Key Points
Question
Does robotic gastrectomy for resectable gastric cancer reduce the incidence of intra-abdominal infectious complications compared with laparoscopic gastrectomy?
Findings
In this randomized clinical trial of 241 patients with gastric cancer, the primary end point of reducing intra-abdominal infectious complications with robotic gastrectomy was not met. Secondary end point results showed good short-term surgical outcomes in both the laparoscopic gastrectomy group and the robotic gastrectomy group.
Meaning
This study suggests that robotic gastrectomy for patients with gastric cancer is unable to reduce postoperative intra-abdominal complications.
Abstract
Importance
Robotic gastrectomy (RG) for gastric cancer may be associated with decreased incidence of intra-abdominal infectious complications, including pancreatic fistula, leakage, and abscess. Prospective randomized clinical trials comparing laparoscopic gastrectomy (LG) and RG are thus required.
Objective
To compare the short-term surgical outcomes of RG with those of LG for patients with gastric cancer.
Design, Setting, and Participants
In this phase 3, prospective superiority randomized clinical trial of RG vs LG regarding reduction of complications, 241 patients with resectable gastric cancer (clinical stages I-III) were enrolled between April 1, 2018, and October 31, 2020.
Interventions
LG vs RG.
Main Outcomes and Measures
The primary end point was the incidence of postoperative intra-abdominal infectious complications. Secondary end points were incidence of any complications, surgical results, postoperative courses, and oncologic outcomes. The modified intention-to-treat population excluded patients who had been randomized and met the postrandomization exclusion criteria. There was also a per-protocol population for analysis of postoperative complications.
Results
This study enrolled 241 patients, with 236 patients in the modified intention-to-treat population (150 men [63.6%]; mean [SD] age, 70.8 [10.7] years). There was no significant difference in the incidence of intra-abdominal infectious complications (per-protocol population: 10 of 117 [8.5%] in the LG group vs 7 of 113 [6.2%] in the RG group). Of 241 patients, 122 were randomly assigned to the LG group, and 119 patients were randomly assigned to the RG group. Two of the 122 patients (1.6%) in the LG group converted from LG to open surgery, and 4 of 119 patients (3.4%) in the RG group converted from RG to open or laparoscopic surgery, with no significant difference. Finally, 117 patients in the LG group completed the procedure, and 113 in the RG group completed the procedure; these populations were defined as the per-protocol population. The overall incidence of postoperative complications of grade II or higher was significantly higher in the LG group (23 [19.7%]) than in the RG group (10 [8.8%]) (P = .02). Even in analysis limited to grade IIIa or higher, the complication rate was still significantly higher in the LG group (19 [16.2%]) than in the RG group (6 [5.3%]) (P = .01).
Conclusions and Relevance
This study found no reduction of intra-abdominal infectious complications with RG compared with LG for gastric cancer.
Trial Registration
umin.ac.jp/ctr Identifier: UMIN000031536
This randomized clinical superiority trial compares the short-term surgical outcomes of robotic gastrectomy with those of laparoscopic gastrectomy for patients with gastric cancer.
Introduction
Minimally invasive surgery, represented by laparoscopic surgery, is widely accepted as curative treatment for gastric cancer (GC). Several multicenter randomized clinical trials (RCTs) have reported lower rates of postoperative complications in patients who underwent laparoscopic gastrectomy (LG) compared with open gastrectomy, but with similar long-term prognosis between them.1,2,3,4,5 Laparoscopic gastrectomy still has several drawbacks, however, including the limited range of movement, amplification of operator hand tremors, and inconvenient surgical positioning.6,7,8,9,10
The robotic surgical system, meanwhile, has several technical advantages compared with laparoscopic instruments, including 3-dimensional (3-D) high-definition vision controlled by the operators, easier instrument movement, tremor filtration, and better ergonomics.11,12,13,14 Robotic gastrectomy (RG) may therefore overcome some of the drawbacks associated with LG. Robotic gastrectomy may be associated with decreased incidence of intra-abdominal infectious complications, including postoperative pancreatic fistulas and abscesses. Prospective RCTs comparing LG and RG are required. The present phase 3 RCT compares the short-term surgical and long-term oncologic safety of RG compared with LG for patients with GC.
Methods
This was a phase 3, two-center, open-label, prospective superiority RCT conducted between April 1, 2018, and October 31, 2020, to demonstrate the benefits of RG compared with LG for resectable GC regarding reduction of complications. The primary end point was the incidence of postoperative intra-abdominal infectious complications of Clavien-Dindo grade II or higher.15 Intra-abdominal infectious complications were defined as anastomotic leakage, pancreatic fistula, and intra-abdominal abscess.6,16 Secondary end points were incidence of any complications of Clavien-Dindo grade II or higher, surgical results (operation time, blood loss, transition rate to open or laparoscopic surgery, and the number of retrieval lymph nodes), postoperative courses, and oncologic outcomes (overall survival and disease-free survival). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines and was conducted according to a protocol reviewed and approved by the Ethical Committee on Human Research at Wakayama Medical University Hospital in Wakayama, Japan. All patients provided written informed consent. The study protocol was registered in the University Hospital Medical Information Network. The design of the trial was reported previously (trial protocol in Supplement 1).16 The study initially began as a single-center RCT at Wakayama Medical University Hospital. In an interim analysis in December 2018, a third-party monitoring committee highlighted the slow speed of patient registration, which was perhaps due to a gradual decrease in the incidence of GC in Japanese people.10 The protocol was therefore revised to become a 2-center RCT.
Participants
Before participation in this study, patients underwent esophagogastroscopy and enhanced computed tomography scans of the chest and abdomen to evaluate the pretreatment tumor stage.17 Inclusion criteria were as follows: histologically proven gastric carcinoma, resectable GC according to the eighth editon of the TNM classification (clinical stages I-III),17 not applicable for endoscopic submucosal dissection according to Japanese classification,18 patients aged between 20 and 90 years, Eastern Cooperative Oncology Group performance status of 0 or 1, body mass index (calculated as weight in kilograms divided by height in meters squared) of less than 35, no history of gastrointestinal surgery that may affect protocol surgery, no history of chemotherapy or radiotherapy, normal function of the major organs (leukocyte count >3000/µL [to convert to × 109 per liter, multiply by 0.001]; platelet count >100 000 × 103/µL [to convert to × 109 per liter, multiply by 1.0]; aspartate aminotransferase and alanine aminotransferase levels <200 U/L [to convert to microkatals per liter, multiply by 0.0167]; and creatinine level <2 mg/dL [to convert to micromoles per liter, multiply by 88.4]), and proven written informed consent. Exclusion criteria were as follows: synchronous or metachronous malignant neoplasms other than carcinoma in situ, pregnant or breastfeeding, severe mental illness, continuous systemic corticosteroid therapy, history of myocardial infarction or unstable angina pectoris within 6 months, uncontrollable hypertension, uncontrollable type 1 or 2 diabetes or administration of insulin, respiratory disease requiring continuous oxygen therapy, and history of deep-vein thrombosis.
