Abstract
The nontransmembrane protein tyrosine phosphatase SHP-2 plays a critical role in growth factor and cytokine signaling pathways. Previous studies revealed that a fraction of SHP-2 moves to focal contacts upon integrin engagement and that SHP-2 binds to SHP substrate 1 (SHPS-1)/SIRP-1α, a transmembrane glycoprotein with adhesion molecule characteristics (Y. Fujioka et al., Mol. Cell. Biol. 16:6887–6899, 1996; M. Tsuda et al., J. Biol. Chem. 273:13223–13229). Therefore, we asked whether SHP2–SHPS-1 complexes participate in integrin signaling. SHPS-1 tyrosyl phosphorylation increased upon plating of murine fibroblasts onto specific extracellular matrices. Both in vitro and in vivo studies indicate that SHPS-1 tyrosyl phosphorylation is catalyzed by Src family protein tyrosine kinases (PTKs). Overexpression of SHPS-1 in 293 cells potentiated integrin-induced mitogen-activated protein kinase (MAPK) activation, and potentiation required functional SHP-2. To further explore the role of SHP-2 in integrin signaling, we analyzed the responses of SHP-2 exon 3−/− and wild-type cell lines to being plated on fibronectin. Integrin-induced activation of Src family PTKs, tyrosyl phosphorylation of several focal adhesion proteins, MAPK activation, and the ability to spread on fibronectin were defective in SHP-2 mutant fibroblasts but were restored upon SHP-2 expression. Our data suggest a positive-feedback model in which, upon integrin engagement, basal levels of c-Src activity catalyze the tyrosyl phosphorylation of SHPS-1, thereby recruiting SHP-2 to the plasma membrane, where, perhaps by further activating Src PTKs, SHP-2 transduces positive signals for downstream events such as MAPK activation and cell shape changes.
Complex processes such as cell growth, differentiation, and migration require the integration of multiple types of extracellular signals, including those delivered by growth factors, cytokines, and hormones (soluble signals), and solid-state signals, transmitted by cell-cell and cell-extracellular matrix (ECM) interactions. Most of these signaling pathways involve changes in cellular tyrosyl phosphorylation. Tyrosyl phosphorylation is regulated by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs). Although many PTKs are implicated in signaling pathways for both soluble and solid-state signals, the roles of specific PTPs are less well defined.
Multiple reverse-genetic studies suggest that the nontransmembrane PTP SHP-2 is a required positive (i.e., signal-enhancing) component of growth factor and cytokine signal transduction pathways (for reviews, see references 35, 56, and 63). Consistent with these studies, fibroblasts from mice containing a deletion of SHP-2 exon 3 (hereafter referred to as SHP-2 mutant mice), which express low levels of a defective SHP-2 protein that lacks its N-terminal SH2 domain (48), exhibit impaired mitogen-activated protein kinase (MAPK) activation in response to fibroblast growth factor (FGF), epidermal growth factor, and insulin-like growth factor I (48, 55). SHP-2 functions similarly in lower organisms. Dominant-negative SHP-2 blocks FGF-induced mesoderm induction in Xenopus ectodermal explants and completion of gastrulation in early embryos, leading to severe tail truncations (38, 57). Likewise, corkscrew (csw), the Drosophila SHP-2 homolog (39, 40), is required for multiple receptor tyrosine kinase (RTK) pathways involved in early development (e.g., Torso, Sevenless, Breathless, and Drosophila EGF receptor) (1, 39, 40), and the recently described Caenorhabditis elegans homolog, Ptp-2, is a component of the Let-23 pathway (13).
The precise mechanism by which SHP-2 orthologs function, as well as their specific target(s), has remained unclear. SHP-2 binds directly to and may dephosphorylate some growth factor and cytokine receptors (for reviews, see references 35 and 63). In other pathways, however, SHP-2 binds to distinct signaling intermediates. One class of SHP-2 binding proteins, exemplified by the Drosophila daughter of sevenless (dos) gene product, consists of an N-terminal pleckstrin homology domain and multiple proline-rich stretches and potential tyrosyl phosphorylation sites (42). Dos is essential for Sevenless signaling (15, 42) and may be a direct substrate for Csw (15). Mammalian cells express several groups of molecules with overall topology similar to that of Dos (for a review, see reference 63). including insulin receptor substrate family members (for a review, see reference 64), Gab-1 (16), P97/Gab-2 (11, 12), and FGF receptor substrate 2/SNT (26).
SHP substrate 1 (SHPS-1/SIRP-α1; hereafter referred to as SHPS-1) is the prototype of a second class of SHP-2 binding proteins which includes the more distantly related PECAM (46, 47) and PIR-B/p91A (60). Initially observed by several groups to be an SHP-2 binding protein whose tyrosyl phosphorylation increases in cells expressing catalytically impaired SHP-2 mutants (for reviews, see references 35 and 63). SHPS-1 contains three immunoglobulin domains and four cytoplasmic tyrosyl residues (8). Several highly related cDNAs have been identified, suggesting that a family of SHPS-1-like molecules (termed SIRPs) may exist (23, 36). The precise function(s) of SHPS-1 like glycoproteins and their relatives remains unclear. Growth factor stimulation of quiescent cells leads to enhanced SHPS-1 tyrosyl phosphorylation and increased SHP-2–SHPS-1 association, suggesting a role for SHPS-1–SHPS-2 complexes in RTK signaling (8, 23). Overexpression of SHPS-1 was found to impair EGF-induced mitogenesis and v-Fms-mediated transformation, which led to the conclusion that SHPS-1-like proteins were negative signaling molecules (23). Conceivably, however, SHP-2 must bind both Dos-like and SHPS-1-like molecules to exert positive signaling functions. Thus, overexpressed SHPS-1 might inhibit signaling gratuitously by sequestering SHP-2 from other signaling complexes. The structure of SHPS-1-like molecules, which is reminiscent of cell adhesion molecules, suggests that they may be involved in other signaling pathways, for example, pathways transducing cell-cell or cell-ECM interactions. Consistent with this notion, SHPS-1 tyrosyl phosphorylation increases upon plating of fibroblasts onto some ECMs (8), and PECAM tyrosyl phosphorylation increases upon plating of rat basophilic leukemia cells onto fibronectin (FN) (47).
The major cellular receptors for most ECM ligands are members of the integrin family (18). Integrin engagement activates several downstream signaling pathways (5, 54), including multiple PTKs, the best studied of which are members of the Src (hereafter termed Src PTKs) and focal adhesion kinase (FAK) families (including FAK and Pyk2/RAFTK). Recent work suggests that Abl also is activated during integrin signaling (28), and integrin signaling in hematopoietic cells involves activation of Syk (9). Although the specific roles of each of these PTKs remain unclear, tyrosyl phosphorylation is absolutely required for all integrin signaling functions (for reviews, see reference 58).
Coordinating Src PTK and FAK function is critical for integrin signaling. Activation of Src and FAK PTKs leads to the tyrosyl phosphorylation of several other proteins, including paxillin, tensin, and p130Cas (58). These initial tyrosyl phosphorylation events result in activation of downstream signaling pathways, including phosphatidylinositol 3-kinase, protein kinase C, and MAPK, which ultimately direct adhesion and cell shape changes, cell motility, changes in gene expression, and the prevention of apoptosis (24, 25, 29) (for reviews, see references 54 and 58).
The order of activation of and the relationship between Src and FAK PTKs remain controversial. Some experiments suggest that FAK activation precedes Src PTK activation, whereas others indicate that Src PTKs are upstream of FAK (21, 49, 50, 59). It also is unclear how initial Src or FAK activation is accomplished. Binding of Src PTKs (via their SH2 domains) to tyrosyl-phosphorylated FAK could lead to Src PTK activation, since Src family PTKs are negatively regulated by interactions of their C-terminal phosphotyrosyl residues and their SH2 domains (for a review, see reference 58). In this model, however, it remains unclear how FAK activation occurs, as well as how and why Src PTKs are activated upon integrin engagement in FAK-deficient cells (19). Alternatively, it is unclear how integrin engagement alone could cause Src PTK activation. Some evidence suggests that a fraction of c-Src is rapidly dephosphorylated at its inhibitory C-terminal phosphotyrosyl residue upon integrin binding (22), but the identity of the responsible phosphatase(s) is unknown. Src PTK binding to FAK may also play a role in integrin-induced activation of Src PTKs. Autophosphorylation of FAK (at Tyr397) creates a binding site for the SH2 domains of Src PTKs (50), whereas Src PTKs phosphorylate other residues on FAK, including Tyr925, which directs binding of FAK to the SH2 domain of Grb2 (52). Thus, full phosphorylation and activation of Src and FAK may involve a FAK-Src positive-feedback loop.
