Abstract
Genetic and environmental factors interact with each other to influence the risk of various psychiatric diseases; however, the intensity and nature of their interactions remain to be elucidated. We established a maternal infection model using polyinosinic‐polycytidylic acid (Poly(I:C)) to determine the relationship between the maternal breeding environment and behavioral changes in the offspring. We purchased pregnant C57BL/6J mice from three breeders and administered Poly(I:C) (2 mg/kg) intravenously in their tail vein on gestation day 15. The offspring were raised to 8‐12 weeks old and subjected to the acoustic startle tests to compare their startle response intensity, prepulse inhibition levels, and degree of the adaptation of the startle response. No statistical interaction between Poly(I:C) administration and sex was observed for prepulse inhibition; thus, male and female mice were analyzed together. There was a statistical interaction between the breeder origin of offspring and prepulse inhibition; the Poly(I:C) challenge significantly decreased prepulse inhibition levels of the offspring born to the pregnant dams from Breeder A but not those from the other breeders. However, we failed to detect significant inter‐breeder differences in Poly(I:C) effects on startle response and on startle adaptation with the given number of mice examined. The rearing environment of mouse dams has a prominent effect on the Poly(I:C)‐induced prepulse inhibition deficits in this maternal immune activation model.
Keywords: gene‐environment, polyinosinic‐polycytidylic acid, prepulse inhibition, schizophrenia, startle response
The maternal immune activation (MIA) model using polyinosinic‐polycytidylic acid [Poly(I:C)] is often employed for animal modeling for schizophrenia and/or autism. Here we found a marked inter‐breeder difference in prepulse inhibition of the offspring mice C57BL/6J.
1. INTRODUCTION
Among various environmental factors, maternal infections or inflammation during the gestation draw our attention which is suggested to increase the risk of schizophrenia or autism of their child.1, 2 Thus, we often employ the rodent offspring whose dams were challenged by viral and bacterial antigens as an animal model for maternal infections or inflammation.3, 4, 5, 6 Previous neuropathological studies on this maternal infection model have reported that the brains of the neonates born to the mothers exhibit the increased cell density or pathological abnormalities of the cerebral cortex.7, 8 Poly(I:C) is the double‐stranded RNA analog for viral genome, which is recognized by toll‐like receptor 3 to trigger the immune inflammatory responses to a viral infection.9 Biological factors that influence the neurobehavioral consequences of the Poly (I:C) challenge have been reported from various perspectives.10, 11, 12, 13 The rearing environment of mice in the laboratory alters the neurobehavioral effect of Poly (I:C).14 The environment has marked impact on the behavioral traits and immune inflammatory responses of mice themselves.15, 16 In this study, we purchased pregnant mice with the same C57Bl/6J genetic background from three Japanese breeders and treated them with Poly(I:C) to investigate how environmental factors (ie, breeder) affected the behavioral traits of their offspring at maturity.
2. MATERIALS AND METHODS
2.1. Animals
The genetic strain of C57Bl/6J mice was used in this experiment. Six to ten pregnant mice were purchased from CLEA Japan Inc and grown in their Fuji breeding factory, from Charles River Laboratories Japan Inc and grown in their Hino breeding factory, and from SLC Japan Inc and grown in their Haruno breeding factory. In the authors' institute, the dams and their pups were kept in a specific pathogen‐free environment, and the room temperature and humidity were maintained at 23℃ and 50%‐70%, respectively, with a constant 12‐hour light‐dark cycle (lights off: 8:00 pm‐8:00 am). All animals were provided the same feed (NMF, Oriental Yeast, Inc) and ad libitum access to water.
2.2. Poly(I:C) administration
Pregnant mice on gestation day 15 underwent intravenous injection with 2.0 mg/kg Poly(I:C) (potassium salt, GE Healthcare) or vehicle (sterile pyrogen‐free 0.9% NaCl).17 The dose of Poly(I:C) was optimized in our preliminary experiment (Figure S1).18 The Poly(I:C) solution (1 mg/mL) or saline was administered to the tail vein under mild physical restraint. The pups were weaned on postnatal day 21, and 2‐3 of each sex was randomly assigned to each group. The number of total offspring born was 142 pups.
