Abstract
Little is known about COVID-19 mRNA vaccine humoral immune responses in patients with central nervous system autoimmune demyelinating diseases, multiple sclerosis (MS) and neuromyelitis optica (NMO), who are on B-cell depleting therapies (BCDT) and other disease modifying therapies (DMTs). We conducted a single center prospective study to identify the clinical and immunological features associated with vaccine-induced antibody response in 53 participants before and after COVID-19 mRNA vaccination. This is the first report on the anti-spike RBD and anti-nucleocapsid antibody response, along with pre- and post-vaccine absolute lymphocyte counts (ALC) and flow cytometry analysis of CD19 and CD20 lymphocytes in patients with MS and NMO. We tested the hypothesis that patients on BCDT may have impaired COVID-19 vaccine humoral responses. Among patients on BCDT, 36.4% demonstrated a positive antibody response to spike RBD, in comparison to 100% in all other groups such as healthy controls, untreated MS, and patients on non-B cell depleting DMTs (p < 0.0001). Immunological data revealed lower baseline (pre-vaccination) levels of IgM in patients on BCDT (p = 0.003). Low CD19 and CD20 counts and a shorter interval from the last B cell depleting therapy infusion to the first vaccine dose were associated with a negative spike RBD antibody response (non-seroconverter) in patients on BCDT. Age, body mass index (BMI) and total treatment duration did not differ between seroconverters and non-seroconverters.
Keywords: Humoral Response, COVID-19, Multiple Sclerosis, Demyelinating Diseases, Immune Therapies
1. Introduction
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system. The past decade has seen dramatic improvements in the clinical management of patients with MS with the advent of high efficacy disease modifying therapies (DMTs) such as B-cell depleting therapy (BCDT). These DMTs modulate or suppress the autoimmune process and have been shown to decrease the incidence of relapses and to slow disability progression. However, patients on high efficacy therapy may be more vulnerable to serious infections [1].
The coronavirus (COVID-19) pandemic has raised multiple health concerns for patients with multiple sclerosis (MS). An early study assessed the risk factors associated with severe COVID-19 infection in patients with MS, and reported age, Expanded Disability Status Scale (EDSS), progressive MS, and obesity as significant risk factors [2]. This study did not assess the effects of individual DMT exposure on COVID-19 disease severity and reported increased COVID-19 disease severity scores in the absence of DMT use. In another study, lymphopenia was independently reported to predict severe COVID-19 infection, and subsequent studies reported a higher risk of severe infection following anti-CD20 therapy [3], [4], [5]. Other reports have also suggested that MS patients on BCDT have a more severe COVID-19 disease course [4], [6]. However, regarding COVID-19 vaccines, the current recommendations from the National MS society (NMSS) are to continue current DMTs without modification of therapeutic regimen [7].
Mitigation of the COVID-19 pandemic crisis is dependent upon timely vaccination of a vast majority of the population. Phase III clinical trials of the mRNA-based SARS-CoV-2 vaccines administered in the US, have demonstrated robust vaccine efficacy against symptomatic illness in 95% of immunocompetent individuals [8]. Recent studies conducted on solid organ transplant recipients receiving immunosuppressive therapy demonstrated a significantly lower likelihood of mounting a positive anti-spike response to mRNA vaccines [9]. Patients with rheumatic diseases on mycophenolate or BCDT have also been shown to have an impaired COVID-19 vaccine response [10]. The immunogenicity of these vaccines in MS patients on immune therapy is not well established. Previously only 2 case reports showed one negative and one positive antibody response in 2 patients on ocrelizumab [11], [12]. Another case study reported decreased humoral immune responses to vaccine in patients on BCDT; however, it did not provide correlation with immunological data [13].
We therefore aimed to study the clinical characteristics, immunological laboratory data, and immunoglobulin levels in MS and NMO patients, with the goal of identifying factors impacting the antibody response to COVID-19 mRNA vaccines. This knowledge is crucial when advising patients regarding the timing of vaccine and risk mitigation strategies. We specifically aimed to test the hypothesis that patients on BCDT may have impaired COVID-19 vaccine humoral immune responses.
2. Methods
We conducted a longitudinal prospective study of participants with MS and other demyelinating diseases at the University of Michigan Multiple Sclerosis Center. Informed consent was obtained prior to participation in the study, which was approved by the University of Michigan Institutional Review Board. Those who completed 2 doses of SARS-CoV-2 mRNA vaccines between December 21, 2020 and May 19, 2021 were included.
