Abstract
Background
Joblessness is common after ARDS, but related risk factors are not fully understood.
Research Question
What is the association between survivors’ pre-ARDS workload and post-ARDS functional impairment, pain, and fatigue with their return to work (RTW) status?
Study Design and Methods
The U.S. Occupational Information Network (O∗NET) was used to determine pre-ARDS workload for participants in the ARDS Network Long-Term Outcomes Study (ALTOS). Post-ARDS functional impairment was assessed using the Mini-Mental State Examination and SF-36 Physical Functioning, Social Functioning, and Mental Health sub-scales, and categorized as either no impairments, only psychosocial impairment, physical with low psychosocial impairment, or physical with high psychosocial impairment. Post-ARDS pain and fatigue were assessed using the SF-36 pain item and Functional Assessment of Chronic Illness Therapy—Fatigue Scale fatigue scale, respectively. Generalized linear mixed modeling methods were used to evaluate associations among pre-ARDS workload, post-ARDS functional impairment, and symptoms of pain and fatigue with post-ARDS RTW.
Results
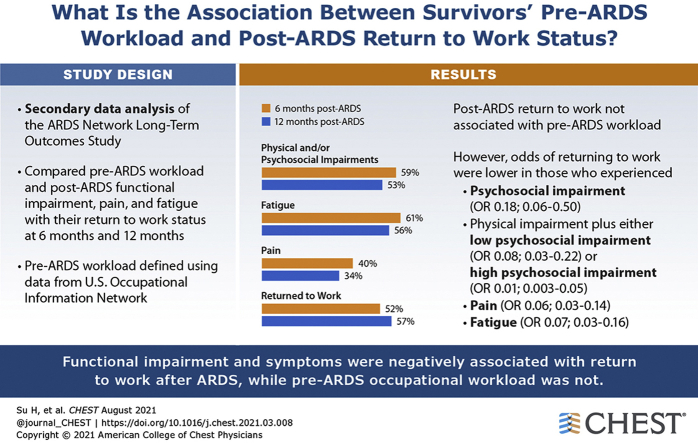
Pre-ARDS workload was not associated with post-ARDS RTW. However, as compared with survivors with no functional impairment, those with only psychosocial impairment (OR [CI]: 0.18 [0.06-0.50]), as well as physical impairment plus either low psychosocial impairment (0.08 [0.03-0.22]) or high psychosocial impairment (0.01 [0.003-0.05]) had lower odds of working. Pain (0.06 [0.03-0.14]) and fatigue (0.07 [0.03-0.16]) were also negatively associated with RTW.
Interpretation
For previously employed survivors of ARDS, post-ARDS psychosocial and physical impairments, pain, and fatigue were negatively associated with RTW, whereas pre-ARDS workload was not associated. These findings are important for designing and implementing vocational interventions for ARDS survivors.
Key Words: employment, functional ability, intensive care unit, outcomes, symptom
Abbreviations: ALTOS, ARDS Network Long-term Outcomes Study; APACHE III, Acute Physiology and Chronic Health Evaluation III; FACIT-F, Functional Assessment of Chronic Illness Therapy—Fatigue Scale; LOS, length of stay; O∗NET, The U.S. Occupational Information Network; RTW, return to work
Graphical abstract
After hospitalization, return to work (RTW) is important for previously employed patients and is positively associated with functional recovery and economic status.1 However, previously employed survivors of ARDS who required intensive care commonly experience joblessness over 5-year follow-up (36%-68%).2, 3, 4 Hence, a greater understanding of occupational and patient factors associated with post-ARDS employment outcomes is urgently needed. Among ARDS survivors, existing patient and hospitalization factors associated with joblessness include older age, non-White race, chronic health conditions, prolonged mechanical ventilation, and longer ICU and hospital lengths of stay.2,5 However, few studies have evaluated joblessness with ARDS survivors’ post-hospitalization status, including aspects of their physical, cognitive, and mental health status.6,7
In other patient populations, post-hospitalization joblessness is associated with pre-hospitalization workload (the minimal ability required to perform a specific job8), post-hospitalization functional impairment (eg, physical, psychological, interpersonal, and cognitive impairment), and symptoms (eg, pain and fatigue).9, 10, 11, 12 Applying these findings to ARDS survivors required a deeper evaluation to assist in designing vocational interventions. Hence, our primary objective was to evaluate the associations of (a) pre-ARDS job workload, (b) post-ARDS functional impairment, and symptoms of (c) pain and (d) fatigue with RTW at 6 and 12 months after ARDS. As an exploratory objective, we also examined the association of these factors, in combination, with RTW at 6 and 12 months.