The role(s) of specific PTPs in integrin signaling are even less well defined. Several, including PTP-PEST (10), PTP-1B (31), and CD45 (44), have been suggested to negatively regulate integrin signaling. The positive role of SHP-2 in growth factor and cytokine signaling pathways, the finding that a fraction of SHP-2 translocates to focal contacts upon integrin engagement, the structure of SHPS-1, and the increased tyrosyl phosphorylation of SHPS-1 upon integrin activation suggested to us that SHPS-1–SHP-2 complexes might serve a positive role in integrin signal transduction. Here we show that SHPS-1 is a target for Src PTKs upon integrin engagement. Moreover, studies of SHP-2 mutant fibroblasts have established an essential role for SHP-2 in integrin signaling, leading to cell spreading, and position SHP-2 action at the earliest times following integrin engagement. We suggest a model in which SHP-2–SHPS-1 complexes promote a positive-feedback loop leading to Src and FAK activation during integrin signaling.
MATERIALS AND METHODS
Cells and cell culture.
Fibroblasts prepared from SHP-2 exon 3−/− (Ex3−/−) mutant mice (B1) and wild-type (J3) littermates (48) were immortalized by following a 3T3 protocol and maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15% fetal bovine serum (FBS), penicillin, and streptomycin. The human embryonic kidney epithelial cell line 293 was obtained from the American Type Tissue Collection. Cell lines derived from mice bearing targeted mutations in Src and/or Fyn, Csk alone or in combination with Src and/or Fyn mutations (59), and Csk mutant cells reconstituted with wild-type or kinase-inactive Csk (17) have been described previously. These cells and 293 cells were maintained in DMEM plus 10% FBS and antibiotics as described above.
Prior to stimulations, cells were starved in DMEM containing 0.2% FBS for 24 h. In some experiments, cells were preincubated with different concentrations of the Src family PTK inhibitor PP1 (Calbiochem) as indicated (see Fig. 2) or with 3 μM cytochalasin D (Sigma) for 20 min (see Fig. 4). The same amount of fresh PP1 or cytochalasin D was added during plating of the cells on FN with serum-free medium (SFM).
FIG. 2.
Basal and integrin-mediated SHPS-1 tyrosyl phosphorylation is regulated by Src PTKs. (A) SHPS-1 tyrosyl phosphorylation is blocked by PP1 treatment. J3 fibroblasts were preincubated for 20 min with different amounts of PP1 as indicated, detached, and replated on FN-coated tissue culture plates containing the same amount of fresh PP1 for 30 min. SHPS-1 and tyrosyl-phosphorylated SHPS1 were analyzed by immunoprecipitation (IP) with anti-SHPS-1 (αSHPS1) followed by immunoblotting as described in the legend to Fig. 1. sus, suspension. (B) Fibroblast cell lines from the indicated mutant mice were lysed, and tyrosyl phosphorylation of SHPS1 was analyzed. KD, kinase negative. (C) SHPS-1 tyrosyl phosphorylation was assessed following plating of the wild-type and the indicated mutant cell lines on FN for the indicated periods of time. WT, wild type.
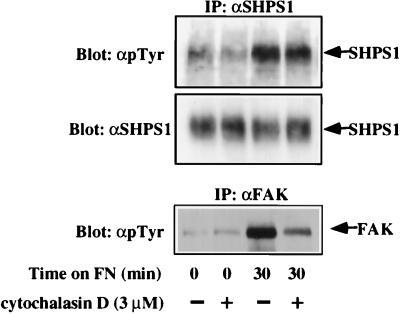
FIG. 4.
FAK activity is not required for SHPS-1 tyrosyl phosphorylation. J3 fibroblasts were left untreated (−) or were treated with cytochalasin D (+) as described in Materials and Methods, detached, and plated on FN in the presence or absence of cytochalasin D. Cell lysates were subjected to immunoprecipitation (IP) with anti-SHPS-1 (αSHPS1) or anti-FAK (αFAK) as indicated and then immunoblotted with anti-pTyr (αpTyr).
Plasmids and plasmid constructions.
Clone pJ3/SHPS-1 consists of full-length wild-type rat SHPS-1 cDNA, generated by reverse transcription-PCR (60), inserted into the expression vector pJ3 (33), which directs transcription under the control of the simian virus 40 promoter. Wild-type SHP-2 (6) and the SHP-2 mutants C459S (3) and ΔP (57) have been described previously. These were excised from pBluescript vectors (Stratagene) as EcoRI fragments, filled in by using the Klenow fragment of DNA polymerase, and ligated into BamHI-digested and blunted pEBB (a generous gift of Bruce Mayer, Children’s Hospital, Boston, Mass.), which directs expression under the control of the elongation factor 1-α promoter.
Plating on ECMs.
Tissue culture plates were coated with different ECMs in accordance with the manufacturer’s instructions (Collaborative Biomedical Products). Briefly, FN, vitronectin (VN), and laminin (LN) were diluted in SFM, added to tissue culture plates (final coating concentration, 5 μg/cm2), and incubated at room temperature for at least 1 h. Following washing with deionized H2O, plates were blocked with 0.1% heat-inactivated bovine serum albumin for 1 h and then washed with SFM. Collagen was diluted in 0.05 N HCl instead of SFM, with coating accomplished as described above. Poly(l-lysine) (10 μg/ml)-coated plates were allowed to dry overnight in a fume hood, then washed one time with water and once with SFM before using.
For plating experiments, cells (∼80 to 90% confluent) were detached with 0.05% trypsin–0.35 mM EDTA and suspended in SFM containing 0.25 mg of soybean trypsin inhibitor/ml. The cells had to be at an appropriate density to allow reproducible activation of Src PTKs; cells that were too dense showed a high basal level of Src PTK activity. After being harvested by centrifugation, the cells were resuspended in SFM, maintained at 37°C for 30 to 40 min, plated onto ECM-coated plates, and incubated for various periods of time at 37°C.
Transient transfections.
Transient transfections of 293 cells were performed essentially as described previously (3). Briefly, cells (2 × 106) were plated on 6-cm-diameter dishes, incubated at 37°C for 24 h, and then transfected with hemagglutinin-tagged MAPK, alone or together with SHP-2 (4 μg for the wild type or the C→S mutant; 8 μg for the ΔP mutant) or SHP-2 and 2 μg of pJ3/SHPS1, in the presence of 25 μM chloroquine. After 10 h at 37°C, the medium was replaced with 4 ml of fresh DMEM containing 10% FBS, and after 12 to 14 h, the cells were starved in DMEM containing 0.2% FBS for an additional 24 h prior to stimulation.
Generation of and infections with recombinant SHP-2-containing adenoviruses.
Wild-type SHP-2 cDNA was cloned into the plasmid pACCMV.pLpA (2) (provided by C. Newgard, University of Texas Southwestern Medical Center, Dallas), generating the plasmid pACCMV.pLpA-SHP-2. Recombinant adenoviruses were generated as previously described (2, 7). Briefly, pACCMV.pLpA-SHP-2 was cotransfected with the plasmid pJM-17 into 80% confluent 293 cells by using a Transfection MBS kit (Stratagene, La Jolla, Calif.) with 0.7 μg of pACCMV.pLpA-SHP-2 and 1 μg of pJM-17 (2) per 35-mm-diameter dish. Cell lysis, indicating a recombination event, occurred 1 to 2 weeks following cotransfection. Several clones of recombinant virus were tested for the successful integration of the SHP-2 coding region by Western blotting with lysates of transduced 293 cells. One clone was amplified further in 293 cells and purified by cesium chloride centrifugation, generating high-titer stocks of a recombinant virus (1 × 1012 to 2 × 1012 PFU/ml, as determined by limiting dilution). A recombinant adenovirus encoding β-galactosidase was provided by C. Newgard and amplified and purified as described above.
Infections of J3 and B1 cells with 50 μl of either SHP-2- or β-galactosidase-encoding recombinant adenoviruses were performed overnight in DMEM plus 15% FBS with constant agitation on a rocking platform. The media were then exchanged for 10-ml volumes of fresh DMEM plus 15% FBS, and following an additional 12-h incubation, the cells were serum starved by incubating them with DMEM containing 0.2% FBS overnight and then used for experiments.
