Abstract
Background
SARS-CoV-2 infection in term placenta is rare. However, growing evidence suggests that susceptibility of the human placenta to infection may vary by gestational age and pathogen. For several viral infections, susceptibility appears to be greatest during early gestation. Peri-implantation placental infections that result in pre-clinical pregnancy loss would typically go undetected. Little is known about the effects of SARS-CoV-2 on the peri-implantation human placenta since this time in pregnancy can only be modeled in vitro.
Methods
We used a human embryonic stem cell (hESC)-derived model of peri-implantation placental development to assess patterns of ACE2 and TMPRSS2 transcription and protein expression in primitive trophoblast. We then infected the same trophoblast cell model with a clinical isolate of SARS-CoV-2 and documented infection dynamics.
Results
ACE2 and TMPRSS2 were transcribed and translated in hESC-derived trophoblast, with preferential expression in syncytialized cells. These same cells supported replicative and persistent infection by SARS-CoV-2, while non-syncytialized trophoblast cells in the same cultures did not.
Conclusions
Co-expression of ACE2 and TMPRSS2 in hESC-derived trophoblast and the robust and replicative infection limited to syncytiotrophoblast equivalents support the hypothesis that increased viral susceptibility may be a defining characteristic of primitive trophoblast.
Keywords: trophoblast, placenta, miscarriage, SARS-CoV-2, COVID-19, spontaneous abortion, stem cell, ACE2, TMPRSS2
While severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pregnancy is known to be associated with severe maternal morbidity and mortality [1–4], fetal risk remains less well delineated. Studies have demonstrated an increased risk of preterm birth [4, 5] and stillbirth [6–8] associated with maternal SARS-CoV-2 infection, but evidence of other adverse pregnancy outcomes is less robust. Similarly, vertical (in utero) transmission of SARS-CoV-2 appears to be rare [9–15], when studied in the setting of maternal infection at or near delivery. Perhaps this is due, in part, to rare maternal viremia and patterns of angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) expression in term placenta, which likely protect against placental infection [12]. Despite the relative rarity of placental infection in SARS-CoV-2 compared to other viral illnesses such as cytomegalovirus [16, 17] and Zika virus (ZIKV) [18], there is evidence that trophoblast can be infected with SARS-CoV-2 [10, 12, 19–21].
Risk of placental infection in the peri-implantation period is unique because the anatomy of the placenta [22] and the immunology at the maternal-fetal interface [22, 23] differ dramatically from that of term placenta, and because pre- and periclinical losses make up the vast majority of all early pregnancy losses [24]. More specifically, for only the first 2 weeks after blastocyst implantation in humans, the leading edge of the placenta is composed of a unique placental cell layer, the primitive trophoblast, that secretes pregnancy hormones and is invasive [22]. The tree-like, fetally-derived villi that are bathed in maternal blood and characterize the maternal-fetal interface of the mature placenta begin to develop thereafter. Unlike the immune environment at the maternal-fetal interface for the vast majority of the remainder of pregnancy, that during the previllous stage of human placentation is highly proinflammatory [23], which may have implications for primitive trophoblast susceptibility to infection. While case-control [25] and cohort studies of women infected in the first trimester [26, 27] have reported that SARS-CoV-2 infection does not increase miscarriage rates, these studies report on pregnancies occurring after the switch from previllous to villous placentation. Moreover, reports of unchanged miscarriage rates in SARS-CoV-2 infection are in contrast to the increased prevalence of early pregnancy loss associated with maternal infection by the related coronaviruses, severe acute respiratory virus syndrome (SARS-CoV-1) and Middle East respiratory syndrome (MERS) [28, 29]. It is possible that the previllous placenta may be more easily infected by maternal SARS-CoV-2 than the placenta later in pregnancy [30, 31] and that the effects of such infections are currently undetected.
The potential for placental infection in early pregnancy remains regrettably understudied, due to difficulties inherent in evaluating pregnancy loss that may occur prior to clinically recognized pregnancy. Very early placental infections that have devastating consequences could go undetected as preclinical pregnancy losses. Alternatively, should infection occur after documentation of pregnancy and the pregnancy continues to term, early placental infections might no longer be detected at the time of delivery. There are concerning reports, however, that placental infection may be more severe in early pregnancy [21, 32–35]. In addition, a series of studies at the RNA and protein levels have shown the major SARS-CoV-2 entry receptor, ACE2 [36], and the postbinding processing protein, TMPRSS2 [36], are transcribed and translated in the outer trophectoderm layer of the human blastocyst, which will become the placenta [37–39]. ACE2 and TMPRSS2 are also expressed in the villous placenta across all 3 trimesters of pregnancy [12, 19–21, 40, 41], although these molecules are very rarely coexpressed in the same cell types, at least in term placenta [12, 42], which would limit susceptibility of the term placenta to SARS-CoV-2 infection. The infectability of trophoblast cell subtypes and placental tissues from the earliest stages of pregnancy has been understudied [35, 38, 39], because of ethical and logistical obstacles to obtaining normal tissues in early human pregnancy, and in maintaining these tissues to allow in vitro exposure to SARS-CoV-2.
