Abstract
Background
Early convalescent plasma transfusion may reduce mortality in patients with nonsevere coronavirus disease 2019 (COVID-19).
Methods
This study emulates a (hypothetical) target trial using observational data from a cohort of US veterans admitted to a Department of Veterans Affairs (VA) facility between 1 May and 17 November 2020 with nonsevere COVID-19. The intervention was convalescent plasma initiated within 2 days of eligibility. Thirty-day mortality was compared using cumulative incidence curves, risk differences, and hazard ratios estimated from pooled logistic models with inverse probability weighting to adjust for confounding.
Results
Of 11 269 eligible person-trials contributed by 4755 patients, 402 trials were assigned to the convalescent plasma group. Forty and 671 deaths occurred within the plasma and nonplasma groups, respectively. The estimated 30-day mortality risk was 6.5% (95% confidence interval [CI], 4.0%–9.7%) in the plasma group and 6.2% (95% CI, 5.6%–7.0%) in the nonplasma group. The associated risk difference was 0.30% (95% CI, −2.30% to 3.60%) and the hazard ratio was 1.04 (95% CI, .64–1.62).
Conclusions
Our target trial emulation estimated no meaningful differences in 30-day mortality between nonsevere COVID-19 patients treated and untreated with convalescent plasma.
Clinical Trials Registration. NCT04545047.
Keywords: coronavirus, COVID-19, SARS-CoV-2, convalescent plasma
This study used electronic health record data to emulate a target trial of early COVID-19 convalescent plasma and 30-day mortality risk. No differences in mortality risk were found between patients with nonsevere COVID-19 who were treated and untreated with plasma.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), currently ranks among the top 3 leading causes of death in the United States among adults aged 45 years and older [1]. Although most infected persons have few or no symptoms, a minority develop severe illness leading to hospitalization with supplemental oxygen support and potentially multiorgan failure and death [2].
Convalescent plasma therapy emerged early on as a promising treatment strategy for COVID-19. The plasma of donors who have recovered from COVID-19 contains a host of antiviral and anti-inflammatory components with potential to confer passive immunity to recipients [3].
Because the proposed mechanism of action is clearance of viremia mediated by neutralizing antibodies, the antiviral properties of convalescent plasma are expected to be most effective during the initial viral replication phase of disease, which peaks during the first week of infection [4]. This claim is supported by reports showing increased viral clearance following convalescent plasma treatment [5]. Furthermore, most patients do not have a readily detectable antibody response until around 10 days after symptom onset, suggesting that convalescent plasma may augment the antiviral immune response when transfused in the early stages of disease [6]. While COVID-19 convalescent plasma therapy has been demonstrated to be safe, the results of randomized trials provide inconclusive evidence about its effectiveness [7, 8].
We assess the effect of convalescent plasma therapy on 30-day mortality in nonsevere COVID-19 patients early in the hospital course. We emulated a hypothetical randomized trial (target trial) using observational data from a large national sample of patients who received care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the largest integrated health care system in the United States.
METHODS
We first describe the protocol of a hypothetical randomized trial (target trial) [9, 10] to estimate the effect of convalescent plasma on 30-day mortality among US veterans with nonsevere COVID-19. Then we describe the procedures enacted to emulate the components of the protocol of the target trial using observational data. Our observational study is registered at ClinicalTrials.gov (NCT04545047). The VA Central Institutional Review Board determined that this study met the criteria for exemption from 45 CFR 46 under category 104d(4)(iii).