Antibodies.
Rabbit polyclonal antibodies against a glutathione S-transferase (GST)–SHPS-1 cytoplasmic domain fusion protein (GST–cytoSHPS-1) (60) and against GST–SHP-2 (27) have been described previously. Antipeptide antibodies against the SHP-2 C terminus were purchased from Santa Cruz Biotechnology, Inc. Polyclonal anti-phospho-specific p44/42 MAPK antibodies were purchased from New England Biolabs. Monoclonal antiphosphotyrosine (anti-pTyr) antibodies (4G10) were obtained from Upstate Biotechnology, Inc. Monoclonal antipaxillin antibodies were from Transduction Laboratories. Polyclonal anti-MAPK antibodies (C1; anti-Erk1/Erk2) were gifts of John Blenis (Harvard Medical School, Boston, Mass.). Monoclonal (2A7) and polyclonal (BC3) anti-FAK antibodies were provided by J. Tom Parsons (University of Virginia Medical School), and monoclonal anti-Src antibodies (327) were from Joan Brugge (Harvard Medical School). Polyclonal anti-p130Cas antibodies were provided by Amy Bouton (University of Virginia Medical School).
Immunoprecipitation and Immunoblotting.
After the plate contents were washed twice with phosphate-buffered saline, the cells were lysed (500 μl/10-cm-diameter plate) in NP-40 buffer (50 mM Tris, pH 8.0; 150 mM NaCl; 1% Nonidet P-40 [NP-40]; 10 mM NaF; 2 mM Na3VO4) containing a protease inhibitor cocktail (1 μg of aprotinin/ml, 1 μg of antipain/ml, 10 μg of leupeptin/ml, 1 μg of pepstatin A/ml, and 20 μg of phenylmethylsulfonyl fluoride/ml). Cell lysates were clarified by centrifugation at 10,000 × g for 15 min at 4°C. For immunoprecipitations, each sample (containing 200 to 1,000 μg of total protein) was incubated with the relevant antibody for 2 h at 4°C; this was followed by the addition of protein A-Sepharose beads (Sigma) and a 1-h incubation. The antibodies were used as follows: antipaxillin, anti-FAK, and anti-Cas, 2 μg; anti-Src, 1 μg; anti-SHPS-1 serum, 15 μl; and anti-GST–SHP-2 antibodies, 30 μg. Immune complexes were collected by centrifugation, washed three times with NP-40 buffer, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, boiled, and analyzed by SDS-PAGE (8% gel). Proteins were electroblotted onto Immobilon P membranes (Millipore) and probed with appropriate primary and peroxidase-conjugated secondary antibodies (Amersham). Signals were detected by enhanced chemiluminescence (ECL; Amersham). The concentrations of primary antibodies used in immunoblotting were as follows: 1:500 for anti-SHPS-1 serum; 1:1,000 for antipaxillin, anti-FAK, anti-p130Cas, anti-Src, anti-SHP-2 C-terminal peptide, and anti-phospho-MAPK; 1:5,000 for anti-MAPK; and 1:7,500 for anti-pTyr. In some experiments, total-cell lysates (30 μg) were used for anti-phospho-MAPK. Immune complex kinase assays for monitoring Src activity were performed as described previously (9).
In vitro phosphorylation and tryptic phosphopeptide analysis.
GST-cytoSHPS1 was prepared and purified on glutathione-agarose beads as described previously (60). GST-FAK C-terminal fusion protein (GST-FAK-CT) was kindly provided by David Schlaepfer (Scripps Institute). Purified fusion proteins (3 μg) were phosphorylated by incubating them with 1.5 μg of purified recombinant c-Src (a generous gift of Michael J. Eck, Dana Farber Cancer Institute) in kinase buffer {30 mM HEPES [pH 7.4], 5 mM MgCl2, 1 mM dithiothreitol, 20 μM ATP (5 μCi of [32P-γ]ATP)} at 37°C for various periods of time. After being washed twice with kinase buffer, GSTicytoSHPS-1 and bound proteins were recovered by brief centrifugation, boiled in SDS-PAGE sample buffer, and analyzed by SDS-PAGE (10% gel).
Radiolabeled bands were excised from the gel, swollen in 50 mM NH4CO3, and homogenized in a disposable 1-ml Eppendorf homogenizer. Two-dimensional tryptic phosphopeptide analysis was carried out essentially as described previously (32), with-thin layer electrophoresis being performed at pH 1.9.
Cell spreading assays.
For cell spreading experiments, cells were plated on FN-coated 60-mm-diameter tissue culture dishes as described above. At various time points, three different fields were photographed. Only cells that were flat and dark were considered to be spread. All bright cells were counted as nonspread cells. Cells were visualized with a Nikon microscope, and images were processed by using a Photometrics digital camera and Phase 3 Imaging software. Quantification was carried out by a blinded observer (i.e., one with no knowledge of the cells used in a given experiment).
RESULTS
SHPS-1 tyrosyl phosphorylation is mediated by Src PTKs.
A previous report revealed that SHPS-1 tyrosyl phosphorylation increased when Chinese hamster ovary (CHO) cells were plated onto FN-coated plates (8). We confirmed that SHPS-1 becomes tyrosyl phosphorylated upon cell-ECM interaction in our mouse embryonic fibroblasts (J3). Plating on FN induced a rapid increase in tyrosyl phosphorylation of SHPS-1 (Fig. 1A). In addition, adhesion to VN, LN, or collagen induced tyrosine phosphorylation of SHPS-1 to various extents (Fig. 1B). The increased tyrosyl phosphorylation of SHPS-1 upon plating on FN was accompanied by an increase in both total and tyrosyl-phosphorylated SHPS-1 associated with SHP-2. SHP-2 itself was also tyrosyl phosphorylated, although weakly, upon plating on FN (data not shown).
FIG. 1.
SHPS-1 tyrosyl phosphorylation on different ECM molecules. (A) J3 fibroblasts were detached and replated on FN-coated tissue culture plates in SFM and were allowed to spread for the indicated periods of time. Anti-SHPS-1 (αSHPS1) immunoprecipitates (IP) were resolved by SDS-PAGE and subjected to either antiphosphotyrosine (αpTyr; top panel) or anti-SHPS-1 (αSHPS1; bottom panel) immunoblotting, as described in Materials and Methods. (B) J3 fibroblasts were detached and replated for the indicated time periods on poly-l-lysine (pLys)-, FN-, VN-, collagen (Col)-, or LN-coated tissue culture plates in SFM. Tyrosyl phosphorylation of SHPS1 was analyzed as described above.
As described above (see the introduction), integrin engagement rapidly induces the activation of Src and FAK family PTKs. The rapid tyrosyl phosphorylation of SHPS-1 following plating on FN suggested that one or more of these PTKs might catalyze SHPS-1 phosphorylation. Src PTKs can be inhibited specifically by the tyrosine kinase inhibitor PP1 (14), so we monitored the effects of PP1 treatment on FN-induced SHPS-1 tyrosyl phosphorylation. In the presence of 250 to 500 nM PP1, FN-induced SHPS-1 tyrosyl phosphorylation was reduced substantially (Fig. 2A, lanes 7 and 8). SHPS-1 tyrosyl phosphorylation in adherent, exponentially growing J3 fibroblasts was inhibited by similar concentrations of PP1 (data not shown). Since the in vitro 50% inhibitory concentrations of PP1 for two major Src PTKs in fibroblasts, Fyn and Src, are 6 and 170 nM, respectively, and significant reduction of SHPS-1 tyrosyl phosphorylation required around 250 nM PP1, our results suggested that either Src specifically or multiple Src PTKs must be inhibited to prevent integrin-induced tyrosyl phosphorylation of SHPS-1. However, these data cannot exclude the possibility that other SHPS-1 kinases exist, since PP1 treatment (at 500 nM) did not abolish tyrosyl phosphorylation of SHPS-1 completely (Fig. 2A, lane 8).