In very early pregnancy, the pregnancy remains clinically undetectable and therefore cannot be studied by using primary cell isolates, nor can it be easily modeled in animals. Several laboratories, including our own, have developed models that mimic early human placental development. These include, among others, extended human blastocyst culture [43, 44] and the use of human embryonic stem cells, induced pluripotent stem cells [45–48], and trophoblast stem cells [49]. We have used human embryonic stem cells to create a model of primitive trophoblast: the transient, invasive, and syncytialized leading edge of the newly implanted blastocyst present only during the first 2 weeks of human pregnancy [45–47]. We previously demonstrated that these cells are highly susceptible to infection by different ZIKV strains, in contrast to trophoblasts isolated from term placentas [30, 31]. Using this model of the earliest events in postimplantation placental development, we assessed coexpression of ACE2 and TMPRSS2 and SARS-CoV-2 infection rates, viral replication, and cytopathic effects in primitive and developing human placenta.
METHODS
Ethics Statement
All research was approved by Institutional Biosafety Committee and performed in the biosafety level 3 laboratory at the University of Missouri Laboratory for Infectious Disease Research.
Cells and Virus
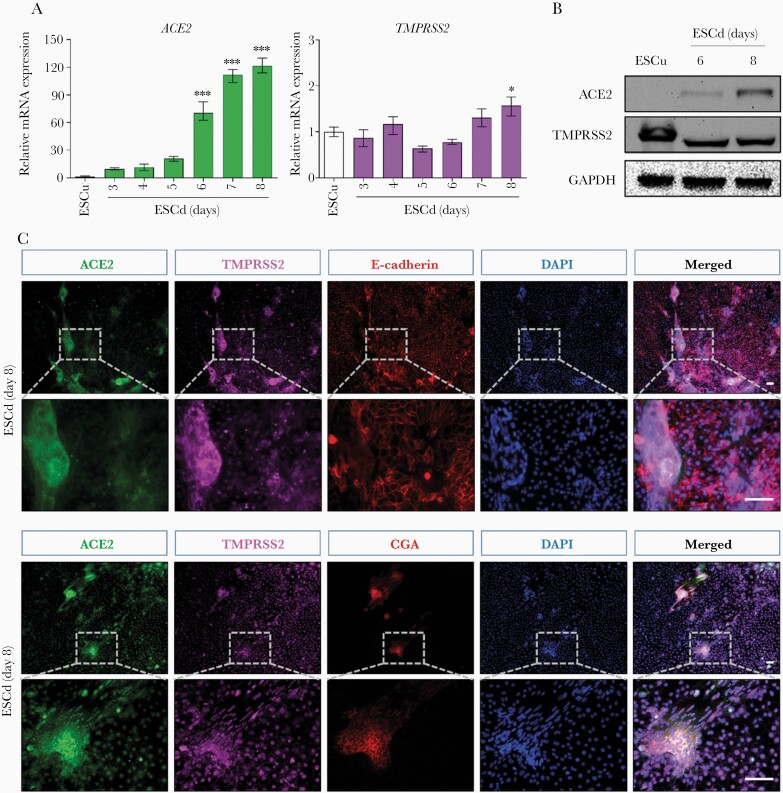
The stem cell-derived primitive trophoblast cell model was created as follows. Human embryonic stem cells (ESC; H1, WA01, obtained from WiCell Research Institute, Madison, WI [50]) were cultured in 6-well tissue culture plates (Thermo Scientific) on Matrigel (BD Bioscience)-coated wells under an atmosphere of 5% (vol/vol) CO2/air at 37°C in mTeSR1 medium (STEMCELL Technologies). The culture medium was changed daily and the cells were passaged every 5–6 days. The method for trophoblast differentiation has been described previously [46] and is briefly reviewed in the Supplementary Methods. Throughout the remainder of the manuscript, differentiated, human ESC-derived trophoblasts will be referred to as ESCd and/or BAP-treated cells, to reflect the ingredients of the media used to create ESCd (BMP4, 10 ng/mL; A83-01, 1 μM; and PD173074, 0.1 μM). BAP-treated differentiated trophoblast cells were cultured for up to 8 days (Figure 1A). Control undifferentiated H1 ESC (ESCu) were cultured for a similar amount of time in mouse embryonic fibroblast-conditioned medium containing only fibroblast growth factor 2 (FGF2) .
Figure 1.
Expression of ACE2 and TMPRSS2 in ESCd trophoblasts. A, H1 ESC were maintained on mTeSR. On the day following passaging, the medium was replaced by mouse embryonic fibroblast-conditioned medium supplemented with 4 ng/mL FGF2. After an additional day, the medium was changed to one containing 10 ng/mL BMP4, 1 μM A83-01, and 0.1 μM PD173074 for 8 days. B, RNAseq data are from Yabe et al [47]. Expression levels (in FPKM) for ACE2 and TMPRSS2 in ESCu, ESCd <40 µm cytotrophoblast cells (ESCd <40), and >70 µm syncytiotrophoblast (ESCd >70) fractions. The bar graphs indicate mean ± SD. The asterisks represent a statistically significant differential expression between groups (*P < .01, Benjamini-Hochberg P value adjustment method). Abbreviations: ACE2, angiotensin-converting enzyme 2; BAP, BMP4, A83-01, and PD173074; ESCd, embryonic stem cell derived; ESCu, embryonic stem cell undifferentiated; FGF2, fibroblast growth factor 2; FPKM, fragments per kilobase of transcript per million mapped reads; TMPRSS2, transmembrane serine protease 2.
