Summary
Sleep disturbances among pregnant women are increasingly linked to suboptimal maternal/birth outcomes. Few U.S. studies investigating sleep by pregnancy status have included racially/ethnically diverse populations, despite worsening disparities in adverse birth outcomes. Using a nationally representative sample of 71,644 (2,349 pregnant) women from the National Health Interview Survey (2004–2017), we investigated relationships between self-reported pregnancy and six sleep characteristics stratified by race/ethnicity. We also examined associations between race/ethnicity and sleep stratified by pregnancy status. We used average marginal predictions from fitted logistic regression models to estimate prevalence ratios (PRs) and 95% confidence intervals (CIs) for each sleep dimension, adjusting for socio-demographic and health characteristics. Pregnant women were less likely than non-pregnant women to report short sleep (PROverall=0.75, 95% CI: 0.68–0.82), and more likely to report long sleep (PROverall=2.06, 95% CI: 1.74–2.43) and trouble staying asleep (PROverall=1.34, 95% CI: 1.25–1.44). The association between pregnancy and sleep duration was less pronounced among women 35–49 years of age compared to those <35 years. Among white women, sleep medication use was less prevalent among pregnant compared to non-pregnant women (PRWhite=0.45, 95% CI: 0.31–0.64), but this association was not observed among black women (PRBlack=0.98, 95% CI: 0.46–2.09) and was less pronounced among Hispanic/Latina women (PRHispanic/Latina=0.82, 95% CI: 0.38–1.77). Compared to pregnant whites, pregnant blacks had a higher short sleep prevalence (PRBlack=1.35, 95% CI: 1.08–1.67). Given disparities in maternal/birth outcomes and sleep, expectant mothers (particularly racial/ethnic minorities) may need screening for sleep disturbances. Our findings should be interpreted in the social context of the U.S.
Keywords: Pregnancy, Sleep, Sleep deprivation, Sleep initiation and maintenance disorders, Race factors, Ethnic groups
INTRODUCTION
Pregnancy may negatively affect sleep health (Mindell et al., 2015). Sleep disruption is common among pregnant women and a recent meta-analysis found that 46% of pregnant women experienced poor sleep quality (Sedov et al., 2018). Factors thought to contribute to sleep disturbance, particularly during late pregnancy, are decreased bladder control, fetal movements, and general physical discomfort (Mindell et al., 2015). Psychological factors, hormones, and sleep disorders such as restless legs syndrome and sleep disordered breathing may also be involved (Bublitz et al., 2018, Oyiengo et al., 2014).
Sleep disturbance during pregnancy is linked to adverse maternal and child health outcomes, such as maternal stress, perinatal and postpartum depression, hypertension, excessive gestational weight gain, gestational diabetes, shorter birth length, preterm birth, and stillbirth (Heazell et al., 2017, Rawal et al., 2017, Bublitz et al., 2018, Facco et al., 2017, Wang et al., 2017, Gay et al., 2017, Okun, 2016). Most prior U.S. studies examining sleep during pregnancy have focused on non-Hispanic whites and/or Hispanic women, while few studies have included black women. There is both a higher incidence of adverse birth outcomes (e.g., preterm birth) and insufficient or suboptimal sleep among racial/ethnic minorities, particularly among black compared to white women (Amyx et al., 2017, Grobman et al., 2018, Johnson et al., 2019).
A prior study has informed the complex relationship between pregnancy, sleep, and race/ethnicity in the U.S. by characterizing sleep duration among both pregnant and non-pregnant women (Amyx et al., 2017). The authors documented disparities in the prevalence of sleep disorders by race/ethnicity among pregnant women, but not among non-pregnant women. This descriptive evidence, along with emerging data from other smaller epidemiologic studies, suggests that the association between pregnancy and sleep may differ by race/ethnicity. However, this research question has yet to be addressed in comprehensive epidemiologic analyses of nationally representative data that adjust for potential confounders, examine multiple measures of sleep health, and assess for modification by key risk factors, such as advanced maternal age.
To address this gap in the literature, we examined the association between pregnancy and multiple sleep characteristics using data from a large, nationally representative sample of white, black, and Hispanic/Latina women in the U.S. using cross-sectional data collected and pooled over 14 survey years. We hypothesized that pregnant women would have a higher prevalence of sleep disturbances compared to non-pregnant women and that the strength of this association would be modified by race/ethnicity. We further hypothesized that pregnant racial/ethnic minority women would have a higher prevalence of sleep disturbances compared to their pregnant white counterparts. In a secondary analysis, we also examined whether the association between pregnancy and sleep disturbances is influenced by advanced maternal age, hypothesizing that pregnant women of advanced maternal age will report more sleep disturbances.
METHODS
Study Population
We used self-reported data from the National Health Interview Survey (NHIS), which is comprised of a series of cross-sectional household interview surveys (Blewett et al., 2019). We pooled 14 years of cross-sectional NHIS data from 2004–2017, which was merged by the Integrated Health Interview Series. A detailed description of NHIS procedures has been published elsewhere (National Center for Health Statistics, 2017). The National Center for Health Statistics approved the NHIS protocol and participants provided written informed consent.
All adult women aged 18–49 years old who self-reported being US-born non-Hispanic white, non-Hispanic black/African American, or Hispanic/Latina were eligible for inclusion in this analysis. We excluded participants with missing data on pregnancy status, sleep characteristics, or potential confounders or modifiers (17%), as well as military personnel (NHIS was not designed to be representative of this population). Our final analytic sample consisted of 71,644 women (49,659 whites, 13,368 blacks, and 8,617 Hispanics/Latinas).
Measurements
Pregnancy.
To identify pregnancy status, all women aged 18–49 years were asked, “Are you currently pregnant? (Yes, No).”
Sleep Duration and Sleep Difficulties.
Interviewers asked respondents, “On average, how many hours of sleep do you get in a 24-hour period?” Responses were categorized according to the National Sleep Foundation recommendations for adults aged 18–64 years as “short sleep” (< 7 hours), “recommended or sufficient sleep” (7–9 hours), and “long sleep” (>9 hours) (Hirshkowitz et al., 2015). For these analyses, we used 7–9 hours as the reference group because it is associated with the lowest levels of morbidity and mortality (Watson et al., 2015). Measures of sleep duration were available from 2004–2017. Several sleep difficulty measures were also assessed, including ‘trouble falling asleep’, ‘trouble staying asleep’, ‘not waking up most days (≥4) feeling rested’, and ‘sleep medication taken ≥1 time’ (all in the previous week). The sleep difficulty measures were available from 2013–2017.
Covariates.
Several sociodemographic, health behavior, and clinical characteristics were considered potential confounders or effect measure modifiers in analyses. Race/ethnicity was assessed in the survey by asking participants, “What race or races do you consider yourself to be?” They could select one or more of the following categories: American Indian/Alaskan native, Asian, non-Hispanic black/African American, white, or one or more of several races. National origin or ancestry refers to the national or cultural group from which the person is descended, and ethnicity is classified as Hispanic or Non-Hispanic. Due to sample size limitations, participant race/ethnicity was ultimately categorized in the analysis as Non-Hispanic white, Non-Hispanic black, or Hispanic/Latina (hereafter, white, black, and Hispanic/Latina, respectively).