2.3. Prepulse inhibition test
The pups' acoustic startle response, startle adaptation, and prepulse inhibition (PPI) were assessed between postnatal days 56 and 84.19 A mouse was placed in a plastic cylinder, and the cylinder was fixed to a testing chamber (SR‐Lab Systems). After completing the acclimatization period with 70 dB background noise (white noise), the main acoustic stimuli (120 dB, white noise; duration, 40 ms) were presented with prepulse stimuli (73, 76, 79, or 82 dB) to mice in an order predetermined by a pseudorandom number generator with 15‐second intervals between stimulus presentations.19 Before and after measuring PPI, a 120‐dB acoustic stimulus was administered to mice five times and the proportion of the startle change was calculated as an adaptation (%).
2.4. Sound startle response test
Mice were placed in a plastic cylinder which was fixed to a testing chamber (SR‐Lab Systems) as described above. Acoustic stimuli (90, 95, 100, 105, 110, 115, and 120 dB, white noise; duration, 40 ms) were presented in a pseudorandom manner with 15‐second intervals. Their mean intensity for 120‐dB stimuli was selected and presented as the intensity of the startle response.
2.5. Statistical analysis
Factorial analysis of variance (ANOVA) was first performed with the main factors of Poly (I:C), breeder, tone intensity, and sex using SPSS ver. 11.0 (SPSS Japan). As there was a significant interaction between breeder and other indices, following statistical analyses were done in each breeder. Interactions between behavioral data and sex were also analyzed (Table S1). In the absence of such interaction, the data from both sexes were combined. Post hoc analyses were performed by planned comparisons of Welch t test with Holm's correction or by multiple comparisons of Tukey HSD test. P < .05 was considered statistically significant.
3. RESULTS
3.1. Prepulse inhibition difference among mouse breeders
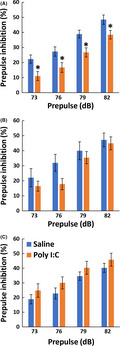
Prepulse inhibition levels are known to decrease in patients with various psychiatric diseases.20 The effects of the maternal Poly(I:C) challenge on PPI were measured when pups reached maturity (Figure 1). First, we performed factorial ANOVA with the between‐subject factors of breeders, Poly(I:C), and sex, and the within‐subject factors of prepulse. We found a significant interaction between breeders and Poly(I:C) (F 2,130 = 3.206, P =.044) and no significant interaction between sex and any other subject factor, suggesting that Poly(I:C) effects varied among breeders but not between the sexes. Thus, we divided the statistical analyses into individual breeders, combining the male and female PPI data (see statistical details in Table S1). PPI levels only decreased significantly in the pups of C57BL/6J dams purchased from CLEA Japan (F 1,52 = 5.659, P = .021). When pregnant C57BL/6J dams purchased from Charles River Japan and SLC Japan, the PPI of their pups was not significantly altered by Poly(I:C) treatment (Charles River: F 1,31 = 0.725, P = .401; SLC: F 1,47 = 1.960, P = .168).
FIGURE 1.