Study Design and Methods
Overview and Participants
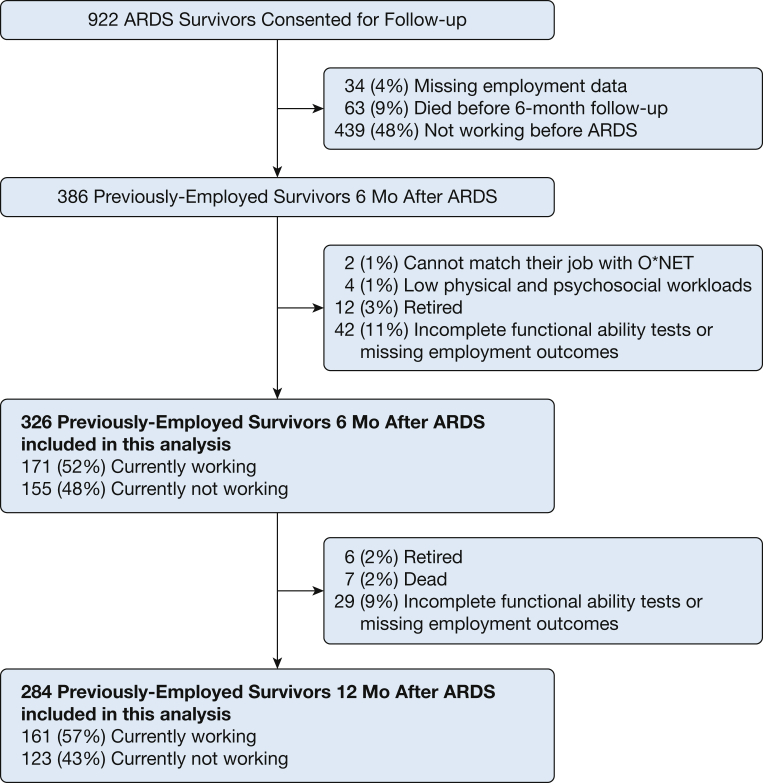
This evaluation was conducted as a secondary data analysis of the ARDS Network Long-Term Outcomes Study (ALTOS).13, 14, 15, 16 ALTOS is prospective cohort study that evaluated 6- and 12-month outcomes of participants from randomized trials conducted by the National Institutes of Health ARDS Network. Participants were enrolled from 43 hospitals during the period of 2008 to 2014.17, 18, 19, 20 In prospective follow-up of participants, treatments from these trials did not have any impact on 6- and 12-month physical, cognitive, or mental health and quality of life outcomes.13, 14, 15, 16 For this analysis, participants in the ARDS Network parent studies were excluded if they (1) reported no employment before hospitalization for ARDS; (2) died or retired during the follow-up period; (3) had incomplete functional assessments; or (4) were missing employment outcome data during follow-up. As required to associate specific jobs with employment workload, we also excluded survivors who had a job title that: (1) could not be matched within the Occupational Information Network (O∗NET) dataset (n = 2), or (2) was classified as having both low physical and psychosocial workloads (because of a sample size too small for meaningful statistical analysis [n = 4]). Approval for ALTOS was obtained from the institutional review boards of all participating study sites. We reported this analysis in accordance with Strengthening the Reporting of Observational Studies in Epidemiology guidelines.21
Demographic and ICU Variables
Demographic and ICU variables were collected in the parent study, including age, sex, race, ZIP Code, ethnicity, Acute Physiology and Chronic Health Evaluation III (APACHE III) severity of illness score,22 duration of mechanical ventilation, and ICU and hospital lengths of stay (LOS). As a measure of socioeconomic status, pre-ARDS household income was estimated using publicly available ZIP Code data, as done previously for this studys.23
Primary Outcome: Return to Work
In the parent study, a previously developed employment questionnaire2,5 was administered to survivors (or their proxies) at 6 and 12 months after ARDS. This in-depth questionnaire included questions regarding pre-ARDS job title, working full-time before ARDS (Y/N), and current employment status (ie, working or not working). For statistical analysis, the primary outcome was evaluated at both 6 and 12 months, and modeled as a binary variable indicating whether the ARDS survivor was working vs not working at the respective time point.