Other sociodemographic characteristics included: marital status (married; divorced, separated, or widowed; single/never married), educational attainment (<high school (HS), HS/general education diploma, some college, college degree or more education), living in poverty (based on annual household income and family size, categorized as ‘yes/no’); occupational class (professional/management, support services, laborers); and annual household income ($0–$34,999, ≥$35,000).
Health behaviors
Health behaviors included: smoking status (never, current, former); alcohol consumption (never, current, former); and leisure-time physical activity (never or unable: unable to do a ≥10 minute time period of moderate or vigorous activity; low: ≥10 minute of moderate activity ≤1 time/week to 4 times/week or vigorous activity 1–3 times/week; high: ≥10 minutes of moderate activity >4 times/week or vigorous activity >3 times/week) (U.S. Department of Health and Human Services, 2015).
Clinical characteristics
Clinical characteristics included: body mass index (BMI) (overweight/obese is defined as a BMI (weight (kg)/height (m)2) value of ≥25 kg/m2; obese is defined as a BMI value of ≥30 kg/m2); hypertension, diabetes, heart disease, or cancer (yes/no); depressive symptoms (most/all of the time, none/little/some of the time); and self-rated health status (fair/poor, good, excellent/very good).
Statistical Analysis
Statistical analyses were completed using the SAS® version 9.4 (SAS Institute Inc., Cary, North Carolina) and SAS-Callable SUDAAN (Research Triangle Institute, Research Triangle Park, North Carolina). All analyses accounted for the NHIS complex survey design and were weighted to account for unbalanced probabilities of selection to participate in the study, nonresponse, and oversampling of certain population subgroups (e.g., elderly, racial/ethnic minorities) (National Center for Health Statistics, 2016). Taylor series linearization was used for variance estimation. We used absolute frequencies and weighted percentages to describe the distribution of sociodemographic, health behavior, and clinical characteristics of the study population. To provide context for covariate-adjusted models, these descriptive analyses were conducted for the overall study population, as well as stratified by race/ethnicity and pregnancy status. In supplementary analyses, we additionally examined these distributions stratified by educational attainment (≤HS vs. >HS).
We used average marginal predictions from fitted logistic regression models to estimate the associations between pregnancy status and each sleep characteristic based on prevalence ratios (PRs) and 95% confidence intervals (CIs) in SAS while accounting for the survey design (Bieler et al., 2010). Non-pregnant participants were used as the reference group and pre-specified covariates were entered into the model in a stepwise manner: model 1 adjusted only for age; model 2 was additionally adjusted for socioeconomic characteristics- marital status, educational attainment, occupational class, and annual household income; model 3 additionally adjusted for health characteristics- smoking status, alcohol consumption, physical activity, self-rated health status, hypertension, diabetes, and heart disease. We examined these associations overall as well as stratified by race/ethnicity (white, black, and Hispanic/Latina) and age category (<35 years vs 35–49 years, chosen to coincide with the definition of advanced maternal age (Facco et al., 2010)).
Using the same stepwise modeling approach described above, we additionally generated PR’s and 95% CI’s to compare the sleep characteristics of blacks and Hispanics/Latinas to whites, stratified by pregnancy status.
RESULTS
Study Population Characteristics
Table 1 shows the age-standardized distribution of sociodemographic, health behavior and clinical characteristics of all 71,644 women by race/ethnicity and pregnancy status. There were 2,349 (3%) pregnant women and 69,295 (97%) non-pregnant women. Compared to non-pregnant women, pregnant women were slightly younger (mean age ± standard error 28 ± 0.14 years vs. 34 ± 0.07 years) and more likely to be married (66% vs. 41%). Compared to pregnant white women, pregnant black women were least likely to be married (29% vs. 74% for white women and 60% for Hispanic/Latina women) and most likely to report an annual household income <$35,000 (64% vs. 23% for white women and 38% for Hispanics/Latina women 38%).
Table 1.
Age-Standardized Sociodemographic, Health Behavior, and Clinical Characteristicsa among Women 18–49 Years of Age (n=71,644) by Race/Ethnicity and Pregnancy Status, National Health Interview Survey, 2004–2017
| Overall | Non-Hispanic whites | Non-Hispanic blacks | Hispanics/Latinas | |||||
|---|---|---|---|---|---|---|---|---|
| Pregnant | Not Pregnant | Pregnant | Not Pregnant | Pregnant | Not Pregnant | Pregnant | Not Pregnant | |
| n=2,349 | n=69,295 | n=1,581 | n=48,078 | n=404 | n=12,964 | n=364 | n=8,253 | |
| Mean age ± SE (unstandardized) | 28 ±0.14 | 34 ± 0.07 | 29 ± 0.16 | 34 ± 0.09 | 27 ± 0.33 | 34 ± 0.12 | 27 ± 0.30 | 32 ± 0.12 |
| Age-Standardized Distribution (%) | ||||||||
| Marital status | ||||||||
| Married | 66 | 41 | 74 | 46 | 29 | 19 | 60 | 39 |
| Divorced/separated/ widowed | 10 | 18 | 9 | 18 | 18 | 20 | 9 | 20 |
| Never married | 24 | 41 | 17 | 36 | 54 | 62 | 31 | 41 |
| Educational attainment | ||||||||
| <High school | 8 | 7 | 7 | 5 | 13 | 11 | 14 | 12 |
| High school graduate/GED | 22 | 21 | 20 | 20 | 28 | 25 | 30 | 25 |
| Some college | 32 | 38 | 30 | 37 | 39 | 42 | 34 | 42 |
| ≥College | 38 | 34 | 43 | 38 | 21 | 22 | 22 | 21 |
| Class of worker | ||||||||
| Private wage | 77 | 78 | 77 | 78 | 81 | 77 | 74 | 77 |
| Government | 17 | 17 | 16 | 17 | 17 | 20 | 24 | 19 |
| Self-employedb | 6 | 5 | 7 | 6 | 2 | 3 | 3 | 4 |
| Occupational class | ||||||||
| Professional/management | 19 | 17 | 20 | 19 | 13 | 12 | 16 | 14 |
| Support services | 67 | 65 | 67 | 64 | 65 | 65 | 72 | 68 |
| Laborers | 15 | 18 | 13 | 17 | 23 | 23 | 12 | 17 |
| Annual household income <$35K (yes) | 30 | 38 | 23 | 33 | 64 | 59 | 38 | 42 |
| Living in poverty (yes) | 16 | 18 | 10 | 15 | 47 | 32 | 18 | 22 |
| Smoking status | ||||||||
| Never | 63 | 63 | 60 | 59 | 71 | 74 | 83 | 72 |
| Current | 12 | 23 | 13 | 24 | 14 | 20 | 4 | 16 |
| Former | 24 | 14 | 27 | 16 | 15 | 6 | 13 | 12 |
| Alcohol consumption | ||||||||
| Never | 16 | 15 | 13 | 12 | 33 | 27 | 23 | 20 |
| Current | 66 | 75 | 68 | 79 | 55 | 63 | 61 | 70 |
| Former | 18 | 10 | 20 | 9 | 11 | 10 | 16 | 10 |
| Physical activity | ||||||||
| None/unable | 35 | 25 | 30 | 21 | 57 | 39 | 47 | 29 |
| Moderate | 26 | 26 | 27 | 26 | 19 | 24 | 26 | 25 |
| High | 39 | 50 | 43 | 53 | 24 | 38 | 27 | 46 |
| Weight, kg/m 2 | ||||||||
| Underweight/normal, <25 | 40 | 47 | 45 | 52 | 18 | 29 | 25 | 37 |
| Overweight, ≥25-<30 | 27 | 25 | 26 | 24 | 23 | 28 | 34 | 28 |
| Obese, ≥30 | 34 | 28 | 29 | 24 | 59 | 43 | 40 | 35 |
| Clinical characteristics | ||||||||
| Hypertension | 11 | 14 | 10 | 12 | 20 | 23 | 11 | 12 |
| Diabetesc | 2 | 3 | 2 | 3 | 6 | 5 | 3 | 5 |
| Heart disease | 4 | 5 | 4 | 6 | 7 | 5 | 4 | 4 |
| Cancer | 3 | 4 | 3 | 5 | 2 | 2 | 6 | 3 |
| Depressive symptoms | ||||||||
| None/little/some | 97 | 97 | 98 | 97 | 93 | 95 | 98 | 95 |
| Most/all of time | 3 | 3 | 2 | 3 | 7 | 5 | 2 | 5 |
| Health status | ||||||||
| Excellent/very good | 76 | 70 | 79 | 73 | 62 | 59 | 70 | 63 |
| Good | 19 | 22 | 17 | 21 | 25 | 28 | 26 | 27 |
| Fair/poor | 6 | 8 | 4 | 7 | 13 | 13 | 4 | 10 |
Abbreviations: GED, General Education Diploma; SE, Standard error
Unweighted frequencies; weighted percentages and means. Some columns may not add to 100% due to rounding.