We collected two blood samples: the first sample was collected before the first dose of the vaccine as a pre-vaccination baseline and the second sample was collected approximately 3 weeks after the second vaccine dose as a post-vaccination sample. These samples were tested for complete blood counts with differential, immunoglobulin levels and flow cytometry profile (which measures CD3, CD4, CD8, CD19, CD20 and CD16/56). These samples were also analyzed using the Roche Elecsys anti-SARS-CoV-2 nucleocapsid and Siemens SARS-CoV-2 Spike RBD total antibody assays which have been previously described [14]. The Roche anti-SARS-CoV-2 nucleocapsid assay works is an electro-chemiluminescent immunoassay (ECLIA) which utilizes recombinant biotinylated and ruthenium-labeled nucleocapsid protein. An index value >1 was recorded as a positive anti-nucleocapsid antibody response, which reflects past infection with COVID-19. The Siemens SARS-CoV-2 Spike RBD total antibody assay works as a chemiluminescent immunoassay (CLIA) by utilizing recombinant S1 subunit receptor-binding domain as a biotinylated and acridinium ester-conjugated antigen. An index value >1 was recorded as a positive anti-Spike RBD antibody response, which indicates a positive vaccine response. Hence both tests give a qualitative assessment of the antibody response as either “positive” or “negative”.
We compared anti-S antibody levels following SARS-CoV-2 mRNA vaccines [either BNT162b2 (Pfizer) or mRNA-1273 (Moderna)] in patients receiving B cell depleting therapy to four other groups including untreated MS patients, patients on non B-cell depleting therapies, patients with prior COVID-19 infection, and healthy controls. Any patient who had previously contracted COVID-19 was studied in the prior infection group. B-cell depleting therapies included ocrelizumab, inebilizumab, and rituximab. Non B-cell depleting therapies included glatiramer acetate, peginterferon beta-1a, dimethyl fumarate, diroximel fumarate, ozanimod, and natalizumab. Since NMSS recommendations at the time of data collection did not recommend alteration of dosing interval around the time of vaccination, this cohort includes patients who received vaccines on a first-come-first-served basis. Therapy duration measured in days, was from the first infusion to the date of last infusion plus 180 days. Time interval was from the date of the last BCDT infusion to the date of first vaccine, measured in days. During the pandemic, some patients chose to cancel or postpone BCDT infusion. We collected random samples of patients who had various time intervals from last infusion to first vaccine. We also collected demographic data including gender, age and ethnicity, and clinical data including current disease course, disease duration, and information on concomitant diseases from patient records. A diagnosis of multiple sclerosis was confirmed by an MS neurologist based on the McDonald criteria [15].
The primary outcome of interest was the presence or absence of an anti-Spike RBD humoral immune response post-COVID-19 vaccination. The secondary outcomes were B lymphocyte and immunoglobulin levels. We compared demographic and disease characteristics across the five groups. The presence of SARS-CoV-2 anti-Spike RBD antibody response was dichotomized as positive (= 1) or negative (= 0) and was analyzed as a dependent variable. For between group comparisons of CD19 and CD20, cell counts were categorized as 0 (for values < 2% or < 20 cells/cmm) and 1 (for values > 2% or > 20 cell/cmm). For the comparison of categorical variables, a chi-square test was used. For continuous variables of normal distribution, parametric t tests or Kruskal-Wallis tests were used. For variables with non-normal distribution, Wilcoxon Rank-Sum nonparametric tests were used for comparison. Logistic regression was used to assess the relationship of independent variables on binary dependent variables. Small sample sizes precluded multiple independent variables from being included in logistic regression models. Odds ratios (ORs) reflect the multiplicative change in the odds of being at a higher level of the dependent variable for every one-unit increase of the independent variable. All tests for statistical significance were performed at two-sided α = 0.05. A p value < 0.05 was considered significant. All analyses were performed using SAS 9.4 or GraphPad Prism 8 software.
3. Results
A total of 53 participants, including 42 patients with MS, 2 with NMO, 2 with optic neuritis and 7 healthy controls (HC), were analyzed (Table 1 ). The HC and three MS groups were predominantly female (p = 0.003) and were similar in mean age (range 41–61 years, p = 0.20) with a Caucasian predominance (p = 0.01). The groups were similar in their comorbidities except for the patients with a prior diagnosis of COVID-19 who were older in age and had significant risk factors such as diabetes mellitus and chronic kidney disease (p = 0.0008 and p = 0.01, respectively).
Table 1.