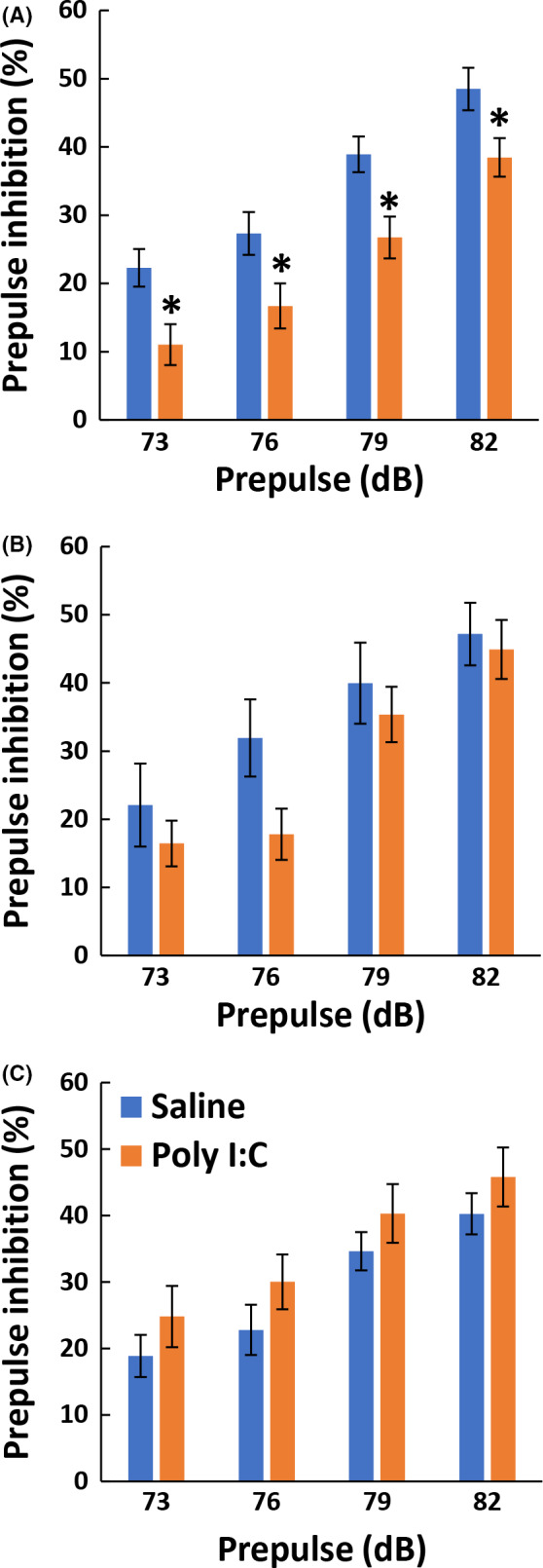
The effect of dams' breeders on prepulse inhibition after administering Poly(I:C) to their dams. The inhibitory effects of prepulse sounds at 73, 76, 79, and 82 dB were assessed as PPI levels in pups born to pregnant C57BL/6J mice after raising them for 2‐3 months. On gestation day 15, pregnant mice from CLEA Japan (A; n = 17 for saline and n = 39 for Poly(I:C) from 3 and 6 dams, respectively), Charles River Japan (B; n = 21 for saline and n = 14 for Poly(I:C) both from 3 dams), and SLC Japan (C; n = 27 for saline and n = 24 for Poly(I:C) both from 5 dams) were challenged with Poly(I:C) or saline. Data are presented as mean ± SEM. PPI was observed in the inhibitory % of the main pulse‐triggered startle. *P < .05, planned multiple comparison was performed between saline and Poly(I:C) groups at each prepulse levels using Welch t test with the Holm's compensation
3.2. Influences of mouse breeders on sound startle response and test adaptation
At maturity, the Poly(I:C) effect on the offspring startle response was also measured with 120‐dB sound alone. Factorial ANOVA was similarly performed with the between‐subject factors of breeders, Poly(I:C), and sex. There were main effects of breeder (F 2,130 = 5.68, P = .004) and sex (F 1,130 = 20.2, P < .001) but not that of Poly(I:C) (F 1,130 = 0.892, P = .347) with no interactions between any subject factors (Table S1). Accordingly, the intensity of sound startle responses was plotted in each breeder and each sex (Figure 2A‐C). Post hoc analyses detected the significant sex difference in all breeders (CLEA: P = .012, Charles: P = .028, SLC: P = .02) and found that the startle response of the offspring from CLEA Japan was significantly higher than that from SLC Japan (P = .002). Although there were differences in basal startle amplitudes among breeders and sex, the behavioral scores were not affected by administering Poly(I:C) to the dam.
FIGURE 2.