Target Trial Specification
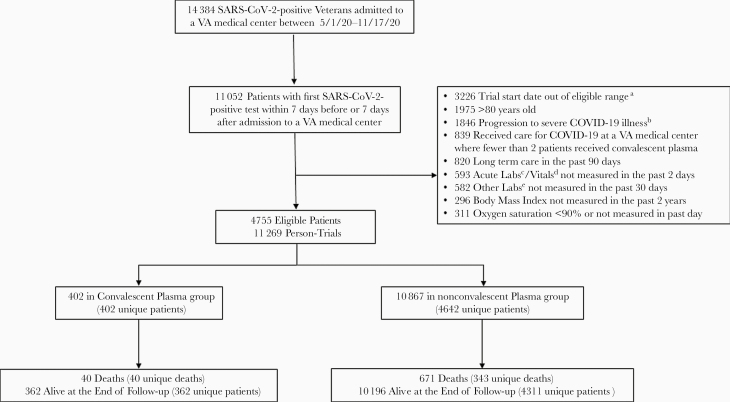
The key components of the target trial protocol are summarized in Table 1 and were specified prior to analysis. The target trial we sought to emulate would include US veterans aged 21–80 years hospitalized between 1 May 2020 and 17 November 2020 at a VA medical center where convalescent plasma was a current practice. Because this study was focused on individuals with nonsevere infections, we would include patients with a first-ever SARS-CoV-2 positive test within 7 days before or after admission, oxygen saturation of ≥90% within the past day, and no severe illness based on VA’s adaptation of the World Health Organization COVID-19 clinical progression scale [11]. This scale defines severe illness as treatment with high-flow oxygen, mechanical ventilation, intubation, dialysis, vasoactive or inotropic infusion, or extracorporeal membrane oxygenation (see procedure codes and medications in Supplementary Table). Patients previously treated with convalescent plasma or residing in a long-term care or domiciliary in the past 90 days would not be eligible. The patient would become eligible at the first SARS-CoV-2–positive test date (if the positive test occurred during hospitalization) or at the hospital admission date (if the positive test occurred before hospitalization). Figure 1 shows a flow diagram of selection of the 4755 eligible patients.
Table 1.
Protocol Components for the Specification and Emulation of a Target Trial of Convalescent Plasma and 30-day Mortality Using Observational Data From the VA Corporate Data Warehouse
| Protocol Component | Target Trial Specification | Target Trial Emulation |
|---|---|---|
| Eligibility criteria | • US veterans aged 21–80 years • Hospitalized between 1 May 2020 and 17 November 2020 with a SARS-CoV-2 positive test, at a VA Medical Center where convalescent plasma had been administered to at least 1 patient and remained a current practice at that VA Medical Center • A SARS-CoV-2 positive test within 7 days before or after hospital admission • No prior treatment with convalescent plasma • No long-term care in a domiciliary or nursing home in the past 90 days • Minimum oxygen saturation (measured within the past day) ≥90% • No prior intubation, ventilation, high flow oxygen, extracorporeal membrane oxygenation, dialysis, or vasopressors during current hospitalization • Vitals (pulse, respiration, temperature, systolic blood pressure) and acute labs (hemoglobin, platelet, white blood cells) measured within the past 2 days • Albumin, alanine aminotransferase, creatinine measured within the past 30 days • Weight measurement recorded in the past 2 years |
Same A list of procedure codes and medications used to assess eligibility from VA corporate data warehouse are shown in the Supplementary Table |
| Treatment strategies | (1) Receipt of convalescent plasma transfusion (2) No receipt of convalescent plasma transfusion |
Same |
| Treatment assignment | Individuals are randomly assigned to a treatment strategy
Individuals and their treating physicians are aware of assigned treatment |
We classified patients according to the treatment with which their data were compatible and attempted to emulate randomization by adjusting for baseline covariates (Table 2) |
| Outcomes | 30-day all-cause mortality | Same |
| Follow-up | Starts at treatment date, which must be within 2 days after both hospitalization and SARS-CoV-2 positive test, and ends at 30 days or death | Same |
| Causal contrasts | Intention-to-treat effect and per-protocol effect | Observational analogue of per-protocol effect |
| Statistical analysis | Intention-to-treat analysis; per-protocol analysis with adjustment for baseline covariates via inverse probability weighting |
Same per-protocol analysis, except that patients may be enrolled in multiple trials for statistical efficiency |
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VA, Veterans Affairs.
Figure 1.
Flowchart for selection of eligible patients for emulation of a target trial assessing the effectiveness of convalescent plasma on reducing mortality among severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–positive veterans hospitalized at a Veterans Affairs (VA) medical center. aEligible trials must start within 2 days after the first day hospitalized with a positive severe acute respiratory syndrome coronavirus 2 test, at a VA medical center where COVID-19 convalescent plasma transfusion was a current practice and at least 1 patient had already received transfusion. bProgression to severe illness was defined as any prior treatment during current hospitalization with high-flow oxygen, mechanical ventilation, dialysis, vasoactive infusion, or extracorporeal membrane oxygenation. cAcute labs included hemoglobin, platelet, white blood cells counts. dVitals included pulse, respiration, temperature, and systolic blood pressure. eOther labs included alanine aminotransferase, albumin, and creatinine.