Randomization
Patients were randomized to undergo either LG or RG. The minimization method with a random component was used to balance the groups regarding institution and planned gastrectomy type (distal gastrectomy and total or proximal gastrectomy).
Interventions and Outcome Measurement
The LG and RG procedures were identical except for the surgical approach. A standard radical gastrectomy with lymphadenectomy was performed according to the clinical T and N stages, based on the fifth edition of the Japanese Gastric Cancer Treatment Guidelines.18
All RG procedures were performed using the da Vinci Si or Xi Surgical System (Intuitive Surgical) with 4 articulating robotic arms: a central arm for a 30° rigid endoscope, a first arm for monopolar scissors, a second arm for fenestrated bipolar forceps, and a third arm for Cadiere forceps.6,13,19,20 We used a robotic vessel-sealing system with articulated function (Vessel Sealer; Intuitive Surgical). The RG procedure did not differ from the LG procedure with regard to lymph node dissection. The robotic lymphadenectomy procedures (without contact with the pancreas) were previously described.6,13
In both the LG group and the RG group, intracorporeal anastomosis using linear staples, such as gastroduodenostomy, gastrojejunostomy, or esophagojejunostomy, was performed.19,20,21,22,23 If an incision exceeding 10 cm was required for the control of intraoperative complications or tumor extension, the procedure was considered to be a conversion to open surgery.
A single abdominal drain was inserted into the left subphrenic cavity after reconstruction in both groups. The amylase level in the drainage fluid was checked on postoperative days 1 and 3 (PODs 1 and 3). Postoperative analgesia with epidural anesthesia was used routinely. Use of analgesics after removal of the epidural anesthesia tube (on POD 3) was recorded.
All surgical and medical complications and mortality events were documented. Postoperative complications were analyzed according to the Clavien-Dindo classification.15 Complications of grade II or higher were regarded as clinically significant. Surgical complications were confined to events that occurred within 90 days after surgery; these included anastomotic leakage, pancreatic fistula, intra-abdominal abscess, intra-abdominal bleeding, intraluminal bleeding, ileus, cholecystitis, anastomotic stenosis, and wound infection. Postoperative pancreatic fistulas of grade II or higher (requiring pharmacologic treatment) were considered to be a complication regardless of amylase levels in serum or drainage fluid. Medical complications included pulmonary, cardiovascular, liver, urinary, and thrombosis events.
Follow-up
Adjuvant chemotherapy with S-1, an oral fluoropyrimidine, for 1 year was included in the protocol treatment for patients with pathologic stage II or III GCs.18
Statistical Analysis
The primary end point of this trial was the determination of the rate of postoperative intra-abdominal infectious complications. The sample size used to determine the number of patients necessary for statistical validity (2-sided test) was based on our retrospective data from January 2011 to December 2017 (n = 899). According to these data, the incidence rate of intra-abdominal infectious complications after LG was 11%. We therefore estimated that there would be an incidence rate of 11% in the LG group in the present trial. Similarly, because the incidence rate in the RG group was 0% in a retrospective study,6 we expected that the incidence rate of intra-abdominal infectious complications in the RG group would be 2% (odds ratio, 0.165; 95% CI, 0.051-0.512). We calculated that 117 patients would be required in each group of this trial, with a significance of α = 0.5 and a power of (1 − β) = 0.8. Anticipating follow-up loss, we calculated that 120 patients would be required in each group of this trial, for a total study population of 240 patients.
We defined 2 different populations for analysis. The modified intention-to-treat population (mITTP) excluded patients who had been randomized and met the postrandomization exclusion criteria. There was also a per-protocol population (PPP) for analysis of postoperative complications. Patients whose procedure was converted to the surgical approach (conversion from LG to open gastrectomy or conversion from RG to LG or open gastrectomy) were excluded from the PPP. Analysis using the PPP thus reflected the pure results after LG or RG.
Quantitative results are expressed as median values and ranges. Statistical comparisons between the LG group and the RG group were performed using χ2 statistics, the Fisher exact test, and the Mann-Whitney test; P < .05 was considered statistically significant. The SPSS, version 24.0 software program (SPSS Inc) was used for all statistical analyses.
Results
Patients
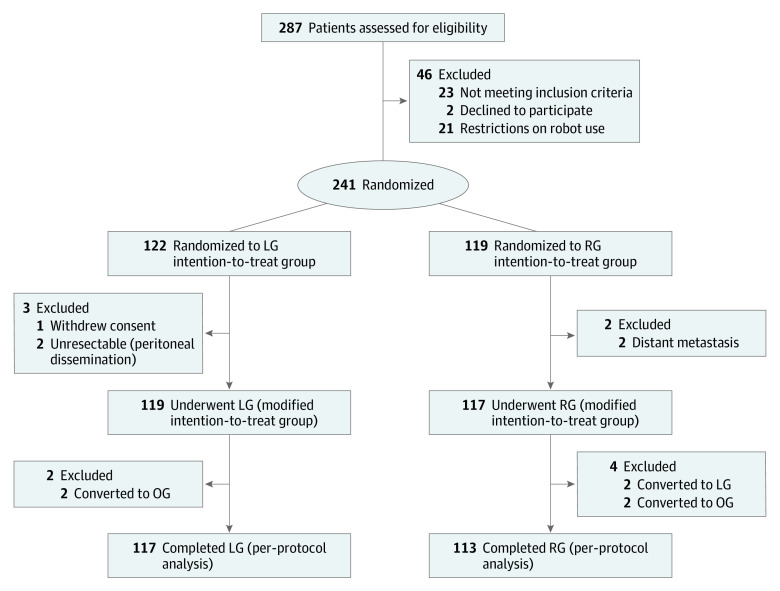
Between April 1, 2018, and October 31, 2020, 287 patients were scheduled to undergo radical gastrectomy for resectable GC. A CONSORT flow diagram of this RCT is shown in Figure 1. Of the 287 patients, 46 were excluded from the study before randomization. The remaining 241 patients were randomized into 2 groups; 122 patients were randomly assigned to undergo LG, and 119 patients were randomly assigned to undergo RG. For the mITTP analysis, 5 patients were excluded. Laparoscopic gastrectomy was performed for 119 patients, and RG was performed for 117 patients. These populations were defined as the mITTP. Six patients had their procedure converted to the approach of the other group. Finally, 117 patients completed LG, and 113 completed RG; these populations were defined as the PPP.