Patient demographics with pre- and post-COVID mRNA vaccine labs and antibody response.
| Healthy Controls | Untreated MS | Other DMT Treated MS | Patients on BCDT | Post COVID-19 | P-value | ||
|---|---|---|---|---|---|---|---|
| Number of Patients | 7 | 13 | 6 | 23 | 4 | ||
| Age [years] | 41.6 (10.5) | 45.4 (14.2) | 44.3 (15.5) | 43.5(12.4) | 61.3 (10.7) | 0.20 | |
| Female [n%] | 71.4 | 92.3 | 100 | 60.9 | 0 | 0.003 | |
| BMI [kg/m2] | 23.6 (5.5) | 27.3 (6.2) | 35.1 (9.9) | 32.4 (8.7) | 33.5 (5.1) | 0.03 | |
| Ethnicity | Caucasian [n%] | 57.1 | 100 | 100 | 82.6 | 75 | 0.01 |
| African American [n%] | 0 | 0 | 0 | 12.5 | 25 | ||
| Others [n%] | 42.9 | 0 | 0 | 4.2 | 0 | ||
| Comorbidities | DM [n%] | 0 | 0 | 16.7 | 8.7 | 75 | 0.0008 |
| HTN [n%] | 0 | 7.7 | 33 | 26.1 | 50 | 0.18 | |
| CKD [n%] | 0 | 0 | 0 | 0 | 25 | 0.01 | |
| Other autoimmune [n%] | 14.3 | 23.1 | 33.3 | 0 | 0 | 0.08 | |
| Diagnosis | MS [n%] | 0 | 100 | 100 | 91.3 | 50 | |
| NMO [n%] | 0 | 0 | 0 | 8.7 | 0 | ||
| Optic neuritis [n%] | 0 | 0 | 0 | 0 | 50 | ||
| Vaccine | |||||||
| [Pfizer n%] | 100 | 84.6 | 66.7 | 60.9 | 0.15 | ||
| Pre-Vaccine | ALC [K/uL] | ^2.0 (0.6) | 2.5 (0.5) | 1.5 (0.6) | 1.7 (0.8) | 0.21 | |
| IGG [mg/dL] | 961.3 (245.5) | 1268.3 (334.5) | 968.5 (348.0) | 913.6(240.3) | 0.30 | ||
| IGA [mg/dL] | 147.8 (48.3) | 243.3 (87.0) | 183.8 (90.0) | 242.3 (191.6) | 0.46 | ||
| IGM [mg/dL] | 176.0 (97.1) | 151.3 (44.5) | 126.5 (75.7) | 67.8(53.1) | 0.003 | ||
| Positive Spike RBD Antibody [n%] | 0 | 0 | 25 | 0 | 0.11 | ||
| Positive Nucleocapsid Antibody [n%] | 0 | 0 | 100 | 0 | |||
| Post-Vaccine | ALC [K/uL] | 1.7 (0.4) | 2.4 (1.0) | 1.6 (1.1) | *1.7 (0.6) | &1.8 (0.6) | 0.25 |
| IGG [mg/dL] | 718.6 (105.3) | 840 (261.3) | 884.4 (209.2) | 1088.7 (143.5) | 0.12 | ||
| IGA [mg/dL] | 163.6 (32.3) | 129.3 (32.0) | 197.9 (103.6) | 308.0 (71.1) | 0.08 | ||
| IGM [mg/dL] | 104.8 (57.4) | 60.0 (31.4) | 125.9 (216.9) | 89.7 (26.9) | 0.41 | ||
| Positive Spike RBD Antibody [n%] | 100 | 100 | 100 | 36.4 | 100 | <0.0001 | |
| Positive Nucleocapsid Antibody [n%] | 0 | 25 | 0 | 4.8 | 100 | 0.002 |
The values listed are mean (standard deviation). Categorical variables have been reported as a percentage and continuous variables have been reported with mean and standard deviation. P-values for categorical variables were calculated using the Chi-Square test. P-values for continuous variables were calculated using the Wilcoxon Rank-Sum test. Nonparametric tests were used due to non-normality of the continuous variables. P < 0.05 was considered significant, P < 0.01 highly significant.
^ For HC, only one time pre-vaccine Ig lab was done. & For post-COVID-19 patients, only one time lab was done (listed under post-vaccine as natural positive controls though these patients did not receive the vaccines). *1 patient in the BCDT group did not have post vaccine labs and was not included in the within-group comparisons.
DM, diabetes mellitus, HTN: hypertension, CKD: chronic kidney disease.
Twenty-three patients on B-cell depleting therapy (BCDT) had a mean disease duration of 17 years. Among them, 36.4% (8/22; 1 with missing data) demonstrated a positive antibody response, in comparison to 100% in all other groups (p = <0.0001) (Table 1). Of the 20 MS patients on ocrelizumab, 30% (6/20) demonstrated a positive antibody response. Of the two aquaporin-4 antibody positive NMO patients, one had a time interval of 62 days between inebilizumab infusion and the first dose of vaccine and the other had 119 days between rituximab infusion and the first dose of vaccine. They both demonstrated a negative antibody response.