Self-employed includes work without pay.
Includes type 1 and 2 diabetes mellitus; does not include gestational diabetes.
Figure 1 shows the age-standardized prevalence of sleep disturbances by race/ethnicity and pregnancy status. Overall, pregnant women compared to non-pregnant women were less likely to report short sleep (<7 hours, 23% vs 32%, p < 0.0001), trouble falling asleep (41% vs 45%, p =0.0203), and sleep medication use (6% vs 15%, p < 0.0001). This pattern was observed across racial/ethnic groups for each sleep measure, with the one exception: black pregnant women were more likely than their non-pregnant black women counterparts (50% vs 41%, p =0.0444) to report trouble falling asleep. Pregnant black women also reported the highest prevalence of not waking up most (≥4) days feeling rested (59%), compared to pregnant white (49%, p =0.0412) and Hispanic/Latina (41%, p =0.0060) women. Pregnant black women also had a non-statistically significant higher prevalence of recent sleep medication use (9% for black, 6% for white, and 5% for Hispanic/Latina pregnant women, p=0.2150 for black vs white and p=0.2304 for black vs Hispanic/Latina).
Figure 1.
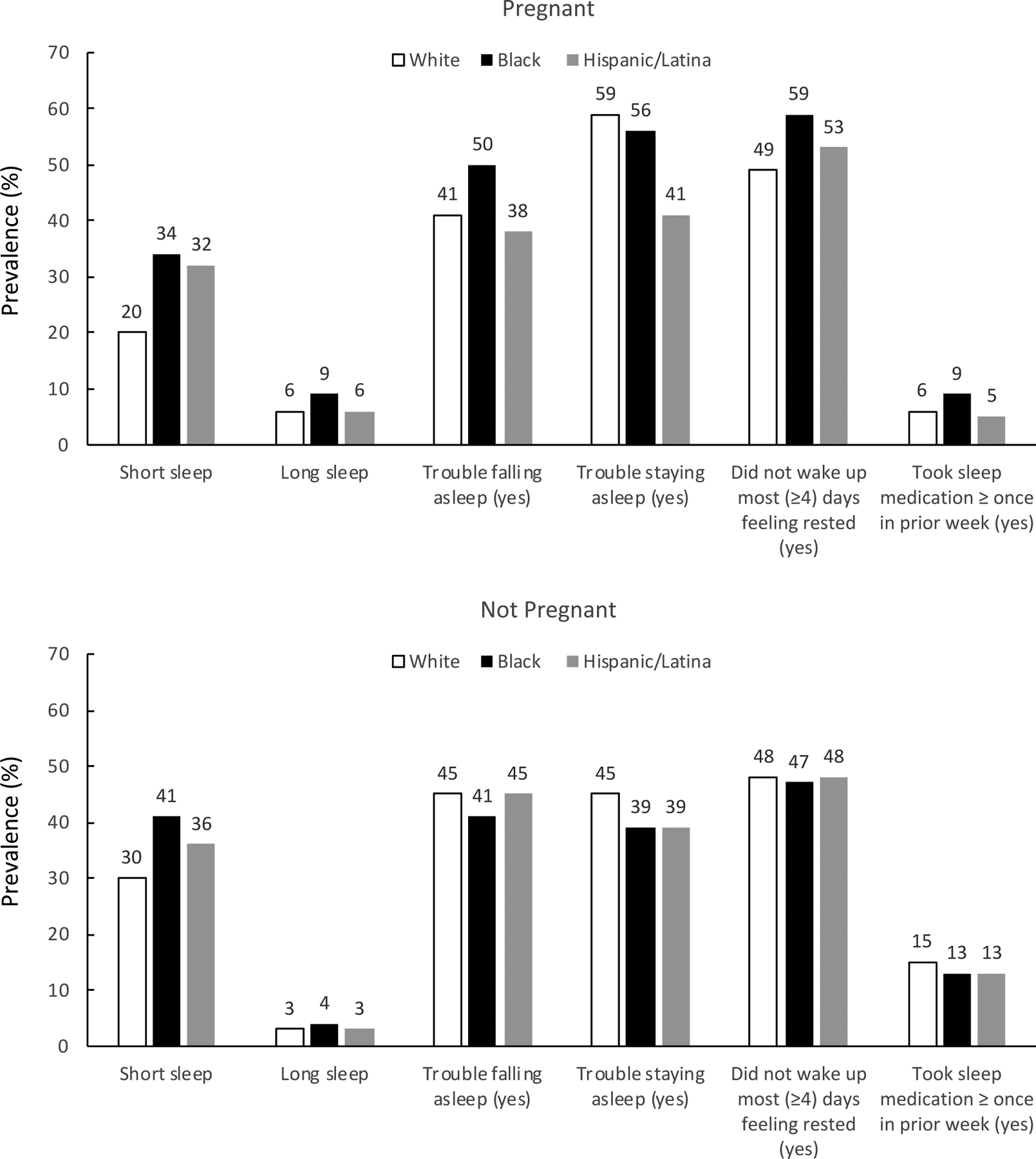
Age-standardized prevalence of sleep disturbances by race/ethnicity and pregnancy status.
Most women (70%) completed more than a high school education (Supplemental Table 1). Compared to women who were more educated, women who completed at most a high school education were more likely to report short or long sleep duration, trouble falling asleep, not waking up most (≥4) days feeling rested, and taking sleep medication. These disparities were small overall, but more pronounced among whites and blacks compared to Hispanics/Latinas.
Association of pregnancy and sleep disturbances, Overall and Stratified by Race/Ethnicity and Age Group
In the section that follows, we present the results from model 3, which included adjustment for all covariates (Figure 2 and Figure 3). Results from partially adjusted models 1 and 2 were similar and are shown in the online supplement (Supplemental Table 2).