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram.
LG indicates laparoscopic gastrectomy; OG, open gastrectomy; and RG, robotic gastrectomy.
Patient Demographic and Tumor Characteristics
Table 117,18 shows the results for the patient demographic characteristics. No significant difference was observed between the 2 groups. There were no differences between the 2 groups in terms of tumor location, tumor size, tumor type, or distribution of tumor stages. Tumor characteristics were well balanced between the groups.
Table 1. Patient Demographic and Tumor Characteristics (Modified Intention-to-Treat Population).
| Variable | LG (n = 119) | RG (n = 117) |
|---|---|---|
| Patient demographic characteristics | ||
| Sex, No. (%) | ||
| Male | 77 (64.7) | 73 (62.4) |
| Female | 42 (35.3) | 44 (37.6) |
| Age, median (range), y | 72 (40-90) | 71 (34-90) |
| Institution, No. (%) | ||
| Wakayama Medical University Hospital | 108 (90.8) | 109 (93.2) |
| Mie University Hospital | 11 (9.2) | 8 (6.8) |
| BMI, median (range) | 22.4 (14.0-31.9) | 21.9 (14.0-32.1) |
| ASA score, No. (%) | ||
| 1 | 44 (37.0) | 39 (33.3) |
| 2 | 72 (60.5) | 74 (63.2) |
| 3 | 3 (2.5) | 4 (3.4) |
| Comorbidity, No. (%) | ||
| Hypertension | 62 (52.1) | 53 (45.3) |
| Type 1 or 2 diabetes | 28 (23.5) | 20 (17.1) |
| Pulmonary | 7 (5.9) | 10 (8.5) |
| Cardiovascular | 13 (10.9) | 14 (12.0) |
| Renal | 7 (5.9) | 4 (3.4) |
| Hepatic | 2 (1.7) | 2 (1.7) |
| Cerebrovascular | 16 (13.4) | 6 (5.1) |
| History of abdominal surgery, No. (%) | 27 (22.7) | 32 (27.4) |
| Open cholecystectomy | 4 (3.4) | 2 (1.7) |
| Laparoscopic cholecystectomy | 3 (2.5) | 3 (2.6) |
| Appendectomy | 14 (11.8) | 18 (15.4) |
| Colorectal surgery | 3 (2.5) | 3 (2.6) |
| Gynecological surgery | 7 (5.9) | 8 (6.8) |
| Hepatectomy | 0 | 1 (0.9) |
| Smoking history, Brinkman index, median (range) | 60 (0-2500) | 400 (0-4000) |
| Daily drinker, No. (%) | 57 (47.9) | 48 (41.0) |
| Tumor characteristics | ||
| Location, No. (%) | ||
| Upper third of the stomach | 24 (20.2) | 31 (26.5) |
| Middle third of the stomach | 43 (36.1) | 35 (29.9) |
| Lower third of the stomach | 50 (42.0) | 46 (39.3) |
| Whole stomach | 2 (1.7) | 5 (4.3) |
| Size, median (range), mm | 32 (3-120) | 35 (8-150) |
| Macroscopic type, No. (%)a | ||
| 0 | 55 (46.2) | 57 (48.7) |
| 1 | 6 (5.0) | 4 (3.4) |
| 2 | 22 (18.5) | 20 (17.1) |
| 3 | 29 (24.4) | 30 (25.6) |
| 4 | 4 (3.4) | 5 (4.3) |
| 5 | 3 (2.5) | 1 (0.9) |
| Histologic type, No. (%)a | ||
| Pap | 1 (0.8) | 1 (0.9) |
| Tub1 | 35 (29.4) | 32 (27.4) |
| Tub2 | 34 (28.6) | 28 (23.9) |
| Por | 42 (35.3) | 46 (39.3) |
| Sig | 2 (1.7) | 6 (5.1) |
| Muc | 2 (1.7) | 1 (0.9) |
| Others | 3 (2.5) | 3 (2.6) |
| pT stage, No. (%)b | ||
| T1a | 23 (19.3) | 25 (21.4) |
| T1b | 40 (33.6) | 43 (36.8) |
| T2 | 14 (11.8) | 10 (8.5) |
| T3 | 26 (21.8) | 25 (21.4) |
| T4a | 15 (12.6) | 11 (9.4) |
| T4b | 1 (0.8) | 3 (2.6) |
| pN stage, No. (%)b | ||
| N0 | 70 (58.8) | 76 (65.0) |
| N1 | 24 (20.2) | 19 (16.2) |
| N2 | 12 (10.1) | 15 (12.8) |
| N3 | 13 (10.9) | 7 (6.0) |
| pM stage, No. (%)b | ||
| M0 | 111 (93.3) | 114 (97.4) |
| M1 | 8 (6.7) | 3 (2.6) |
| p Stage, No. (%)b | ||
| IA | 52 (43.7) | 52 (44.4) |
| IB | 19 (16.0) | 16 (13.7) |
| IIA | 12 (10.1) | 18 (15.4) |
| IIB | 9 (7.6) | 13 (11.1) |
| IIIA | 9 (7.6) | 10 (8.5) |
| IIIB | 5 (4.2) | 2 (1.7) |
| IIIC | 5 (4.2) | 3 (2.6) |
| IV | 8 (6.7) | 3 (2.6) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); LG, laparoscopic gastrectomy; Muc, mucinous adenocarcinoma; Pap, papillary adenocarcinoma; Por, poorly differentiated adenocarcinoma; RG, robotic gastrectomy; Sig, signet-ring cell carcinoma; Tub1, well differentiated tubular adenocarcinoma; Tub2, moderately differentiated tubular adenocarcinoma.