To further probe the role of Src PTKs in SHPS-1 tyrosyl phosphorylation, we examined the latter in fibroblast cell lines generated by Csk−/− mice. Since Csk negatively regulates Src PTKs by catalyzing phosphorylation of their C-terminal tyrosyl residues, Csk−/− fibroblasts exhibit elevated activity of multiple Src PTKs (20, 34, 37). Consistent with a role for Src PTKs in SHPS-1 phosphorylation, SHPS-1 was hyperphosphorylated on tyrosyl residues in Csk−/− fibroblasts (Fig. 2B) and in Csk−/− fibroblasts placed in suspension and then plated onto FN (data not shown). Notably, SHPS-1 tyrosyl phosphorylation was decreased by reconstituting expression of wild-type, but not kinase-negative, Csk in Csk−/− fibroblasts (Fig. 2B), indicating that SHPS-1 hyperphosphorylation in the Csk−/− cells was due to the absence of Csk per se rather than to clonal variation.
Previous studies suggested that some targets of Src family PTKs are phosphorylated preferentially by individual family members (59). The concentration of PP1 required to inhibit SHPS-1 phosphorylation (Fig. 2A) suggested that Src itself or multiple Src PTKs must be inhibited to block SHPS-1 tyrosyl phosphorylation. To determine whether a particular Src PTK is required preferentially for SHPS-1 tyrosyl phosphorylation, we examined SHPS-1 tyrosyl phosphorylation in fibroblasts from mice bearing homozygotic mutations in Csk alone, Csk and Src, Csk and Fyn, or Csk, Src, and Fyn. Loss of Src or Fyn in the Csk−/− background partially suppressed the SHPS-1- hyper-tyrosyl phosphorylation observed in fibroblasts mutant for Csk alone, whereas loss of both Src and Fyn in the Csk−/− background almost completely suppressed SHPS-1 phosphorylation (Fig. 2B). These data suggest that multiple Src PTKs regulate SHPS-1 tyrosyl phosphorylation, at least in fibroblasts. To determine if integrin-induced tyrosyl phosphorylation of SHPS-1 also is regulated by Src PTKs, we analyzed SHPS-1 tyrosyl phosphorylation in wild-type, Src−/−, Fyn−/−, or Src−/− Fyn−/− fibroblasts plated on FN. Integrin-induced tyrosyl phosphorylation of SHPS-1 was partially suppressed in the absence of either of the individual Src PTKs and was more dramatically reduced in the absence of both Src and Fyn (Fig. 2C).
The above experiments strongly indicate that Src family PTKs are required for proper SHPS-1 tyrosyl phosphorylation, but they do not imply that Src PTKs directly phosphorylate SHPS-1. We asked whether recombinant Src could phosphorylate SHPS-1 in vitro and, if so, how this compared to the phosphorylation of other known Src substrates. Recombinant Src efficiently phosphorylated GST–cytoSHPS-1, causing a quantitative mobility shift into at least two more-slowly migrating forms. In addition, a fraction of Src associated with phosphorylated SHPS-1 in vitro (Fig. 3A). Comparison of GST–cytoSHPS-1 with two other known Src substrates, enolase and GST-FAK CT (Fig. 3B), revealed much higher levels of phosphorylation of GST–cytoSHPS-1 (about 25-fold higher than for either of the other two substrates). Tryptic phosphopeptide analysis indicated that Src catalyzes phosphorylation of SHPS-1 on two major and two minor sites (Fig. 3C), consistent with the four available sites present in GST–cytoSHPS-1. Together with the quantitative mobility shift produced by Src-catalyzed phosphorylation, these data argue that at least 2 mol of phosphate is incorporated per mol of GST–cytoSHPS-1. Our results indicate that SHPS-1 is a much better substrate for Src than either enolase or the FAK C-terminal domain and suggest that Src PTKs directly catalyze the tyrosyl phosphorylation of SHPS-1 at multiple sites.
FIG. 3.
In vitro phosphorylation of SHPS-1 by c-Src. (A) Src phosphorylates SHPS-1 in vitro. Purified GST–cytoSHPS-1 bound to glutathione-agarose beads was phosphorylated by purified recombinant Src in the absence (no ATP, lane 3) or presence of [32P]ATP for the indicated time periods at 37°C. After incubation, GST–cytoSHPS-1 and bound proteins were recovered by centrifugation. The lane labeled Src alone contains the same amount of Src used in the other lanes in the absence of any exogenous substrate. Coomassie staining and autoradiographs are shown. Note that the entire mass of the GST–SHPS-1 fusion protein shifts to at least two more-slowly migrating forms, implying stoichiometric phosphorylation, and that about 5 to 10% of input Src becomes bound to SHPS-1 in a phosphorylation-dependent manner. (B) SHPS-1 is a better substrate than enolase or FAK. Comparison of Src-catalyzed incorporation of 32P into GST–cytoSHPS-1, enolase, and GST-FAK-CT (GST-FAK C-term). Saturating amounts of each substrate (3 μg) were phosphorylated with 0.05 μg of purified recombinant Src for 5 min at 30°C in an assay buffer containing 30 mM HEPES (pH 7.4), 100 mM NaCl, 10 mM MgCl2, 10 mM MnCl2, 7.5 μM unlabeled ATP, and 10 μCi of [32P]ATP. The kinetics of incorporation were linear over this time period (data not shown). Reactions were terminated with 5× sample buffer, and the proteins were separated by SDS-PAGE. Coomassie staining and autoradiographs are shown. Arrows indicate GST–cyto-SHPS-1. The numbers shown represent incorporation (in counts per minute per microgram of protein) after the bands were cut out and subjected to Cherenkov counting. No incorporation into GST alone was observed (data not shown). (C) Two-dimensional phosphotryptic analysis of the in vitro phosphorylation reaction. Note the stoichiometric phosphorylation on two sites (inferred from mobility shift data; see above) and the high-level substoichiometric phosphorylation on two others. TLC, thin-layer chromatography; TLE, thin-layer electrophoresis.
Although these in vitro data argue for a direct role for Src PTKs in SHPS-1 phosphorylation, the loss of SHPS-1 tyrosyl phosphorylation in Src PTK-deficient cells could also be due to FAK, since integrin-induced FAK phosphorylation is also strongly reduced in Src−/− Fyn−/− cells (data not shown). Therefore, we treated cells with cytochalasin D, which prevents integrin-induced FAK phosphorylation (30) but does not affect integrin-induced Src activation (32a). Pretreatment of cells with cytochalasin D (3 μM) substantially diminished (>80%) integrin-mediated FAK phosphorylation, but SHPS-1 tyrosyl phosphorylation was largely unaffected (Fig. 4). Although it remains possible that FAK phosphorylates SHPS-1 on some sites, both the in vitro and in vivo data strongly argue that SHPS-1 is a direct substrate of Src PTKs.
SHP-2 regulates integrin-evoked tyrosyl phosphorylation and MAPK activation.
The above-described studies indicated that upon plating on FN, Src PTKs directly phosphorylate SHPS-1 and cause the assembly of an SHPS-1–SHP-2 complex at the plasma membrane (where SHPS-1 resides). These data suggested that SHP-2 might play a functionally important role in integrin signaling. To test this possibility, we analyzed integrin-evoked signaling events in fibroblast cell lines derived from an SHP-2 mutant mouse or its wild-type littermate.
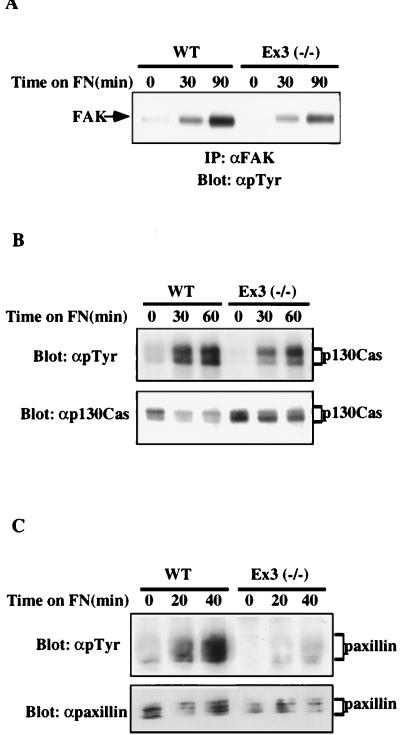
A number of focal adhesion proteins, including FAK, paxillin, and p130Cas, are tyrosyl phosphorylated upon plating on FN (for a review, see reference 58). We assessed FN-induced tyrosyl phosphorylation of these focal adhesion proteins in wild-type and SHP-2 mutant fibroblasts (Fig. 5). Total cellular tyrosyl phosphorylation was reduced in SHP-2 mutant cells plated on FN (data not shown). More specifically, tyrosyl phosphorylation of FAK, paxillin, and p130Cas were diminished in the SHP-2 mutant cells (Fig. 5).