Vero E6 cells were maintained in the growth medium including 1 × Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum and 1% antibiotics (Invitrogen). The SARS-CoV-2 USA-WA1/2020 strain was acquired from BEI Resources (Manassas, VA) and passaged 3 times in Vero E6 cells using the infecting medium including 1 × DMEM with 2% fetal bovine serum and 1% antibiotics (Invitrogen) to establish a stock virus for experiments.
Growth Curves
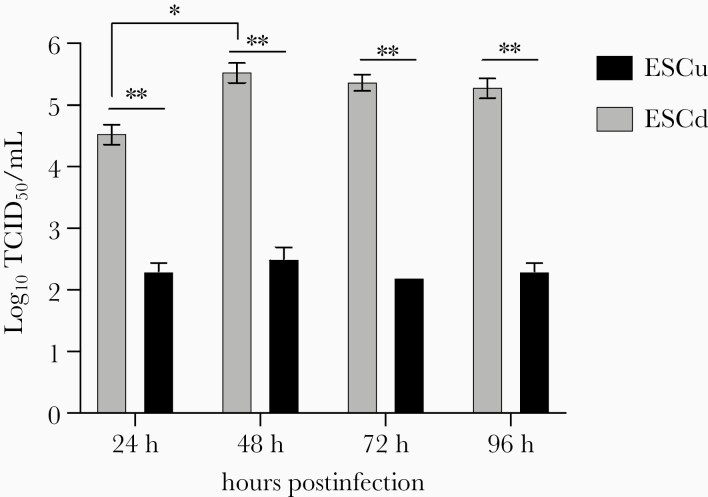
To determine growth dynamics of SARS-CoV-2, ESCu and ESCd at day 4 were mock infected (negative control) or infected with SARS-CoV-2 at a multiplicity of infection (MOI, ratio of the number of virus particles to the number of host cells) of 0.5. Vero E6 cells in 6-well plates were mock infected and infected with the SARS-CoV-2 at an MOI of 0.1 for 48 hours as negative and positive controls, respectively. After a 1-hour incubation, the virus inoculum was removed and replaced with the infection medium. The infected cells were incubated at 37°C with 5% CO2 and media samples collected at 24, 48, 72, and 96 hours postinoculation (hpi). Virus titers of the collected supernatants were determined by inoculating confluent monolayers of Vero E6 cells in 96-well plates, and the 50% tissue culture infective dose per mL (TCID50/mL) was calculated by the method of Reed and Muench [51] based on the presence of cytopathic effects.
Other Methods
RNAseq analyses, RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR), western blotting, and immunocytochemistry and the immunofluorescence assay are described in detail in Supplementary Methods.
RESULTS
Expression of ACE2 and TMPRSS2 in ESC-Derived Trophoblast
To determine the expression of ACE2 and TMPRSS2 in human ESC-derived trophoblasts, bulk RNAseq data from H1 ESCd in vitro to primitive trophoblast by an 8-day exposure to BMP4 (10 ng/mL), A83-01 (1 μM), and PD173074 (0.1 μM), that is BAP treated, was compared to that from H1 ESC maintained in culture media plus FGF2 (undifferentiated control, ESCu) (Figure 1A). ACE2 transcripts were expressed in all populations of ESCd, including: (1) the population that passed through a 40-µm screen (mononucleated cytotrophoblasts); and (2) the population of larger syncytialized cells that failed to pass through a wider-mesh 70-µm screen (syncytiotrophoblast) [47], but were undetectable in ESCu (Figure 1B). ACE2 transcript levels were higher in the larger multinucleated cells (ESCd >70 µm, syncytiotrophoblast) than in the largely mononucleated ESCd <40 µm (cytotrophoblast) fraction (P = .034, adjusted P = .071). TMPRSS2 was robustly expressed in ESCu and cytotrophoblast (ESCd <40 µm) fractions but highly enriched in the syncytiotrophoblast (ESCd >70 µm) fraction when compared to the other 2 cell types.
We next investigated the time course of ACE2 and TMPRSS2 transcript and protein expression in ESCu and BAP-differentiated trophoblast (ESCd) using qPCR, western blotting, and immunocytochemistry. ACE2 transcription began on day 3 of BAP exposure and increased through completion of the experiment on day 8 in ESCd; mRNA expression of ACE2 was barely detectable in ESCu (Figure 2A). On the other hand, the transcript concentrations of TMPRSS2 were similar between ESCu and ESCd and remained unchanged throughout culture, indicating that ESC have a constitutive expression of TMPRSS2 that is not unique to trophoblast. Consistent with gene expression, ACE2 protein was detectable by western immunoblotting on day 6 of BAP treatment and increased by day 8 in ESCd. However, ACE2 remained undetectable in ESCu (Figure 2B). Consistent with the positive detection of its transcript, TMPRSS2 protein levels were similar in ESCu and ESCd throughout culture.
Figure 2.