Figure 2. Prevalence ratios and 95% confidence intervals of sleep characteristics for pregnant compared to non-pregnant women, overall and by race/ethnicity, National Health Interview Survey, 2004–2017.
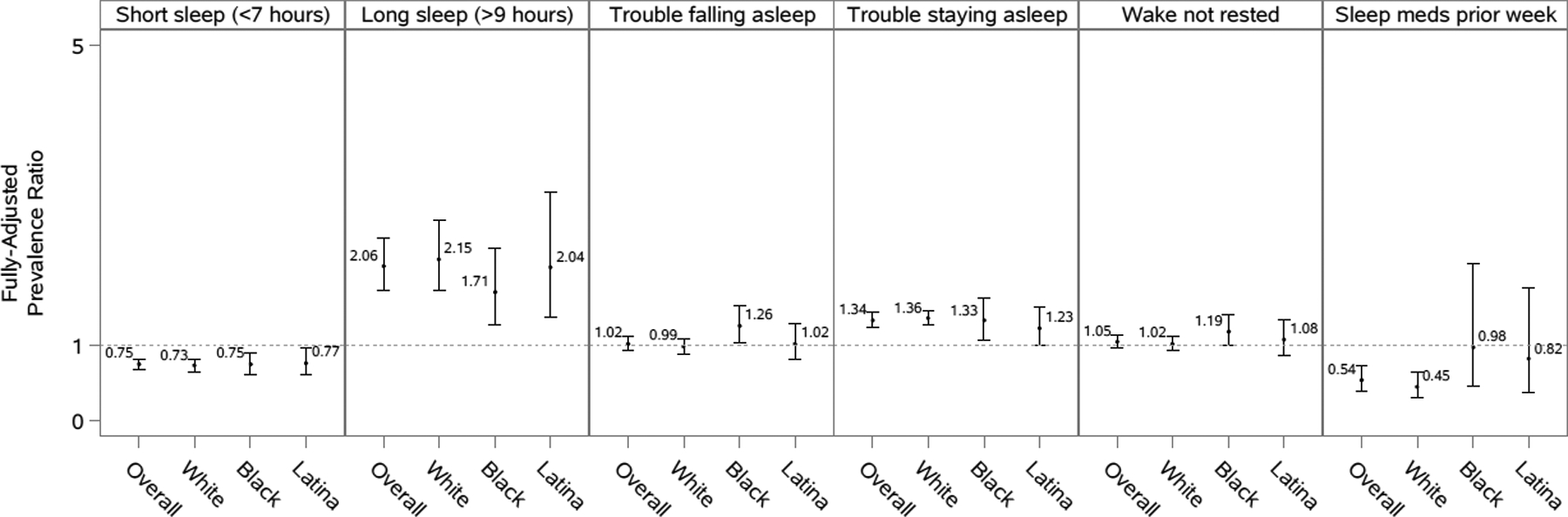
Models are adjusted for age, marital status, educational attainment, occupational class, household income, smoking status, alcohol consumption, physical activity, self-rated health status, hypertension, diabetes, heart disease, cancer, and depressive symptoms. Overall models are additionally adjusted for race/ethnicity. Analyses of short sleep duration excluded those who reported >9 hours of sleep. Analyses of long sleep duration excluded those who reported <7 hours of sleep. Analyses of sleep medication use included fewer than 50 participants in a cell of the crosstab between pregnancy status and the sleep outcome.
Figure 3. Prevalence ratios and 95% confidence intervals of sleep characteristics for pregnant compared to non-pregnant women, stratified by age (<35 vs. 35–49 years of age), overall and by race/ethnicity, National Health Interview Survey, 2004–2017.
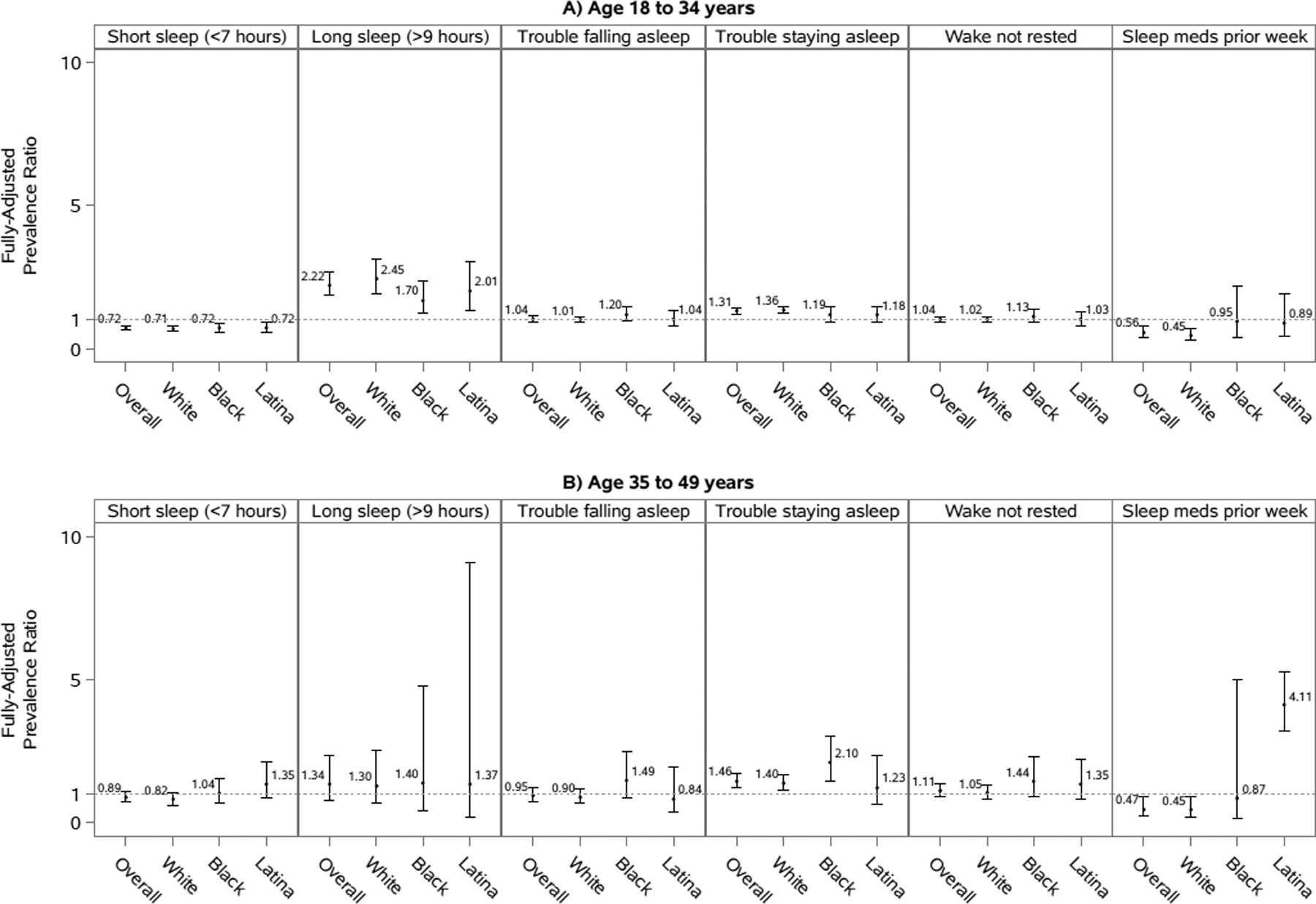
Models are adjusted for marital status, educational attainment, occupational class, household income, smoking status, alcohol consumption, physical activity, self-rated health status, hypertension, diabetes, heart disease, cancer, and depressive symptoms. Overall models are additionally adjusted for race/ethnicity. Analyses of short sleep duration excluded those who reported >9 hours of sleep. Analyses of long sleep duration excluded those who reported <7 hours of sleep. Some analyses included fewer than 50 participants in a cell of the crosstab between pregnancy status and the sleep outcome.