FIG. 5.
SHP-2 mutant fibroblasts show decreased tyrosyl phosphorylation upon plating on FN. Wild-type (WT) or mutant (Ex3−/−) fibroblasts were detached and replated for the indicated periods of time on FN-coated tissue culture plates in SFM. (A) FAK tyrosyl phosphorylation in wild-type and SHP-2 mutant cells was analyzed by immunoprecipitation (IP) with anti-FAK (αFAK) followed by immunoblotting with anti-pTyr (αpTyr). This experiment was repeated five times with similar results. (B) p130Cas tyrosyl phosphorylation was analyzed by immunoprecipitation with anti-Cas (αp130Cas) followed by immunoblotting with anti-pTyr. This experiment was repeated three times with similar results. (C) Paxillin tyrosyl phosphorylation was assessed by immunoprecipitation with antipaxillin followed by immunoblotting with anti-pTyr. Shown is a representative blot from three experiments.
As discussed above, Src PTKs have been implicated in integrin-mediated signaling. To determine whether SHP-2 plays a role in regulation of Src, we measured basal and integrin-activated Src immune complex kinase activity in wild-type or SHP-2 mutant cells (Fig. 6A and B). The specific activity of Src was diminished in both adherent, exponentially growing SHP-2 mutant cells and SHP-2 mutant cells plated on fibronectin.
FIG. 6.
SHP-2 mutant fibroblasts have decreased basal and integrin-induced Src activity. Src immune complex kinase assays were performed, with enolase as an exogeneous substrate, on adherent, exponentially growing (A) or detached and FN-plated (B) wild-type (WT) or Ex3−/− fibroblasts. The levels of Src present in each immune complex were determined by immunoblotting with anti-Src monoclonal antibody 327 (α cSrc). (C and D) SHP-2 mutant cells were infected with a recombinant adenovirus directing the expression of β-galactosidase (β-gal) or wild-type SHP-2 (SHP-2), as described in Materials and Methods, or left uninfected [Ex 3(−/−)]. Adherent, exponentially growing (C) or detached and FN-plated (D) cells were assayed for Src kinase activity, using enolase as an exogeneous substrate. Total cell lysates were prepared and immunoblotted for determination of SHP-2 levels. Control immunoblots indicate that under these conditions, SHP-2 expression is comparable to that in wild-type cells (data not shown).
The above-described differences in multiple integrin signaling events could simply reflect clonal variation between the wild-type and SHP-2 mutant cell lines rather than a specific effect of loss of SHP-2 function. To address this issue, we reconstituted SHP-2 expression in the mutant cell line by infecting it with a recombinant adenovirus that directs SHP-2 expression. Reexpression of SHP-2 to levels comparable to those found endogenously in wild-type cells (data not shown) rescued Src kinase activation and the defects in tyrosyl phosphorylation (Fig. 6C and D and data not shown). These results strongly suggest that the defects in integrin signaling observed in SHP-2 mutant cells specifically reflect the absence of wild-type SHP-2 function in these cells.
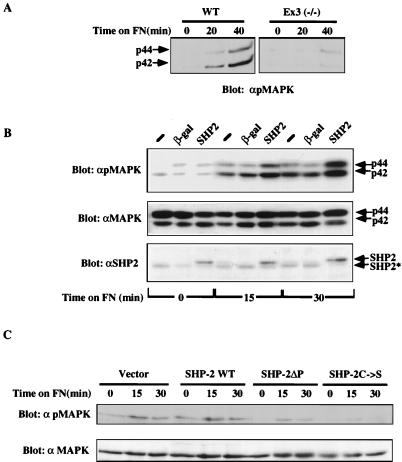
One downstream consequence of integrin engagement is MAPK activation. MAPK (Erk-1 and Erk-2) activation, as monitored by immunoblotting with phospho-specific antibodies, was dramatically impaired in SHP-2 mutant fibroblasts (Fig. 7A). Again, reconstituting SHP-2 expression by infection with the SHP-2 adenovirus increased FN-induced MAPK activation (Fig. 7B). Further evidence that integrin-induced MAPK activation requires SHP-2 function was provided by transient-transfection assays. Two different catalytically inactive forms of SHP-2 (C459S and ΔP; see Materials and Methods) were cotransfected with epitope-tagged MAPK into 293 cells. Following transfection, the cells were detached and replated on FN, and MAPK activation was assessed with phosphospecific antibodies. Both catalytically inactive SHP-2 mutants impaired MAPK activation in this assay, consistent with a requirement for SHP-2 in integrin-stimulated MAPK activation (Fig. 7C).
FIG. 7.
Integrin-induced MAPK activation requires SHP-2. (A and B) SHP2 mutant cell experiments. (A) SHP-2 mutant (Ex3−/−) and wild-type (WT) cells were detached and replated on FN. MAPK activation was assessed by using phospho-specific antibodies (αpMAPK). (B) MAPK activation is enhanced by reconstituting SHP-2 expression. SHP-2 mutant fibroblasts were infected with recombinant β-galactosidase (β-gal) or SHP-2 adenovirus, and integrin-mediated MAPK activation was analyzed as described above. Successful reconstitution of SHP-2 expression is shown by anti-SHP-2 (αSHP2) immunoblotting (bottom panel). Control experiments indicated that the level of SHP-2 expression in the adenovirus-infected cells was comparable to that in wild-type cells (data not shown). (C) Transient transfection assays. A Myc epitope-tagged MAPK expression construct was cotransfected into 293 cells with a vector directing expression of wild-type SHP-2, one of two different catalytically impaired SHP-2 mutants (C/S [SHP-2C→S] and ΔP [SHP-2ΔP), or the expression vector alone. Transfected cells were detached and replated on FN-coated plates, and activated and total MAPK were detected by immunoblotting as indicated.
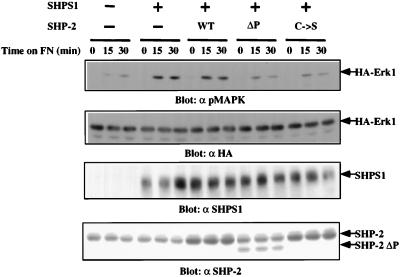
Since engagement of integrins induces tyrosine phosphorylation of SHPS-1 and its association with SHP-2, the role of this complex in integrin-induced MAPK activation was analyzed. 293 cells were cotransfected with epitope-tagged SHPS-1 and MAPK or SHPS-1, MAPK, and SHP2 mutants. Compared to controls, cells expressing elevated levels of SHPS-1 exhibited increased MAPK activation upon plating on FN (Fig. 8). Potentiation of integrin-induced MAPK activity by SHPS-1 was mediated by SHP-2, since cotransfection of C495S or ΔP blocked SHPS-1-mediated enhancement of MAPK activation. These results suggest that the SHPS-1–SHP-2 complex transmits a positive signal to activate MAPK.
FIG. 8.
SHPS-1 potentiates MAPK activation via SHP-2. 293T cells were transfected with the indicated plasmids as described in Materials and Methods. Transfected cells were detached and replated on FN-coated plates for the indicated periods of time. Total-cell lysates were prepared and assayed by immunoblotting for MAPK activation (with anti-pMAPK [α pMAPK]) and expression of SHPS-1 (with anti-SHPS-1 [α SHPS1]), hemagglutinin (HA)-tagged MAPK (using anti-HA-MAPK [α HA]), and SHP-2 (using anti-SHP-2 [α SHP-2]).
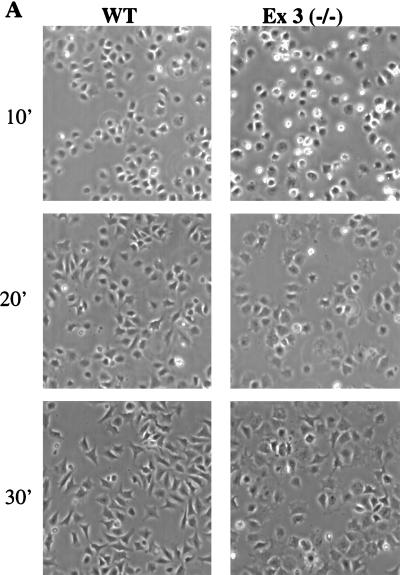
One of the earliest phenotypic consequences of integrin signaling is cell spreading. Given the biochemical changes in the SHP-2 mutant cells, we asked whether cell spreading might be altered in these cells. Following detachment and replating onto FN-coated plates, wild-type fibroblasts attached and began to spread within 5 min and were essentially completely spread within 20 to 30 min. Although SHP-2 mutant cells attached at the same rate as wild-type cells, a significant number of SHP-2 mutant cells remained round at 10 min (bright cells in Fig. 9A), indicating that spreading was delayed and impaired in the absence of SHP-2. Restoring SHP-2 expression rescued the spreading defect as well (Fig. 9B and C). SHP-2 deficiency also affected other integrin-evoked biological functions, such as prevention of apoptosis (unpublished observations). Taken together, our results indicate that SHP-2 is globally required for early and late biochemical and biological events in integrin signaling.
