Abstract
Aim
Cannabis‐based medicinal products (CBMPs) are prescribed with increased frequency, despite a paucity of high‐quality randomized controlled trials. The aim of this study is to analyze the early outcomes of the first series of patients prescribed CBMPs in the UK with respect to effects on health‐related quality of life and clinical safety.
Methods
A prospective case series was performed using the UK Medical Cannabis Registry. Primary outcomes were change in patient‐reported outcomes measures (EQ‐5D‐5L, General Anxiety Disorder‐7 (GAD‐7) and Single‐Item Sleep Quality Scale (SQS)) at 1 and 3 months from baseline. The secondary outcome was the incidence of adverse events. Statistical significance was defined by a P‐value <.050.
Results
There were 129 patients included in the final analysis with a mean age of 46.23 (±14.51) years. The most common indication was chronic pain of undefined etiology (n = 48; 37.2%). The median initial cannabidiol and (−)‐trans‐Δ⁹‐tetrahydrocannabinol daily dose was 20.0 mg (Range: 0.0‐768.0 mg) and 3.9 mg (Range: 0.0‐660.0 mg), respectively. Statistically significant improvements in health‐related quality of life were demonstrated at 1 and 3 months in GAD‐7, SQS, EQ‐5D‐5L pain and discomfort subscale, EQ‐5D‐5L anxiety and depression subscale, EQ‐VAS and EQ‐5D‐5L index values(P < .050). There were 31 (24.03%) total reported adverse events.
Conclusion
This study suggests that CBMP therapy may be associated with an improvement in health‐related quality‐of‐life outcomes as self‐reported by patients. CBMPs are also demonstrated to be relatively safe in the short to medium‐term. These findings must be treated with caution given the limited scope of this initial analysis, with no placebo or an active comparator, with further research required.
Keywords: cannabinoids, medical cannabis, sapphire clinic, UK registry
This study described the early outcomes of the first case series of patients prescribed CBMPs in the UK. Early data suggests improvement in health‐related quality of life and acceptable safety outcomes.
1. INTRODUCTION
Cannabis‐based medicinal products (CBMPs) are prescribed with increasing frequency for selected conditions, with more countries and jurisdictions legalizing their use for a range of different medical indications.1 This is also true of the UK, since the introduction of legislative reform in November 2018 legalized CBMPs prescribed for medicinal purposes.2 However, the National Institute for Health and Care Excellence (NICE) currently recommends the use of CBMPs for just four conditions, only when other treatments have proven ineffective or inappropriate: spasticity in adults with multiple sclerosis, severe treatment‐resistant epilepsy in Lennox‐Gastaut and Dravet Syndromes and chemotherapy‐induced nausea and vomiting.3 The NICE review additionally demonstrated improvements in chronic pain clinical outcomes, however, it exceeded the incremental cost‐effectiveness ratio threshold of £20 000 to £30 000 per quality adjusted life years to recommend prescribing in the National Health System (NHS).3 Physicians in the United Kingdom may prescribe CBMPs for a condition if licensed treatments, both on and off‐license, have been trialed without providing benefit or if there is a clinically appropriate reason why licensed treatments would be inappropriate.4
In spite of the limited indications for accessing CBMPs in the NHS in the UK, there is a growing body of literature that suggests cannabinoids, and other active pharmaceutical ingredients within the Cannabis plant, have potent effects on neurotransmission, neuroendocrine signaling, and inflammatory processes.5 Such discoveries open a plethora of possibilities for the potential use of CBMPs in chronic primary pain, cancer pain, and neuropathic pain management.6, 7, 8 Possible benefits have also been shown for neurological and some psychiatric conditions, such as anxiety‐predominant disorders.9 There is a paucity of high‐quality evidence to guide best practices and optimal therapeutic regimes, however.
Cannabis‐based medicinal products represent a broad spectrum of pharmaceutical products with complex pharmacology. Available formulations range from single isolate cannabinoids to full‐spectrum products containing a range of cannabinoids, terpenes, flavonoids, and other compounds with individual pharmacokinetic and pharmacodynamic properties, which increases the potential for interactions.10, 11, 12 Therefore, it is challenging to study the potential outcomes of these medications within the context of individual clinical trials due to time, resource, and financial constraints. Due to these methodological limitations, conclusions of most existing clinical trials report the benefits of CBMPs as ‘probably beneficial’ or ‘unclear’. Systematic reviews highlight the inconsistencies between reported efficacy, tolerability, and safety in published randomized controlled trials.1, 13 Consequently, the evidence base, while broad, is inconclusive and as such insufficient for informing licensing agencies and guidelines.
Observational studies are a potential alternative to clinical trials, which could be a complementary source of high‐quality data on CBMPs. The analysis of the first 400 patients receiving prescribed cannabidiol (CBD) in New Zealand demonstrated an overall increase in quality of life, with a reduction in non‐cancer pain, anxiety, and depression symptoms.14 Additionally, no major adverse events were reported.14 A 12‐month longitudinal study of self‐medicating cannabis users in Spain exhibited no mental health deterioration or personality change.15 Despite encouraging early evidence, questions about optimal treatment regimens, potential adverse events, the effectiveness of formulations, and patient selection remain.
Formalized patient registries are emerging as a source of high‐quality, comprehensive observational data that can assist in addressing these questions.16, 17, 18 They are an important tool for post‐approval monitoring of drugs, especially for innovative and off‐license medications, for which conventional outcomes data collection methods might not be feasible or practical.19 By collecting prospective data, patient registries help to answer clinically relevant questions on safety and effectiveness, whilst being reflective of real‐world prescribing and usage patterns.20 The UK Medical Cannabis Registry was developed to capture the overwhelming majority of patients treated with CBMPs, which has occurred outside of the NHS, and to help in bridging the knowledge gap and provide valuable insight.