Short Sleep.
Pregnant women, overall and by race/ethnicity, had a lower short sleep prevalence compared to non-pregnant women in fully-adjusted models, with statistically significant PRs ranging from 0.73 to 0.77 (Figure 2). These associations were less pronounced and less consistent across racial/ethnic groups among women 35–49 years of age, with an overall PR (95% CI) of 0.89 (0.73–1.10) (Figure 3).
Long Sleep.
Pregnant women, overall and by race/ethnicity, generally had approximately twice the prevalence of long sleep compared to non-pregnant women in fully-adjusted models, with statistically significant PRs ranging from 1.71 to 2.15 (Figure 2). These associations appeared to be modified by age, with an overall PR (95% CI) of 2.22 (1.86–2.66) for women <35 years of age compared to 1.34 (0.76–2.35) for women 35–49 years of age (Figure 3). The p-value for the interaction term for pregnancy and age, however, was not statistically significant at an alpha level of 0.05 in fully adjusted models (Supplemental Table 3).
Trouble Falling Asleep.
The association between pregnancy status and trouble falling asleep varied by race/ethnicity (Figure 2). In fully-adjusted models, there was a significantly higher prevalence of trouble falling asleep among pregnant compared to non-pregnant black women (PR=1.26, 95%CI: 1.03–1.53). However, no association was observed for white and Hispanic/Latina women. No major differences in these associations were observed when stratifying by age group (Figure 3 and Supplemental Table 3).
Trouble Staying Asleep.
In fully-adjusted models, pregnant women were more likely than non-pregnant women to report trouble staying asleep (Figure 2). The association was slightly stronger for whites (PR=1.36, 95% CI: 1.27–1.46) and blacks (PR=1.33, 95% CI: 1.08–1.64) compared to Hispanics/Latinas (PR=1.23, 95% CI: 1.00–1.51). Among black women, the association between pregnancy status and trouble falling asleep appeared to be largely driven by women >35 years of age (Figure 3).
Not waking up most (≥4) days feeling rested.
There were no strong differences between pregnant and non-pregnant women in not waking up most (≥4) days feeling rested, overall, by race/ethnicity (Figure 2), or by age group (Figure 3). The possible exception to this was again among black and Hispanic/Latina women >35 years of age, for whom we observed an elevated but non-statistically significant association indicating that black and Hispanic/Latina pregnant women >35 years of age may be more likely to not wake up most (≥4) days feeling rested compared to non-pregnant black and Hispanic/Latina women >35 years of age (Figure 3).
Recent Sleep Medication Use.
Overall, the prevalence of medication use among pregnant women was about half that of non-pregnant women (PR: 0.54; 95% CI: 0.40–0.74) (Figure 2). However, when stratified by race/ethnicity, this association was only strong among white women (PR=0.45, 95% CI: 0.31–0.64). Although the association was not as strong and non-significant, this trend was also observed for Hispanic/Latina women (PR=0.82, 95% CI: 0.38–1.77). In contrast, this association was not observed for black women, with pregnant and non-pregnant women reporting similar sleep medication use. Some of the associations between pregnancy and sleep medication use were modified by age, particularly among Hispanic/Latina women (Figure 3). While the association for Hispanic/Latina women <35 years of age was similar to the overall association for Hispanic/Latina women, the association for Hispanic/Latina women >35 years of age was in the opposite direction and statistically significant (PR= 4.11; 95% CI: 3.19–5.29).
Association of race/ethnicity and sleep disturbances, overall and stratified by pregnancy status (fully adjusted model 3 is shown in Figure 4; partially adjusted models 1–2 are additionally shown in Supplemental Table 4)
Figure 4. Prevalence ratios and 95% confidence intervals of sleep characteristics comparing non-Hispanic black and Hispanic/Latina to non-Hispanic white participants by pregnancy status, National Health Interview Survey, 2004–2017.
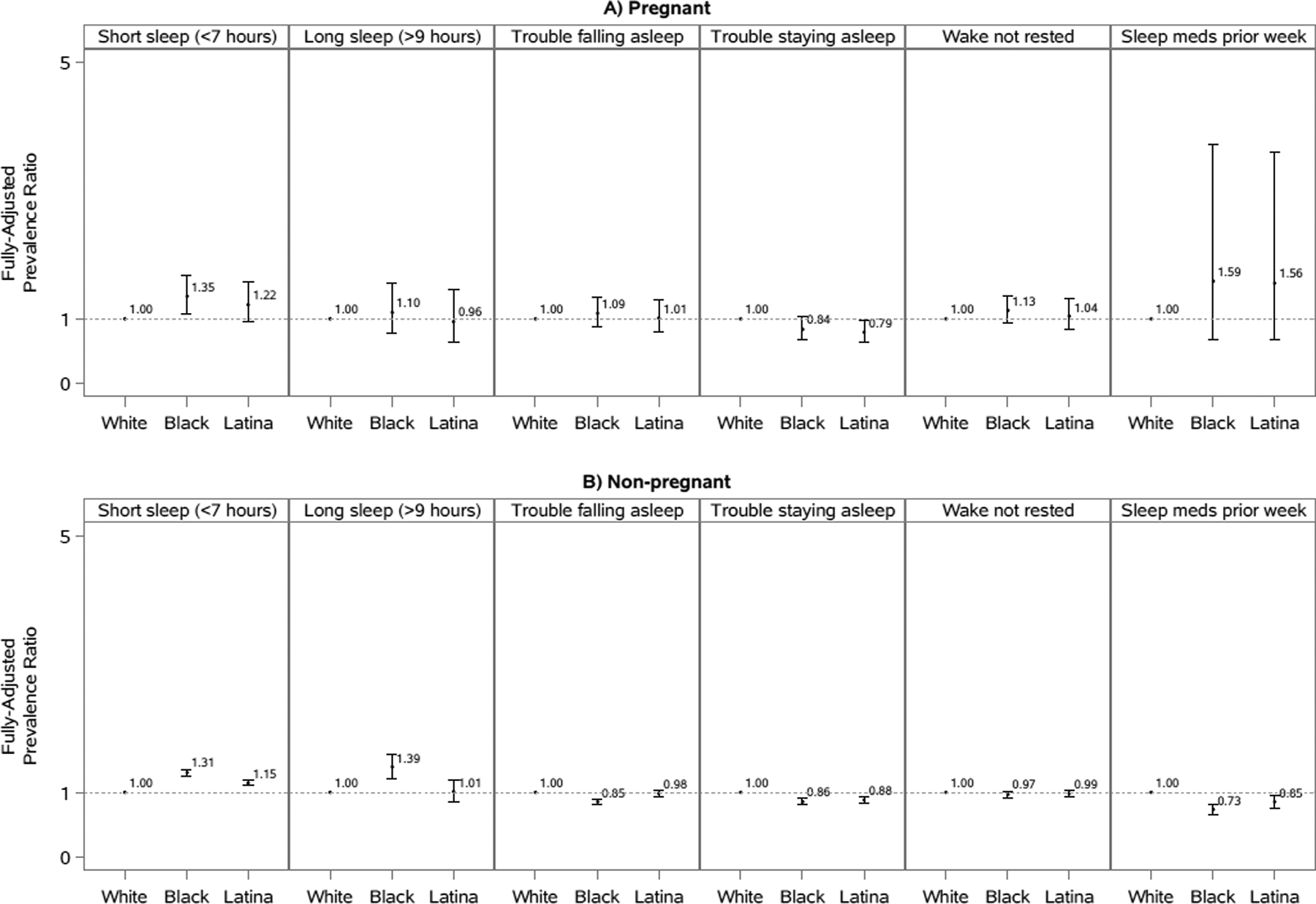
Models are adjusted for age, marital status, educational attainment, occupational class, household income, smoking status, alcohol consumption, physical activity, self-rated health status, hypertension, diabetes, heart disease, cancer, and depressive symptoms. Overall models are additionally adjusted for race/ethnicity. Analyses of short sleep duration excluded those who reported >9 hours of sleep. Analyses of long sleep duration excluded those who reported <7 hours of sleep. Some analyses included fewer than 50 participants in a cell of the crosstab between pregnancy status and the sleep outcome.