The identification of ACE2 and TMPRSS2 expression in ESCd trophoblasts. A, qPCR analysis of mRNA levels of ACE2 and TMPRSS2 in ESCu and ESCd at day 3, 4, 5, 6, 7, and 8. The bar graphs indicate mean ± SD (n = 3). The asterisks represent a statistically significant difference versus ESCu (ANOVA, P = 5.79 × 10–13 for ACE2 expression, P = 6.53 × 10–5 for TMPRSS2 expression; Tukey post-hoc test, *P < .05, ***P < .0001). B, Western blotting analysis with the indicated antibodies in ESCu and ESCd at day 6 and 8. C, Immunocytochemistry with the indicated antibodies in ESCd at day 8. Scale bars, 100 μm. Abbreviations: ACE2, angiotensin-converting enzyme 2; CGA, chorionic gonadotropin α; DAPI, 4′,6-diamidino-2-phenylindole; ESCd, embryonic stem cell derived; ESCu, embryonic stem cell undifferentiated; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TMPRSS2, transmembrane serine protease 2.
Immunocytochemistry of BAP-derived trophoblast/ESCd allowed assessment of cellular colocalization of ACE2 and TMPRSS2, which was noted on day 8 of differentiation (Figure 2C). E-cadherin and chorionic gonadotropin-α (CGA) were used as markers for the identification of cytotrophoblast cells (Figure 2C, upper panel) and syncytiotrophoblast (Figure 2C, lower panels), respectively. Staining for ACE2 and TMPRSS2 was colocalized and strongly overlapped with CGA-positive and E-cadherin–negative areas of the colonies, which represent syncytiotrophoblast in BAP-differentiated colonies (ESCd). As expected, ACE2 was present in both the cytoplasm and on the cell surface and TMPRSS2 was largely expressed in the cytoplasm and nucleoplasm of syncytiotrophoblast.
Human ESC-Derived Trophoblast Supports SARS-CoV-2 Replication
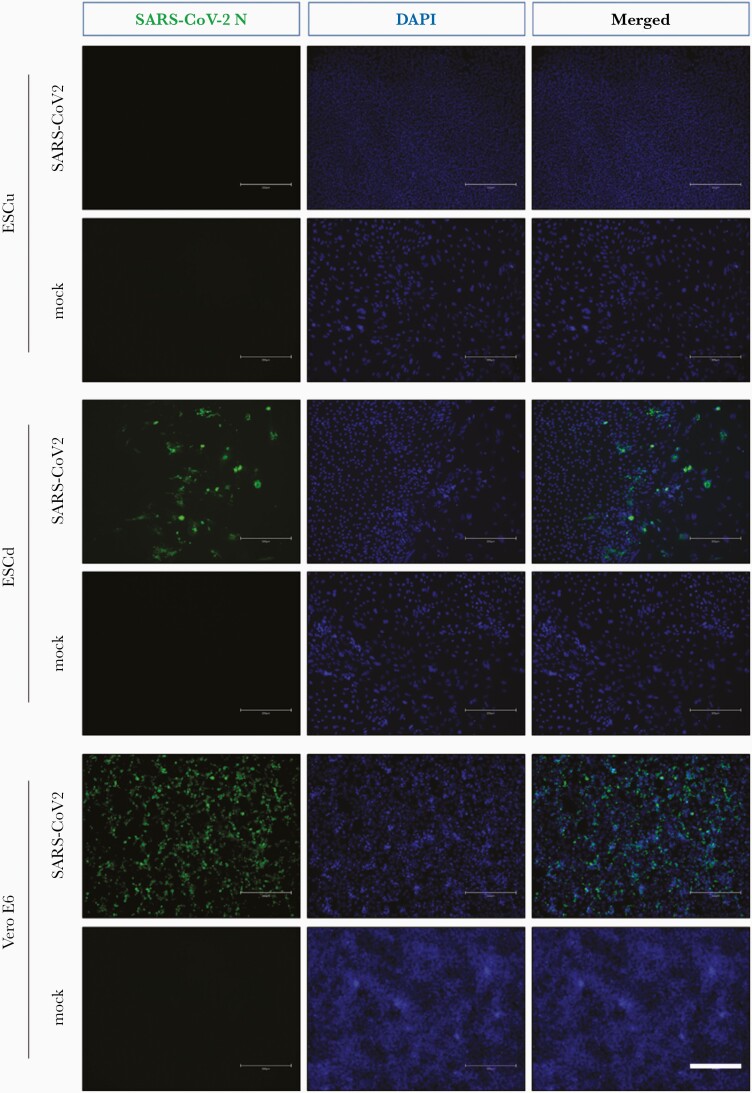
Both ESCu and ESC-derived trophoblast (ESCd) could be infected with SARS-CoV-2 in vitro, although significantly higher virus titers (at least 2 log-fold) were detected in infected differentiated trophoblast at all tested time points. Furthermore, SARS-CoV-2 viral titers in infected ESCd increased markedly between 24 and 48 hpi but plateaued at later time points (72 and 96 hpi) (Figure 3), indicating that the virus successfully replicated in differentiated trophoblast. In contrast, viral titers did not change in infected ESCu controls with increasing incubation time (Figure 3). Moreover, SARS-CoV-2 nucleocapsid (N) protein was detected in infected ESCd at all tested time points (Figure 4 and Supplementary Figure 1), further demonstrating that ESC-derived trophoblast supports both entry and replication of SARS-CoV-2. Noticeably, SARS-CoV-2 N protein was only detected in infected ESCu at the earliest time point (24 hpi) but not at later time points (48 and 96 hpi) (Figure 4 and Supplementary Figure 1). Interestingly, cytopathic effects were not observed in BAP-differentiated trophoblast nor in undifferentiated ECS controls infected with SARS-CoV-2, in stark contrast to Vero 6 cells infected with SARS-CoV-2 (Supplementary Figure 1).