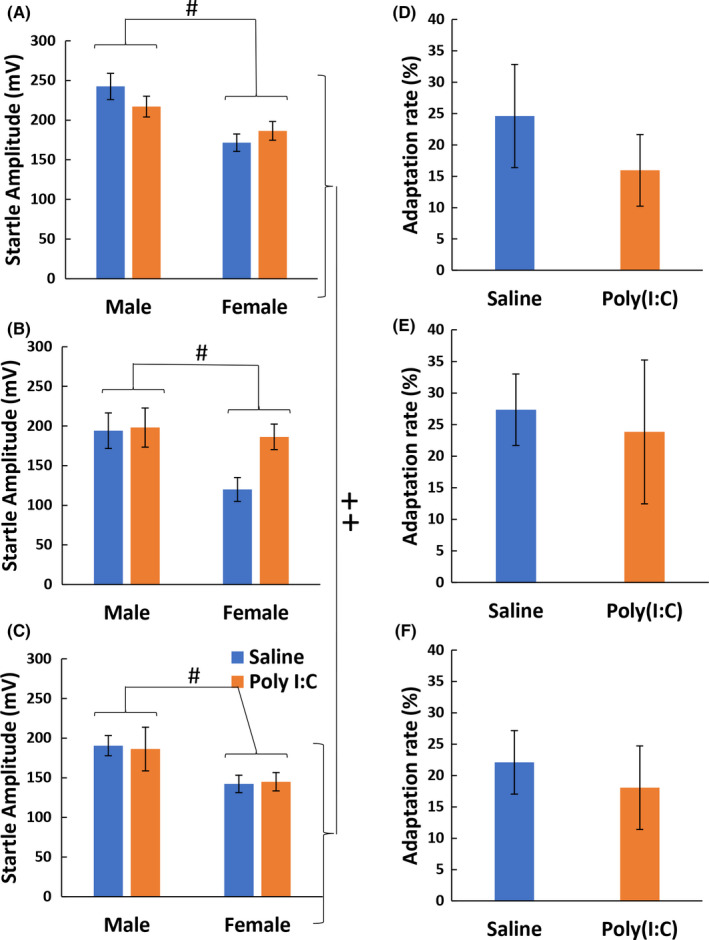
Inter‐breeder differences in sound startle response and adaptation. Employing the same sets of mice in Figure 1, we determined the intensity of their startle response (mV) to 120‐dB white noises (A‐C) and adaptation rates of the startle responses (%) to the 120‐dB noises (D‐F). The pregnant mice (C57BL/6J) were purchased from CLEA Japan (A, D), Charles River Japan (B, E), and SLC Japan (C, F). Data are presented as mean ± SEM. See statistical details in Table S1. In the startle intensity, there were an inter‐breeder difference between CLEA Japan and SLC Japan (++ P < .01, Tukey HSD) and a sex difference in all breeders (# P < .05, Welch t test with the Holm's compensation), which was irrespective of the Poly(I:C) effects
It has been reported that the ability to adapt to external stimuli is reduced in patients with psychiatric disorders.21 The effects of Poly(I:C) on the adaptation of pups for PPI test paradigm were assessed at their maturity (Figure 2D‐F). Factorial ANOVA found no significant main effects of breeders, Poly(I:C), or sex without their interactions (Table S1).
4. DISCUSSION
In the present study, we focused on one of the suggested environmental factors, maternal breeding environment, to investigate how this environmental factor affects the sound startle feature of this model; PPI levels, sound startle responses, and their startle adaptation.3, 17, 22 We purchased pregnant dam mice with the same genetic background that had been raised in three different environments and observed the following behavioral similarity and difference of their offspring across dam's breeders: (a) Maternal Poly (I:C) challenge significantly disrupted PPI levels of their offspring only when pregnant mice were obtained from CLEA Japan; (b) the Poly (I:C) effects on the startle responses of their offspring were undetectable in and indistinguishable among all companies, and (c) there was no significant inter‐breeder difference in acoustic adaptation scores with the given number of animals. (d) Irrespective of the Poly (I:C) effects, the basal behavioral feature of sound startle response varied among breeders and between the sexes.