Eligible patients would be randomly assigned to either receive or not receive convalescent plasma within 2 days of assignment. The exact timing of transfusion and determination of suitability for use (based on antibody levels or donor characteristics of the plasma units) would be left to the discretion of treating physicians. The period of 0–2 days was chosen to evaluate early intervention of convalescent plasma use and (in the subsequent emulation) to reduce confounding by progression of disease. Treatment assignment would occur at the first SARS-CoV-2–positive test date (if the positive test occurred during hospitalization) or at the hospital admission date if the positive test occurred before hospitalization. The primary outcome would be 30-day mortality. The causal contrasts of interest would be the intention-to-treat effect and the per-protocol effect.
Analysis of the Target Trial
To estimate the intention-to-treat effect (the effect of assignment to convalescent plasma on 30-day mortality), we would estimate the 30-day mortality risk difference and hazard ratio comparing patients assigned to each group. The risk (cumulative incidence) of death could be estimated nonparametrically using the Kaplan-Meier method or under parametric smoothing assumptions via a pooled logistic regression model including a time-varying intercept, an indicator for treatment group, and a product (interaction) term between time and treatment group [12]. The hazard ratio could be estimated from the pooled logistic regression model without the product term. In the case of a prespecified imbalance in baseline characteristics between intervention arms, relevant covariates could be added to the model.
To estimate the per-protocol effect (the effect of treatment with convalescent plasma on 30-day mortality if all patients had adhered to the protocol), we would restrict the above intention-to-treat analysis to patients who adhered to their assigned treatment (convalescent plasma or no convalescent plasma) and adjust for the 22 a priori baseline prognostic factors in Table 2, as well as trial start day relative to first eligibility. The validity of this per-protocol analysis requires that no other prognostic factors are strongly associated with adherence to the assigned treatment strategy. The adjustment for these covariates would be carried out via inverse probability weighting, with all continuous variables flexibly modeled using restricted cubic splines and estimated weights truncated at the 99.9th percentile to prevent extreme weights from affecting the analyses. In a secondary analysis, the adjustment for these covariates would instead be carried out via standardization. In all analyses, we would use nonparametric bootstrapping with 1500 samples, which yields unbiased estimates of standard error, to calculate percentile-based confidence intervals (CI) for survival difference and hazard ratio estimates.
Table 2.
Baseline Characteristics of Eligible Patients When Emulating a Target Trial of Convalescent Plasma and 30-day Mortality in SARS-CoV-2–Positive Veterans Hospitalized at a VA Medical Center Between 1 May 2020 and 17 November 2020
| Characteristica | Convalescent Plasma Group (n = 402) | Nonconvalescent Plasma Group (n = 10 867) |
|---|---|---|
| Age, y, mean (SD) | 65.0 (11.3) | 64.1 (12.0) |
| Sex, male | 370 (92) | 10 101 (93) |
| Race | ||
| White | 258 (64) | 6194 (57) |
| Black | 109 (27) | 3814 (35) |
| Other | 35 (9) | 859 (9) |
| Ethnicity, Hispanic or Latino | 52 (13) | 1123 (10) |
| Region | ||
| Central | 114 (28) | 2584 (24) |
| Northeast | 69 (17) | 2367 (22) |
| Pacific | 27 (7) | 1527 (14) |
| Southeast | 192 (48) | 4389 (40) |
| Body mass index, kg/m2, mean (SD) | 32.2 (7.1) | 31.0 (7.2) |
| Smoking status | ||
| Never | 109 (27) | 2791 (26) |
| Current | 81 (20) | 2836 (26) |
| Former | 74 (18) | 1923 (18) |
| Unknown | 138 (34) | 3317 (31) |
| Cardiovascular disorder within the past 5 y | 156 (39) | 4663 (43) |
| Chronic obstructive pulmonary diseaseb | 119 (30) | 2463 (23) |
| Dementiab | 29 (7) | 816 (8) |
| Diabetesb | 219 (55) | 5145 (47) |
| Hypertensionb | 307 (76) | 7747 (71) |
| Estimated glomerular filtration rate within the past 30 d, mL/min/1.73m2, median (IQR)c | 79.3 (59.1–94.8) | 77.3 (54.9–93.6) |
| In intensive care unitd | 153 (38) | 2204 (20) |
| Glucocorticoid used | 215 (54) | 2258 (21) |
| Remdesivir used | 187 (47) | 1476 (14) |
| Supplemental non–high-flow oxygend | 9 (2) | 161 (2) |
| Minimum oxygen saturation in the past d, %, mean (SD)d | 93.3 (2.8) | 94.6 (3.1) |
| First oxygen saturation during hospitalization, %, mean (SD)d | 93.8 (3.8) | 95.5 (3.3) |
| Maximum white blood cell count in past 2 d, 1000 cells/µL, median (IQR)c,d | 6.6 (4.6–9.0) | 5.9 (4.5–8.1) |
| Systemic inflammatory response syndromed | 243 (60) | 4739 (44) |
| Calendar day of trial start, mean (SD) | 249 (52) | 231 (52) |
Data shown in this table are number and proportion (%) except where indicated.