Herein we describe the analysis of outcomes of the first cohort of enrolled patients to the UK Medical Cannabis Registry, which was set up in December 2019, the first of its kind in the UK. We aimed to investigate prescribed formulations, adverse events, and patient‐reported outcome measures.
2. METHODS
2.1. Study overview
A case series of patients prescribed CBMPs for a number of indications was conducted using the UK Medical Cannabis Registry and herein reported in accordance with the STROBE statement for reporting observational studies.21 In accordance with the NHS Health Research Authority and Research Ethics Committees’ guidance, this study was adjudged to not require formal ethical approval. All participants completed written, informed consent prior to enrolment in the registry.
2.2. Setting and participants
The UK Medical Cannabis Registry is the first such prospective registry launched in the UK and captures prospective pseudonymized data on patients treated with CBMPs produced according to Good Manufacturing Practice criteria. The UK Medical Cannabis Registry is privately owned and managed by Sapphire Medical Clinics. Clinicopathological information, comorbidities, drug, and alcohol history and medication information are entered prospectively by clinical staff following an initial consultation. Patient‐reported outcome measures (PROMs), clinical effectiveness measures, and adverse event questionnaires are remotely administered to patients through an online web‐based platform at baseline, 1 month, 3 months, 6 months, and then 6 monthly intervals thereafter. To date, the only clinic to routinely require registration of patients with the UK Medical Cannabis Registry is Sapphire Medical Clinics, which, through utilizing remote consulting, treats patients across all four nations in the United Kingdom and the Channel Islands. Prior to registration participants are required to give informed consent otherwise their data is withheld from the registry.
2.3. Patient and data selection
For this analysis data were extracted for the initial participants of the UK Medical Cannabis Registry who were prescribed CBMPs and had recorded PROMs at baseline with follow‐up at 1 and/or 3 months from that date. Regulations in the UK stipulate that treatment with CBMPs can only be considered once licensed treatments have proven ineffective or inappropriate.4
Primary, secondary and tertiary conditions, as identified by the treating clinician, included those for which CBMPs were prescribed with the intention of treating associated symptoms or sequelae of disease. These included anxiety, autistic spectrum disorder, cancer pain, complex regional pain syndrome, Crohn's disease, depression, Ehlers–Danlos, epilepsy, fibromyalgia, headaches, insomnia, migraine, multiple sclerosis, neuropathic pain, obsessive‐compulsive disorder, palliative care, Parkinson's Disease, and post‐traumatic stress disorder. Palliative care was defined using World Health Organization terminology as ‘the prevention and relief of suffering of adult and pediatric patients and their families facing the problems associated with life‐threatening illness.22 Patients with chronic pain caused by an alternative pathology, which could not be described by the terminology previously listed, were defined as ‘chronic pain of undefined etiology’. This, therefore, describes a heterogeneous group of patients with both chronic primary and secondary pain. Palliative care was defined as those patients treated for physical and psychological symptoms caused by life‐threatening or life‐limiting conditions, or the treatments for those illnesses.
Data regarding demographic details including age, sex, and occupation were recorded. Participant body mass index (BMI) was also extracted. Patient comorbidities were recorded, including hypertension, depression and/or anxiety, arthritis, epilepsy, and endocrine dysfunction. In addition, the relevant co‐morbidities contributing to the Charlson comorbidity index, a widely used prognostic scoring model for 10‐year mortality, were collected and a score calculated for each patient.23, 24, 25
Drug and alcohol data on patients were extracted and analyzed including smoking status, smoking pack years, alcohol units per week, and cannabis status. For those who had previously or were presently taking non‐prescription cannabis a novel metric of ‘gram years’ was calculated.
Cannabis gram years = average cannabis consumption in grams per day x years of use
The gram year metric was devised to quantify the potential of prior cannabis use on developing biological tolerance to the effects of cannabis which is known to be related to the quantity of cannabis consumed and the length of time over which it has been repeatedly consumed.26, 27 This is not a validated metric, however.
Medication data were also recorded for prescriptions in the following British National Formulary (BNF) chapters:
Analgesics
Anticoagulants and protamine
Antidepressants
Antidiabetic Drugs
Antiplatelets
Hypnotics and Anxiolytics
Oral morphine equivalents were calculated for prescribed opioid medications in accordance with conversion factors quoted by the BNF.28
All CBMP prescriptions were recorded and analyzed including company, formulation, method of administration, CBD concentration, (−)‐trans‐Δ⁹‐tetrahydrocannabinol (THC) concentration, and strain.