Pre-ARDS Workload Phenotype Exposure Variable
To estimate survivors’ pre-ARDS workload, we matched their pre-ARDS job title from their ALTOS employment questionnaire2,5 with the O∗NET system.24 O∗NET is a regularly updated US Department of Labor database that provides, in detail, specific domains (ie, abilities, interests, knowledge, skills, work activities, work context, and work values) of nearly 1,000 occupations. Each domain contains several standardized descriptors, on a 1 to 5 scale, with higher scores identifying attributes more vital for specific occupations. In our study, descriptor scores >2 indicated a high workload, as defined by O∗NET.25 Because impairments in memory, attention, and executive function are frequently reported cognitive issues in ICU survivors,26 we used the descriptors “memorization and complex problem-solving” to capture cognitive workload. Additionally, we used descriptors “level of competition” and “social perceptiveness” to capture survivors’ emotional and interpersonal workload, respectively. We then collapsed cognitive, emotional, and interpersonal workloads into a single variable, psychosocial workload, to identify jobs that had a high level of demand in this area (range, 0-3, with a higher number indicating greater demands). Finally, we used the descriptor “performing general physical activities” to capture survivors’ physical workload.
Using these psychosocial and physical workload data, we defined three pre-ARDS workload phenotypes based on actual RTW patterns observed in ALTOS: (1) only high psychosocial workload (ie, an O∗NET descriptor score >2 in >1 of psychosocial category [ie, cognitive, emotional, or interpersonal]; eg, telemarketer or software developer), (2) high physical (descriptor score >2) and low psychosocial (descriptor score >2 in ≤1 category) workload (eg, custodian or grocery store clerk), and (3) high physical and psychosocial (descriptor score >2 in >1 category) workload (eg, nurse or basketball coach).
Functional Impairments Exposure Variable
Functional impairment, an outcome encompassing psychosocial impairment (comprising cognitive, emotional, and interpersonal impairment) and physical impairment, was measured at 6 and 12 months post-ARDS. More specifically, cognitive function was measured by the Mini-Mental State Examination,27 with a score <24 indicating cognitive impairment.28 Emotional, interpersonal, and physical impairment were measured by Short Form 36 (SF-36) mental health, social functioning, and physical function subscales, respectively. Each normalized subscale ranges from 0 to 100 (mean [SD] = 50 [10]), with a higher score indicating better status,29 and emotional, interpersonal, and physical impairments, respectively, defined as mental health, social functioning, and physical functioning normalized scores ≤40 (≥1 SD lower). Using these variables, we defined four post-ARDS functional impairment phenotypes based on observed RTW patterns: (1) no functional impairment, (2) only psychosocial impairments, (3) physical and low psychosocial impairment, and (4) physical and high psychosocial impairment.
Pain and Fatigue Exposure Variables
Pain and fatigue symptoms were measured at 6 and 12 months post-ARDS. Pain was evaluated by the pain interference question of the SF-36. This question asks the individual to rate the extent to which pain interferes with normal work, with responses ranging from “Not at all,” “A little bit,” “Moderate,” “Quite a bit,” to “Extremely,”29 with responses of “Moderate” or higher defined as experiencing pain. Fatigue was measured by Functional Assessment of Chronic Illness Therapy- Fatigue Scale, which measures an individual’s level of fatigue during their usual daily activities over the past week. A transformed score (range, 0-100) ≤68 was defined as experiencing fatigue.30
Statistical Analysis
We summarized data using means and SDs for continuous variables, and proportions for categorical variables. We compared data using Student t tests for continuous variables, and χ2 or Fisher exact tests, as appropriate, for categorical variables.
For the primary objective, we used a generalized linear mixed model with a random intercept to evaluate, separately, the cross-sectional association of each exposure factor (pre-ARDS workload phenotype, post-ARDS functional impairment phenotype, pain, and fatigue) with RTW. Furthermore, we used logistic regression to evaluate the associations of individual exposures at 6 months with RTW at 12 months.
For the secondary objective, we included all factors (pre-ARDS workload phenotype, post-ARDS functional impairment phenotype, pain, and fatigue) in separate generalized linear mixed-model and logistic regression models, evaluating their cross-sectional and prospective association with RTW, respectively. The following covariates were included in all regression models, chosen a priori based on prior publications2,5: age, sex, race, APACHE III, and hospital LOS. Because socioeconomic status also may influence RTW, all models were adjusted for pre-ARDS estimated household income.23 In all models, multicollinearity was evaluated using variance inflation factors and was not detected (ie, all variance inflation factors <10). All analyses were performed using STATA 16 statistical software (StataCorp). A two-sided P < .05 denoted statistical significance, with no adjustment for multiple comparisons. To address the potential impact of missing data on our primary analyses, we conducted sensitivity analyses using multiple imputation methods (e-Appendix 1).