FIG. 9.
SHP-2 mutant fibroblasts exhibit delayed spreading (A) Wild-type (WT) and SHP-2 mutant (Ex3−/−) fibroblasts were detached, replated on FN-coated plates for the indicated time periods (in minutes), and photographed under phase-contrast optics. (B) Restoring SHP-2 expression rescues the spreading defect. SHP-2 mutant fibroblasts were infected with a β-galactosidase (βgal)- or SHP-2-expressing adenovirus or left uninfected (Ex3−/−), detached, and allowed to spread on FN-coated plates for the indicated times. Shown is a representative experiment from three independent infections. (C) Quantification of the results of reconstitution experiments. Shown are mean percentages of spread cells per field ± the standard errors of the means. A total of approximately 300 cells were counted for each bar.
DISCUSSION
Integrin engagement activates a signal transduction cascade that leads to changes in cellular tyrosyl phosphorylation, activation of MAPKs, alterations in the cytoskeletal architecture, changes in gene expression, and prevention of apoptosis. Relatively little is known about how specific PTPs help regulate these processes. The results presented here implicate SHP-2, acting via one of its specific binding proteins, SHPS-1, as a critical positive component in integrin signaling. We have shown that (i) Src PTKs catalyze tyrosyl phosphorylation of SHPS-1 upon plating on FN; (ii) SHP-2 is required for normal integrin-induced activation of Src (and probably other Src PTKs), tyrosyl phosphorylation of FAK, paxillin, and p130Cas, and MAPK activation; (iii) increasing SHPS-1 expression can potentiate integrin-induced MAPK activation and this requires SHP2 function; and (iv) biological responses, such as spreading on FN, require normal SHP-2 function.
These results and those of previous studies suggest a possible model for the role of SHP-2 and SHPS-1 in integrin signal transduction (Fig. 10). We propose that upon integrin engagement by appropriate ECM ligands, Src PTKs catalyze the phosphorylation of SHPS-1 on multiple tyrosyl residues. A recent report indicates that Fyn can be recruited to β1 integrins by the adapter protein caveolin and that a fraction of the caveolin-associated Fyn is activated. While the mechanism of Fyn activation is unclear, this population could catalyze the phosphorylation of SHPS-1. At least two of the SHPS-1 phosphorylation sites presumably function to recruit SHP-2. SHP-2 would then act in a positive-feedback loop to sustain high levels of Src PTK activity and/or to activate additional Src PTK molecules, potentially by catalyzing dephosphorylation of their inhibitory C-terminal phosphotyrosyl residues (e.g., Y527 in Src). The high level of Src PTK activation is necessary to promote effective phosphorylation of FAK, binding of Src PTKs to the FAK autophosphorylation site, and further phosphorylation of FAK (50, 52) and downstream targets. This model may also apply to complexes formed by SHP-2 with other transmembrane glycoproteins (e.g., PECAM and PIR-B/P91A; see the introduction). In addition to tyrosine dephosphorylation and activation of Src upon integrin engagement, activation of Src by tyrosyl dephosphorylation following treatment of platelets with thrombin (4) or of Swiss 3T3 cells with bombesin (45) has also been reported. It will be interesting to determine whether ligand-inducible complexes of SHP-2- and SHPS-1-like molecules (eg., PECAM in platelets) play a role in c-Src activation by these G protein-coupled receptors as well.
FIG. 10.
Model for regulation of integrin signaling by SHP2. For details, see the text.
Previous work showed that SHPS-1 tyrosyl phosphorylation increases upon plating of CHO cells on FN. Our results, along with recent work of others, confirm and extend these findings to other fibroblast cell lines and other ECM ligands (62). Not all ECMs evoke tyrosyl phosphorylation of SHPS-1 to equal extents, with FN and VN causing strong tyrosyl phosphorylation of SHPS-1 and Col and LN evoking only weak induction (Fig. 1B). These differences in ECM effects could reflect intrinsic differences in signal transduction from different integrins. However, we cannot exclude the trivial possibility that such differences reflect differential levels of integrin expression in the cell lines we have studied. Further work is required to distinguish among these possibilities.
Integrin engagement is known to activate FAK and Src family PTKs (see the introduction). The following multiple lines of evidence strongly implicate Src family rather than FAK family PTKs in SHPS-1 tyrosyl phosphorylation: (i) SHPS-1 tyrosyl phosphorylation is dramatically increased in Csk−/− fibroblasts, in which Src PTK activity is elevated, whereas it is decreased in Csk−/− cells in which Src PTK deficiency is superimposed; (ii) integrin-induced SHPS-1 tyrosyl phosphorylation is decreased in cells lacking one or more Src PTKs; (iii) treatment of cells with the Src PTK inhibitor PP1 dramatically decreases SHPS-1 phosphorylation (Fig. 2); (iv) recombinant Src phosphorylates the cytoplasmic domain of SHPS-1 in vitro (better than FAK or enolase), and transient cotransfection of Src and SHPS-1 expression constructs leads to increased tyrosyl phosphorylation of SHPS-1 (Fig. 3 and data not shown); and (v) blocking phosphorylation of FAK by pretreating cells with cytochalasin D has little effect on Src activation (32a) or SHPS-1 tyrosyl phosphorylation (Fig. 4). While our manuscript was under review, Tsuda and coworkers also reported decreased SHPS-1 phosphorylation in Src- and Fyn-deficient cells. However, they observed a decrease in SHPS-1 tyrosyl phosphorylation in FAK-deficient cells and in cells treated with cytochalasin D (62). It is unclear to us why our results with cytochalasin D differ from those of Tsuda and coworkers; nevertheless, their in vitro studies suggest that Src, but not FAK, can phosphorylate SHPS-1. Thus, our data, along with these recent results, argue that while FAK may contribute to the regulation of SHPS-1 tyrosyl phosphorylation, Src PTKs directly phosphorylate SHPS-1 early upon integrin engagement.
The precise order in which Src family PTKs and FAK are activated during integrin signaling has been a subject of controversy. One school of thought holds that FAK is activated directly upon integrin engagement and that autophosphorylated FAK then recruits and activates Src and other Src family PTKs by engaging their SH2 domains (50). In this model, activated Src family PTKs bound to FAK then further phosphorylate FAK on additional sites and phosphorylate other substrates. Consistent with this model, Src PTKs can be coimmunoprecipitated with FAK, and Src-FAK association is dependent on the FAK autophosphorylation site. However, other evidence suggests that Src PTK activation precedes FAK activation. For example, FAK is hyperactivated in Csk−/− cells (59) in which Src PTK activity is high (20, 34), as well as in v-Src-transformed cells (21, 50), and kinase-negative FAK can be phosphorylated upon integrin engagement (43, 53). In addition, FAK tyrosyl phosphorylation is absent in cells deficient for both Src and Fyn (data not shown).
Although we favor the latter activation order, our proposed role for SHPS-1–SHP-2 complexes can be accommodated within either model. If FAK is upstream of Src, then initial FAK activation upon integrin engagement could lead to the recruitment and activation of a small amount of Src (and other Src family PTKs), which in turn could phosphorylate SHPS-1. Tyrosyl-phosphorylated SHPS-1 would then recruit SHP-2, which could then either activate additional molecules of Src or promote sustained Src activation. Interestingly, previous studies have shown that Src activation persists far longer than Src-FAK complexes are detectable by coimmunoprecipitation (51), suggesting that sustained Src activation results from a mechanism other than SH2 domain engagement. Alternatively, basal levels of Src activity could phosphorylate SHPS-1, leading to a positive-feedback loop consisting of further Src activation, FAK activation, and downstream signals. Either model also can accommodate previous suggestions that SHPS-1 is a substrate of SHP-2 (8, 23): by promoting SHPS-1 dephosphorylation, SHP-2 would turn off the very process that it initiates, thereby limiting PTK activation following integrin engagement.