Abbreviations: IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VA, Veterans Affairs.
aEach individual patient may contribute to multiple target trials.
bWithin the past 2 years.
cMedian and IQR presented due to nonnormal distribution.
dDuring COVID-19 hospitalization prior to trial start.
Target Trial Emulation
We emulated the above target trial using the VA Corporate Data Warehouse (CDW), a database that integrates all VA electronic health records (EHR) nationwide. Lists of convalescent plasma recipients were obtained from 2 sources, the VA Pathology and Laboratory Medicine Service and the Mayo Clinic Expanded Access Program (EAP) database. Each patient was matched to the EHR using unique identifiers. Data on SARS-CoV-2 tests were obtained from the VA COVID-19 Shared Data Resource. Information on the baseline prognostic factors in Table 2 was extracted from CDW (see procedure codes, diagnosis codes, and medications in Supplementary Table). Laboratory and vitals measurements during the hospitalization were recorded daily, with the last observation carried forward 2 days if no new measurements were obtained.
Patients received COVID-19 convalescent plasma through several mechanisms. From April to August 2020, plasma treatments were made available to VHA patients under the Mayo Clinic EAP [13]. Patients may have also received convalescent plasma through enrollment in clinical trials and emergency single-patient new drug applications. Following the US Food and Drug Administration’s (FDA) issuance of an emergency use authorization of investigational convalescent plasma on 23 August 2020, health care providers could administer the treatment to hospitalized patients with COVID-19 based on their assessment of an individual’s potential for risk and benefit [13].
We used the VA data to emulate the eligibility criteria, treatment strategies, outcome, and follow-up of the target trial as described above. The per-protocol analysis was identical to the one described for the target trial with one modification: In the target trial, we would assign treatment at time zero to each eligible patient on a single day (0, 1, or 2) of first eligibility. In the observational analysis, however, we may observe eligible patients on multiple days and include them in a trial on each day of eligibility (person-trial). Therefore, we used the observational data to emulate 3 separate target trials starting on each of the 3 eligible days (0, 1, 2) and pooled their estimates for computational efficiency, as described elsewhere [14]. Each patient could be assigned to the nonplasma group at most 3 times (if they never initiated plasma) but to the plasma group only once on the day of treatment initiation. Plasma patients could be included in nonplasma person trials on eligible days prior to receipt of plasma.
We conducted 2 sensitivity analyses to explore the magnitude and direction of confounding. First, we repeated the main analysis with partial adjustment for only age, race, sex, and region. Second, we expanded the window during which convalescent plasma could be administered to 0–7 days. Because later administration of convalescent plasma is expected to be associated with a worsening clinical course, the ability to adjust for confounding decreases as the window is increased. All analyses were conducted using R studio version 1.3.1093.
RESULTS
Characteristics of the Study Population
Table 2 shows the baseline characteristics of the 11 269 eligible person-trials, among which 402 initiated convalescent plasma (plasma group) and 10 867 did not (nonplasma group). Compared with the nonplasma group, the patients in the plasma group were more likely to be white, Hispanic/Latino, and live in the US central or southeast regions; have chronic obstructive pulmonary disease, diabetes, and hypertension; have had an intensive care unit admission; have received treatment with glucocorticoids or remdesivir; have higher white blood cell counts; and have lower oxygen saturation levels both upon hospital admission and immediately prior to trial start date. After inverse probability weighting, all measured baseline characteristics were reasonably well balanced between the 2 groups (Supplementary Figure 1).