Challenging mouse dams with Poly(I:C) had different effects on pups' PPI levels depending on the breeder that the dam was purchased from. This presumably represents a result of the biological interaction between genetic factors and breeding environment (ie, environmental factors). This finding appears to agree with the report that the cognitive‐behavioral changes such as sociability and exploratory behavior of this model varied between the breeders that provided the dams.23 The present inter‐breeder difference is consistent with the fact that the blood levels of Poly(I:C)‐induced inflammatory cytokines differ depending on the dam's breeding environment.15, 23 Gut microbiota is known to regulate such immune responses and homeostasis24, 25, 26 and reported to markedly differ among the mouse breeders in Japan.27, 28, 29 Therefore, the inter‐breeder difference in gut microbiota of mouse dams can be the candidate factor that affects PPI levels of their offspring.
Our present results may be in agreement with the previous study that PPI levels were decreased in the pups of pregnant mice (the genetic strain of C57BL/6J) challenged intraperitoneally with Poly(I:C) on the late gestation,17 although some controversy still remains.10 Such controversy might stem from the variation of the experimental conditions or procedures such as Poly(I:C) doses, injection sites, pregnancy periods. With this respect, therefore, we do not rule out the possibility that a higher or lower dose of Poly(I:C) might result in distinct breeder‐dependency of PPI deficits.18
Although the reproducibility of this maternal immune activation model is debated,30 this study revealed that the breeder from which mice are procured is an important factor for the establishment of PPI deficits in this model. Further investigations on the specific aspects of the breeding environment underlying our findings are reflected in mice in vivo are warranted.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
YK performed the experiments and coordinated the work presented. HNawa designed the experiments and wrote the manuscript. HNamba, YI, HN, HS, YM and KI provided technical assistances and commented on the manuscript.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
N/A.
INFORMED CONSENT
N/A.
REGISTRY AND THE REGISTRATION
N/A.
ANIMAL STUDIES
All experiments adopted in this study were approved by and conducted under the guidance of the Niigata University Animal Ethics Committee. Efforts were made to minimize animal suffering.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by MEXT Grant‐in‐Aid for Scientific Research on Innovative Areas “Multiscale Brain” (HNawa: 18H05429, 18H05428, KI: 18H05430), AMED (KI: JP20dm0207074) and by The Uehara Memorial Foundation to HN.
Kobayashi Y, Inaba H, Iwakura Y, et al. Inter‐breeder differences in prepulse inhibition deficits of C57BL/6J mice in a maternal immune activation model. Neuropsychopharmacol Rep. 2021;41:416–421. 10.1002/npr2.12178
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary materials of this article.
REFERENCES
- 1.Brown AS, Derkits EJ. Prenatal Infection and Schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64(3):217–30. [DOI] [PubMed] [Google Scholar]
- 3.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59(6):546–54. [DOI] [PubMed] [Google Scholar]
- 4.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28(10):1778–89. [DOI] [PubMed] [Google Scholar]
- 5.Hida H, Mouri A, Noda Y. Behavioral phenotypes in schizophrenic animal models with multiple combinations of genetic and environmental factors. J Pharmacol Sci. 2013;121(3):185–91. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto K, Hitora‐Imamura N, Hioki H, Ikegaya Y. GABAergic malfunction in the anterior cingulate cortex underlying maternal immune activation‐induced social deficits. J Neuroimmunol. 2018;321:92–6. [DOI] [PubMed] [Google Scholar]
- 7.Yim YS, Park A, Berrios J, Lafourcade M, Pascual LM, Soares N, et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature. 2017;549(7673):482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SEP, Elliott RM, Anderson MP. Maternal immune activation increases neonatal mouse cortex thickness and cell density. J Neuroimmune Pharmacol. 2012;7(3):529–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double‐stranded RNA and activation of NF‐kappa B by toll‐like receptor 3. Nature. 2001;413(6857):732–8. [DOI] [PubMed] [Google Scholar]