At first glance, the positive role for SHPS-1–SHP-2 complexes in integrin-mediated signaling suggested by our results seems to conflict with the previous suggestion that SHPS-1 negatively regulates growth factor receptor-induced signaling and mitogenesis (23). It is conceivable that SHPS-1–SHP-2 complexes have distinct effects on different signaling pathways—for example, inhibiting RTK signaling while activating ECM signaling. However, if distinct SHP-2 complexes are required in multiple signaling pathways, then the previous experiments, in which the role of SHPS-1 was inferred on the basis of the results of overexpression experiments, may have been misleading: overexpression of SHPS-1 could titrate SHP-2 needed for other positive signaling functions (e.g., direct binding to RTKs or binding to downstream scaffolding molecules such as IRS family members, Gab1, or FRS-2; see the introduction). Since SHPS-1 is implicated in both RTK and integrin signaling, a particularly attractive possibility, and one that we strongly favor, is that SHPS-1–SHP-2 complexes help explain one or more well-known modulatory effects of ECM on growth factor and cytokine receptor signaling. In this way, SHPS-1–SHP-2 complexes could serve as integrators of these two types of signaling pathways. The ability of SHPS-1 to potentiate MAPK activation in an SHP-2-dependent manner is consistent with this hypothesis (Fig. 8).
Although our data implicate SHP-2 in integrin-induced Src family kinase regulation, other PTPs also play roles in Src PTK regulation. Two receptor PTPs, PTPα and CD45, have been proposed to regulate Src family PTKs. Overexpression of PTPα results in dephosphorylation of the negative regulatory tyrosine of Src PTKs (66), whereas CD45 can have either negative or positive effects on Src PTK activation, depending on the cellular context. In lymphocytes, CD45 has been proposed to play a positive role in activation of Lck and, to a lesser extent, Fyn (for a review, see reference 61). In contrast, recent results indicate that in macrophages, CD45 is targeted to focal contacts and the absence of CD45 results in elevated levels of Src PTK activity, suggesting that CD45 is a negative regulator of integrin signaling (44). Accordingly, we cannot exclude the possibility that SHP-2’s effects on Src PTK activity are indirect, e.g., via effects on HPTPα or other PTPs.
As a consequence of the essential role of SHP-2 in integrin signaling, cytoskeletal changes (e.g., spreading) (Fig. 9) and apoptosis prevention (unpublished observations) are impaired in SHP-2 mutant fibroblasts. Since the exon 3−/− SHP-2 mutant protein has elevated PTP activity (41), it remains possible that defects we have discussed are due to increased SHP-2 activity. However, the rescue of these defects upon reconstitution of SHP-2 expression suggests that the defects in integrin signaling most likely arise as a consequence of the diminished SHP-2 function. Consistent with our findings, while this article was under review, Yu and coworkers also reported that SHP-2 mutant cells have defects in migration as well as spreading and have an increased number of focal adhesions (65). Our results confirm and extend their study by showing that SHP-2’s effects on MAPK activation are mediated by its association with SHPS-1 and that SHP-2 either directly or indirectly regulates Src kinase activity and downstream tyrosyl phosphorylation events.
Defects in cell adhesion and migration may account for the developmental effects of defective SHP-2 function. Dominant-negative SHP-2 blocks FGF-induced elongation of Xenopus animal caps and prevents completion of gastrulation (57), a process which is nonmitogenic and involves the reorganization of existing cells. SHP-2 mutant mice also fail to gastrulate properly, and many of the defects in mutant embryos have been postulated to be a result of improper organization or migration of mesodermal cells (48). The results presented here support this hypothesis by demonstrating a role for SHP-2 in integrin biology, including cell spreading and survival.
Src PTKs have been implicated in the regulation of both of these processes; thus, it is interesting to postulate that the defects observed both in vivo and in cultured cells could be due primarily to effects of SHP-2 on Src PTKs. For example, Src−/− cells, like SHP-2 mutant cells, show a reduced rate of spreading (22). Thus, the suppression of Src PTK activation observed in SHP-2 mutant cells could be the primary mechanism contributing to the phenotypic defects in vivo and in culture. Additional genetic and biochemical studies will be performed to test this hypothesis.
Finally, given that attachment to an appropriate ECM is critical to allow growth factors to promote mitogenesis, it is conceivable that some or even all of the previously observed effects on loss of SHP-2 function on growth factor-induced signaling instead reflect interference with what would otherwise be a constitutive signal from the ECM. Further study is required to delineate just how defective SHP-2 function affects growth factor and ECM signaling pathways and their integration and the role of SHP-2 in the regulation of complex biological responses.
ACKNOWLEDGMENTS
E.-S.O., H.G., T.M.S., and J.F.T. contributed equally to this work.
This work was supported by NIH grants R01 CA49152 (to B.G.N.), R01 CA75621 (to S.M.T.), and R01 DK43051 (to B.B.K.) and by grants from the Medical Research Council of Canada and the National Cancer Institute of Canada (to T.P.). T.P. is an International Scholar of the Howard Hughes Medical Institute. H.G. was supported by NIH NRSA CA72144, J.F.T. was supported by a postdoctoral fellowship from the Leukemia Society, and S.H. was the recipient of a postdoctoral fellowship from The Medical Foundation. E.U.F. was supported by a Physician Scientist Award (NIA no. AG 00294).
We are grateful to C. Newgard for providing adenovirus plasmids, Mike Eck for the kind gift of recombinant c-Src, Karen Zoller and Cindy Miranti for helpful discussions, and Joan S. Brugge and Alana M. O’Reilly for insightful comments and critical reading of the manuscript.
REFERENCES
- 1.Allard J D, Chang H C, Herbst R, McNeill H, Simon M A. The SH2-containing tyrosine phosphatase corkscrew is required during signaling by sevenless, Ras1 and Raf. Development. 1996;122:1137–1146. doi: 10.1242/dev.122.4.1137. [DOI] [PubMed] [Google Scholar]
- 2.Becker T C, Noel R J, Coats W S, Gomez-Foix A M, Alam T, Gerard R D, Newgard C B. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 3.Bennett A M, Hausdorff S F, O’Reilly A M, Freeman R M, Jr, Neel B G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark E A, Brugge J S. Redistribution of activated pp60c-src to integrin-dependent cytoskeletal complexes in thrombin-stimulated platelets. Mol Cell Biol. 1993;13:1863–1871. doi: 10.1128/mcb.13.3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 6.Freeman R M, Jr, Plutzky J, Neel B G. Identification of a human src-homology 2 (SH2) containing tyrosine phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci USA. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frevert E U, Kahn B B. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol Cell Biol. 1997;17:190–198. doi: 10.1128/mcb.17.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao J, Zoller K E, Ginsberg M H, Brugge J S, Shattil S J. Regulation of the pp72syk protein tyrosine kinase by platelet integrin alpha IIb beta 3. EMBO J. 1997;16:6414–6425. doi: 10.1093/emboj/16.21.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garton A J, Tonks N K. PTP-PEST: a protein tyrosine phosphatase regulated by serine phosphorylation. EMBO J. 1994;13:3763–3771. doi: 10.1002/j.1460-2075.1994.tb06687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu H, Griffin J D, Neel B G. Characterization of two SHP-2-associated binding proteins and potential substrates in hematopoietic cells. J Biol Chem. 1997;272:16421–16430. doi: 10.1074/jbc.272.26.16421. [DOI] [PubMed] [Google Scholar]
- 12.Gu H, Pratt J C, Burakoff S J, Neel B G. Cloning and characterization of the major SHP-2 binding protein in hematopoietic cells (p97) reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 13.Gutch M J, Flint A J, Keller J, Tonks N K, Hengartner M O. The Caenorhabditis elegans SH2 domain-containing protein tyrosine phosphatase PTP-2 participates in signal transduction during oogenesis, embryogenesis and vulval development. Genes Dev. 1998;12:571–585. doi: 10.1101/gad.12.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent and Src family selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 15.Herbst R, Carroll P M, Allard J D, Schilling J, Raabe T, Simon M A. Daughter of Sevenless is a substrate of the phosphotyrosine phosphatase corkscrew and functions during Sevenless signaling. Cell. 1996;85:899–909. doi: 10.1016/s0092-8674(00)81273-8. [DOI] [PubMed] [Google Scholar]
- 16.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 17.Howell B W, Cooper J A. Csk suppression of Src involves movement of Csk to sites of Src activity. Mol Cell Biol. 1994;14:5402–5411. doi: 10.1128/mcb.14.8.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes R O. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 19.Illc D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 20.Imamoto A, Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993;73:1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
- 21.Kanner S B, Reynolds A B, Vines R R, Parsons J T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci USA. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan K B, Swedlow J R, Morgan D O, Varmus H E. c-Src enhances the spreading of src−/− fibroblasts on fibronectin by a kinase-independent mechanism. Genes Dev. 1995;9:1505–1517. doi: 10.1101/gad.9.12.1505. [DOI] [PubMed] [Google Scholar]
- 23.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signaling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 24.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kouhara H, Hadari Y, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 27.Lechleider R J, Freeman R M, Neel B G. Tyrosyl phosphorylation and growth factor receptor association of the human corkscrew homologue, SH-PTP2. J Biol Chem. 1993;268:13434–13438. [PubMed] [Google Scholar]
- 28.Lewis J M, Baskaran R, Taagepera S, Schwartz M A, Wang J Y. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc Natl Acad Sci USA. 1996;93:15174–15179. doi: 10.1073/pnas.93.26.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao L, Jaken S. Effect of alpha-protein kinase C neutralizing antibodies and the pseudosubstrate peptide on phosphorylation, migration, and growth of REF52 cells. Cell Growth Differ. 1993;4:309–316. [PubMed] [Google Scholar]
- 30.Lipfert L, Haimovich B, Schaller M D, Cobb B S, Parsons J T, Brugge J S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992;119:905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu F, Sells M A, Chernoff J. Protein tyrosine phosphatase 1B negatively regulates integrin signaling. Curr Biol. 1998;8:173–176. doi: 10.1016/s0960-9822(98)70066-1. [DOI] [PubMed] [Google Scholar]
- 32.Lorenz U, Ravichandran K S, Pei D, Walsh C T, Burakoff S J, Neel B G. Lck-dependent tyrosyl phosphorylation of the phosphotyrosine phosphatase SH-PTP1 in murine T cells. Mol Cell Biol. 1994;14:1824–1834. doi: 10.1128/mcb.14.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Mirante, C., and J. Brugge. Personal communication.