All participants completed three quality‐of‐life PROMs, including EQ‐5D‐5L, General Anxiety Disorder‐7 (GAD‐7) and Single‐Item Sleep Quality Scale (SQS).29, 30, 31 The EQ‐5D‐5L is a two‐part tool that initially measures the quality of life across five domains (mobility, self‐care, usual activities, pain or discomfort, anxiety or depression) with five levels of severity (1–no problems; 2–slight problems; 3–moderate problems; 4–severe problems; 5–extreme problems). Each of these levels and dimensions is combined to create a 5‐digit code representing one of 3125 health states.29 The combined health state is mapped to EQ‐5D‐5L index values for analysis utilizing the mapping function described by Van Hout et al, the preferred mapping function of NICE for measuring health‐related quality of life of UK populations.32, 33 An index score of 1, represents full health (health state 11 111), whilst a score below 0 represents a health state worse than death.32 The second part of the EQ‐5D‐5L is the EQ‐visual analog scale (EQ‐VAS) which consists of a vertical scale of 0 to 100, whereby “100” corresponds to the “best health you can imagine” and “0” corresponds to the “worst health you can imagine”.29 For the GAD‐7 score registry participants are asked about how often over the past two weeks they had been bothered by the core symptoms of generalized anxiety disorder, generating a score from 0 to 21.30 Mild, moderate, and severe anxiety symptoms are defined by thresholds of ≥5, ≥10, and ≥15, respectively. A general population validation suggests that mean GAD‐7 scores for men and women are 2.66 and 3.20, respectively.30 The SQS is a validated question of sleep quality over the past seven days only with sleep quality rated from 0 to 10, wherein “10” signifies “excellent” and “0” denotes “terrible”.31
Participants reported adverse events at one and three months from baseline, either through self‐reporting or during routine follow‐up with a clinician. These were subsequently inputted into the registry. Adverse events were recorded in accordance with the common terminology criteria for adverse events version 3.0.34
Primary outcome measures for this initial analysis were change in quality‐of‐life metrics at 1 and 3 months from baseline. The registry also contains data relating to condition‐specific effectiveness measures and PROMs however the reporting of these data will be the subject of future peer‐reviewed publications, once sufficient condition‐specific data is available.
2.4. Statistical analysis
Demographic variables, conditions, tobacco and alcohol use, cannabis status, medication data, and adverse events were analyzed using descriptive analysis. Data from PROMs were analyzed compared to baseline at 1 and 3 months. Each data set was confirmed to be parametric or non‐parametric via a Shapiro–Wilk Test. Parametric data are presented as a mean value ± (standard deviation (±SD)), whilst non‐parametric data is presented as median (range). Analysis was performed with paired t‐test or Wilcoxon rank‐sum test depending on whether the data was parametric or non‐parametric, respectively. Statistical significance was defined using P‐value <.050. All statistics were performed using Statistical Package for Social Sciences (SPSS) [IBM Statistics version 27 SPSS Inc].
3. RESULTS
Initial data extraction included the first 210 patients who had been registered on the UK Medical Cannabis Registry. On excluding those without completion of baseline PROMs, 129 patients were included in the final data analysis. Of these, 70 patients had recorded PROMs at one month, whilst 50 patients had recorded PROMs at 3 months.
Demographic details are presented in Table 1. The mean age of patients was 46.23 (±14.51). Sixty‐three (48.8%) of patients were female. The highest occupation category registered was other occupations (n = 65; 50.4%). Of these 36 (27.9%) were unemployed. The mean BMI of participants was 26.02 (±6.22).
TABLE 1.
Demographic details of study participants
| Demographic details | n (%)/mean (±SD) |
|---|---|
| Sex | |
| Female | 63 (48.8%) |
| Male | 66 (51.2%) |
| Age | 46.23 ± 14.51 |
| Occupation | |
| Clerical support workers | 1 (0.8%) |
| Craft and related trades workers | 4 (3.1%) |
| Elementary occupations | 5 (3.1%) |
| Managers | 11 (8.5%) |
| Plant and machine operators, and assemblers | 3 (2.3%) |
| Professional | 20 (15.5%) |
| Service and sales workers | 3 (2.3%) |
| Skilled agricultural, forestry and fishery workers | 2 (1.6%) |
| Technicians and associate professionals | 4 (3.1%) |
| Other occupations* | 65 (50.4%) |
| Body Mass Index | 26.02 ± 6.22 |
Other Occupations – Unemployed (n = 36), Retired (n = 14), all else (n = 1) [not described to avoid indirect personal identification].
Table 2 outlines the primary diagnosis for which treatment was initiated. The most common primary diagnosis was chronic pain of undefined etiology (n = 48; 37.2%), followed by neuropathic pain (n = 22; 17.1%) and anxiety (n = 11; 8.5%). A total of 86 (66.7%) patients had a primary diagnosis of a condition associated with chronic pain. Forty‐four (34.1%) and eight (6.2%) patients, respectively, also had a secondary or tertiary indication of pain for CBMP therapy. The median Charlson comorbidity index was 0 (Range: 0‐11). The incidence of hypertension (n = 9; 7.0%), depression and/or anxiety (n = 39; 30.2%), arthritis (n = 22; 17.1%); epilepsy (n = 5; 3.9%); endocrine dysfunction (n = 4; 3.1%) was also recorded.
TABLE 2.
