin, symptoms such as chest anguish, chills, insomnia, loss of appetite, and depressed mood improved promptly. After 7 days, all symptoms that appeared after discontinuation of pregabalin were improved; therefore, psychiatric follow‐up was terminated. He was subsequently prescribed to continue 600 mg/day of pregabalin, and no recurrence was observed.
3. LITERATURE REVIEW
This qualitative systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) recommendations for reporting systematic reviews and meta‐analyses.7
3.1. Literature search
A PubMed search was conducted (last search: April 4, 2021) with combinations of the following keywords: """pregabalin""[Title/Abstract] AND (""Abuse""[Title/Abstract] OR ""withdraw*""[Title/Abstract] OR ""Discontinuation""[Title/Abstract])" Studies in any language that met the following criteria were included; (a) patients taking a regular dose of pregabalin up to 600 mg/d: (b) patients experiencing pregabalin withdrawal (psycho pathogenic symptoms, vegetative symptoms, or neurologic and physical complications) after discontinuation or taper of a regular dose of pregabalin: (c) studies detailing the following information on cases of pregabalin withdrawal: age, sex, pregabalin doses, withdrawal symptoms, and the course of pregabalin withdrawal: 4) case report or case series in full text.
3.2. Process of study selection
The screening of eligible publications was carried out independently by Ha.I and MT First, the titles and abstracts of all publications were reviewed. In the first screening, we included studies with designs other than case reports or case series to detect studies that may have been missed in our systematic literature search. Next, the full text of potentially relevant publications was reviewed, and ineligible publications were excluded. Discrepancies were resolved by consensus, and any disagreement was resolved by a systematic and thorough discussion with Hi.I.
3.3. Data extraction and risk of bias assessment
The following data were extracted by Ha.I and cross‐checked by M.T: (a) author, year of publication; (b) patients’ key characteristics, such as age, sex, and comorbid psychiatric disorders; (c) Pregabalin dosage and duration; (d) withdrawal symptoms of pregabalin (psycho pathogenic symptoms, vegetative symptoms, and neurologic and physical complications); (6) discontinuation or taper period of pregabalin, the onset of symptoms after discontinuation of pregabalin.
We decided not to conduct a risk of bias assessment because important information about pregabalin withdrawal was included in the inclusion criteria.
3.4. Results of the literature review
Figure 1 shows a flow diagram of the literature search process. We identified 463 articles through the systematic literature search. Of these, six case reports (six cases) were identified in which withdrawal symptoms occurred in patients administered pregabalin at the usual dose of 600 mg/d or lower.8, 9, 10, 11, 12, 13 Table 1 shows the demographic data and clinical symptoms of the six cases. Among these cases, three patients had no comorbid mental or substance use disorders. Among all patients, the dose of pregabalin ranged from 150 to 600 mg/d, and the duration of pregabalin use ranged from a few weeks to many years. Of these, five had psychopathological symptoms, three had vegetative symptoms, and three had neurologic and physical complications.
FIGURE 1.
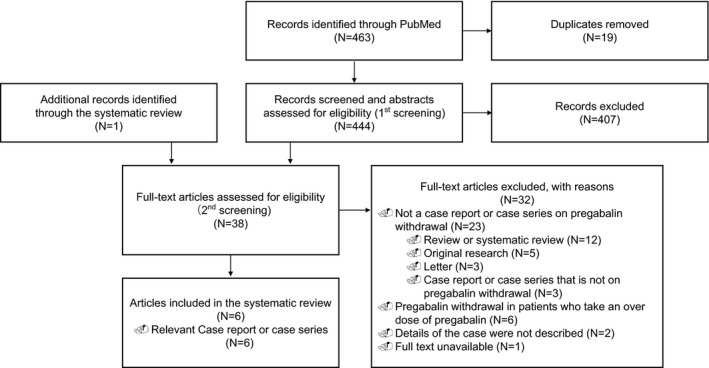
Flowchart of the study selection process for the studies included in the review
TABLE 1.