Results
Patient Characteristics
A total of 326 and 284 previously employed survivors, respectively, were included in our 6- and 12-month post-ARDS analyses (Fig 1). Demographic and ICU variables and employment situations are summarized in Table 1 and e-Table 1, respectively. There were no significant differences in demographic or ICU factors among survivors who did or did not meet inclusion criteria for the 6-month analysis. However, as compared with those included, survivors excluded from the 12-month analysis tended to have longer mechanical ventilation duration and ICU LOS (e-Table 2).
Figure 1.
Patient flow chart. O∗NET= The U.S. Occupational Information Network.
Table 1.
Demographic and ICU Variables, by Employment Status at 6 and 12 Months After ARDSa
| Variable | Working at 6 Mo (n = 171) | Not Working at 6 Mo (n = 155) | Pb | Working at 12 Mo (n = 161) | Not Working at 12 Mo (n = 123) | Pb |
|---|---|---|---|---|---|---|
| Demographic factors | ||||||
| Age, mean (SD), y | 44 (13) | 46 (12) | .16 | 44 (12) | 47 (12) | .04 |
| Female | 69 (40) | 76 (49) | .12 | 64 (40) | 61 (50) | .12 |
| White race | 143 (84) | 114 (74) | .03 | 138 (86) | 87 (71) | .002 |
| Hispanic ethnicity | 12 (7) | 17 (11) | .25 | 11 (7) | 13 (11) | .28 |
| Pre-ARDS estimated household income, mean (SD), in thousands of dollarsc | 55 (21) | 52 (1.7) | .08 | 56 (21) | 51 (18) | .03 |
| ICU Factors | ||||||
| APACHE III severity of illness | 84 (26) | 85 (27) | .75 | 82 (25) | 85 (27) | .39 |
| ARDS primary risk factor: | ||||||
| Pulmonary | 119 (70) | 102 (66) | .48 | 106 (66) | 90 (73) | .20 |
| Sepsis | 27(16) | 31 (20) | .39 | 27 (17) | 19 (16) | .87 |
| Others | 25 (15) | 25 (16) | .76 | 28 (17) | 17 (14) | .51 |
| Mechanical ventilation, mean (SD) d | 9 (8) | 13 (12) | <.001 | 9 (9) | 12 (12) | .02 |
| ICU LOS, mean (SD) d | 12 (9) | 17 (12) | <.001 | 12 (9) | 15 (12) | .005 |
| Hospital LOS, mean (SD) d | 18 (11) | 27(17) | <.001 | 19 (13) | 24 (16) | .005 |
| Pre-ARDS Workloadd | ||||||
| High psychosocial workload only | 63 (37) | 57 (37) | .99 | 62 (39) | 42 (34) | .45 |
| High physical & low psychosocial workload | 34 (20) | 38 (25) | .35 | 28 (17) | 34 (28) | .04 |
| High physical & high psychosocial workload | 74 (43) | 60 (39) | .43 | 71 (44) | 47 (39) | .39 |
| Functional Impairment after ARDSe | ||||||
| No impairment | 99 (58)f | 36 (23)f | <.001 | 113 (71)g | 21 (17)g | <.001 |
| Only psychosocial impairments | 30 (17)f | 18 (12)f | .16 | 18 (11)g | 24 (20)g | .062 |
| Physical & low psychosocial impairment | 28 (16)f | 50 (32)f | .001 | 24 (15)g | 37 (30)g | .002 |
| Physical & high psychosocial impairment | 14 (8)f | 51 (33)f | <.001 | 6 (4)g | 41 (34)g | <.001 |
| Symptoms after ARDS | ||||||
| Pain | 33 (19)f | 98 (63)f | <.001 | 30 (19)g | 67 (55)g | <.001 |
| Fatigue | 74 (44)f | 126 (81)f | <.001 | 61 (38)g | 97 (85)g | <.001 |
APACHE III = Acute Physiology and Chronic Health Evaluation III; LOS = length of stay.
Data presented as No. (%) unless otherwise noted and may not add to 100% because of rounding.