Patient primary, secondary and tertiary diagnoses
| Diagnosis |
Primary n (%) |
Secondary n (%) |
Tertiary n (%) |
|---|---|---|---|
| Chronic pain of undefined etiology | 48 (37.2%) | 12 (9.3%) | 3 (2.3%) |
| Neuropathic pain | 22 (17.1%) | 13 (10.1%) | 2 (1.6%) |
| Anxiety | 11 (8.5%) | 13 (10.1%) | 4 (3.1%) |
| Fibromyalgia | 8 (6.2%) | 11 (8.5%) | 1 (0.8%) |
| Ehlers–Danlos | 7 (5.4%) | 5 (3.9%) | 2 (1.6%) |
| PTSD | 7 (5.4%) | 1 (0.8%) | 0 (0.0%) |
| Palliative care | 6 (4.7%) | 0 (0.0%) | 0 (0.0%) |
| Epilepsy adult | 4 (3.1%) | 1 (0.8%) | 0 (0.0%) |
| Migraine | 3 (2.3%) | 2 (1.6%) | 5 (3.9%) |
| Depression | 3 (2.3%) | 2 (1.6%) | 4 (3.1%) |
| Insomnia | 2 (1.6%) | 3 (2.3%) | 2 (1.6%) |
| Headache | 2 (1.2%) | 0 (0.0%) | 0 (0.0%) |
| Autistic spectrum disorder | 1 (0.8%) | 2 (1.6%) | 1 (0.8%) |
| Crohn's Disease | 1 (0.8%) | 1 (0.8%) | 0 (0.0%) |
| OCD | 1 (0.8%) | 1 (0.8%) | 0 (0.0%) |
| Complex regional pain syndrome | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) |
| Multiple sclerosis | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) |
| Parkinson's | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) |
| Agoraphobia | 0 (0.0%) | 1 (0.8%) | 0 (0.0%) |
| Cancer pain | 0 (0.0%) | 3 (2.3%) | 0 (0.0%) |
| Cluster headaches | 0 (0.0%) | 1 (0.8%) | 0 (0.0%) |
| Ulcerative colitis | 0 (0.0%) | 1 (0.8%) | 0 (0.0%) |
| Chemotherapy induced nausea and vomiting | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) |
| Eating Disorder | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) |
Seventy‐one (55.0%) participants had never smoked, 26 (20.2%) were ex‐smokers and 27 (20.9%) were current smokers on registration. The mean alcohol consumption per week was 5.93 (±14.61) units. Fifty‐eight (45%) participants had never used cannabis, 14 (10.9%) participants were ex‐cannabis consumers, and 52 (40.3%) were current cannabis users. The median oral morphine equivalent of those prescribed opioid medication at baseline was 25.5 mg (Range 3.0 mg–750.0 mg). The median oral morphine equivalent for the entire patient cohort was 0.0 mg (Range: 0.0 mg–750.0 mg).
3.1. Cannabis‐based medicinal products dosing and mode of administration
The median number of CBMPs prescribed at the initiation of therapy was 2, with 33 (25.6%), 86 (66.7%), 8 (6.2%), and 2 (1.6%) being prescribed 1 to 4 different CBMPs, respectively. The majority of patients were on at least one oil preparation with only 17 (13.2%) patients not prescribed an oil preparation, which is prescribed for oral or sublingual administration. In regard to vaporized dry flower preparations (flos or granulate), 29 (22.5%) were prescribed one preparation, and 5 (3.9%) were prescribed two preparations. The median initial CBD dose was 20.0 mg (Range: 0.0‐768.0 mg). The median initial THC dose was 3.9 mg (Range: 0.0‐660.0 mg).
3.2. Patient‐reported outcome measures
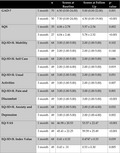
Table 3 outlines in full paired results from baseline to 1 month and 3 months. Statistically significant improvements in health‐related quality of life were demonstrated at 1 and 3 months in GAD‐7, SQS, EQ‐5D‐5L pain and discomfort subscale, EQ‐5D‐5L anxiety and depression subscale, EQ‐VAS scores, and EQ‐5D‐5L index values (P < .050). Statistically significant improvements were demonstrated at 3 months only in the EQ‐5D‐5L usual activities subscale (P < .050).
TABLE 3.
Paired baseline and follow‐up patient‐reported outcome measures
| n | Scores at baseline | Scores at follow up | P‐value | ||
|---|---|---|---|---|---|
| GAD‐7 | 1 month | 70 | 6.50 (0.00‐24.00) | 5.00 (0.00‐22.00) | .001 |
| 3 month | 50 | 7.50 (0.00‐24.00) | 4.50 (0.00‐19.00) | <.001 | |
| SQS | 1 month | 35 | 4.06 ± 2.78 | 5.57 ± 2.54 | .002 |
| 3 month | 27 | 4.04 ± 2.46 | 5.78 ± 2.52 | <.001 | |
| EQ‐5D‐5L mobility | 1 month | 68 | 3.00 (1.00‐5.00) | 2.00 (1.00‐5.00) | .102 |
| 3 month | 49 | 2.00 (1.00‐5.00) | 2.00 (1.00‐5.00) | .140 | |
| EQ‐5D‐5L self care | 1 month | 68 | 2.00 (1.00‐5.00) | 2.00 (1.00‐5.00) | .686 |
| 3 month | 49 | 2.00 (1.00‐4.00) | 2.00 (1.00‐5.00) | .819 | |
| EQ‐5D‐5L usual activities | 1 month | 68 | 3.00 (1.00‐5.00) | 2.00 (1.00‐5.00) | .051 |
| 3 month | 49 | 3.00 (1.00‐5.00) | 2.00 (1.00‐5.00) | .007 | |
| EQ‐5D‐5L pain and discomfort | 1 month | 68 | 3.00 (1.00‐5.00) | 3.00 (1.00‐5.00) | .001 |
| 3 month | 49 | 3.00 (1.00‐5.00) | 3.00 (1.00‐5.00) | <.001 | |
| EQ‐5D‐5L anxiety and depression | 1 month | 68 | 2.00 (1.00‐5.00) | 2.00 (1.00‐4.00) | .032 |
| 3 month | 49 | 3.00 (1.00‐5.00) | 2.00 (1.00‐4.00) | .002 | |
| EQ‐VAS | 1 month | 66 | 46.98 ± 20.53 | 53.57 ± 22.47 | <.001 |
| 3 month | 49 | 45.41 ± 22.25 | 59.59 ± 25.49 | <.001 | |
| EQ‐5D‐5L index value | 1 month | 68 | 0.41 ± 0.33 | 0.4747 ± 0.33 | .039 |
| 3 month | 49 | 0.43 ± 0.31 | 0.53 ± 0.30 | .005 |
Abbreviations: EQ‐VAS, EQ‐visual analogue scale; GAD‐7, General Anxiety Disorder‐7; SQS, Sleep Quality Scale.