Japanese Classification of Gastric Carcinoma.18
UICC 8th edition.17
Postoperative Intra-abdominal Infectious Complications
The primary end point of the study was the incidence of postoperative intra-abdominal infectious complications of Clavien-Dindo grade II or higher, including anastomotic leakage, pancreatic fistula, and intra-abdominal abscess.15 There was no significant difference in the overall incidence of intra-abdominal infectious complications of Clavien-Dindo grade II or higher in either the LG group (10 of 119 [8.4%] in the mITTP and 10 of 117 [8.5%] in the PPP) or in the RG group (7 of 117 [6.0%] in the mITTP and 7 of 113 [6.2%] in the PPP) (Table 2). Even in analysis limited to complications of grade IIIa or higher, the complication rate was not significantly different between the 2 groups (PPP: 9 of 117 [7.7%] in the LG group vs 5 of 113 [4.4%] in the RG group; Table 2). Comparing the incidence of grade II or higher anastomotic leakage, the LG group (5 of 119 [4.2%] in the mITTP and 5 of 117 [4.3%] in the PPP) and RG group (4 of 117 [3.4%] in the mITTP and 4 of 113 [3.5%] in the PPP) were similar (P = .99; Table 3).18 In our consecutive series, grade II or higher postoperative pancreatic fistulas were not found in the RG group but were found in 2 of 119 patients (1.7%) in the LG group, so there was no significant difference (P = .50; Table 3).18 There was also no significant difference in the incidence of grade II or higher intra-abdominal abscess between the LG group (3 of 119 [2.5%] in the mITTP and 3 of 117 [2.6%] in the PPP) and the RG group (3 of 117 [2.6%] in the mITTP and 3 of 113 [2.7%] in the PPP) (P = .99; Table 3).18
Table 2. Postoperative Intra-abdominal Infectious Complications.
| Variable | Modified intention-to-treat population, No. (%) | Per-protocol population, No. (%) | ||||
|---|---|---|---|---|---|---|
| LG (n = 119) | RG (n = 117) | P value | LG (n = 117) | RG (n = 113) | P value | |
| Intra-abdominal infectious complication, ≥grade IIa,b | 10 (8.4) | 7 (6.0) | .47 | 10 (8.5) | 7 (6.2) | .50 |
| Intra-abdominal infectious complication, ≥grade IIIa | 9 (7.6) | 5 (4.3) | .29 | 9 (7.7) | 5 (4.4) | .30 |
Abbreviations: LG, laparoscopic gastrectomy; RG, robotic gastrectomy.
Including anastomotic leakage, pancreatic fistula, and intra-abdominal abscess.
Surgical complications were classified into 5 categories according to the Clavien-Dindo classification.
Table 3. Surgical Results, Postoperative Recovery, and Postoperative Complications.
| Variable | Modified intention-to-treat population | Per-protocol population | ||||
|---|---|---|---|---|---|---|
| LG (n = 119) | RG (n = 117) | P value | LG (n = 117) | RG (n = 113) | P value | |
| Surgical results | ||||||
| Operative procedure, No. (%) | ||||||
| DG | 80 (67.2) | 69 (59.0) | .08 | 80 (68.4) | 67 (59.3) | .07 |
| TG | 24 (20.2) | 38 (32.5) | 22 (18.8) | 36 (31.9) | ||
| PG | 15 (12.6) | 10 (8.5) | 15 (12.8) | 10 (8.8) | ||
| Lymph node dissection, No. (%)a | ||||||
| D1 | 11 (9.2) | 3 (2.6) | .05 | 11 (9.4) | 3 (2.7) | .06 |
| D1+ | 59 (49.6) | 51 (43.6) | 59 (50.4) | 50 (44.2) | ||
| D2 | 49 (41.2) | 62 (53.0) | 47 (40.2) | 59 (52.2) | ||
| D2 and PAND | 0 (0) | 1 (0.9) | 0 | 1 (0.9) | ||
| Reconstruction, No. (%) | ||||||
| BI | 51 (42.8) | 35 (29.9) | .14 | 51 (43.6) | 34 (30.1) | .13 |
| BII | 10 (8.4) | 14 (12.0) | 10 (8.5) | 13 (11.5) | ||
| RY | 43 (36.1) | 58 (49.6) | 41 (35.0) | 56 (49.6) | ||
| EG | 6 (5.0) | 4 (3.4) | 6 (5.1) | 4 (3.5) | ||
| DT | 9 (7.6) | 6 (5.2) | 9 (7.7) | 6 (5.3) | ||
| Combined resection, No. (%) | 7 (5.9) | 17 (14.5) | 7 (6.0) | 16 (14.2) | ||
| Gall bladder | 4 (3.4) | 10 (8.5) | .03 | 4 (3.4) | 10 (8.8) | .04 |
| Spleen | 1 (0.8) | 7 (6.0) | 1 (0.9) | 6 (5.3) | ||
| Pancreas | 0 | 3 (2.6) | 0 | 2 (1.8) | ||
| Colon | 1 (0.8) | 1 (0.9) | 1 (0.9) | 1 (0.9) | ||
| Intestine | 1 (0.8) | 0 | 1 (0.9) | 0 | ||
| Renal (partial) | 0 | 1 (0.9) | 0 | 1 (0.9) | ||
| Operation time, median (range), min | 246 (131-598) | 298.5 (179-654) | .001 | 245 (131-534) | 297 (179-654) | .001 |
| Robot type, No. (%) | ||||||
| da Vinci Xi | NA | 95 | NA | NA | 93 | NA |
| da Vinci Si | NA | 22 | NA | NA | 20 | NA |
| Console time, median (range), min | NA | 249 (115-586) | NA | NA | 250 (124-586) | NA |
| Blood loss, median (range), mL | 25 (5-1920) | 25 (5-2540) | .66 | 25 (5-1405) | 25 (5-475) | .18 |
| Intraoperative transfusion, No. (%) | 4 (3.4) | 2 (1.7) | .68 | 3 (2.6) | 1 (0.9) | .62 |
| Retrieved lymph nodes, median (range), No. | 35 (5-129) | 31.5 (10-103) | .05 | 35 (5-92) | 30 (10-103) | .09 |
| No. of involved lymph nodes | ||||||
| Median | 0 | 0 | .23 | 0 | 0 | .45 |
| Mean (range) | 2.28 (0-41) | 1.54 (0-21) | 1.97 (0-28) | 1.57 (0-21) | ||
| R classification, No. (%)a | ||||||
| R0 | 110 (92.4) | 114 (97.4) | .10 | 108 (92.3) | 110 (97.3) | .11 |
| R1 | 5 (4.2) | 3 (2.6) | 5 (4.3) | 3 (2.7) | ||
| R2 | 4 (3.4) | 0 | 4 (3.4) | 0 | ||
| Postoperative recovery | ||||||
| Time to ambulation, median (range), POD | 1 (1-2) | 1 (1-2) | .78 | 1 (1-2) | 1 (1-2) | .96 |