- 10.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22(4):469–86. [DOI] [PubMed] [Google Scholar]
- 11.Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism‐like behaviors in mice. Transl Psychiatry. 2013;3:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller FS, Richetto J, Hayes LN, Zambon A, Pollak DD, Sawa A, et al. Influence of poly(I:C) variability on thermoregulation, immune responses and pregnancy outcomes in mouse models of maternal immune activation. Brain Behav Immun. 2019;80:406–18. [DOI] [PubMed] [Google Scholar]
- 13.Bundo M, Toyoshima M, Okada Y, Akamatsu W, Ueda J, Nemoto‐Miyauchi T, et al. Increased L1 retrotransposition in the neuronal genome in schizophrenia. Neuron. 2014;81(2):306–13. [DOI] [PubMed] [Google Scholar]
- 14.Mueller FS, Polesel M, Richetto J, Meyer U, Weber‐Stadlbauer U. Mouse models of maternal immune activation: mind your caging system! Brain Behav Immun. 2018;73:643–60. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov II, Frutos RD, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, et al. Specific microbiota direct the differentiation of IL‐17‐oroducing T‐helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, van Praag H. Maternal immune activation differentially impacts mature and adult‐born hippocampal neurons in male mice. Brain Behav Immun. 2015;45:60–70. [DOI] [PubMed] [Google Scholar]
- 18.Aoki H, Mizuno M, Kakita A, Nawa H. Experimental evaluation of the maternal viral infection hypothesis of schizophrenia; contribution of cytokine induction. Annu Rep Pharmacopsychiatry Res Found. 2006;38:204–9. [Google Scholar]
- 19.Tohmi M, Tsuda N, Mizuno M, Takei N, Frankland PW, Nawa H. Distinct influences of neonatal epidermal growth factor challenge on adult neurobehavioral traits in four mouse strains. Behav Genet. 2005;35(5):615–29. [DOI] [PubMed] [Google Scholar]
- 20.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156(2–3):234–58. [DOI] [PubMed] [Google Scholar]
- 21.Chang WP, Arfken CL, Sangal MP, Boutros NN. Probing the relative contribution of the first and second responses to sensory gating indices: a meta‐analysis. Psychophysiology. 2011;48(7):980–92. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa K, Yoshino H, Ogawa Y, Yamamuro K, Kimoto S, Noriyama Y, et al. Maternal immune activation affects hippocampal excitatory and inhibitory synaptic transmission in offspring from an early developmental period to adulthood. Front Cell Neurosci. 2020;14:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, et al. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549(7673):528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu HJ, Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. 2012;3:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sadler R, Singh V, Benakis C, Garzetti D, Brea D, Stecher B, et al. Microbiota differences between commercial breeders impacts the post‐stroke immune response. Brain Behav Immun. 2017;66:23–30. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi Y, Hiraguchi M, Ushida K. The composition of intestinal bacteria affects the level of luminal IgA. Biosci Biotechnol Biochem. 2006;70:3031–5. [DOI] [PubMed] [Google Scholar]
- 27.Hirayama K, Endo K, Kawamura S, Mitsuoka T. Comparison of the intestinal bacteria in specific pathogen free mice from different breeders. Jikken Dobutsu. 1990;39:263–7. [DOI] [PubMed] [Google Scholar]
- 28.Nozu R, Ueno M, Hayashimoto N. Composition of fecal microbiota of laboratory mice derived from Japanese commercial breeders using 16S rRNA gene clone libraries. J Vet Med Sci. 2016;78:1045–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakita Y, Shimomura Y, Kitada Y, Yamamoto H, Ohashi Y, Matsumoto M. Taxonomic classification for microbiome analysis, which correlates well with the metabolite milieu of the gut. BMC Microbiol. 2018;18:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kentner AC, Bilbo SD, Brown AS, Hsiao EY, McAllister AK, Meyer U, et al. Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology. 2019;44:245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available in the supplementary materials of this article.