3.3. Adverse events
Reported adverse events are described in full in Table 4. There were 31 (24.03%) total reported adverse events. The most commonly experienced adverse events were somnolence and constipation, experienced by 4 (3.10%) participants each. There was 1 (0.78%) disabling adverse event of insomnia, which lasted 4 days in duration.
TABLE 4.
Reported adverse events by participants (n = 129)
| Adverse events | Mild | Moderate | Severe | Life‐threatening /disabling | Total (%) |
|---|---|---|---|---|---|
| Amnesia (memory loss) | 1 | 0 | 0 | 0 | 1 (0.78%) |
| Anorexia (lack of appetite) | 0 | 1 | 0 | 0 | 1 (0.78%) |
| Anxiety | 1 | 0 | 0 | 0 | 1 (0.78%) |
| Blurred vision | 1 | 0 | 0 | 0 | 1 (0.78%) |
| Cognitive disturbance | 0 | 0 | 1 | 0 | 1 (0.78%) |
| Concentration impairment | 0 | 1 | 0 | 0 | 1 (0.78%) |
| Constipation | 3 | 1 | 0 | 0 | 4 (3.10%) |
| Coordination/balance/speech | 1 | 1 | 0 | 0 | 2 (1.55%) |
| Dizziness | 0 | 2 | 1 | 0 | 3 (2.33%) |
| Dry mouth | 1 | 0 | 0 | 0 | 1 (0.78%) |
| Fall | 0 | 2 | 0 | 0 | 2 (1.55%) |
| Fatigue | 0 | 0 | 2 | 0 | 2 (1.55%) |
| Headache | 0 | 0 | 1 | 0 | 1 (0.78%) |
| Increased seizures | 0 | 1 | 0 | 0 | 1 (0.78%) |
| Insomnia (inability to sleep) | 0 | 0 | 0 | 1 | 1 (0.78%) |
| Nausea | 3 | 0 | 0 | 0 | 3 (2.33%) |
| Somnolence (sleepy/drowsy) | 0 | 4 | 0 | 0 | 4 (3.10%) |
| Spasticity | 0 | 0 | 1 | 0 | 1 (0.78%) |
| Total | 11 (8.53%) | 13 (10.08%) | 6 (4.65%) | 1 (0.78%) | 31 (24.03%) |
4. DISCUSSION
This analysis presents the initial findings of a section of the first cohort of patients prescribed CBMPs in the UK and captured on the UK Medical Cannabis Registry. Early results demonstrate CBMPs may be associated with improved health‐related quality of life across a broad spectrum of conditions. In particular, there are statistically significant improvements in symptoms of anxiety and depression, sleep quality, pain, and discomfort. The adverse event rate within this analysis was 24.03%, with constipation being the most frequently experienced adverse event (3.10%). These findings, however, must be treated with caution given the limited scope of this initial analysis.
In the present study, patients prescribed CBMPs demonstrated the improved patient‐reported quality of life as illustrated by an increase in mean paired EQ‐5D‐5L index values and EQ‐VAS scores at both 1 and 3 months. This is consistent with results from an audit of 400 patients prescribed CBD oil in New Zealand, which found a mean increase in EQ‐VAS score of 13.6.14 However, the median follow‐up of this study was 36 days.14 The earlier realization of improved quality of life as reported by the EQ‐VAS scale may be secondary to higher initial doses of CBD in the New Zealand‐based study.14 Furthermore, THC was also prescribed to participants of this study which is recognized to have a higher incidence of adverse events in comparison to CBD.35 A study of Spanish patients with chronic disease who self‐medicated with cannabis failed to demonstrate an improvement in quality of life with cannabis with 12 months follow‐up.15 However, these results may be subsequent to patients self‐medicating prior to enrolment, biasing results to the null. Furthermore, the cannabis they were ingesting was unregulated and therefore may have been inconsistent in cannabinoid concentration and therefore dose.
There was a modest improvement in anxiety symptoms within participants in this study as demonstrated by improved GAD‐7 and EQ‐5D‐5L anxiety and depression subscale scores. There is increasing pre‐clinical data to support the role of the endocannabinoid system in modulating anxiety.36 However, there is a paucity of randomized control trial data to support prescribing in generalized anxiety disorder. There is however a small clinical trial demonstrating beneficial effects in social anxiety.37 A comprehensive systematic review and meta‐analysis by Black et al on cannabinoids in the treatment of mental health disorders concluded that there is a paucity of evidence across the spectrum of psychiatric disease.38 However, some very‐low quality evidence suggested that THC improves anxiety symptoms in those with chronic medical conditions.38 These results suggest that there may be a role for the utilization of CBMPs in anxiety symptoms and therefore anxiety disorders, however, this requires further investigation.