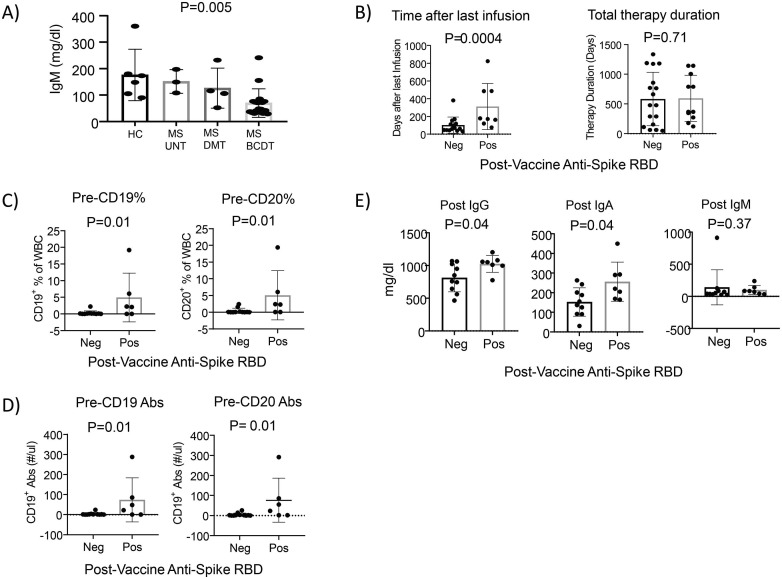
Immunological laboratory data revealed lower levels of serum immunoglobulin IgM in patients on BCDT (p = 0.003) prior to vaccination (Table 1; Fig. 1 A). Unadjusted cox proportional hazard ratios revealed that for each one unit increase in IgM levels, the rate of having a positive antibody response to vaccination increased by 1.9% (p = 0.03). Unadjusted outcomes revealed higher odds of having a positive antibody response [OR 1.05; 95% confidence interval (CI) 1.01–1.09; p value = 0.01] with each unit increase in IgM levels.
Fig. 1.
Immunological parameters of patients in response to COVID mRNA vaccines. A) Pre-vaccination IgM levels are lower in patients on BCDT. The p values are presented above the boxplots. P < 0.05 was considered significant. B) The time interval between infusion and vaccine, and total infusion therapy duration comparison between post-vaccination anti-spike positive and negative groups in patients on BCDT. A shorter time interval between infusion and vaccine was more likely to result in a negative antibody response. Comparisons were performed by Wilcoxon's two-sample test for continuous variables. The p values are presented above the boxplots. P < 0.05 was considered significant. C) Pre-vaccine percentage of CD19 and CD20 comparison between anti-spike positive and negative groups. For the comparison, the percentage of CD19 and CD20 levels were categorized as 0 (for values < 2%) and 1 (for values > 2%). Chi-Square test was used for categorical variables. Lower percentage levels of CD19 and CD 20 were seen for both pre- and post-vaccination in the anti-spike negative subgroup (non-seroconverter). Graphs shown are for pre vaccination data. The p values are presented above the boxplots. P < 0.05 was considered significant. D) Pre-vaccine absolute CD19 and CD20 (Abs) comparison between anti-spike positive and negative groups. CD19 Abs and CD20 Abs were categorized as 0 (for values < 20 cells/cmm) and 1 (for values > 20 cells/cmm). A Chi-Square test was used for categorical variables. Lower Abs CD19 and Abs CD20 levels were seen for both pre- and post-vaccination in the anti-spike negative subgroup (non-seroconverter). Graphs shown are for pre vaccination data. The p values are presented above the boxplots. P < 0.05 was considered significant. E) Post-vaccine IgG, IgA and IgM comparison between anti-spike antibody positive and negative groups. Lower IgG and IgA levels were seen post--vaccination anti-spike antibody negative group (non-seroconverter). Comparisons were performed by Wilcoxon's two-sample test for continuous variables. The p values are presented above the boxplots, and P < 0.05 was considered significant.
We further compared patients on BCDT with negative (non-seroconverter) vs. positive (seroconverter) anti-spike RBD antibodies post-vaccination (Table 2 ). The two subgroups had similar distributions to each other in age, BMI and gender. The total duration of treatment was not different between the two groups. The mean time interval between last infusion of BCDT and the first dose of vaccination was 313 (range: 77–823) days for those with positive response, as compared to 102 (range: 33–381) days amongst those with negative antibody response (p = 0.0037) (Fig. 1B). There was a trend toward higher odds of having a positive antibody response [OR 1.009 [95% confidence interval (CI) 1.00–1.02; p value = 0.06] with each day of increase in time interval between the last infusion and vaccination.
Table 2.