Calculated by Student t test for continuous variables and χ2 or Fisher exact tests, as appropriate, for categorical variables.
Estimated household income is based on ZIP code of residence.22
Psychosocial workload combines cognitive, emotional, and interpersonal workload categories, with a job exhibiting low (<2 categories), or high (≥2 categories) number of these categories.
Psychosocial impairment combines cognitive, emotional, and interpersonal impairment categories, with survivors having impairments in low (<2 categories), or high (≥2 categories) number of these categories.
Analyzed using functional impairments, pain, and fatigue at 6 months.
Analyzed using functional impairments, pain, and fatigue at 12 months.
RTW, Impairments, and Symptoms
At 6 and 12 months after ARDS, respectively, 171 (52%) and 161 (57%) previously employed survivors had returned to work, with 191 (59%) and 150 (53%) reporting impairments (ie, physical or psychosocial), 200 (61%) and 158 (56%) reporting fatigue, and 131 (40%) and 97 (34%) reporting pain (Table 1). Among survivors with psychosocial impairments, 33%, 54%, and 44% reported cognitive, emotional, and interpersonal impairments at 6 months, respectively, and 48%, 71%, and 33% reported these impairments at 12 months. Those who were not working tended to be older and non-White, with longer hospital and ICU LOS, mechanical ventilation duration, and reporting greater impairments (physical or psychosocial) and symptoms (fatigue or pain).
In cross-sectional models involving individual exposure factors, post-ARDS functional impairments, pain, and fatigue were independently associated with not returning to work post-ARDS (Table 2). Compared with ARDS survivors with no functional impairments, those with only psychosocial impairments (OR [95%CI], 0.18 [0.06-0.50], P = .001), as well as physical plus either low psychosocial impairments (0.08 [0.03-0.22], P < .001) or high psychosocial impairments (0.01 [0.003-0.05], P < .001) had lower odds of working (Table 2, individual models). Compared with ARDS survivors without symptoms, those with pain or fatigue symptoms (0.06 [0.03-0.14], P < .001; 0.07 [0.03-0.16], P < .001) had lower odds of working, respectively. Similar results were found in the prospective model examining exposure factors at 6 months with RTW at 12 months (Table 3, individual models).
Table 2.
Cross-sectional Associations of Return to Work After ARDS With Pre-ARDS Workload Phenotypes, Functional Impairments, Pain, and Fatiguea
| Variable | Individual Models |
Combined Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Return to Work |
Return to Work |
Return to Work |
Return to Work |
Return to Work |
||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Pre-ARDS workloadb | ||||||||||
| Only high psychosocial workload | Reference | Reference | ||||||||
| High physical and low psychosocial workload | 0.76 (0.25, 2.35) | .64 | … | … | … | … | … | … | 0.40 (0.13, 1.20) | .10 |
| High physical and high psychosocial workload | 0.92 (0.35, 2.40) | .86 | … | … | … | … | … | … | 1.17 (0.47, 2.89) | .74 |
| Functional impairmentsc | ||||||||||
| No impairment | Reference | Reference | ||||||||
| Only psychosocial impairments | … | … | 0.18 (0.06, 0.50) | .001 | … | … | … | … | 0.40 (0.14, 1.16) | .09 |
| Physical and low psychosocial impairment | … | … | 0.08 (0.03, 0.22) | <.001 | … | … | … | … | 0.28 (0.10, 0.78) | .015 |
| Physical and high psychosocial impairment | … | … | 0.01 (0.003, 0.05) | <.001 | … | … | … | … | 0.07 (0.02, 0.27) | <.001 |
| Pain | … | … | … | … | 0.06 (0.03, 0.14) | <.001 | … | … | 0.21 (0.09, 0.52) | .001 |
| Fatigue | … | … | … | … | … | … | 0.07 (0.03, 0.16) | <.001 | 0.39 (0.16, 0.96) | .041 |
The intraclass correlation (ICC) for the combined model was 62%.
Each column represents one model. Generalized linear mixed model with a random intercept evaluating the association of working status with the variable named in that row, adjusted for age, sex, race, pre-ARDS median household income, APACHE III (Acute Physiology and Chronic Health Evaluation III), hospital length of stay, and follow-up time.
Psychosocial workload combines cognitive, emotional, and interpersonal workload categories, with a job exhibiting low (<2 categories), or high (≥2 categories) numbers of these categories.