Moreover, these results suggest the effectiveness of CBMPs in improving sleep in a cohort of medical cannabis patients. The endocannabinoid system has been implicated in the regulation of the sleep‐wake cycle.39 It is thought to regulate the interaction between the suprachiasmatic nucleus, which drives circadian rhythm in response to light, and sleep.39 A systematic review and meta‐analysis of placebo‐controlled trials of CBMPs demonstrated an improvement in sleep quality and sleep disturbance, when prescribed in the setting of other chronic conditions.35
As described, CBMPs demonstrated improvements in psychological domains such as sleep, anxiety/depression, and discomfort/pain, rather than direct motor function. These results suggest that the general improvements in HRQOL are more closely related to the psychoactive effects of CBMPs, rather than functional improvements. Whilst the psychotropic effects of cannabis are often described in both scientific and lay literature, there is clear pre‐clinical evidence of cannabinoids acting upon peripheral pain receptors.25 Real‐world outcomes of chronic pain patients will be individually investigated within the UK Medical Cannabis Registry, and other settings in the future once sufficient data is available. Whilst central processing is universally recognized as a determinant of overall health within a biopsychosocial model of health,40 further disease‐specific analysis will provide further clarity on whether CBMPs improve motor function in addition to psychological distress in clinical settings. Particular attention in future studies should focus on CBMPs’ psychoactive properties is the risk of developing a cannabis use disorder, particularly in comparison to opioids and benzodiazepines which present a greater risk for dependence and personal harm.41
The adverse event rate of patients in this registry was 24.03%. One patient (0.78%) suffered a disabling adverse event, which was insomnia. An open‐label post‐marketing surveillance study of nabiximols for treatment‐resistant spasticity found an all‐cause adverse event incidence of 31.3%.42 Another post‐marketing safety analysis of nabiximols similarly found a 27.9% all‐cause adverse event rate.43 This suggests that the reported adverse event rate in this registry is close to the true population rate. Whilst self‐reporting of adverse events is a potential limitation it confirms that the methodology applied by the UK Medical Cannabis Registry is satisfactory in capturing adverse events in this setting.
The UK Medical Cannabis Registry is the first UK‐based CBMP registry allowing for prospective longitudinal data capture and observational analysis. The most notable limitation of this dataset is the lack of a comparison arm to account for any potential placebo effect influencing outcomes. In addition, a serious limitation of the study is the high rate at which baseline PROMs were not completed, representing a limitation of this study and remote data collection more broadly. As an observational study, it is also subject to confounding. This is further affected as access to treatment is through a private prescription, therefore limiting inclusion to those able to afford the associated costs. CBMPs are not available via private insurance and are therefore self‐funded by individuals. However, 36 (27.9%) and 14 (10.9%) of the studied population were unemployed or retired suggesting that an absolute bias towards high wealth individuals was not clearly present. The median follow‐up length was 36 days, which limits inferences which can be drawn on long‐term effects of CBMPs. Moreover, 51.2% of participants were either currently or had formerly used cannabis increasing the likelihood of selection bias. This is also represented in a median oral morphine equivalent dose of 0.0, suggesting that a number of patients had also successfully weaned from opioid medications prior to receiving a prescription for medical cannabis. Treatment was administered via a single clinic, however, due to limited prescribing of medical cannabis in the UK on the NHS and more broadly in the UK, this will likely be representative of present CBMP prescribing outcomes in the UK. Whilst this deepens selection bias and decreases internal validity, this study design increases the external validity of determined results to reflect real‐world clinical practice.
Study quality is reliant upon the accurate collection of demographic and clinical data. To limit the effect of inaccurate or missing data was restricted to those with baseline and follow‐up PROMs at one and/or three months. The absence of coded data may subsequently bias the results to the null and therefore fail to identify significant changes where they exist or underestimate the effect size of treatment. However, in the context of adverse event capture, which is completed remotely by participants in the UK Medical Cannabis Registry, the results are similar to previously published post‐marketing studies of nabiximols. Like all registry studies, the extent of missing data is likely to impact the results presented and must be accounted for in their interpretation. The present study is also limited by the heterogeneity of the studied population which, whilst providing an overview of outcomes for all conditions where CBMPs have been prescribed.
Subsequent studies of the UK Medical Cannabis Registry will aim to undertake a disease‐specific approach to analyze short and long‐term outcomes. For chronic pain, specifically, outcomes of patients prescribed CBMPs will be analyzed to identify the effectiveness and safety of its use, with particular attention paid to disease‐specific PROMs and longitudinal opioid requirements. These results suggest that across a broad spectrum of chronic diseases, CBMPs may be associated with a modest, yet statistically significant improvement in health‐related quality of life at 1 month and 3 month follow‐up. This analysis, which is limited in providing insights on long‐term effects of CBMPs, shall be built upon in future publications as these and other patients are followed up longitudinally.