| Time to first flatus, median (range), POD | 2 (1-6) | 2 (1-4) | .01 | 2 (1-6) | 2 (1-4) | .01 |
| Time to first liquid intake, median (range), POD | 1 (1-26) | 1 (1-5) | .47 | 1 (1-26) | 1 (1-4) | .49 |
| Time to first solid intake, median (range), POD | 3 (2-27) | 4 (3-18) | .91 | 3 (2-27) | 4 (3-18) | .93 |
| Postoperative hospital stay, median (range), POD | 13 (6-45) | 12 (7-43) | .99 | 13 (6-45) | 12 (7-43) | .93 |
| Doses of analgesic, median (range), No. | 2 (0-21) | 1 (0-12) | .001 | 2 (0-21) | 1 (0-12) | .001 |
| Postoperative complications, No. (%) | ||||||
| Overall complications, ≥grade IIb | 23 (19.3) | 10 (8.5) | .02 | 23 (19.7) | 10 (8.8) | .02 |
| Overall complications, ≥grade IIIa | 19 (16.0) | 6 (5.1) | .01 | 19 (16.2) | 6 (5.3) | .01 |
| Reoperation, grade IIIb | 3 (2.5) | 1 (0.9) | .62 | 3 (2.6) | 1 (0.9) | .62 |
| Mortality | 0 | 0 | .99 | 0 | 0 | .99 |
| Surgical complications, No. (%) | ||||||
| Anastomotic leakage, ≥grade II | 5 (4.2) | 4 (3.4) | .99 | 5 (4.3) | 4 (3.5) | .99 |
| Anastomotic leakage, ≥grade IIIa | 5 (4.2) | 3 (2.6) | .72 | 5 (4.3) | 3 (2.7) | .72 |
| Pancreatic fistula, ≥grade II | 2 (1.7) | 0 | .50 | 2 (1.7) | 0 | .50 |
| Pancreatic fistula, ≥grade IIIa | 1 (0.8) | 0 | .99 | 1 (0.9) | 0 | .99 |
| Intra-abdominal abscess, ≥grade II | 3 (2.5) | 3 (2.6) | .99 | 3 (2.6) | 3 (2.7) | .99 |
| Intra-abdominal abscess, ≥grade IIIa | 3 (2.5) | 2 (1.7) | .99 | 3 (2.6) | 2 (1.8) | .99 |
| Intra-abdominal bleeding, ≥grade II | 0 | 0 | .99 | 0 | 0 | .99 |
| Intra-luminal bleeding, ≥grade II | 0 | 0 | .99 | 0 | 0 | .99 |
| Ileus, ≥grade IIIa | 2 (1.7) | 1 (0.9) | .99 | 2 (1.7) | 1 (0.9) | .99 |
| Cholecystitis, ≥grade II | 3 (2.5) | 0 | .25 | 3 (2.6) | 0 | .25 |
| Cholecystitis, ≥grade IIIa | 2 (1.7) | 0 | .50 | 2 (1.7) | 0 | .50 |
| Hepatic portal venous gas, ≥grade IIIa | 1 (0.8) | 0 | .99 | 1 (0.9) | 0 | .99 |
| Stenosis, ≥grade IIIa | 3 (2.5) | 0 | .25 | 3 (2.6) | 0 | .25 |
| Wound infection, ≥grade II | 1 (0.8) | 1 (0.9) | .99 | 1 (0.9) | 1 (0.9) | .99 |
| Wound infection, ≥grade IIIa | 0 (0) | 1 (0.9) | .50 | 0 | 1 (0.9) | .50 |
| Medical complications, No. (%) | ||||||
| Pneumonia, ≥grade II | 5 (4.2) | 1 (0.9) | .21 | 5 (4.3) | 1 (0.9) | .21 |
| Pneumonia, ≥grade IIIa | 2 (1.7) | 0 | .50 | 2 (1.7) | 0 | .50 |
| Pneumothorax, ≥grade IIIa | 1 (0.8) | 0 | .99 | 1 (0.9) | 0 | .99 |
| Cardiovascular system, ≥grade II | 0 | 0 | .99 | 0 | 0 | .99 |
| Liver system, ≥grade II | 0 | 0 | .99 | 0 | 0 | .99 |
| Urinary system, ≥grade II | 1 (0.8) | 0 | .99 | 1 (0.9) | 0 | .99 |
| Thrombosis, ≥grade II | 0 | 0 | .99 | 0 | 0 | .99 |
Abbreviations: BI, Billroth-I reconstruction; BII, Billroth-II reconstruction; D1, D1 lymph node dissection; D1+, D1 plus lymph node dissection; D2, D2 lymph node dissection; DG, distal gastrectomy; DT, double-tract reconstruction; EG, esophago-gastrostomy; LG, laparoscopic gastrectomy; NA, not applicable; PAND, para-aortic nodal dissection; PG, proximal gastrectomy; POD, postoperative day; RG, robotic gastrectomy; RY, Roux-en-Y reconstruction; TG, total gastrectomy.
Japanese Classification of Gastric Carcinoma.18
Surgical complications were classified into 5 categories according to the Clavien-Dindo classification.
Converted Cases
The rate of conversion to open or laparoscopic surgery was a secondary end point of the study. Two of 119 patients (1.7%) had their procedure converted from LG to RG, and 4 of 117 patients (3.4%) converted from RG, 2 to LG and 2 to open gastrectomy, with no significant difference between the 2 groups (P = .44; eTable 1 in Supplement 2). Detailed characteristics of the 6 patients are listed in eTable 1 in Supplement 2.
Surgical Results
Surgical results were also stratified by the 2 surgical groups (Table 3).18 There were no significant differences between the 2 groups in terms of the ratio of distal gastrectomy to total or proximal gastrectomy or in terms of the range of lymphadenectomy. There was no bias between the 2 groups regarding the reconstruction procedure. The rates of simultaneous combined resection were significantly higher in the RG group (17 of 117 [14.5%] in the mITTP and 16 of 113 [14.2%] in the PPP) than in the LG group (7 of 119 [5.9%] in the mITTP and 7 of 117 [6.0%] in the PPP) (mITTP, P = .03; PPP, P = .04). Operation time, blood loss, and the number of retrieved lymph nodes were also secondary end points of the study. The median operation time was significantly longer in the RG group (298.5 minutes [95% CI, 309-345 minutes] in the mITTP and 297 minutes [95% CI, 307-342 minutes] in the PPP) than in the LG group (246 minutes [95% CI, 253-282 minutes] in the mITTP and 245 minutes [95% CI, 250-276 minutes] in the PPP) (mITTP: difference, 59.8 minutes [95% CI, 36.8-82.7 minutes]; P = .001; PPP: difference, 61.8 minutes [95% CI, 39.8-83.7 minutes]; P = .001). The median intraoperative blood loss was 25 mL in both groups (PPP: difference, –22.3 mL [95% CI, –55.2 to 10.6 mL]; P = .18). There was no significant difference in the number of retrieved or involved lymph nodes between the 2 groups, and the rates of surgical curability were also similar.