Comparison of immunological parameters of MS patients on BCDT with positive vs. negative anti-Spike RBD antibody response after two doses of COVID mRNA vaccines.
| Reference Range | Negative Anti-Spike RBD Antibody | Positive Anti-Spike RBD Antibody | P-Value | |
|---|---|---|---|---|
| Number of Patients | 14 | 8 | ||
| Age [years] | 43.5 (12.9) | 42.5 (12.7) | 0.95 | |
| Time interval last infusion to the 1st dose of vaccine [days] | 101.9 (91.3) | 312.9 (260.1) | 0.004 | |
| Total duration of therapy [days] | 647.9 (463.5) | 527.6 (394.0) | 0.71 | |
| BMI [kg/m2] | 33.1 (9.1) | 32.2(8.4) | 0.92 | |
| Pre-Vaccine | ||||
| CD3 [%] | 61–79 | 85.3 (8.1) | 79.7 (12.6) | 0.34 |
| CD4 [%] | 38–54 | 61.9 (7.6) | 56.8 (9.2) | 0.16 |
| CD8 [%] | 14–28 | 21.5 (7.5) | 21.0 (8.4) | 0.65 |
| CD19 [%] | 7–24 | 0.26 (0.7) | 4.9 (7.3) | 0.01 |
| CD20 [%] | 7–20 | 0.41 (0.8) | 5.07 (7.4) | 0.01 |
| CD16/56 [%] | 6–12 | 13.5 (8.1) | 14.7 (9.6) | 0.96 |
| C4: C8 [ratio] | 1.2–2.6 | 3.3 (1.4) | 3.2 (1.8) | 0.76 |
| CD3 [cells/cmm] | 732–3160 | 1514.6 (603.5) | 1288.8 (407.9) | 0.65 |
| CD4 [cells/cmm] | 456–2160 | 1093.8 (417.5) | 941.5 (382.3) | 0.39 |
| CD8 [cells/cmm] | 168–1120 | 385.4 (207.5) | 320.3 (109.4) | 0.65 |
| CD16/56 [cells/cmm] | 84–960 | 239.5 (163.3) | 240.7 (203.1) | 0.84 |
| CD19 [cells/cmm] | 84–800 | 3.4 (7.0) | 73.8 (109.8) | 0.01 |
| CD20 [cells/cmm] | 1–354 | 4.6 (8.1) | 76.3 (110.0) | 0.01 |
| ALC [K/ul] | 1.2–4.0 | 1.9 (0.8) | 1.4 (0.7) | 0.25 |
| IGG [mg/dL] | 620–1520 | 865.0 (256.6) | 1010.7 (185.8) | 0.24 |
| IGA [mg/dL] | 40–350 | 225.1 (218.8) | 276.7 (131.6) | 0.15 |
| IGM [mg/dL] | 50–370 | 50.1 (20.2) | 103.3 (80.1) | 0.19 |
| Post-Vaccine | ||||
| CD3 [%] | 61–79 | 84.0 (7.2) | 85.3 (8.2) | 0.96 |
| CD4 [%] | 38–54 | 60.9 (9.4) | 61.5 (4.3) | 0.79 |
| CD8 [%] | 14–28 | 21.3 (8.1) | 22.7 (10.2) | 0.87 |
| CD19 [%] | 7–24 | 1.0 (2.2) | 2.9 (2.1) | 0.07 |
| CD20 [%] | 7–20 | 1.3 (2.2) | 3.0 (2.0) | 0.01 |
| CD16/56 [%] | 6–12 | 13.7 (6.4) | 11.0 (6.4) | 0.32 |
| C4:C8 [ratio] | 1.2–2.6 | 3.5 (1.8) | 3.6 (2.7) | 0.96 |
| CD3 [cells/cmm] | 732–3160 | 1488.1 (587.3) | 1722.8 (451.5) | 0.43 |
| CD4 [cells/cmm] | 456–2160 | 1092.2 (475.4) | 1249.6 (350.3) | 0.49 |
| CD8 [cells/cmm] | 168–1120 | 367.1 (196.3) | 452.2 (247.2) | 0.56 |
| CD16/56 [cells/cmm] | 84–960 | 220.8 (101.5) | 224.8 (147.2) | 1.0 |
| CD19 [cells/cmm] | 84–800 | 19.9 (40.5) | 58.4 (49.6) | 0.03 |
| CD20 [cells/cmm] | 1–354 | 22.8 (41.4) | 59.8 (48.1) | 0.03 |
| ALC [K/ul] | 1.2–4.0 | 1.7 (0.6) | 2.0 (0.5) | 0.31 |
| IGG [mg/dL] | 620–1520 | 800.3 (206.3) | 1028.5 (140.6) | 0.04 |
| IgA [mg/dL] | 40–350 | 152.5 (69.0) | 249.7 (108.1) | 0.04 |
| IgM [mg/dL] | 50–370 | 131.9 (260.5) | 100.7 (85.7) | 0.37 |
The values listed are mean (standard deviation). P-values for continuous variables were calculated using the Wilcoxon Rank-Sum nonparametric tests due to non-normality of the continuous variables. For these comparisons, the percentage of CD19 and CD20 levels were categorized as 0 (for values < 2%) and 1 (for values ≥ 2%) and similarly CD19 Abs and CD20 Abs were categorized as 0 (for values < 20 cells/cmm) and 1 (for values ≥ 20 cell/cmm) respectively. Between group comparisons were done using chi-square tests. P < 0.05 was considered significant. ALC, Absolute lymphocyte count.