CONFLICT OF INTERESTS
Simon Erridge is a junior doctor and undertakes paid consultancy work at Sapphire Medical Clinics. Simon Erridge is an honorary clinical research fellow at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS. Oliver Salazar and Michal Kawka is a medical student at Imperial College London. Carl Holvey is the chief clinical pharmacist at Sapphire Medical Clinics. Ross Coomber is a consultant orthopedic surgeon, a director, and a shareholder at Sapphire Medical Clinics and a consultant at St George's Hospital, London. The views expressed are those of the author(s) and not necessarily those of the NHS. Azfer Usmani is a pain specialist at Sapphire Medical Clinics (London) and a consultant at Dartford and Gravesham NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS. Mohammed Sajad is a pain specialist at Sapphire Medical Clinics (London) and a consultant at Dudley Group of Hospitals NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS. Sushil Beri is a consultant in pediatrics and a director and a shareholder at Sapphire Medical Clinics (London). The views expressed are those of the author(s) and not necessarily those of the NHS. Jonathan Hoare is a consultant in gastroenterology and a director and a shareholder at Sapphire Medical Clinics (London). The views expressed are those of the author(s) and not necessarily those of the NHS. Shaheen Khan is a consultant in palliative medicine and a director and a shareholder at Sapphire Medical Clinics (London). The views expressed are those of the author(s) and not necessarily those of the NHS. Mark Weatherall is a consultant in neurology and a director and a shareholder at Sapphire Medical Clinics (London). The views expressed are those of the author(s) and not necessarily those of the NHS. Michael Platt is a consultant in pain services and a director and a shareholder at Sapphire Medical Clinics (London). The views expressed are those of the author(s) and not necessarily those of the NHS. James Rucker is a consultant psychiatrist, a director, and a shareholder at Sapphire Medical Clinics (London). James Rucker is an honorary consultant psychiatrist at The South London & Maudsley NHS Foundation Trust, and an NIHR Clinician Scientist Fellow at the Centre for Affective Disorders at King's College London. James Rucker is funded by a fellowship (CS‐2017‐17‐007) from the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health. James Rucker leads the Psychedelic Trials Group at King's College London. King's College London receives grant funding from COMPASS Pathways PLC to undertake phase 1 and phase 2 trials with psilocybin. COMPASS Pathways PLC has paid for James Rucker to attend trial related meetings and conferences to present the results of research using psilocybin. James Rucker has undertaken paid consultancy work for Beckley PsyTech and Clerkenwell Health. Payments for consultancy work are received and managed by King's College London and James Rucker does not benefit personally. James Rucker reviewed this article and made comments. Mikael Sodergren is a consultant hepatopancreatobiliary surgeon, a director, and a shareholder at Sapphire Medical Clinics and a consultant at Imperial College NHS Trust, London. He is an honorary senior clinical lecturer at Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS. Mikael Sodergren is the research director at EMMAC Life Sciences. All authors have contributed to and approved the final manuscript. The authors confirm that the PI for this paper is Mikael H Sodergren and that he had direct clinical responsibility for patients. Simon Erridge, Oliver Salazar, Michal Kawka, Carl Holvey, Ross Coomber, Azfer Usmani, Mohammed Sajad, Sushil Beri, Jonathan Hoare, Shaheen Khan, Mark Weatherall, Michael Platt and James Rucker: had no shareholdings in pharmaceutical companies. SE, CH, RC, SB, JH, SK, MWW, MP, JJR, and MHS are the founding clinicians of Sapphire Medical Clinics, which is the first clinic registered with the CQC to evaluate patients for medical cannabis in England.
AUTHOR CONTRIBUTIONS
Study conception and design: SE, CH, RC, SB, JH, SK, MWW, MP, JRR, MHS; Acquisition of data: SE, OS, CH, RC, AU, MS, SB, JH, SK, MWW, MP, JRR, MHS; Analysis and interpretation of data: SE, OS, MK, MHS; Drafting of manuscript: SE, OS, MK, MHS; Critical revision: SE, OS, MK, CH, RC, AU, MS, SB, JH, SK, MWW, MP, JRR, MHS.
ETHICS APPROVAL
In the UK, formal ethics approval is not required for research database analysis as detailed by the UK Health Research Authority.
PATIENT CONSENT STATEMENT
All participants completed written, informed consent prior to enrolment in the registry. Permission to reproduce material from other sources: N/A.
Erridge S, Salazar O, Kawka M, et al. An initial analysis of the UK Medical Cannabis Registry: Outcomes analysis of first 129 patients. Neuropsychopharmacol Rep. 2021;41:362–370. 10.1002/npr2.12183
Twitter Institutional Handle: @Sapphireclinics
Contributor Information
Simon Erridge, @Simon_Erridge.
Mikael H. Sodergren, Email: m.sodergren@imperial.ac.uk, @DrMSodergren.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. The raw data belonged to the present study cannot be made publicly available, because the disclosure of personal data was not included in the research protocol of the present study.
REFERENCES
- 1.Montero‐Oleas N, Arevalo‐Rodriguez I, Nuñez‐González S, Viteri‐García A, Simancas‐Racines D. Therapeutic use of cannabis and cannabinoids: An evidence mapping and appraisal of systematic reviews. BMC Complementary Med Ther. 2020;20(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torjesen I. Medical cannabis will be available on prescription in UK from autumn. BMJ. 2018;362:k3290. 10.1136/bmj.k3290 [DOI] [PubMed] [Google Scholar]
- 3.Guideline NG144 N . Cannabis‐based medicinal products; 2019.