Convalescent Plasma Therapy and 30-Day Mortality
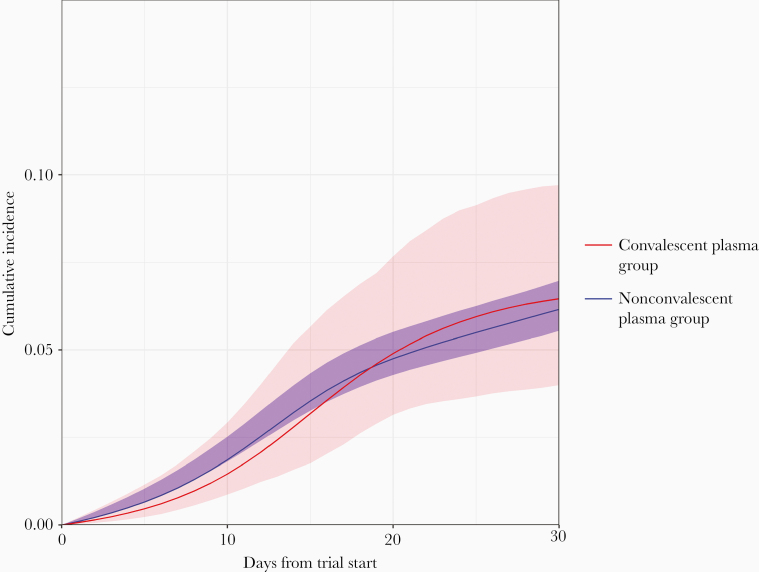
Over the 30-day follow-up period, there were 40 deaths among the plasma group and 671 deaths among the nonplasma group. The cumulative incidence curves are shown in Figure 2. The estimated 30-day mortality risk was 6.5% (95% CI, 4.0%–9.7%) in the plasma group and 6.2% (95% CI, 5.6%–7.0%) in the nonplasma group, resulting in a risk difference of 0.30% (95% CI, −2.30% to 3.60%) and hazard ratio of 1.04 (95% CI, .64–1.62). Supplementary Figure 2 shows the overlay of the inverse probability weighted, standardized, and unadjusted cumulative incidence curves. Adjustment via standardization yielded similar estimates. The corresponding unadjusted risk difference was 3.76% (95% CI, .91%–6.94%) and the unadjusted hazard ratio was 1.64 (95% CI, 1.14–2.18).
Figure 2.
Inverse probability weighted cumulative incidence of 30-day mortality (with 95% confidence intervals) for the target trial of convalescent plasma among severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–positive veterans hospitalized at a Veterans Affairs medical center.
In our sensitivity analyses, when repeating the analysis with adjustment for only age, race, sex, and region, the estimated 30-day mortality risk difference was 3.05% (95% CI, .38%–6.08%) and the hazard ratio was 1.52 (95% CI, 1.43–2.36) (Supplementary Figure 3), which suggests that substantial confounding by clinical factors exist. When extending eligibility to 7 days, we identified 4837 eligible patients, corresponding to 598 person-trials in the plasma group and 18 451 in the nonplasma group. Over the 30-day follow-up period, there were 64 deaths in the plasma group and 1126 deaths in the nonplasma group. The estimated 30-day mortality risk difference was 4.81% (95% CI, 1.40%–8.52%) and the hazard ratio was 1.83 (95% CI, 1.23–2.44) for the plasma group compared with the nonplasma group (Supplementary Figure 4), which suggests that the ability to adjust for confounding decreases as the window of eligibility increases.
DISCUSSION
We used observational data to emulate a target trial of early intervention with convalescent plasma in US veterans recently hospitalized with nonsevere COVID-19. Our findings do not show evidence of an effect of convalescent plasma treatment on 30-day mortality.