Postoperative Recovery
Secondary end points of the study considered the postoperative course, including times to resumption of drinking and eating and postoperative hospital stay. In postoperative recovery, the median time to ambulation, first drinking, first oral intake, and postoperative stay were not significantly different between the 2 groups (Table 3).18 The median time to first flatus was significantly shorter in the RG group (2 days [95% CI, 1.84-2.17 days] in the mITTP and 2 days [95% CI, 1.81-2.15 days] in the PPP) than in the LG group (2 days [95% CI, 2.15-2.49 days] in the mITTP and 2 days [95% CI, 2.13-2.47 days] in the PPP) (mITTP: difference, −0.31 days [95% CI, −0.55 to −0.08 days]; P = .001; PPP: difference, –0.32 days [95% CI, –0.55 to –0.08 days]; P = .001). The number of postoperative analgesic doses was significantly lower in the RG group (1 dose [95% CI, 0.97-1.71 doses] in the mITTP and 1 dose [95% CI, 0.96-1.73 doses] in the PPP) than in the LG group (2 doses [95% CI, 2.88-4.53 doses] in the mITTP and 2 doses [95% CI, 2.90-4.58 doses] in the PPP) (mITTP: difference, –2.36 doses [95% CI, –3.27 to –1.46 doses]; P = .001; PPP: difference, −2.40 doses [95% CI, −3.32 to −1.48 doses]; P = .001) (Table 3).18
Postoperative Laboratory Data
There were no differences between the 2 groups in maximum body temperature on PODs 1, 2, 3, and 4. There were also no differences between the groups in white blood cell count, C-reactive protein levels, and albumin levels on PODs 1, 3, and 5 (eTable 2 in Supplement 2).
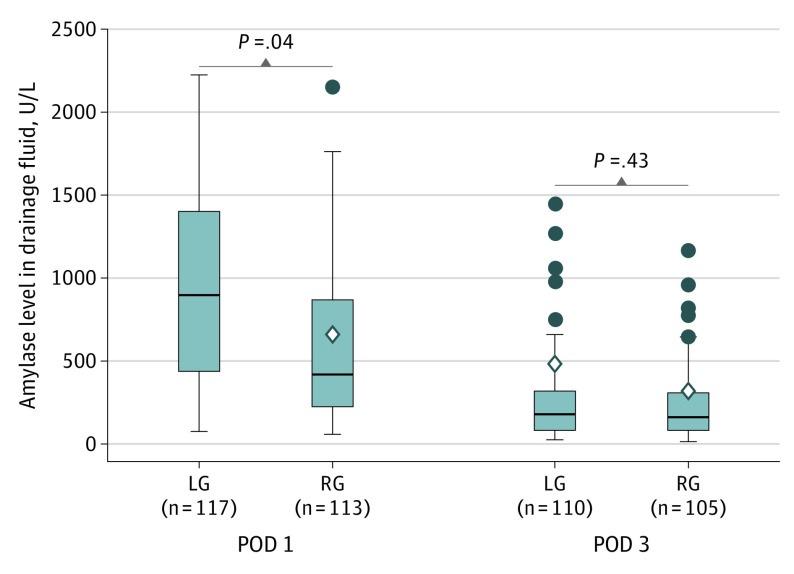
Amylase levels in drainage fluid on POD 1 were significantly lower in the RG group (438 U/L [95% CI, 519-838 U/L] [to convert to microkatals per liter, multiply by 0.0167] in the PPP) than in the LG group (925 U/L [95% CI, 693-4655 U/L] in the PPP) (PPP: difference, −1986 U/L [95% CI, −3860 to −110 U/L]; P = .04) (Figure 2). On POD 3, however, these values were not significantly different (171 U/L [95% CI, 97-853 U/L] in the LG group and 144 U/L [95% CI, 179-449 U/L] in the RG group) (difference, 161 U/L [95% CI, −240 to 561 U/L]; P = .43) (Figure 2).
Figure 2. Amylase Level in Drainage Fluid.
Per-protocol population was used for analysis (Mann-Whitney test). The horizontal lines inside the boxes indicate mean (SD) values, the circles indicate outliers, the diamonds indicate median values, and the error bars indicate 95% CIs. LG indicates laparoscopic gastrectomy; POD, postoperative day; and RG, robotic gastrectomy. To convert amylase levels to microkatals per liter, multiply by 0.0167.
Postoperative Complications
Table 318 summarizes the postoperative complications. The incidence of complications of Clavien-Dindo grade II or higher was a secondary end point of the study.15 The overall incidence of postoperative complications of grade II or higher was significantly higher in the LG group (23 of 119 [19.3%] in the mITTP and 23 of 117 [19.7%] in the PPP) than in the RG group (10 of 117 [8.5%] in the mITTP and 10 of 113 [8.8%] in the PPP) (P = .02). Clinically serious complications of grade IIIa or higher occurred in 19 of 117 patients (16.2%) in the LG group in the PPP and 6 of 113 patients (5.3%) in the RG group in the PPP (P = .01). In comparisons for each subtype of complication, no difference was observed in surgical and medical complications. Three of the 117 patients (2.6%) who underwent LG and 1 of the 113 patients (0.9%) who underwent RG required a reoperation (grade IIIb) (P = .62). The mortality rate in both groups in this trial was zero. The detailed characteristics of these 4 patients are listed in eTable 3 in Supplement 2.
Discussion
The primary end point of reducing intra-abdominal infectious complications in RG was not met. The results of the secondary end points showed good short-term surgical outcomes in both the LG and RG groups. Robotic gastrectomy did not change the rate of intra-abdominal infections but performed better in other outcomes.