Short Sleep.
Compared to white women, black and Hispanic/Latina women had a higher prevalence of short sleep in fully-adjusted models regardless of pregnancy status. However, the association comparing pregnant Hispanic/Latina women to pregnant white women did not reach statistical significance.
Long Sleep.
Associations were less pronounced for long sleep duration, with only non-pregnant black women having a higher prevalence of long sleep compared to white non-pregnant women in fully-adjusted models (PR=1.39, 95% CI: 1.22–1.59).
Trouble Falling Asleep.
We did not observe strong disparities between white, black, and Hispanic/Latina women when comparing trouble falling asleep regardless of pregnancy status. We did observe a relatively weak association suggesting that black non-pregnant women may have more trouble falling asleep than non-pregnant white women (PR=0.85, 95% CI: 0.81–0.90).
Trouble Staying Asleep.
Compared to white women, black and Hispanic/Latina women reported a higher prevalence of trouble staying asleep in fully-adjusted models regardless of pregnancy status. However, the association comparing pregnant black women to pregnant white women was not statistically significantly different.
Not waking up most (≥4) days feeling rested.
Overall, no strong associations were observed comparing not waking up most (≥4) days feeling rested between black and Hispanic/Latina women to white women regardless of pregnancy status. The possible exception to this was the association comparing pregnant black women to pregnant white women (PR=1.13, 95% CI: 0.93–1.36), which indicated that black pregnant women may have a higher prevalence of not waking up most (≥4) days feeling rested noting that this association was not statistically significant.
Recent Sleep Medication Use.
Pregnancy status appeared to modify the association between race/ethnicity and medication use. Among non-pregnant women, blacks (PR=0.73, 95% CI: 0.65–0.81) and Hispanics/Latinas (PR=0.85, 95% CI: 0.75–0.96) were less likely to report recent medication use compared to whites. However, the opposite trend was observed among pregnant women, with black and Hispanic/Latina pregnant women more likely to report recent medication use compared to pregnant white women. However, these latter associations were not statistically significant.
DISCUSSION
In this large, nationally-representative study of women in the U.S. of reproductive age, we found a high prevalence of sleep disturbances, with only 71% of pregnant and 65% of non-pregnant women reporting getting the recommended 7–9 hours of daily sleep. We noted differences in the pregnancy-sleep relationship by race/ethnicity, with black pregnant women more likely than black non-pregnant women to report trouble falling asleep, which was not observed among white and Hispanic/Latina women. Finally, we found that maternal age may contribute to the complex relationship between race/ethnicity, pregnancy, and sleep in some contexts, but not uniformly in the direction we hypothesized. More research is needed.
Racial disparities in sleep health that likely contribute to health conditions like cardiovascular disease have been documented (Jackson et al., 2015). Compared to whites, blacks and Hispanics/Latinas generally have a higher prevalence of experiencing both short and long sleep durations, which have been associated with early mortality (Johnson et al., 2019). Furthermore, polysomnographic studies have provided objective evidence of worse sleep quality, measured by continuity of sleep and percentage of slow wave sleep, among blacks and Hispanics/Latinas compared to whites (Ruiter et al., 2011). Our study highlights that these disparities persist among women of reproductive age, including during pregnancy.
Pregnancy was associated with longer sleep across all racial/ethnic groups among women aged <35 years, but not among women 35–49 years, with black pregnant women having the highest prevalence of long sleep. Long sleep has been associated with higher rates of mortality, stroke, cardiovascular disease, and many other health consequences (Jike et al., 2018). The mechanisms for this association are not well established, although increased sleep fragmentation, negative effects on immune function, undiagnosed or poorly controlled conditions like sleep apnea and cardiovascular disease, for instance, have been hypothesized contributors (Grandner and Drummond, 2007). The specific effects of long sleep on pregnancy are unclear, but a recent study demonstrated that sleep durations >9 hours were associated with higher rates of stillbirth (O’Brien et al., 2019). There are no official recommendations for sleep duration specific to pregnant women, and the amount of necessary sleep may differ between pregnant and non-pregnant women as physiological changes associated with pregnancy may result in longer, necessary sleep durations, especially in the first trimester. On the other hand, the presence of undetected sleep disorders during pregnancy, e.g., obstructive sleep apnea, could be related to longer sleep times (Louis et al., 2014). Further studies are warranted to better distinguish in which circumstances prolonged sleep is beneficial versus a sign of an undiagnosed sleep disorder or other health condition. Future research also should seek to corroborate and better understand why pregnant women <35 years of age had a lower prevalence of short sleep compared to non-pregnant women of the same age, with no difference among older women still of reproductive age. One potential explanation is that older women may be more likely to have other children that may be contributing to less sleep.
We also found that pregnant compared to non-pregnant women had a higher prevalence of trouble staying asleep, which has been associated with various health consequences, including cognitive deficits, mental health disorders, cardiovascular disease, etc. (Medic et al., 2017). Although the impact of sleep fragmentation on pregnancy has not been well studied in humans, animal models suggest that fragmented sleep in mothers may be associated with higher rates of metabolic syndrome in their offspring (Khalyfa et al., 2014). One study conducted among adult women also found that poor sleep was associated with a higher prevalence of metabolic syndrome, particularly among pre-menopausal women (Gaston et al., 2019). We also observed that the association between pregnancy and trouble staying asleep was strongest among black women of advanced maternal age, a finding that warrants further investigation considering the likelihood of the effect of social stressors (e.g., discrimination) accumulating over time and contributing to poorer health. For instance, older Black pregnant women tend to have higher body weights that could lead to higher risk of sleep disorders like obstructive sleep apnea, which may be exacerbated by pregnancy in this older age group.