Psychosocial impairment combines cognitive, emotional, and interpersonal impairment categories, with survivors having impairments in low (<2 categories) or high (≥2 categories) number of these categories.
Table 3.
Prospective Association of Return to Work 12 Months After ARDS With Pre-ARDS Workload Phenotypes, Functional Impairments, Pain, and Fatigue at 6 Monthsa
| Variable | Individual Models |
Combined Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Return to Work at 12 Mo |
Return to Work at 12 Mo |
Return to Work at 12 Mo |
Return to Work at 12 Mo |
Return to Work at 12 Mo |
||||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Pre-ARDS Workloadb | ||||||||||
| Only high psychosocial workload | Reference | Reference | ||||||||
| High physical and low psychosocial workload | 0.79 (0.42, 1.47) | .45 | … | … | … | … | … | … | 0.61 (0.28, 1.33) | .22 |
| High physical and high psychosocial workload | 0.87 (0.51, 1.49) | .61 | … | … | … | … | … | … | 1.04 (0.54, 2.00) | .91 |
| Functional impairments at 6 moc | ||||||||||
| No impairment | Reference | Reference | ||||||||
| Only psychosocial impairments | … | … | 0.38 (0.17, 0.84) | .02 | … | … | … | … | 0.62 (0.26, 1.47) | .28 |
| Physical and low psychosocial impairment | … | … | 0.16 (0.08, 0.32) | <.001 | … | … | … | … | 0.31 (0.13, 0.72) | .006 |
| Physical and high psychosocial impairment | … | … | 0.07 (0.03, 0.15) | <.001 | … | … | … | … | 0.15 (0.05, 0.42) | <.001 |
| Pain at 6 mo | … | … | … | … | 0.24 (0.15, 0.41) | <.001 | … | … | 0.66 (0.32, 1.36) | .26 |
| Fatigue at 6 mo | … | … | … | … | … | … | 0.16 (0.09, 0.28) | <.001 | 0.43 (0.19, 0.93) | .03 |
The pseudo-r2 for the combined model was 23%.
Each column represents one model. Multivariable logistic regression model evaluating the association of working status with the variable named in that row, adjusted for age, sex, race, pre-ARDS median household income, APACHE III (Acute Physiology and Chronic Health Evaluation III), hospital length of stay, and follow-up time.
Psychosocial workload combines cognitive, emotional, and interpersonal workload categories, with a job exhibiting low (<2 categories) or high (≥2 categories) numbers of these categories.
Psychosocial impairment combines cognitive, emotional, and interpersonal impairment categories, with survivors having impairments in low (<2 categories) or high (≥2 categories) numbers of these categories.
Pre-ARDS Workload and RTW
Pre-ARDS workload was not associated with returning to work after ARDS (Tables 2 and 3, individual models). When simultaneously evaluating all factors, the associations among functional impairment, pain, and fatigue variables were attenuated (Table 2, combined model). Physical impairments, with either low or high psychosocial impairments (0.28 [0.10-0.78], P = .015 and 0.07 [0.02-0.27], P < .001), pain (0.21 [0.09-0.52], P = .001) and fatigue (0.39 [0.16-0.96], P = .04) symptoms had strong negative associations for return to work post-ARDS (Table 2, combined model). In the logistic regression model simultaneously evaluating all exposure factors at 6 months, only fatigue (0.43 [0.19-0.93], P = .03) and physical impairments with either low or high psychosocial impairments (0.31 [0.13-0.72], P = .006 and 0.15 [0.05-0.42], P < .001) had strong negative associations for RTW at 12 months (Table 3, combined model).
Sensitivity analyses using multiple imputation for missing demonstrate similar findings as the primary analysis using complete case analysis (e-Tables 3, 4), except for the combined cross-sectional association model. This combined model, with multiple imputation, demonstrated that ARDS survivors with only psychosocial impairment had lower odds of working (0.37 [0.15-0.94], P = .04), compared with survivors with no functional impairment (e-Table 3, combined model).
Discussion
Six months after ARDS, only 52% of previously employed survivors of ARDS had returned to work; 12 months after ARDS, this proportion rose minimally, to 57%. At 6- and 12-month follow-up, more than half of survivors had psychosocial and physical impairments and commonly reported symptoms of pain and fatigue. Notably, the presence of these impairments and symptoms were independently associated with not working after ARDS, whereas patients’ pre-ARDS occupational workload was not associated with RTW.