- 4.Case P. The NICE guideline on medicinal cannabis: keeping pandora’s box shut tight? Medical Law Rev. 2020;28(2):401–11. [DOI] [PubMed] [Google Scholar]
- 5.Huang W, Chen W, Zhang X. Endocannabinoid system: role in depression, reward and pain control. Mol Med Rep. 2016;14(4):2899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee G, Grovey B, Furnish T, Wallace M. Medical cannabis for neuropathic pain. Curr Pain Headache Rep. 2018;22(1):1–12. [DOI] [PubMed] [Google Scholar]
- 7.Blake A, Wan BA, Malek L, DeAngelis C, Diaz P, Lao N, et al. A selective review of medical cannabis in cancer pain management. Ann Palliat Med. 2017;6(suppl 2):s215–22. [DOI] [PubMed] [Google Scholar]
- 8.Romero‐Sandoval EA, Fincham JE, Kolano AL, Sharpe BN, Alvarado‐Vázquez PA. Cannabis for chronic pain: challenges and considerations. Pharmacotherapy. 2018;38(6):651–62. [DOI] [PubMed] [Google Scholar]
- 9.Lim K, See YM, Lee J. A systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disorders. Clin Psychopharmacol Neurosci. 2017;15(4):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namdar D, Voet H, Ajjampura V, Nadarajan S, Mayzlish‐Gati E, Mazuz M, et al. Terpenoids and phytocannabinoids co‐produced in cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules. 2019;24(17):3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andre CM, Hausman J, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci. 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand U, Pacchetti B, Anand P, Sodergren MH. Cannabis‐based medicines and pain: A review of potential synergistic and entourage effects. Pain Manag. 2021;11(4):395–403. [DOI] [PubMed] [Google Scholar]
- 13.Häuser W, Petzke F, Fitzcharles MA. Efficacy, tolerability and safety of cannabis‐based medicines for chronic pain management–An overview of systematic reviews. Eur J Pain. 2018;22(3):455–70. [DOI] [PubMed] [Google Scholar]
- 14.Gulbransen G, Xu W, Arroll B. Cannabidiol prescription in clinical practice: An audit on the first 400 patients in New Zealand. BJGP open. 2020;4(1):bjgpopen20X101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouso JC, Jiménez‐Garrido D, Ona G, Woźnica D, Santos RG, Hallak JEC, et al. Quality of life, mental health, personality and patterns of use in Self‐Medicated cannabis users with chronic diseases: a 12‐Month longitudinal study. Phytother Res. 2020;34(7):1670–7. [DOI] [PubMed] [Google Scholar]
- 16.Camm AJ, Fox KA. Strengths and weaknesses of ‘real‐world’ studies involving non‐vitamin K antagonist oral anticoagulants. Open Heart. 2018;5(1):e000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nabhan C, Klink A, Prasad V. Real‐world evidence—what does it really mean? JAMA Oncol. 2019;5(6):781–3. [DOI] [PubMed] [Google Scholar]
- 18.Nelson EC, Dixon‐Woods M, Batalden PB, Homa K, Van Citters AD, Morgan TS, et al. Patient focused registries can improve health, care, and science. BMJ. 2016;354:i3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonker CJ, van den Berg H, Marijke KMS, Hoes AW, Mol PG. Registries supporting new drug applications. Pharmacoepidemiol Drug Saf. 2017;26(12):1451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee R, Prasad V. Are observational, real‐world studies suitable to make cancer treatment recommendations? JAMA Netw Open. 2020;3(7):e2012119. [DOI] [PubMed] [Google Scholar]
- 21.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . Integrating palliative care and symptom relief into primary health care: A WHO guide for planners, implementers and managers; 2018.
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 24.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. [DOI] [PubMed] [Google Scholar]
- 25.Radovanovic D, Seifert B, Urban P, Eberli FR, Rickli H, Bertel O, et al. Validity of charlson comorbidity index in patients hospitalised with acute coronary syndrome. insights from the nationwide AMIS plus registry 2002–2012. Heart. 2014;100(4):288–94. [DOI] [PubMed] [Google Scholar]
- 26.Colizzi M, Bhattacharyya S. Cannabis use and the development of tolerance: a systematic review of human evidence. Neurosci Biobehav Rev. 2018;93:1–25. [DOI] [PubMed] [Google Scholar]
- 27.Ramaekers JG, Mason NL, Theunissen EL. Blunted highs: pharmacodynamic and behavioral models of cannabis tolerance. Eur Neuropsychopharmacol. 2020;36:191–205. [DOI] [PubMed] [Google Scholar]
- 28.Joint Formulary Committee . British national formulary (BNF) 66; Vol 66. Pharmaceutical Press; 2013. [Google Scholar]
- 29.Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20(10):1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. Validation and standardization of the generalized anxiety disorder screener (GAD‐7) in the general population. Med Care. 2008;46(3):266–74. [DOI] [PubMed] [Google Scholar]
- 31.Snyder E, Cai B, DeMuro C, Morrison MF, Ball W. A new single‐item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med. 2018;14(11):1849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hout B, Janssen MF, Feng Y‐S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ‐5D‐5L: mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value Health. 2012;15(5):708–15. [DOI] [PubMed] [Google Scholar]
- 33.National Institute for Health and Care Excellence . Position statement on the use of the EQ‐5D‐5L value set for England; 2019.
- 34.Trotti A, Colevas A, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3. 0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13(3):176–81. [DOI] [PubMed] [Google Scholar]
- 35.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. Cannabinoids for medical use: a systematic review and meta‐analysis. JAMA. 2015;313(24):2456–73. [DOI] [PubMed] [Google Scholar]
- 36.Sarris J, Sinclair J, Karamacoska D, Davidson M, Firth J. Medicinal cannabis for psychiatric disorders: a clinically‐focused systematic review. BMC Psychiatry. 2020;20(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergamaschi MM, Queiroz RHC, Chagas MHN, de Oliveira DC, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment‐naive social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta‐analysis. Lancet Psychiatry. 2019;6(12):995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babson KA, Sottile J, Morabito D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. 2017;19(4):1–12. [DOI] [PubMed] [Google Scholar]
- 40.Lehman BJ, David DM, Gruber JA. Rethinking the biopsychosocial model of health: understanding health as a dynamic system. Soc Pers Psychol Compass. 2017;11(8):e12328. [Google Scholar]
- 41.Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369(9566):1047–53. [DOI] [PubMed] [Google Scholar]
- 42.Etges T, Karolia K, Grint T, Taylor A, Lauder H, Daka B et al. An observational postmarketing safety registry of patients in the UK, Germany, and Switzerland who have been prescribed sativex®(THC: CBD, nabiximols) oromucosal spray. Ther Clin Risk Manag. 2016;12:1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oreja‐Guevara C, Casanova B, Ordás CM, Silván CV, Asensio D, Massana M. Observational safety study of THC: CBD oromucosal spray (sativex) in multiple sclerosis patients with spasticity. Clin Exp Pharmacol. 2015;5(184). 10.4172/2161-1459.1000184 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. The raw data belonged to the present study cannot be made publicly available, because the disclosure of personal data was not included in the research protocol of the present study.