In our study, we observed an association between pregnancy and trouble falling asleep among black women but not in whites or Hispanics/Latinas. Trouble falling asleep has been associated with higher rates of cardiovascular disease and other medical problems, medication use, healthcare utilization, and missed work (Leger et al., 2002, Spiegelhalder et al., 2010). Our understanding of why black pregnant women are disproportionately affected is limited and future research on the role of social (e.g., discrimination; trauma) and environmental (e.g., endocrine disruptors) determinants are warranted based on prior literature identifying their influence on various health outcomes among women (Ertel et al., 2012, Gaston et al., 2018, Jackson, 2017, McWhorter et al., 2019, Ncube et al., 2016).
Overall, pregnant women were less likely than non-pregnant women to report recent sleep medication use. However, this association varied by race/ethnicity, with black pregnant women just as likely as non-pregnant black women to report recent sleep medication use. A variety of medications are used to treat insomnia during pregnancy, including antihistamines, benzodiazepines, non-benzodiazepine hypnotic agents, as well as anti-depressants (Okun et al., 2015). The overall reduction in sleep medication use among pregnant compared to non-pregnant women may partially be explained by concerns for infant safety given that not all hypnotics are considered safe. The safety of sleep-promoting medications in pregnancy varies widely depending on the drug and the risk-benefit ratio of sleep medications during pregnancy should involve shared clinical decision making between the pregnant women and their clinicians. For example, the antihistamine doxylamine is used widely and considered safe in pregnancy (Federal Drug Administration (FDA) pregnancy category A). Other drug classes, such as benzodiazepines, may also be indicated for individual women, despite conferring greater risk (FDA pregnancy categories of D or even X). More pregnant women also appear to be using marijuana to address pregnancy-related symptoms (Young-Wolff et al., 2019), perhaps considering it a safer alternative to sleep medications despite data showing increased risk of preterm birth with marijuana use (Corsi et al., 2019).
It is unclear why differences in the use of sleep medications during pregnancy differs by race/ethnicity, although they may partially relate to underlying racial disparities in the frequency of sleep disturbances. Among Hispanic/Latina women, we also observed modification of the association between pregnancy and sleep medication use by age, with a strong statistically significant association in the opposite direction observed among Hispanic/Latina women aged 35–49 years. However, given the smaller sample size of women who took sleep medications, particularly when stratified by race/ethnicity and/or age, these findings should be interpreted with caution and warrant confirmation by future studies. Given the widely varying safety of different sleep medications in pregnancy, it is unclear if lower medication use has harmful or positive effects on infant outcomes. Future studies should determine which sleep medications are most commonly used among pregnant women and whether the use of these medications differs by race/ethnicity.
Of note, the goal of the sleep-related questions in the NHIS is to assess overall sleep patterns on a population level rather than to diagnose or screen for specific sleep disorders. Although there is general consensus on how to objectively measure sleep disorders in the clinical setting, assessing the broader concept of “sleep health” (which is relevant to individuals with and without a clinical sleep disorder) on the population level has not been established although some dimensions commonly agreed to be important include sleep duration, sleep continuity, the circadian timing of sleep, and sleep quality (Buysse, 2014). An important limitation of this particular study is that the sleep-related questions in the NHIS have not been validated. A recent qualitative study consisting of cognitive interviewing evaluated the performance of the NHIS sleep-related questions and found that accuracy may have been lowered due to respondent uncertainty (Wheeler and Massey, 2018). For example, respondents had issues with accurately quantifying the amount of sleep they had in the prior 24 hours due to not remembering when they went to bed and due to inconsistent sleep schedules. When asked to quantify how many days in the past week they felt “well rested”, respondents also had difficulties with responding accurately due to the subjective nature of the question. Respondents had less difficulties determining how often they took sleep medications, as most took them daily or not at all. The incorporation of validated tools for measuring sleep health in nationally-representative surveys may offer improvements; however, currently validated instruments such as the Pittsburgh Sleep Quality Index also have their limitations, pointing to the need for more research to adequately define and validate measures of sleep health (Manzar et al., 2018, Pilz et al., 2018). Studies such as this one, while not without limitations, provide preliminary evidence that should be replicated with objective sleep measures.
Other limitations of our study include the cross-sectional study design, which precludes causal inference due to non-temporality. Some results could be due to chance because we tested for multiple associations and because multiple levels of stratification resulted in small sample sizes in certain instances; however, we sought to capture any possible associations that offer opportunities for future research given the limited research that has been conducted on this topic. Additionally, all NHIS data were self-reported and future studies that include objective measures of sleep health are warranted (Herring et al., 2013, Jackson et al., 2018, Jackson et al., 2019). Although we categorized long and short sleep duration based on commonly applied cut points, the ideal sleep duration for pregnant women is currently unknown. Finally, although our analysis accounted for relevant socioeconomic, behavioral and clinical confounders, unmeasured confounders such as timing of gestation, gestational weight gain and maternal parity may remain. Despite these limitations, our study has important strengths. In particular, our data came from a nationally representative sample of pregnant and non-pregnant women with multiple dimensions of sleep health and up to 14 years of data, permitting robust stratification by race/ethnicity and age.
Sleep is critical to health and well-being, and our research underscores the connection between pregnancy and sleep disturbances. Although routine prenatal visits offer opportunities (albeit unequal) for medical interventions, we found that sleep disturbances remain common among pregnant women in the U.S., particularly among black women. Future research should determine whether this racial/ethnic disparity may be contributing to the disproportionate burden of adverse birth outcomes experienced among black and other racial/ethnic minority women. Our findings should be interpreted in the social context of the U.S.
Supplementary Material
Acknowledgements
This work was funded by the Intramural Program at the NIH, National Institute of Environmental Health Sciences (Z1AES103325-01). The funding sources were not involved in the data collection, data analysis, manuscript writing nor publication. These results were, in part, presented at the 33rd Annual Meeting of the Associated Professional Sleep Societies, LLC joint meeting of the American Academy of Sleep Medicine and the Sleep Research Society in San Antonio, Texas from June 8-12, 2019.
Footnotes
Conflict of interests: None declared.