Lymphocyte immune profiling were performed using flow cytometry. No significant difference was found in the frequency of CD4 T cells, CD8 T cells, NK cells as well as CD4/CD8 ratio between anti-Spike RBD antibody negative subjects and anti-Spike RBD antibody positive subjects (Table 2). Among anti-Spike RBD antibody negative subjects (non-seroconverters), 88.9% had CD19 and CD20 B cell counts <20 cell/cmm or <2% vs. the antibody positive subjects (seroconverter) where only 40% had low levels of CD19 and CD20 (p = 0.01) in pre-vaccination samples (Fig. 1C-D). Post-vaccination, CD19 and CD20 percentage and absolute counts remained statistically different between seroconverters and non-seroconverters (p = 0.01) (Table 2). Lower IgG and IgA levels were seen in the anti-spike antibody negative group (non-seroconverter) than the positive group (seroconverter) (p = 0.04) (Fig. 1E).
Only minimal to mild side effects from mRNA COVID-19 vaccines were reported in all participants; common symptoms included mild body ache, headache, local muscle pain, and low-grade fever which mostly lasted less than a day. Only two people reported new or worsening MS symptoms.
4. Discussion
Our study is the first prospective study on COVID-19 mRNA vaccine humoral immune responses in patients with MS and other demyelinating diseases on BCDT compared to other DMTs. We analyzed clinical features, antibody status, pre- and post-vaccine ALC, CD19 and CD20 counts and immunoglobin levels. Both anti-spike RBD and anti-nucleocapsid total antibodies were negative in most patients before vaccination. After vaccination, the rate of seroconversion to positive anti-spike RBD was 100% in all groups (HC, MS no treatment, and MS on other DMTs) except patients on BCDT, in which it was 36.4% (Table 1). Our results showed a markedly blunted vaccine response in patients on BCDT highlighting the need for immunization prior to treatment.
Our results are consistent with the VELOCE study [16] which showed that Ocrelizumab-treated individuals were half as likely to mount an antibody response against tetanus toxoid vaccine (23.9% ocrelizumab vs. 54.5% controls) and almost two thirds less likely to mount an antibody response to 12 or more pneumococcal serotypes (37.3% ocrelizumab vs. 97.1% controls). One implication of our data is a possible benefit of postponing the initiation of B cell depleting therapies or increasing the time interval between infusion and immunization as others have proposed [17]. Of course, clinicians also need to weigh the risks of potential relapse and worsening disease in such scenarios. Other cell depleting DMTs such as alemtuzumab could also potentially affect vaccine efficacy. It might be particularly challenging to vaccinate patients on ofatumumab which is administered monthly as opposed to ocrelizumab given every 6 months.
While the NMSS recommends continuation of current DMTs in MS patients, the likelihood of a protective immune response has not yet been correlated with individual DMT and patient profiles. Cell depleting therapy may interfere with the mounting of a protective immune response to SARS-CoV-2, hence optimizing the timing of vaccinations relative to the use of ongoing DMTs is critical for effective vaccination against this virus. Our data demonstrate that a time interval of <4 months between the last infusion and the first vaccination results in a lower likelihood of mounting a positive humoral response. Age, BMI, and total treatment duration in our cohort did not differ between the antibody positive and negative group. In addition, we found that CD19 and CD20 lymphocyte counts and immunoglobulin levels (but not absolute lymphocyte counts) around the time of vaccination impacted the antibody response in patients on BCDT. We also demonstrate a significant reduction of IgG and IgA levels in the antibody negative groups (Table 2). Previously, low levels of IgG and IgM have been shown to correlate with longer effective treatment durations of ocrelizumab and an increased risk of infections [18].
Our study reports qualitative results with total antibodies to spike RBD and subgroup antibody responses of IgG, IgA and IgM to spike RBD were not measured. Although the specific subtype of Ig and quantitative results may be desirable, it is currently unknown what quantitative antibody titers are required to provide sufficient protection (i.e. a correlate of protection). A standardized quantitative assay may allow us to understand this more precisely in the future. Another limitation of our study is the small sample size, therefore any direct comparisons between BCDT to other DMTs needs to be further validated. In our small study, non-cell depleting DMTs were shown to unlikely negatively impact the COVID-19 vaccine antibody response. Further longitudinal studies with individual DMTs may provide more information. We are unable to generalize our findings in NMO patients owing to small numbers of patients included. However our study has provided a glimpse into the vaccine response of NMO patients on BCDT and larger future studies in NMO population are needed. In addition, we did not address any differences between the two mRNA vaccines in our study.