Figure 3.
Growth kinetics of SARS-CoV-2 in hESCd trophoblasts. At day 4, ESCu and ESCd were infected with SARS-CoV-2 at a multiplicity of infection of 0.5. The viral titers depicted are presented as means ± SDs (n = 3). The asterisks represent a statistically significant difference between groups (*P < .05; **P < .001, unpaired t test). Abbreviations: ESCd, embryonic stem cell derived; ESCu, embryonic stem cell undifferentiated; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TCID50, 50% tissue culture infective dose.
Figure 4.
Detection of SARS-CoV-2 N protein in hESCd trophoblasts by immunofluorescence assay. At day 4, ESCu and ESCd were infected with SARS-CoV-2 at MOI of 0.5 for 96 hours. The Vero E6 cells infected with SARS-CoV-2 at an MOI of 0.1 for 48 hours were used for the positive control. To detect SARS-CoV-2 N protein, the fixed cells were incubated with the rabbit anti-SARS-CoV-2 N monoclonal antibody and the fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G second antibody. Nuclei were stained with DAPI. Scale bars, 300 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ESCd, embryonic stem cell derived; ESCu, embryonic stem cell undifferentiated; MOI, multiplicity of infection; N, nucleocapsid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
Reports of associations between maternal SARS-CoV-2 infection and adverse fetal outcomes have been inconsistent [12, 14, 25]. Data supporting the rarity of vertical transmission of SARS-CoV-2 after infection in late pregnancy are strong [12], although the hypothesized reasons for this do not necessarily apply in early pregnancy, when receptor expression patterns and trophoblast response to infection can vary dramatically [9, 10, 12–15, 30, 31, 35, 42]. Whether SARS-CoV-2 infects early pregnancy tissue remains largely unknown, due to difficulties inherent in studying the earliest pregnancies, including miscarriages that might go clinically undetected. We were specifically interested in the effects of SARS-CoV-2 infection on early (previllous) pregnancy and early placental development, because while several papers have suggested that placental infection can occur in all trimesters of pregnancy [20, 21, 33], infection risk may be higher in earlier stages of gestation [20, 21, 35]. Key anatomic and gene/protein expression differences between the early postimplantation previllous placenta and the full-term villous placenta may elucidate any increased early pregnancy susceptibility to SARS-CoV-2 infection [22, 30, 31, 35].
The previllous placenta (from the time of implantation on or about day 7 postfertilization to the third week of pregnancy when it degenerates) is composed of an inner layer of cytotrophoblast cells and an invasive, leading edge syncytiotrophoblast that interacts directly with the maternal decidua and its resident specialized immune cells, as well as with decidual glandular secretions and the maternal arteriolar and venous blood of the canalized structures that it invades [22, 23]. Pregnancy in humans at the immediate postimplantation stage is preclinical and can only be studied by in vitro modeling. In one such human model, extended blastocyst culture, coexpression of ACE2 and TMPRSS2, the proteins necessary for SARS-CoV-2 entry and infection [13], was demonstrated in the trophectoderm and early trophoblast cells, including syncytiotrophoblast [44].
The evolution of the mature villous structure begins at approximately 3 weeks postfertilization. The mature placental villi will be composed of a core containing fetal vessels that are separated from maternal blood by several layers of protection, including maternally derived stromal cells, proliferative cytotrophoblast cells, and a single, multinucleated, nonproliferative layer of syncytiotrophoblast. Single-cell RNAseq [42], RNA in situ hybridization [12, 19] and immunohistochemical analyses [12, 19] of term placenta suggest that this villous structure helps to protect the fetus from vertical transmission of SARS-CoV-2 in pregnancy. While ACE2 and TMPRSS2 are expressed in the placenta, anatomic separation in the third trimester placenta makes transmission from mother to fetus through these proteins unlikely [12]. Furthermore, while SARS-CoV-2 viremia occurs at the low rate of 10%–27% of infected nonpregnant patients with severe/critical illness [52–54], viremia may be exceedingly rare in pregnant women at term [12].
Using a stem cell-derived model of early trophoblast development, we demonstrated coexpression of ACE2 and TMPRSS2 in vitro in modeled multinucleated syncytiotrophoblast. In vivo, these cells interact directly with the maternal decidua in the previllous placenta, and therefore may be susceptible to maternal infection. We demonstrated that viral infection with SARS-CoV-2 was followed by viral replication, but we did not detect cytopathic effects during the 96-hour postexposure culture period. Infection and virus production in this model was limited to the syncytialized areas that express both ACE2 and TMPRSS2. One other group demonstrated SARS-CoV-2 infection in tissues from very early, preimplantation stage, human pregnancy, although these findings have not yet undergone peer review [38]. In that study, human blastocyst trophectoderm biopsies were used for RNAseq analyses, and human blastocysts were used for immunohistochemistry to show transcription and protein coexpression of ACE2 and TMPRSS2. The authors then used SARS-CoV-2 spike protein-pseudotyped viruses to demonstrate viral entry in trophectoderm cells of discarded human blastocysts. Viral replication and cytopathic effects were not assessed.