In the present RCT, the total operation time was longer in the RG group than in the LG group, which was consistent with previous studies.24,25,26 The reason might be attributed to the setting and docking of the robotic arms, which results in longer operation times. The surgical resection time was similar between the 2 groups (245 minutes in the LG group and 250 minutes in the RG group). Meanwhile, because there were more patients with proximal GC invading the greater curvature in the RG group, there were 7 additional splenectomies in the RG group (6.0%) compared with just 1 in the LG group (0.8%). The high rates of combined resection of the spleen in the RG group may be associated with prolonged operation time. Previous studies indicated lower intraoperative blood loss during RG than during LG.6,24 In our RCT, however, the median intraoperative blood loss was 25 mL (interquartile range, 34-90 mL) in both groups. When skilled surgeons in our team performed LG using a recent 3-D or 4K laparoscope, the amount of bleeding was equivalent to that in an RG. Of the 122 patients assigned to the LG group, surgery was performed using a 3-D monitor for 59 of the patients (48.3%) and a 4K monitor for 47 of the patients (38.5%). During laparoscopic or robotic surgery, a clear surgical field of view helps surgeons better control bleeding in small blood vessels. The number of retrieved lymph nodes is assocated with surgical technique and a clear surgical field of view.24,27,28 Contrary to various reports, however, the number of retrieved lymph nodes were similar in both groups; we suggest that this is because of the surgeon’s skill and the use of a high-definition laparoscopic surgical system.
This RCT was established based on the hypothesis that robotic GC surgery has a lower rate of intra-abdominal infectious complications, including postoperative pancreatic fistulas, anastomotic leakage, and abscess, than laparoscopic GC surgery. The rate of intra-abdominal infectious complications in the RG group (6.2%) was lower than that in the LG group (8.5%), but it was not a statistically significant difference. The primary end point of this RCT was not met. Incidences of anastomotic leakage and intra-abdominal abscess could not be reduced using RG, as previously reported.29,30,31 We hypothesized that RG might reduce the incidence of postoperative pancreatic fistulas by avoiding unnecessary pancreatic injury. Amylase levels in drainage fluid on POD 1 were significantly lower in the RG group than in the LG group. The cited reports had similarly low levels of amylase in drainage fluid in the RG group compared with those in the LG group.32,33 However, the incidence of postoperative pancreatic fistulas was not significantly different between the 2 groups.
Robotic gastrectomy reduced the incidence of overall postoperative complications, including surgical and medical issues, which were the secondary end points of the study. These results were consistent with those of a recent RCT based in China, where the decrease in the incidence of complications in the RG group was caused mainly by a decrease in medical complications, not by surgical complications.14 Surgical tissue damage and the accompanying inflammatory response may lead to increased systemic complications, such as pneumonia. However, there were no differences between the LG and RG groups in inflammatory marker levels, including maximum body temperature, white blood cell count, and C-reactive protein levels. Further research into the inflammatory response after robotic surgery is needed in a larger-scale RCT.
Regarding postoperative recovery, the median time to first flatus was shorter in our RG group than in our LG group, similar to the results of a recent RCT.14 Rapid recovery of gastrointestinal peristalsis in patients undergoing robotic surgery might be associated with the stable and flexible movements of the robotic forceps, avoiding excessive traction on the tissue and accidental injury to the blood vessels and decreasing surgical trauma to the patients.34,35 In addition, the number of postoperative analgesic administrations was lower in the RG group than in the LG group. We hypothesize that rapid recovery of gastrointestinal peristalsis and milder surgical stress after RG may result in postoperative pain relief.
As we enter this new era of value-based medicine, an investment in this technology can be justified only if the costs are reasonable and a significant benefit is demonstrated regarding patient outcomes. A previous RCT compared the total costs of RG and LG and showed the high costs of RG.14 In practice, widespread use of RG may be limited owing to the high costs involved.
Limitations
This study had several limitations. First, we did not show the long-term oncologic outcome in this RCT, which might confirm the final impact of robotic GC surgery. Second, blinding was not applied regarding postoperative management of the patients. Third, owing to the relatively small sample size, subgroup analyses would decrease statistical power. The findings from this study could not establish conclusive evidence but rather serve to inform the need for larger randomized multicenter phase 3 clinical trials comparing LG and RG.
Conclusions
In this RCT of 241 patients with GC, contrary to expectation, there was no reduction of intra-abdominal infectious complications with RG compared with LG.
Trial Protocol
eTable 1. Converted Cases (Secondary End Points)
eTable 2. Postoperative Laboratory Data
eTable 3. Reoperation Cases
Data Sharing Statement
References
- 1.Katai H, Mizusawa J, Katayama H, et al. Survival outcomes after laparoscopy-assisted distal gastrectomy versus open distal gastrectomy with nodal dissection for clinical stage IA or IB gastric cancer (JCOG0912): a multicentre, non-inferiority, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol. 2020;5(2):142-151. doi: 10.1016/S2468-1253(19)30332-2 [DOI] [PubMed] [Google Scholar]
- 2.Kim W, Kim HH, Han SU, et al. ; Korean Laparo-endoscopic Gastrointestinal Surgery Study (KLASS) Group . Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01). Ann Surg. 2016;263(1):28-35. doi: 10.1097/SLA.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 3.Kim HH, Han SU, Kim MC, et al. ; Korean Laparoendoscopic Gastrointestinal Surgery Study (KLASS) Group . Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019;5(4):506-513. doi: 10.1001/jamaoncol.2018.6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350-1357. doi: 10.1200/JCO.2015.63.7215 [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Huang C, Sun Y, et al. ; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group . Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. 2019;321(20):1983-1992. doi: 10.1001/jama.2019.5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojima T, Nakamura M, Nakamori M, et al. Robotic radical lymphadenectomy without touching the pancreas during gastrectomy for gastric cancer. Medicine (Baltimore). 2019;98(13):e15091. doi: 10.1097/MD.0000000000015091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang X, Hiki N, Nunobe S, et al. Postoperative pancreatic fistula and the risk factors of laparoscopy-assisted distal gastrectomy for early gastric cancer. Ann Surg Oncol. 2012;19(1):115-121. doi: 10.1245/s10434-011-1893-y [DOI] [PubMed] [Google Scholar]