Summary of pregabalin withdrawal in cases taking a regular dose of pregabalin
| Case report citation | Case | Psychiatric disorders | PGB maximum dosage | PGB duration | Discontinuation or taper period | Onset of symptoms after discontinuation of PGB | Withdrawal symptoms | ||
|---|---|---|---|---|---|---|---|---|---|
| Psychopathologic symptoms | Vegetative symptoms | Neurologic and physical complications | |||||||
| Uçar 20208 | 30 F | None | 150 mg/d | 45 days | Discontinuation | 48 hours | Insomnia, dysphoria, delusions, visual hallucinations, and the idea of harm | ND | ND |
| Naveed 20189 | 62 F | GAD and DD | 450 mg/d | ND | ND | ND | Anxiety, fear, insomnia, suicidal ideations, and feeling of going crazy. | Tremors, dyspnea, palpitations, and dizziness. | Chest pain, extreme weakness of legs, and aches. |
| Du 201710 | 52 F | None | 150 mg/d | Many years | Discontinuation | 4 days | ND | ND | Tonic‐clonic seizure |
| Driot 201611 | 24 F | GAD, DD, PD, and anorexia | 300 mg/d | A few weeks | No tapering or discontinuation | ND | Asthenia, insomnia, and suicidal ideas | Tremors, sweating | ND |
| Gahr 201312 | 38 F | PD and DD | 600 mg/d | 2 months | 25 mg/d | 10 days | Psychomotor agitation and craving | Hypertension, tachycardia, and tremor | ND |
| Oaklander 200513 | 80 F | None | 375 mg/d | 49 weeks | 0 | 30 hours | Visual and auditory hallucinations, alexia, distorted color perception, delirium, terror | ND | Nausea, headache, “imbalance,” |
| Case 1 | 39 M | None | 600 mg/d | 2 months | 0 | 2 days | Insomnia, anxiety, and restlessness | Palpitations | difficulty in breathing, and loss of appetite |
| Case 2 | 74 F | None | 150 mg/d | 10 years | 0 | 7 days | Insomnia, depressed mood, anxiety, restlessness, suicidal ideation, and psychological discomfort | Tremor, cold sweats, lacrimation | Physical discomfort, headache, appetite loss, dyspnea, and epigastric discomfort |
| Case 3 | 69 M | None | 600 mg/d | 19 years | 0 | 1 day | Insomnia and depressed mood | None | Chest tightness, suffocation, chills, and loss of appetite |
Abbreviations: DD, depressive disorder; GAD, generalized anxiety disorder; ND, not described; PD, personality disorder; PGB, pregabalin.
4. DISCUSSION
In this case series, we present three cases of pregabalin withdrawal experienced after discontinuation in patients without a history of psychiatric or substance use disorders taking regular doses of pregabalin. Our case series sheds light on the scant evidence from previous research on physical dependence in patients taking regular doses of pregabalin. Furthermore, our cases were also valuable in demonstrating that pregabalin withdrawal can occur even after a relatively short period of 2 months.
On pregabalin withdrawal, our cases experienced psychopathological symptoms such as anxiety, restlessness, and insomnia; vegetative symptoms such as palpitations, sweating, and shedding tears were also observed, along with neurologic and physical complications, such as headache, dyspnea, and loss of appetite. The symptoms experienced by our patients after discontinuation of the regular dose of pregabalin were similar to those reported in previous studies of pregabalin and gabapentin, which has the same pharmacological mechanism as pregabalin.9, 12, 13, 14, 15, 16, 17, 18 Considering that our patients had no psychiatric disorders, our diagnosis was reasonable because the symptoms were caused by pregabalin withdrawal. The package insert contains a warning that the abrupt discontinuation of pregabalin can induce symptoms such as insomnia, nausea, headache, anxiety, hyperhidrosis, and diarrhea;19 however, it should be noted that other symptoms such as tremors, suicidal ideation, and hallucinations can also occur as withdrawal symptoms of pregabalin.
Possible mechanisms by which withdrawal from pregabalin may result in psychiatric symptom is unclear. However, considering that pregabalin and gabapentin have a common pharmacological mechanism of binding to the α2δ subunit of voltage‐gated Ca2+ channels and consequently reducing the release of excitatory neurotransmitters by inhibiting Ca2+ influx into the nerve4 and that the withdrawal symptoms of pregabalin and gabapentin are similar,9, 12, 13, 14, 15, 16, 17, 18 this pharmacological feature may directly or indirectly cause withdrawal symptoms.
It is also interesting to note that our cases developed pregabalin withdrawal after discontinuing a regular dose of pregabalin. The intake of pregabalin in two patients was 600 mg/d, while that in one patient was 150 mg/d. As shown in Table 1, there have been only six prior cases of pregabalin withdrawal due to the discontinuation of regular doses of pregabalin. This study suggests that withdrawal can occur even in cases without pregabalin dependence or abuse. The dose and duration of pregabalin use were previously found to correlate with pregabalin withdrawal significantly.20 Therefore, clinicians must be aware of the possibility of physical dependence with the long‐term use of pregabalin, even at regular doses. It is also important to consider the duration of pregabalin use. Case 1 showed pregabalin withdrawal after only 2 months of pregabalin use, and previous reports have also shown pregabalin withdrawal in cases after a few weeks or 45 days of short‐term pregabalin use.8, 11 Case 1 was particularly worth reporting because it suggested that even a short period of a regular dose of pregabalin can cause physical dependence. Since the risk factors for physical dependence during the intake of a regular dose of pregabalin have not been fully elucidated, it may be necessary to gradually taper off the dose of pregabalin while paying close attention to pregabalin withdrawal symptoms, even if a regular dose is used for a short period of time. Further well‐designed prospective studies are warranted to examine the incidence and risk factors of pregabalin withdrawal in patients taking regular doses of pregabalin.