REFERENCES
- Amyx M, Xiong X, Xie Y and Buekens P Racial/Ethnic Differences in Sleep Disorders and Reporting of Trouble Sleeping among Women of Childbearing Age in the United States. Maternal and child health journal, 2017, 21: 306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieler GS, Brown GG, Williams RL and Brogan DJ Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. American Journal of Epidemiology, 2010, 171: 618–23. [DOI] [PubMed] [Google Scholar]
- Blewett LA, Rivera Drew JA, King ML and Williams KCW IPUMS Health Surveys: National Health Interview Survey, Version 6.4 [dataset]. In. IPUMS 2019, Minneapolis, MN, 2019. [Google Scholar]
- Bublitz MH, Bourjeily G, D’angelo C and Stroud LR Maternal Sleep Quality and Diurnal Cortisol Regulation Over Pregnancy. Behav. Sleep Med, 2018, 16: 282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ Sleep health: can we define it? Does it matter? Sleep, 2014, 37: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi DJ, Walsh L, Weiss D et al. Association Between Self-reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes. JAMA, 2019, 322: 145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel KA, James-Todd T, Kleinman K et al. Racial discrimination, response to unfair treatment, and depressive symptoms among pregnant black and African American women in the United States. Ann Epidemiol, 2012, 22: 840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facco FL, Grobman WA, Reid KJ et al. Objectively measured short sleep duration and later sleep midpoint in pregnancy are associated with a higher risk of gestational diabetes. Am J Obstet Gynecol, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facco FL, Kramer J, Ho KH, Zee PC and Grobman WA Sleep disturbances in pregnancy. Obstet Gynecol, 2010, 115: 77–83. [DOI] [PubMed] [Google Scholar]
- Gaston SA, Jackson WB 2nd, Williams DR and Jackson CL Sleep and cardiometabolic health by government-assisted rental housing status among Black and White men and women in the United States. Sleep health, 2018, 4: 420–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston SA, Park YM, Mcwhorter KL, Sandler DP and Jackson CL Multiple poor sleep characteristics and metabolic abnormalities consistent with metabolic syndrome among white, black, and Hispanic/Latina women: modification by menopausal status. Diabetol Metab Syndr, 2019, 11: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CL, Richoux SE, Beebe KR and Lee KA Sleep disruption and duration in late pregnancy is associated with excess gestational weight gain among overweight and obese women. Birth-Issues in Perinatal Care, 2017, 44: 173–80. [DOI] [PubMed] [Google Scholar]
- Grandner MA and Drummond SP Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep medicine reviews, 2007, 11: 341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobman WA, Parker CB, Willinger M et al. Racial Disparities in Adverse Pregnancy Outcomes and Psychosocial Stress. Obstet. Gynecol, 2018, 131: 328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazell A, Li M, Budd J et al. Association between maternal sleep practices and late stillbirth - findings from a stillbirth case-control study. BJOG: An International Journal of Obstetrics and Gynaecology, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring SJ, Foster GD, Pien GW et al. Do pregnant women accurately report sleep time? A comparison between self-reported and objective measures of sleep duration in pregnancy among a sample of urban mothers. Sleep & breathing = Schlaf & Atmung, 2013, 17: 1323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep health, 2015, 1: 40–43. [DOI] [PubMed] [Google Scholar]
- Jackson CL Determinants of racial/ethnic disparities in disordered sleep and obesity. Sleep health, 2017, 3: 401–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Patel SR, Jackson WB 2nd, Lutsey PL and Redline S Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep, 2018, 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Redline S and Emmons KM Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health, 2015, 36: 417–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CL, Ward JB, Johnson DA, Sims M, Wilson J and Redline S Concordance between Self-Reported and Actigraphy-Assessed Sleep Duration among African-American Adults: Findings from the Jackson Heart Sleep Study. Sleep, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jike M, Itani O, Watanabe N, Buysse DJ and Kaneita Y Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep medicine reviews, 2018, 39: 25–36. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Jackson CL, Williams NJ and Alcantara C Are sleep patterns influenced by race/ethnicity - a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep, 2019, 11: 79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalyfa A, Mutskov V, Carreras A, Khalyfa AA, Hakim F and Gozal D Sleep fragmentation during late gestation induces metabolic perturbations and epigenetic changes in adiponectin gene expression in male adult offspring mice. Diabetes, 2014, 63: 3230–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger D, Guilleminault C, Bader G, Levy E and Paillard M Medical and socio-professional impact of insomnia. Sleep, 2002, 25: 625–9. [PubMed] [Google Scholar]
- Louis JM, Mogos MF, Salemi JL, Redline S and Salihu HM Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep, 2014, 37: 843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzar MD, Bahammam AS, Hameed UA et al. Dimensionality of the Pittsburgh Sleep Quality Index: a systematic review. Health Qual Life Outcomes, 2018, 16: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcwhorter KL, Parks CG, D’aloisio AA, Rojo-Wissar DM, Sandler DP and Jackson CL Traumatic childhood experiences and multiple dimensions of poor sleep among adult women. Sleep, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medic G, Wille M and Hemels ME Short- and long-term health consequences of sleep disruption. Nat Sci Sleep, 2017, 9: 151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA, Cook RA and Nikolovski J Sleep patterns and sleep disturbances across pregnancy. Sleep medicine, 2015, 16: 483–8. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics Variance Estimation and Other Analytic Issue, NHIS 2006–2015. In, 2016.
- National Center for Health Statistics Survey Description, National Health Interview Survey, 2016. In, Hyattsville, MD, 2017. [Google Scholar]
- Ncube CN, Enquobahrie DA, Albert SM, Herrick AL and Burke JG Association of neighborhood context with offspring risk of preterm birth and low birthweight: A systematic review and meta-analysis of population-based studies. Soc Sci Med, 2016, 153: 156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’brien LM, Warland J, Stacey T, Heazell AEP, Mitchell EA and Consortium, S. Maternal sleep practices and stillbirth: Findings from an international case-control study. Birth, 2019, 46: 344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML Disturbed Sleep and Postpartum Depression. Current psychiatry reports, 2016, 18: 66. [DOI] [PubMed] [Google Scholar]
- Okun ML, Ebert R and Saini B A review of sleep-promoting medications used in pregnancy. Am J Obstet Gynecol, 2015, 212: 428–41. [DOI] [PubMed] [Google Scholar]
- Oyiengo D, Louis M, Hott B and Bourjeily G Sleep disorders in pregnancy. Clinics in chest medicine, 2014, 35: 571–87. [DOI] [PubMed] [Google Scholar]
- Pilz LK, Keller LK, Lenssen D and Roenneberg T Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep, 2018, 41 [DOI] [PubMed] [Google Scholar]
- Rawal S, Hinkle SN, Zhu Y, Albert PS and Zhang C A longitudinal study of sleep duration in pregnancy and subsequent risk of gestational diabetes: findings from a prospective, multiracial cohort. American Journal of Obstetrics and Gynecology, 2017, 216: 399.e1–99.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiter ME, Decoster J, Jacobs L and Lichstein KL Normal sleep in African-Americans and Caucasian-Americans: A meta-analysis. Sleep medicine, 2011, 12: 209–14. [DOI] [PubMed] [Google Scholar]
- Sedov ID, Cameron EE, Madigan S and Tomfohr-Madsen LM Sleep quality during pregnancy: A meta-analysis. Sleep medicine reviews, 2018, 38: 168–76. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder K, Scholtes C and Riemann D The association between insomnia and cardiovascular diseases. Nat Sci Sleep, 2010, 2: 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Step It Up! The Surgeon General’s Call to Action to Promote Walking and Walkable Communities. In: O. O. T. S. G. U.S. DEPT OF HEALTH AND HUMAN SERVICES (Ed, Washington, DC, 2015. [PubMed] [Google Scholar]
- Wang W, Zhong C, Zhang Y et al. Shorter sleep duration in early pregnancy is associated with birth length: a prospective cohort study in Wuhan, China. Sleep medicine, 2017, 34: 99–104. [DOI] [PubMed] [Google Scholar]
- Watson NF, Badr MS, Belenky G et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med, 2015, 11: 591–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler N and Massey M Results of Cognitive Testing of Questions on Stress, Sleep, and other topics for the National Health Interview Survey (NHIS). In, 2018.
- Young-Wolff KC, Sarovar V, Tucker LY et al. Self-reported Daily, Weekly, and Monthly Cannabis Use Among Women Before and During Pregnancy. JAMA Netw Open, 2019, 2: e196471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