Here, we report that in stem cell-derived trophoblast, ACE2 and TMPRSS2 transcripts and proteins were preferentially expressed or solely expressed in multinucleated, syncytialized cells and viral infection was similarly restricted. This pattern of viral infection mimics that seen in our ZIKV [30, 31] and Dengue virus (Sheridan et al, unpublished observation) models, suggesting susceptibility of early syncytiotrophoblast to viral infection. It also is consistent with the limited available reports on in vitro infection of human blastocysts [38, 39] and in vivo patterns of placental infection. In situ hybridization and immunohistochemistry of infected placentas shows infection of extravillous cytotrophoblast cells (which were not specifically evaluated in our model) and of villous trophoblast [10, 12, 19–21]. Among the villous trophoblast subpopulations, cytotrophoblast cell infection was seen at a much lower level than infection of syncytiotrophoblast [10, 12, 19–21].
The reason for such preferential infection of syncytiotrophoblast in vitro and in vivo remains unclear. For in vivo infections, it is possible that the syncytiotrophoblast, as the first trophoblast subtype to encounter virus in a viremic mother, is the most likely to become infected. If such infection were not lytic, infection might remain mostly localized to the syncytiotrophoblast layer. While ACE2/TMPRSS2 expression patterns and anatomic separation of cells in the placental villi may help prevent robust spread of viral infection in term placenta, these mechanisms cannot explain infection specificity for syncytiotrophoblast in our model of primitive trophoblast because BAP exposure generates a mixture of trophoblast cell types in the colonies and allows for equal virus access to all trophoblast types. Rather, in our model of primitive trophoblast, a combined expression of ACE2 and TMPRSS2 likely explains the viral tropism. It is also possible that in vitro and in vivo, the complex process of syncytialization, which involves endogenous retroviral envelope proteins and their association with their binding partners, may also make syncytializing and syncytialized cells more susceptible to SARS-CoV-2 and to other viral infections.
In contrast to Vero cells, ESC-derived trophoblast cells did not exhibit SARS-CoV-2–related cytopathic effects over 96 hours, despite robust viral replication and maintenance in these cells. The reasons for a lack of lytic effects are unclear but may be specific to early placenta and deserve further investigation. We hypothesize that this phenomenon may be partly responsible for the rarity of vertical transmission of SARS-CoV-2 even in the presence of detectable placental infection. In addition, persistence of SARS-CoV-2 in the placenta could have effects far removed from the time of maternal infection. A report of a pregnant woman with known exposure and asymptomatic infection in the first trimester of pregnancy who suffered an early second trimester loss of a karyotypically normal fetus [21] is consistent with this possibility. At the time of this loss, the patient was negative for SARS-CoV-2 in the nasopharynx but had virus detectable by RT-PCR and immunohistochemistry in the placenta. Infection was found in both cytotrophoblast and syncytiotrophoblast of this placenta but was much more robust in the latter [21].
In our experiments, control, undifferentiated ESC could be infected in vitro, despite the absence of ACE2 (Supplementary Figure 1; conditioned medium plus FGF2 24-hour data). On the other hand, infection levels were several log-fold lower than those in ESC-derived trophoblast that coexpressed ACE2 and TMPRSS2. There was no evidence of replication or persistence of the virus in control, undifferentiated ESCu cells. This suggests that SARS-CoV-2 is able to enter the undifferentiated control cells, likely through endocytosis, as demonstrated previously [30, 31, 55]. However, the lack of viral replication in undifferentiated ESC contrasts with reports from ACE2-negative human airway cells, where replication followed passive infection [55]. The underlying mechanisms allowing undifferentiated ESC to permit SARS-CoV-2 entry but preventing replication require further investigation.
Finally, detectable maternal viremia in SARS-CoV-2–infected pregnant women [12] is seldom reported. This raises the question of how the primitive trophoblast could become infected by SARS-CoV-2, an event we speculate might occur but typically go undetected. Possibly, the immunomodulatory milieu of placental hormones may protect from infection, with protection increasing with gestational age. Many of the immunomodulatory pregnancy-related hormones found in high levels in maternal serum, and higher levels at the maternal-fetal interface at the end of pregnancy, are present locally and systemically at the time of implantation, but at much lower levels. This may allow viremic events (and placental infection) in early gestation at levels resembling those in the nonpregnant population [52–54]. Levels of viremia in early pregnancy, particularly during preclinical pregnancy, have never been studied. The cells of the maternal endometrium in pregnancy (decidua) coexpress ACE2 and TMPRSS2 [56], and SARS-CoV-2 can be detected in semen of infected men [57, 58]. Infection of decidual cells could allow for transmission from decidua to trophoblast at the time of implantation, and semen could mediate infection at the time of fertilization. ACE2 and TMPRSS2 coexpression has been demonstrated in single-cell RNAseq analysis in human zygotes, and 2- and 4-cell embryos; after that, ACE2 expression moves to the trophectoderm and primitive endoderm [37], demonstrating that early embryos are theoretically susceptible to infection by SARS-CoV-2. This outcome has been verified in extended human blastocyst cultures in vitro [44] and may have important implications for transmission during assisted reproduction, particularly via artificial insemination.