Our findings are compatible with those of the RECOVERY trial, the largest trial randomized trial of COVID-19 convalescent plasma whose results were reported after our study was completed [15]. RECOVERY found a 28-day mortality risk of 24% in both groups (rate ratio, 1.00; 95% CI, .93–1.07). Other randomized trials were small and reported imprecise and inconclusive effect estimates for mortality [5, 16–24]. For example, a meta-analysis of 4 earlier randomized trials [5, 18, 19, 21] estimated an odds ratio of 0.79 (95% CI, .52–1.19; I2 28%) for convalescent plasma versus no convalescent plasma [7]. However, only 3 trials focused on patients with less-severe illness [18, 19, 22]: 1 trial with 81 patients in Spain was halted early due to recruitment obstacles (interim results were inconclusive [19]) and the 2 other trials which were too small to estimate effects on mortality, as we now describe. A randomized double-blind trial in Argentina assigned 160 elderly patients with mild COVID-19 within 72 hours of symptom onset to either high-titer convalescent plasma or placebo [22] and found a lower risk of progression to severe illness (Relative Risk [RR], 0.52; 95% CI, .29–.94) and mortality (RR, 0.50; 95% CI, .09–2.65) in the convalescent plasma group. The PLACID trial, an open-label randomized trial with 464 moderately ill hospitalized patients with COVID-19 in India, reported a RR of 1.04 (95% CI, .66–1.63) for 28-day mortality in patients treated with plasma containing detectable antibody levels (median, 1:40; interquartile range, 1:30 to 1:80) relative to controls [18].
In contrast to the randomized controlled trial data, most observational studies reported a lower mortality risk in patients treated with COVID-19 convalescent plasma [7, 25], particularly when transfused early in the disease course [26, 27]. An observational study of 3082 patients from a US national registry of hospitalized adults with COVID-19 found a reduced risk of 30-day mortality with transfusion of high-titer plasma relative to transfusion of plasma with medium-titer (RR, 0.90; 95% CI, .78–1.05) and low-titer (RR, 0.82; 95% CI, .67–1.00) plasma, especially when analyses were restricted to patients who received convalescent plasma within 3 days of diagnosis [26]. An interim analysis from another recent study of 316 patients enrolled in the Houston Methodist Hospitals observed higher 28-day mortality in nonplasma patients compared with patients transfused with high-titer plasma within 72 hours of admission (RR, 5.92; 95% CI, .90–38.84) but not compared with those transfused after that window (RR, 0.89; 95% CI, .24–3.30) [27]. A follow-up analysis identified the first 44 hours of hospitalization to be the optimal window for administering high-titer COVID-19 convalescent plasma [28]. However, it is noteworthy that the Houston Methodist Hospitals were more likely to treat healthier patients with convalescent plasma, whereas a different pattern of confounding by indication was observed in the VA health care system where less-healthy patients were more likely to be treated with plasma.
Results from many observational studies may not be directly comparable to ours due to differences in causal questions and potential biases. Bias may be introduced by initiating follow-up at different times for different treatment groups. As demonstrated by our sensitivity analyses, bias from residual confounding by indication may result from adjustment for baseline confounders when time-varying confounding by indication is expected. Additionally, results are difficult to interpret if the study population is restricted based on propensity scores rather than a priori defined eligibility criteria. Differences in results across studies may also be explained by effect measure modification. Finally, differing results could be explained by slight dissimilarities in causal questions due to variations in study population, antibody concentration levels, and geographic pandemic timing. We explicitly specified and emulated a target trial to account for and reduce the bias in true causal estimates through use of strict eligibility criteria and clear specification of the causal question. As such, the results of our study are aligned with findings from randomized controlled trials.
Our study has several limitations. First, like any observational analysis, the emulation of randomization using baseline covariates would result in biased estimates if important confounding factors were not included. We attempted to adjust for known and available indications for convalescent plasma therapy, which varied over time and across facilities. Early in the pandemic, the primary means for receiving convalescent plasma was under a compassionate use protocol, with many patients receiving it as salvage therapy. We incorporated knowledge of this by adjusting for calendar time. In addition, our sensitivity analyses show that lack of adjustment for the full set of clinical factors and poorly specified eligibility criteria in analyses that may be prone to time-varying confounding yield biased effect estimates.