- 8.Park JM, Jin SH, Lee SR, et al. Complications with laparoscopically assisted gastrectomy: multivariate analysis of 300 consecutive cases. Surg Endosc. 2008;22(10):2133-2139. doi: 10.1007/s00464-008-9962-4 [DOI] [PubMed] [Google Scholar]
- 9.Washio M, Yamashita K, Niihara M, Hosoda K, Hiki N. Postoperative pancreatic fistula after gastrectomy for gastric cancer. Ann Gastroenterol Surg. 2020;4(6):618-627. doi: 10.1002/ags3.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida K, Honda M, Kumamaru H, et al. Surgical outcomes of laparoscopic distal gastrectomy compared to open distal gastrectomy: a retrospective cohort study based on a nationwide registry database in Japan. Ann Gastroenterol Surg. 2017;2(1):55-64. doi: 10.1002/ags3.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Kim JK, Kim YN, et al. Safety and feasibility of reduced-port robotic distal gastrectomy for gastric cancer: a phase I/II clinical trial. Surg Endosc. 2017;31(10):4002-4009. doi: 10.1007/s00464-017-5435-y [DOI] [PubMed] [Google Scholar]
- 12.Kim YM, Son T, Kim HI, Noh SH, Hyung WJ. Robotic D2 lymph node dissection during distal subtotal gastrectomy for gastric cancer: toward procedural standardization. Ann Surg Oncol. 2016;23(8):2409-2410. doi: 10.1245/s10434-016-5166-7 [DOI] [PubMed] [Google Scholar]
- 13.Ojima T, Hayata K, Yamaue H. Robotic complete lymphadenectomy at the splenic hilum during total gastrectomy for advanced gastric cancer (with video). J Visc Surg. 2019;156(2):173-174. doi: 10.1016/j.jviscsurg.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Zheng CH, Xu BB, et al. Assessment of robotic versus laparoscopic distal gastrectomy for gastric cancer: a randomized controlled trial. Ann Surg. 2021;273(5):858-867. doi: 10.1097/SLA.0000000000004466 [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojima T, Nakamura M, Nakamori M, et al. Robotic versus laparoscopic gastrectomy with lymph node dissection for gastric cancer: study protocol for a randomized controlled trial. Trials. 2018;19(1):409. doi: 10.1186/s13063-018-2810-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 8th ed. Wiley-Blackwell; 2017. [Google Scholar]
- 18.Japanese Gastric Cancer Association . Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1-21. doi: 10.1007/s10120-020-01042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ojima T, Nakamura M, Hayata K, Yamaue H. Robotic double tract reconstruction after proximal gastrectomy for gastric cancer. Ann Surg Oncol. 2021;28(3):1445-1446. doi: 10.1245/s10434-020-09015-2 [DOI] [PubMed] [Google Scholar]
- 20.Ojima T, Nakamura M, Hayata K, Yamaue H. Laparoscopic Roux-en-Y reconstruction using conventional linear stapler in robotic total gastrectomy for gastric cancer. Surg Oncol. 2020;33:9-10. doi: 10.1016/j.suronc.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 21.Ojima T, Nakamura M, Hayata K, et al. Laparoscopic Billroth I gastroduodenostomy in robotic distal gastrectomy for gastric cancers: fusion surgery. Surg Laparosc Endosc Percutan Tech. 2019;29(6):520-523. doi: 10.1097/SLE.0000000000000720 [DOI] [PubMed] [Google Scholar]
- 22.Ojima T, Nakamura M, Yamaue H. Full robotic Roux-en-Y reconstruction after gastrectomy for gastric cancer: a loop reconstruction technique. Updates Surg. 2020;72(4):1279-1281. doi: 10.1007/s13304-020-00889-1 [DOI] [PubMed] [Google Scholar]
- 23.Ojima T, Nakamori M, Nakamura M, Hayata K, Maruoka S, Yamaue H. Fundoplication with 180-degree wrap during esophagogastrostomy after robotic proximal gastrectomy for early gastric cancer. J Gastrointest Surg. 2018;22(8):1475-1476. doi: 10.1007/s11605-018-3765-2 [DOI] [PubMed] [Google Scholar]
- 24.Ye SP, Shi J, Liu DN, et al. Robotic- versus laparoscopic-assisted distal gastrectomy with D2 lymphadenectomy for advanced gastric cancer based on propensity score matching: short-term outcomes at a high-capacity center. Sci Rep. 2020;10(1):6502. doi: 10.1038/s41598-020-63616-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Kinoshita T, Tonouchi A, Kaito A, Tokunaga M. What are the reasons for a longer operation time in robotic gastrectomy than in laparoscopic gastrectomy for stomach cancer? Surg Endosc. 2019;33(1):192-198. doi: 10.1007/s00464-018-6294-x [DOI] [PubMed] [Google Scholar]
- 26.Chen K, Pan Y, Zhang B, Maher H, Wang XF, Cai XJ. Robotic versus laparoscopic gastrectomy for gastric cancer: a systematic review and updated meta-analysis. BMC Surg. 2017;17(1):93. doi: 10.1186/s12893-017-0290-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu J, Wang W, Zheng CH, et al. Influence of total lymph node count on staging and survival after gastrectomy for gastric cancer: an analysis from a two-institution database in China. Ann Surg Oncol. 2017;24(2):486-493. doi: 10.1245/s10434-016-5494-7 [DOI] [PubMed] [Google Scholar]
- 28.Strong VE, Russo AE, Nakauchi M, et al. Robotic gastrectomy for gastric adenocarcinoma in the USA: insights and oncologic outcomes in 220 patients. Ann Surg Oncol. 2021;28(2):742-750. doi: 10.1245/s10434-020-08834-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrini GP, Esposito G, Magistri P, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: the largest meta-analysis. Int J Surg. 2020;82:210-228. doi: 10.1016/j.ijsu.2020.07.053 [DOI] [PubMed] [Google Scholar]
- 30.Shin HJ, Son SY, Wang B, Roh CK, Hur H, Han SU. Long-term comparison of robotic and laparoscopic gastrectomy for gastric cancer: a propensity score-weighted analysis of 2084 consecutive patients. Ann Surg. 2020. doi: 10.1097/SLA.0000000000003845 [DOI] [PubMed] [Google Scholar]
- 31.Kim KM, An JY, Kim HI, Cheong JH, Hyung WJ, Noh SH. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg. 2012;99(12):1681-1687. doi: 10.1002/bjs.8924 [DOI] [PubMed] [Google Scholar]
- 32.Suda K, Nakauchi M, Inaba K, Ishida Y, Uyama I. Robotic surgery for upper gastrointestinal cancer: current status and future perspectives. Dig Endosc. 2016;28(7):701-713. doi: 10.1111/den.12697 [DOI] [PubMed] [Google Scholar]
- 33.Seo HS, Shim JH, Jeon HM, Park CH, Song KY. Postoperative pancreatic fistula after robot distal gastrectomy. J Surg Res. 2015;194(2):361-366. doi: 10.1016/j.jss.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Li X, Zhao S, Zhang R, Yang D. Robotic versus laparoscopic gastrectomy for gastric cancer: a systematic review and meta-analysis. World J Surg Oncol. 2020;18(1):306. doi: 10.1186/s12957-020-02080-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JY, Jo MJ, Nam BH, et al. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg. 2012;99(11):1554-1561. doi: 10.1002/bjs.8887 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Converted Cases (Secondary End Points)
eTable 2. Postoperative Laboratory Data
eTable 3. Reoperation Cases
Data Sharing Statement