- 33.Morgenstern J P, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990;18:1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nada S, Yagi T, Takeda H, Tokunaga T, Nakagawa H, Ikawa Y, Okada M, Aizawa S. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993;73:1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- 35.Neel B G, Tonks N K. Protein tyrosine phosphatases in signal transduction. Curr Opin Cell Biol. 1997;9:193–204. doi: 10.1016/s0955-0674(97)80063-4. [DOI] [PubMed] [Google Scholar]
- 36.Ohnishi H, Kubota M, Ohtake A, Sato K, Sano S. Activation of protein-tyrosine phosphatase SH-PTP2 by a tyrosine-based activation motif of a novel brain molecule. J Biol Chem. 1996;271:25569–25574. doi: 10.1074/jbc.271.41.25569. [DOI] [PubMed] [Google Scholar]
- 37.Okada M, Nada S, Yamanishi Y, Yamamoto T, Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1991;266:24249–24252. [PubMed] [Google Scholar]
- 38.O’Reilly A M, Neel B G. Structural determinants of SHP-2 function and specificity in Xenopus mesoderm induction. Mol Cell Biol. 1998;18:161–177. doi: 10.1128/mcb.18.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins L A, Johnson M R, Melnick M B, Perrimon N. The nonreceptor protein tyrosine phosphatase Corkscrew functions in multiple receptor tyrosine kinase pathways in Drosophila. Dev Biol. 1996;180:63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- 40.Perkins L A, Larsen I, Perrimon N. corkscrew encodes a putative protein tyrosine phosphatase that functions to transduce the terminal signal from the receptor tyrosine kinase torso. Cell. 1992;70:225–236. doi: 10.1016/0092-8674(92)90098-w. [DOI] [PubMed] [Google Scholar]
- 41.Qu C-K, Shi Z-Q, Shen R, Tsai F-Y, Orkin S H, Feng G-S. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raabe T, Riesgo-Escovar J, Liu X, Bausenwein B S, Deak P, Maroy P, Hafen E. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell. 1996;85:911–920. doi: 10.1016/s0092-8674(00)81274-x. [DOI] [PubMed] [Google Scholar]
- 43.Richardson A, Malik R K, Hildebrand J D, Parsons J T. Inhibition of cell spreading by expression of the C-terminal domain of focal adhesion kinase (FAK) is rescued by coexpression of Src or catalytically inactive FAK: a role for paxillin tyrosine phosphorylation. Mol Cell Biol. 1997;17:6906–6914. doi: 10.1128/mcb.17.12.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roach T, Slater S, Koval M, White L, McFarland E, Okumura M, Thomas M, Brown E. CD45 regulates src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol. 1997;7:408–417. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez-Fernandez J L, Rozengurt E. Bombesin, bradykinin, vasopressin, and phorbol esters rapidly and transiently activate src family tyrosine kinases in Swiss 3T3 cells. J Biol Chem. 1996;271:27895–27901. doi: 10.1074/jbc.271.44.27895. [DOI] [PubMed] [Google Scholar]
- 46.Sabe H, Okada M, Nakagawa H, Hanafusa H. Activation of c-Src in cells bearing v-Crk and its suppression by Csk. Mol Cell Biol. 1992;12:4706–4713. doi: 10.1128/mcb.12.10.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sagawa K, Kimura T, Sweiter M, Siraganian R P. The protein-tyrosine phosphatase SHP-2 associates with tyrosine phosphorylated adhesion molecule PECAM-1 (CD31) J Biol Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- 48.Saxton T M, Henkemeyer M, Gasca S, Shen R, Rossi D J, Shalaby F, Feng G-S, Pawson T. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase SHP-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaller M D, Borgman C A, Cobb B S, Vines R R, Reynolds A B, Parsons J T. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlaepfer D D, Broome M A, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase–c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlaepfer D D, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlaepfer D D, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz M A, Schaller M D, Ginsberg M H. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 55.Shi Z-Q, Lu W, Feng G-S. The Shp-2 tyrosine phosphatase has opposite effects in mediating the activation of extracellular signal-regulated and c-Jun NH2-terminal mitogen-activated protein kinases. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 56.Streuli M. Protein tyrosine phosphatases in signaling. Curr Opin Cell Biol. 1996;8:182–188. doi: 10.1016/s0955-0674(96)80064-0. [DOI] [PubMed] [Google Scholar]
- 57.Tang T L, Freeman R M, O’Reilly A M, Neel B G, Sokol S Y. The SH2-containing protein tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 58.Thomas S M, Brugge J S. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 59.Thomas S M, Soriano P, Imamoto A. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature. 1995;376:267–271. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- 60.Timms J F, Carlberg K, Gu H, Chen H, Kamatkar S, Nadler M J S, Rohrschneider L R, Neel B G. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18:3838–3850. doi: 10.1128/mcb.18.7.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trowbridge I S, Thomas M L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 62.Tsuda M, Matozaki T, Fukunaga K, Fujioka Y, Imamoto A, Noguchi T, Takada T, Yamao T, Takeda H, Ochi F, Yamamoto T, Kasuga M. Integrin-mediated tyrosine phosphorylation of SHPS-1 and its association with SHP-2. J Biol Chem. 1998;273:13223–13229. doi: 10.1074/jbc.273.21.13223. [DOI] [PubMed] [Google Scholar]
- 63.Van Vactor D, O’Reilly A O, Neel B G. Genetic analysis of protein tyrosine phosphatases. Curr Opin Genet Dev. 1998;8:112–126. doi: 10.1016/s0959-437x(98)80070-1. [DOI] [PubMed] [Google Scholar]
- 64.White M F, Yenush L. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Curr Top Microbiol Immunol. 1998;228:179–208. doi: 10.1007/978-3-642-80481-6_8. [DOI] [PubMed] [Google Scholar]
- 65.Yu D-H, Qu C-K, Henegariu O, Lu X, Feng G-S. Protein-tyrosine phosphatase SHP-2 regulates cell spreading, migration and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 66.Zheng X M, Wang Y, Pallen C J. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase. Nature. 1992;359:336–339. doi: 10.1038/359336a0. [DOI] [PubMed] [Google Scholar]