Second, no data were available on the characteristics of the transfused plasma units, such as the concentration of neutralizing antibodies or the donors’ duration or severity of disease. Research shows that the concentration of anti-SARS-CoV-2 antibodies varies widely in recovered patients, with a large proportion showing low neutralization activity [29]. However, the most promising therapeutic use of convalescent plasma is among patients transfused with plasma containing high levels of antibody early in the COVID-19 disease course [8]. After initially authorizing the use of convalescent plasma from all donors, in February 2021, the US FDA limited its authorization to high-titer plasma in hospitalized patients early in the disease course [30]. Because our study took place prior to this, patients in our sample likely received convalescent plasma units with varying degrees of immune activity, which would dilute any beneficial effects of the therapy and bias results toward the null.
In addition, with the lack of data on timing of symptom onset, we calculated the number of days from both SARS-Cov-2 positive test and hospital admission to convalescent plasma treatment to approximate the timing of transfusion in the disease course. However, this approach could not account for a given patient’s disease progression prior to hospitalization or the COVID-19 incubation period of approximately 5 days on average [31].
Third, the analyses were conducted on veterans using VHA health care services, thus our results may not be generalizable to the entire US population. Our study included nearly exclusively males. Relative to the US population, this overrepresentation of males may obscure understanding of key sex influences on outcomes associated with convalescent plasma treatment in the context of COVID-19. Recent evidence suggests that the mortality benefit observed with convalescent plasma treatment may be blunted for males [28] or not apparent in males [32].
Conclusions
Our analyses do not provide support for the use of convalescent plasma therapy in the management of COVID-19. Further research is warranted to definitively determine the effect of early use of high-titer COVID-19 convalescent plasma therapy in limiting morbidity and preventing mortality.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgements. We would like to thank the Veterans Affairs (VA) health care providers, employees, and volunteers for their dedication to caring for our Veterans through these unprecedented challenges. A special thanks goes to those VA providers who delivered convalescent plasma therapy and collected data under the Mayo Clinic EAP. A list of these investigators is provided in the supplementary text in alphabetical order (Supplementary Material). We would also like to acknowledge the contributions of the VA Informatics and Computing Infrastructure team behind the creation of the VA COVID-19 Shared Data Resource.
Author contributions. The study was conceptualized by KC, SCK, KEK, ALM, HG, KJ, MK, GDH, JPC, DRG, MAH, NLS, and JMG. Full access to all the data in the study and responsibility for the integrity of the data and the accuracy of the data analysis are affirmed by KK and HMW. Data curation was performed by KC, SCK., KEK, ALM, HG, HMW, AD, ERT, JP, AH, and DRG. Analyses were performed by KC, SCK, KEK, ALM, HG, HMW, AD, ERT, JP, AH, ERG, MAH, NLS, and JMG. Funding was acquired by KJ, MK, RR, GDH, NLS, and JMG. Investigation was done by KC, SCK, KEK, ALM, HG, JDS, KPM, ACP, KG, KJ, MK, RR, GDH, JPC, MAH, NLS, and JMG. Study methodology implementation was done by KC, SCK, KEK, ALM, HG, AD, ERT, JP, AH, JDS, KPM, ACP, KG, KJ, MK, RR, GDH, JPC, DRG, MAH, NLS, and JMG. Project administration was done by KC, SCK, CH, KJ, MK, RR, GDH, JPC, NLS, and JMG. Resources were provided by KC, SCK, CH, KJ, MK, RR, GDH, JPC, NLS, and JMG. Supervision was done by KC, SCK, MAH, NLS, and JMG. Validation was conducted by KC, SCK, KEK, ALM, HG, HMW, AD, ERT, JP, AH, JDS, KPM, ACP, KG, and MAH. Visualization was prepared by KC, KEK, ALM, HG, DRG, and MAH. Initial manuscript was drafted by KC, SCK, and KEK. All authors participated in manuscript writing and critical revision; all authors approve of the manuscript submission in its current form. Cofirst authors are listed alphabetically by last name. All authors participated in manuscript writing and critical revision; all authors approve of the manuscript submission in its current form. Cofirst authors are listed alphabetically by last name.
Disclaimer. The contents do not represent the views of the US Department of Veterans Affairs or the US Government. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Health and Human Services and its agencies, including Biomedical Advanced Research and Development Authority and the Food and Drug Administration, as well as any other agency of the US Government. Assumptions made within and interpretations from the analysis do not necessarily reflect the position of any US Government entity.