Although our study demonstrated a decreased antibody response to COVID-19 mRNA vaccines with BCDT, it remains possible that an adequate T cell vaccine response could still occur that leads to protection. Depletion of CD8 + T cells in convalescent macaques was reported to partially abrogate the protective efficacy of natural immunity against rechallenge with SARS-CoV-2, which suggests a role for cellular immunity in the context of waning or sub-protective antibody titers [19]. This data demonstrates that relatively low antibody titers are sufficient for protection against SARS-CoV-2 in rhesus macaques, and that cellular immune responses may contribute to protection if antibody responses are suboptimal. Currently no data exists regarding the impact of BCDT on cellular responses to COVID-19 vaccines. It is noteworthy that CD4+ and CD8+ T cell counts remain relatively unchanged after BCDT, which was also seen in the flow cytometry data from our study. After ocrelizumab infusion, CD19+ B cell levels begin to recover after ~ 40 weeks, while memory B cells remain low and naïve B cells roughly follow the recovery trajectory of CD19+ cells [20]. Memory helper T cells, along with cytotoxic T cells, and memory B cells are all important in immunologic memory. We therefore plan to conduct a follow up study to examine polyclonal as well as SARS-CoV-2 antigen-specific T cell COVID vaccine responses in patients on BCDT.
Our study also raises the important question of whether additional boosters in non-seroconverters may be needed to generate a vaccine response. Such a strategy has been shown to significantly increase the antibody response rate in highly immunosuppressed solid organ transplant patients [20]. Further assessment of long-term responses (i.e. 6–12 months post-booster) will also be critical in addressing the impact of immunotherapy on vaccine immune response durability. Such knowledge is crucial when advising patients regarding timing of vaccine, recommendations for booster shots, and risk mitigation strategies for the MS population as discussed previously [21]. Notwithstanding the importance of these future directions for additional studies, our results provide the first prospective data on the humoral response to COVID-19 mRNA vaccines in MS patients.
Author Contributions
Biostatistical analysis was performed by AA, YMD and Q Wu. AA, Q Wang and DD served as clinical coordinators and collected the data. YMD and AA contributed to study design. AA, DD, Q Wu, Q Wang, DAF, DK, GAP, CAD and YMD wrote the manuscript.
Author Disclosure
AA, DD, Q Wu, Q Wang, CAD and DAF has nothing to disclose. DK reports personal fees from Acceleron, Actelion, CSL Behring, Horizon, Mitsubishi Tanabe, Talaris Rx, Prometheus, and grants and personal fees from Bayer, Bristol Myers Squibb and Genentech/Roche. He is Chief Medical Officer of Eicos Sciences and has stock option. GAP is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. He offers consultative advice on vaccine development to Merck & Co., Medicago, GlaxoSmithKline, Sanofi Pasteur, Emergent Biosolutions, Dynavax, Genentech, Eli Lilly and Company, Janssen Global Services LLC, Kentucky Bioprocessing, AstraZeneca, and Genevant Sciences, Inc. GAP holds patents related to vaccinia and measles peptide vaccines. He has received grant funding from ICW Ventures for preclinical studies on a peptide-based COVID-19 vaccine. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. YMD has served as a consultant and/or received grant support from: Acorda, Bayer Pharmaceutical, Biogen Idec, Celgene/Bristol Myers Squibb, EMD Serono, Sanofi-Genzyme, Roche-Genentech, Novartis, Questor, Janssen, and Teva Neuroscience. YMD was supported by grants from NIH NIAID Autoimmune Center of Excellence: UM1-AI110557-05, UM1 AI144298-01, PCORI, Roche-Genentech, Novartis, Sanofi-Genzyme, and Chugai.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Luna G., Alping P., Burman J., Fink K., Fogdell-Hahn A., Gunnarsson M., et al. Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA neurology. 2020;77(2):184. doi: 10.1001/jamaneurol.2019.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sormani M.P. An Italian programme for COVID-19 infection in multiple sclerosis. The Lancet. Neurology. 2020;19(6):481–482. doi: 10.1016/S1474-4422(20)30147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louapre C., Collongues N., Stankoff B., Giannesini C., Papeix C., Bensa C., et al. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA neurology. 2020;77(9):1079. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan L.i., Wang Q.i., Zhang D., Ding J., Huang Q., Tang Y.