In summary, we hypothesize that the unique characteristics of the placenta and local maternal-fetal immune microenvironment during and soon after human blastocyst implantation may result in preclinical and early clinical pregnancy loss in maternal SARS-CoV-2 infection. The immune microenvironment at the maternal-fetal interface at the time of implantation is proinflammatory and differs from the protolerogenic environment otherwise characteristic of pregnancy [23]. This proinflammatory microenvironment may control viral infection locally and could exacerbate the intense inflammation seen in other SARS-CoV-2–infected tissues [59], thereby promoting pregnancy loss. Our in vitro studies have shown that a model of peri-implantation placenta supports SARS-CoV-2 infection and replication. There are no reports on the effects of SARS-CoV-2 infection on very early pregnancy loss, even in early clinical pregnancy. These could be undertaken in assisted reproduction clinics, in which pregnancies are followed very closely with repeated and early testing. In light of the known risk of early pregnancy loss associated with other coronaviruses, including those that also use ACE2 for entry [28, 29], and the widespread prevalence of SARS-CoV-2 infection that continues, such studies are urgently needed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Drs Jeff Adamovicz and Jeff Whyte for providing the SARS-CoV-2 USA-WA1/2020 strain; and Dr Lisa Bebell for her careful and critical review of the manuscript.
Supplement sponsorship. This supplement is sponsored by the Harvard University Center for AIDS Research.
Financial support. This work was supported by the University of Missouri Start-up Grant (to W. M. A.); and the National Institutes of Health (grant numbers 3R01HD094937 and 1R21AI145071 to D. J. S., T. E., and R. M. R., and 3R01HD100022-02S2 and 1R01HD100022 to A. G. E.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Delahoy MJ, Whitaker M, O’Halloran A, et al. ; COVID-NET Surveillance Team . Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19—COVID-NET, 13 states, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zambrano LD, Ellington S, Strid P, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team . Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Durusoy S, Akdoğan V, Paksoy AE. Do three-dimensional modeling and printing technologies have an impact on the surgical success of percutaneous transsacral screw fixation? Jt Dis Relat Surg 2020; 31:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galang RR, Chang K, Strid P, et al. . Severe coronavirus infections in pregnancy: a systematic review. Obstet Gynecol 2020; 136:262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Woodworth KR, Olsen EO, Neelam V, et al. ; CDC COVID-19 Response Pregnancy and Infant Linked Outcomes Team; COVID-19 Pregnancy and Infant Linked Outcomes Team (PILOT) . Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 Jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mullins E, Hudak ML, Banerjee J, et al. ; PAN-COVID investigators and the National Perinatal COVID-19 Registry Study Group . Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol 2021; 57:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khalil A, von Dadelszen P, Draycott T, Ugwumadu A, O’Brien P, Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA 2020; 324:705–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kc A, Gurung R, Kinney MV, et al. . Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health 2020; 8:e1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flaherman VJ, Afshar Y, Boscardin J, et al. . Infant outcomes following maternal infection with SARS-CoV-2: first report from the PRIORITY study [published online ahead of print 18 September 2020]. Clin Infect Dis doi: 10.1093/cid/ciaa1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. . Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020; 11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Islam MM, Poly TN, Walther BA, et al. . Clinical characteristics and neonatal outcomes of pregnant patients with COVID-19: a systematic review. Front Med 2020; 7:573468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edlow AG, Li JZ, Collier AY, et al. . Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open 2020; 3:e2030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alamar I, Abu-Arja MH, Heyman T, et al. . A Possible case of vertical transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a newborn with positive placental in situ hybridization of SARS-CoV-2 RNA. J Pediatric Infect Dis Soc 2020; 9:636–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kotlyar AM, Grechukhina O, Chen A, et al. . Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol 2021; 224:35–53.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fenizia C, Biasin M, Cetin I, et al. . Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun 2020; 11:5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 2000; 74:6808–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Satosar A, Ramirez NC, Bartholomew D, Davis J, Nuovo GJ. Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn. Hum Pathol 2004; 35:536–45. [DOI] [PubMed] [Google Scholar]
- 18. Miner JJ, Diamond MS. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 2017; 21:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hecht JL, Quade B, Deshpande V, et al. . SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol 2020; 33:2092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hosier H, Farhadian SF, Morotti RA, et al. . SARS-CoV-2 infection of the placenta. J Clin Invest 2020; 130:4947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shende P, Gaikwad P, Gandhewar M, et al. . Persistence of SARS-CoV-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum Reprod 2021; 36:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med 2001; 345:1400–8. [DOI] [PubMed] [Google Scholar]
- 23. Mor G, Aldo P, Alvero AB. The unique immunological and microbial aspects of pregnancy. Nat Rev Immunol 2017; 17:469–82. [DOI] [PubMed] [Google Scholar]
- 24. Chard T. Frequency of implantation and early pregnancy loss in natural cycles. Baillieres Clin Obstet Gynaecol 1991; 5:179–89. [DOI] [PubMed] [Google Scholar]