Financial support. This work was supported by the Department of Veterans Affairs Office of Research and Development Cooperative Studies Program (CSP). The study was carried out by the CSP No. 2030 VA Convalescent Plasma Study Team at 2 CSP Epidemiology Centers in Boston, MA and Seattle, WA, with support from CSP No. 2032 VA-CAUSAL Methods Core. In addition, this work was supported using resources and facilities of the VA Boston Healthcare System, VA Puget Sound Healthcare System, and VA Informatics and Computing Infrastructure (grant number VA HSR RES 13–457), all within the Department of Veterans Affairs.
Potential conflicts of interests. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Woolf SH, Chapman DA, Lee JH. COVID-19 as the leading cause of death in the United States. JAMA 2021; 325:123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun 2020; 114:102506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rojas M, Rodríguez Y, Monsalve DM, et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev 2020; 19:102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020; 323:2249–51. [DOI] [PubMed] [Google Scholar]
- 5.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 2020; 324:460–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest 2020; 130:5235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Huo P, Dai R, et al. Convalescent plasma may be a possible treatment for COVID-19: a systematic review. Int Immunopharmacol 2021; 91:107262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojas M, Anaya JM. Why will it never be known if convalescent plasma is effective for COVID-19. J Transl Autoimmun 2020; 3:100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology 2008; 19:766–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol 2016; 183:758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngwa JS, Cabral HJ, Cheng DM, et al. A comparison of time dependent Cox regression, pooled logistic regression and cross sectional pooling with simulations and an application to the Framingham heart study. BMC Med Res Methodol 2016; 16:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration. FDA issues emergency use authorization for convalescent plasma as potential promising COVID-19 treatment, another achievement in administration’s fight against pandemic. Available at: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment. Accessed 26 October 2020.
- 14.Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol 2016; 79:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet 2021; 397:2049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AlQahtani M, Abdulrahman A, Almadani A, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci Rep 2021; 11:9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett-Guerrero E, Romeiser JL, Talbot LR, et al. Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in New York: a double-blind randomized trial. Crit Care Med 2021; 49:1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P; PLACID Trial Collaborators . Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020; 371:m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avendano-Sola C, Ramos-Martinez A, Munez-Rubio E, et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. medRxiv, doi: 10.1101/2020.08.26.20182444, 29 September 2020, preprint: not peer reviewed. [DOI] [Google Scholar]
- 20.Balcells ME, Rojas L, Le Corre N, et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med 2021; 18:e1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gharbharan A, Jordans CCE, Geurtsvankessel C, et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Commun 2021; 12:3189.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libster R, Pérez Marc G, Wappner D, et al. ; Fundación INFANT–COVID-19 Group . Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021; 384:610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasheed AM, Fatak DF, Hashim HA, et al. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med 2020; 28:357–66. [PubMed] [Google Scholar]
- 24.Simonovich VA, Burgos Pratx LD, Scibona P, et al. ; PlasmAr Study Group . A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 2021; 384:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klassen SA, Senefeld JW, Johnson PW, et al. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin Proc 2021; 96:1262–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med 2021; 384:1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salazar E, Christensen PA, Graviss EA, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol 2020; 190:2290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salazar E, Christensen PA, Graviss EA, et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol 2021; 191:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luchsinger LL, Ransegnola BP, Jin DK, et al. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID-19 patients. J Clin Microbiol 2020; 58:e02005–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration. FDA in brief: FDA updates emergency use authorization for COVID-19 convalescent plasma to reflect new data.https://www.fda.gov/news-events/fda-brief/fda-brief-fda-updates-emergency-use-authorization-covid-19-convalescent-plasma-reflect-new-data. Accessed 5 February 2021.
- 31.Wang Y, Cao Z, Zeng DD, Zhang Q, Luo T. The collective wisdom in the COVID-19 research: comparison and synthesis of epidemiological parameter estimates in preprints and peer-reviewed articles. Int J Infect Dis 2021; 104:1–6. [DOI] [PubMed] [Google Scholar]
- 32.Budhiraja S, Dewan A, Aggarwal R, et al. Effectiveness of convalescent plasma in Indian patients with COVID-19. Blood Cells Mol Dis 2021; 88:102548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.