5. CONCLUSION
Since pregabalin withdrawal can occur even with regular doses and short‐term use, clinicians must carefully reduce the dose of pregabalin when reducing or discontinuing treatment, paying close attention to withdrawal symptoms.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the writing of the paper. M.T and Ha.I conducted a systematic literature search. All authors agree to be accountable for all aspects of this study.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
Since this study was a case series and systematic review, it was exempted from the approval of the Ethics Committee. The Ethics Committee Akita University Graduate School of Medicine and Faculty of Medicine determined that there was no need to review this case.
INFORMED CONSENT
Written informed consent was obtained from each patient for the publication of this case series.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.jp) for English language editing.
Ishikawa H, Takeshima M, Ishikawa H, Ayabe N, Ohta H, Mishima K. Pregabalin withdrawal in patients without psychiatric disorders taking a regular dose of pregabalin: A case series and literature review. Neuropsychopharmacol Rep. 2021;41:434–439. 10.1002/npr2.12195
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Pregabalin for neuropathic pain in adults. Cochrane Database Syst Rev. 2019;1(1):CD007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Generoso MB, Trevizol AP, Kasper S, Cho HJ, Cordeiro Q, Shiozawa P. Pregabalin for generalized anxiety disorder: an updated systematic review and meta‐analysis. Int Clin Psychopharmacol. 2017;32(1):49–55. [DOI] [PubMed] [Google Scholar]
- 3.Pulman J, Hemming K, Marson AG. Pregabalin add‐on for drug‐resistant partial epilepsy. Cochrane Database Syst Rev. 2014;12(3):CD005612. [DOI] [PubMed] [Google Scholar]
- 4.Patel R, Dickenson AH. Mechanisms of the gabapentinoids and α 2 δ‐1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. 2016;27(4):e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet U, Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol. 2017;27(12):1185–215. [DOI] [PubMed] [Google Scholar]
- 6.Evoy KE, Sadrameli S, Contreras J, Covvey JR, Peckham AM, Morrison MD. Abuse and misuse of pregabalin and gabapentin: a systematic review update. Drugs. 2021;81(1):125–56. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uçar H, Gundogmus İ, Bolu A, Çelik C. Transient psychosis after low‐dose pregabalin discontinuation. Am J Ther. 2020. [DOI] [PubMed] [Google Scholar]
- 9.Naveed S, Faquih AE, Chaudhary AMD. Pregabalin‐associated discontinuation symptoms: a case report. Cureus. 2018;10(10):e3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du YT, Roberts AP, Torpy DJ. Seizure induced by sudden cessation of pregabalin in a patient with chronic kidney disease. BMJ Case Rep. 2017:bcr‐2016‐219158. 10.1136/bcr-2016-219158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driot D, Chicoulaa B, Jouanjus E, Dupouy J, Oustric S, Lapeyre‐Mestre M. Pregabalin use disorder and secondary nicotine dependence in a woman with no substance abuse history. Therapie. 2016;71(6):575–8. [DOI] [PubMed] [Google Scholar]
- 12.Gahr M, Franke B, Freudenmann RW, Kölle MA, Schönfeldt‐Lecuona C. Concerns about pregabalin: further experience with its potential of causing addictive behaviors. J Addict Med. 2013;7(2):147–9. [DOI] [PubMed] [Google Scholar]
- 13.Oaklander AL, Buchbinder BR. Pregabalin‐withdrawal encephalopathy and splenial edema: a link to high‐altitude illness? Ann Neurol. 2005;58(2):309–12. [DOI] [PubMed] [Google Scholar]
- 14.Aldemir E, Altıntoprak AE, Coşkunol H. Pregabalin dependence: a case report. Turk Psikiyatri Derg. 2015;26(3):217–20. [PubMed] [Google Scholar]
- 15.Grosshans M, Mutschler J, Hermann D, et al. Pregabalin abuse, dependence, and withdrawal: a case report. Am J Psychiatry. 2010;167(7):869. [DOI] [PubMed] [Google Scholar]
- 16.Gundogmus İ, Karagöz A, Algül A. First‐episode psychosis induced by pregabalin withdrawal: a case report. Psychiatr Clin Psychopharmacol. 2018;28(4):461–3. [Google Scholar]
- 17.Nordgaard J, Jurgens G. A case of pregabalin abuse. XXXIII International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT). 2013. Copenhagen, Denmark, Clinical Toxicology, 51(4):320. [conference abstract]. Available from: https://www.tandfonline.com/doi/full/10.3109/15563650.2013.785188 [Google Scholar]
- 18.Mah L, Hart M. Gabapentin withdrawal: case report in an older adult and review of the literature. J Am Geriatr Soc. 2013;61(9):1635–7. [DOI] [PubMed] [Google Scholar]
- 19.Inc P [internet]. Highlights of prescribing information [cited 28 April 2021]. Available from: http://labeling.pfizer.com/showlabeling.aspx?id=561
- 20.İlhanlı İ. Gabapentinoids in penitentiaries: an abuse and addiction research. Turk J Phys Med Rehabil. 2017;63(4):318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