- 25. Cosma S, Carosso AR, Cusato J, et al. . Coronavirus disease 2019 and first-trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am J Obstet Gynecol 2021; 224:391.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. la Cour Freiesleben N, Egerup P, Hviid KVR, et al. . SARS-CoV-2 in first trimester pregnancy: a cohort study. Hum Reprod 2021; 36:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rotshenker-Olshinka K, Volodarsky-Perel A, Steiner N, Rubenfeld E, Dahan MH. COVID-19 pandemic effect on early pregnancy: are miscarriage rates altered, in asymptomatic women? Arch Gynecol Obstet 2021; 303:839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong SF, Chow KM, Leung TN, et al. . Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004; 191:292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kimura T, Takeda A, Sanuki N, et al. . Multicenter prospective study of stereotactic body radiotherapy for previously untreated solitary primary hepatocellular carcinoma: The STRSPH study. Hepatol Res 2021; 51:461–71. [DOI] [PubMed] [Google Scholar]
- 30. Sheridan MA, Yunusov D, Balaraman V, et al. . Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A 2017; 114:E1587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheridan MA, Balaraman V, Schust DJ, Ezashi T, Roberts RM, Franz AWE. African and Asian strains of Zika virus differ in their ability to infect and lyse primitive human placental trophoblast. PLoS One 2018; 13:e0200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamazou F, Oger P, Dieli-Crimi R, et al. . COVID-19 infection in first trimester of pregnancy marked by a liver cytolysis in a woman previously treated by hydroxychloroquine for repeated implantation failure: a case report. BMC Infect Dis 2020; 20:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baud D, Greub G, Favre G, et al. . Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA 2020; 323:2198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu-Culligan A, Chavan AR, Vijayakumar P, et al. . SARS-CoV-2 infection in pregnancy is associated with robust inflammatory response at the maternal-fetal interface. medRxiv, doi: 10.1101/2021.01.25.21250452, 26. January 2021, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roberts DJ, Bebell LM, Edlow AG. SARS-CoV-2 ACE2 and TMPRSS2 receptor protein expression patterns throughout gestation [published online ahead of print 21 April 2021]. J Infect Dis. 2021;224(S6):S642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoffmann M, Kleine-Weber H, Schroeder S, et al. . SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen W, Yuan P, Yang M, et al. . SARS-CoV-2 entry factors: ACE2 and TMPRSS2 are expressed in peri-implantation embryos and the maternal-fetal interface. Engineering 2020; 6:1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Montano M, Victor AR, Griffin DK, et al. . Human pre-implantation embryos are permissive to SARS-CoV-2 entry. bioRxiv, doi: 2021.01.21.427501, 21. January 2021, preprint: not peer reviewed. [Google Scholar]
- 39. Viotti M, Montano M, Victor A, et al. . Human pre-implantation embryos are permissive to SARS-COV-2 entry. Fertil Steril 2020; 114:e526-e. [Google Scholar]
- 40. Cui D, Liu Y, Jiang X, et al. . Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet Gynecol 2021; 57:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh M, Bansal V, Feschotte C. A Single-cell RNA expression map of human coronavirus entry factors. Cell Rep 2020; 32:108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pique-Regi R, Romero R, Tarca AL, et al. . Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? Elife 2020; 9:e58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Logsdon DM, Kile RA, Schoolcraft WB, Krisher RL, Yuan Y. Single cell collection of trophoblast cells in peri-implantation stage human embryos. J Vis Exp 2020; ( 160): doi: 10.3791/61476. [DOI] [PubMed] [Google Scholar]
- 44. Weatherbee BAT, Glover DM, Zernicka-Goetz M. Expression of SARS-CoV-2 receptor ACE2 and the protease TMPRSS2 suggests susceptibility of the human embryo in the first trimester. Open Biol 2020; 10:200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sheridan MA, Yang Y, Jain A, et al. . Early onset preeclampsia in a model for human placental trophoblast. Proc Natl Acad Sci U S A 2019; 116:4336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Amita M, Adachi K, Alexenko AP, et al. . Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc Natl Acad Sci U S A 2013; 110:E1212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yabe S, Alexenko AP, Amita M, et al. . Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas. Proc Natl Acad Sci U S A 2016; 113:E2598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Horii M, Morey R, Bui T, et al. . Modeling preeclampsia using human induced pluripotent stem cells. Sci Rep 2021; 11:5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Okae H, Toh H, Sato T, et al. . Derivation of human trophoblast stem cells. Cell Stem Cell 2018; 22:50–63.e6. [DOI] [PubMed] [Google Scholar]
- 50. Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. . Embryonic stem cell lines derived from human blastocysts. Science 1998; 282:1145–7. [DOI] [PubMed] [Google Scholar]
- 51. Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints12. Am J Epidemiol 1938; 27:493–7. [Google Scholar]
- 52. Chen X, Zhao B, Qu Y, et al. . Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis 2020; 71:1937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang C, Wang Y, Li X, et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fajnzylber J, Regan J, Coxen K, et al. ; Massachusetts Consortium for Pathogen Readiness . SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun 2020; 11:5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Puray-Chavez M, LaPak KM, Schrank TP, et al. . Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell. Cell Reports 2021;109364.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li M, Chen L, Xiong C, Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One 2020; 15:e0230295.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 2020; 3:e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pan F, Xiao X, Guo J, et al. . No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril 2020; 113:1135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol 2021: 21:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.