-Q., et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal transduction and targeted therapy. 2020;5(1) doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes R., Whitley L., Fitovski K., Schneble H.-M., Muros E., Sauter A., et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Multiple sclerosis and related disorders. 2021;49:102725. doi: 10.1016/j.msard.2020.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sormani M.P., De Rossi N., Schiavetti I., Carmisciano L., Cordioli C., Moiola L., et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.v89.410.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-19 Vaccine Considerations for MS Healthcare Providers. (n.d.). Retrieved from https://www.nationalmssociety.org/For-Professionals/Clinical-Care/COVID-19/COVID-19-Vaccine
- 8.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. The New England journal of medicine. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325(21):2204. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyarsky, B. J., Ruddy, J. A., Connolly, C. M., Ou, M. T., Werbel, W. A., Garonzik-Wang, J. M., Segev, D. L., & Paik, J. J. (2021). Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Annals of the rheumatic diseases, annrheumdis-2021-220289. Advance online publication. https://doi.org/10.1136/annrheumdis-2021-220289 [DOI] [PMC free article] [PubMed]
- 11.Khayat-Khoei, M., Conway, S., Rubinson, D. A., Jarolim, P., & Houtchens, M. K. (2021). Negative anti-SARS-CoV-2 S antibody response following Pfizer SARS-CoV-2 vaccination in a patient on ocrelizumab. Journal of neurology, 1–3. Advance online publication. https://doi.org/10.1007/s00415-021-10463-3 [DOI] [PMC free article] [PubMed]
- 12.Mado, H., & Adamczyk-Sowa, M. (2021). Comment on the paper Negative anti-SARS-CoV-2 S antibody response following Pfizer SARS-CoV-2 vaccination in a patient on ocrelizumab: the likely explanation for this phenomenon based on our observations. Journal of neurology, 1–2. Advance online publication. https://doi.org/10.1007/s00415-021-10547-0 [DOI] [PMC free article] [PubMed]
- 13.Achiron, A., Mandel, M., Dreyer-Alster, S., Harari, G., Magalashvili, D., Sonis, P., Dolev, M., Menascu, S., Flechter, S., Falb, R., & Gurevich, M. (2021). Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Therapeutic advances in neurological disorders, 14, 17562864211012835. https://doi.org/10.1177/17562864211012835 [DOI] [PMC free article] [PubMed]
- 14.Manthei, D. M., Whalen, J. F., Schroeder, L. F., Sinay, A. M., Li, S. H., Valdez, R., Giacherio, D. A., & Gherasim, C. (2021). Differences in Performance Characteristics Among Four High-Throughput Assays for the Detection of Antibodies Against SARS-CoV-2 Using a Common Set of Patient Samples. American journal of clinical pathology, 155(2), 267–279. https://doi.org/10.1093/ajcp/aqaa200 [DOI] [PMC free article] [PubMed]
- 15.Thompson A.J., Banwell B.L., Barkhof F., Carroll W.M., Coetzee T., Comi G., et al. revisions of the McDonald criteria, The Lancet Neurology, 17(2), 2018. ISSN. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Or A., Calkwood J.C., Chognot C., Evershed J., Fox E.J., Herman A., et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology. 2020;95(14):e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smets I., Reyes S., Baker D., Giovannoni G. Blunted vaccines responses after ocrelizumab highlight need for immunizations prior to treatment. Multiple sclerosis and related disorders. 2021;50 doi: 10.1016/j.msard.2021.10285114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oksbjerg N.R., Nielsen S.D., Blinkenberg M., Magyari M., Sellebjerg F. Anti-CD20 antibody therapy and risk of infection in patients with demyelinating diseases. Mult. Scler. Relat. Disord. 2021;52:102988. doi: 10.1016/j.msard.2021.102988. [DOI] [PubMed] [Google Scholar]
- 19.McMahan K., Yu J., Mercado N.B., Loos C., Tostanoski L.H., Chandrashekar A., et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. doi: 10.1038/s41586-020-03041-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordero, E., Roca-Oporto, C., Bulnes-Ramos, A., Aydillo, T., Gavaldà, J., Moreno, A., … Pérez-Romero, P.; for the TRANSGRIPE 1–2 Study Group (2017). Two Doses of Inactivated Influenza Vaccine Improve Immune Response in Solid Organ Transplant Recipients: Results of TRANSGRIPE 1–2, a Randomized Controlled Clinical Trial. Clinical Infectious Diseases, 64(7):829–38. DOI: 10.1093/cid/ciw855. [DOI] [PubMed]
- 21.Baker D., Roberts C.A.K., Pryce G., Kang A.S., Marta M., Reyes S., et al. COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases. Clin Exp Immunol. 2020;202(2):149–161. doi: 10.1111/cei.v202.210.1111/cei.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]