To our knowledge, this is the most comprehensive study to date to examine the association of pre-hospitalization workload along with post-ARDS functional impairments and symptoms on RTW status at 6- and 12-month follow-up. Although our results suggest no association between pre-ARDS workload and return to work after ARDS, studies in other populations have yielded both negative10,31, 32, 33 and positive32,34,35 results. As a potential explanation, previous studies explored the association of workload and RTW as single domain (eg, physical or cognitive). However, we assessed workload using multiple domains, categorizing them into different phenotypes. Additionally, we evaluated job workload using detailed O∗NET descriptors, whereas prior studies relied on self-report31 or job content questionnaires.33 However, there is potential for measurement error in our mapping of survivors’ pre-ARDS work with O∗NET descriptors that could have attenuated a potential association.
Consistent with prior studies in ARDS6,7 and general ICU1,36 populations, we found that post-ARDS functional impairments (particularly the combination of physical and psychosocial impairments) were negatively associated with RTW at 6 and 12 months. Although prior RTW studies examined function as a single domain (eg, physical, emotional, or cognitive),1,7 we observed that most survivors reported multiple functional impairments, motivating our approach to analyze function in the context of co-occurring, rather than singular, impairments.
In this analysis, 50% to 70% of psychosocial impairments were related to emotional functional impairment (ie, SF-36 MH score <40).37 This finding is consistent with prior reports demonstrating that anxiety,38 depression,39 and post-traumatic stress symptoms40,41 are common and often co-occur42, 43, 44 in ICU survivors. Given that psychosocial impairments, alone or combined with physical impairments, were negatively associated with RTW after ARDS, specific emphasis on early recognition and management of psychosocial impairments (ie, cognitive, interpersonal, and mental health issues) may be important in assisting with subsequent RTW.
Notably, approximately one third and one half of survivors, respectively, reported pain and fatigue symptoms that were negatively associated with RTW. Similar symptoms contributing to functional disability and RTW have been shown in low back pain/radiculopathy,45 cancer,46,47 and heart failure48 populations. In ICU49,50 and ARDS51 survivors, such symptoms commonly co-exist with physical, cognitive, and mental impairments. Hence, in post-ICU follow-up clinics, early recognition of pain or fatigue symptoms may help prompt a broad exploration of other impairments, and a multidisciplinary and proactive management approach to assist with RTW efforts and potentially reduce chronic and ongoing symptoms in ARDS survivors.
This study had many strengths, including its multicenter longitudinal design, relatively large sample size of previously employed ARDS survivors, high participant retention rate, and detailed evaluation of pre-ARDS workload and post-ARDS impairments and symptoms with return to work. However, our study also has several limitations. First, as an observational study, we cannot make causal inferences between impairments, pain, fatigue, and return to work. Thus, some of the psychosocial impairment may be related to not returning to work. Second, baseline status for these factors is lacking to inform which impairments and symptoms are new or worse after ARDS. Third, we used the SF-36 and Mini-Mental State Examination to measure aspects of functional impairment, which do fully capture functional ability in comparison with physical performance-based tests or more detailed cognitive test batteries.52 Moreover, functional capacity evaluations, work ability indices, and work limitation questionnaires could be applied in future research to build on the findings of this evaluation. Fourth, we dichotomized workload, impairments, and symptoms, rather than analyzing as continuous variables. This approach can lead to loss of information. However, given the novelty and complexity of these analyses, this approach was used for purposes of feasibility and for assisting in easy interpretation of findings. Moreover, in using O∗NET, dichotomization is consistent with the system’s standard scoring. Finally, the RTW follow-up data were collected between 2008 and 2015. Although there are ongoing changes in ICU clinical practice, there is no strong and consistent evidence, as of yet, that such changes have impacted long-term physical, cognitive, and psychological outcomes.53 However, the timing of these study data and potential impact of such ICU changes should be considered when interpreting the study findings.
Interpretation
Almost half of previously employed ARDS survivors had not returned to work at 6 months, with only modest improvement at 12 months. Moreover, at 12 months, 53% reported physical or psychosocial impairments, and 34% and 56% reported pain and fatigue symptoms, respectively. These impairments and symptoms were negatively associated with RTW, whereas survivors’ pre-ARDS workload phenotype was not. These findings are important for designing and implementing vocational rehabilitation interventions to help address a crisis of joblessness among ARDS survivors.
Take-home Points.
Study Question: What are the associations among survivors’ pre-ARDS workload, post-ARDS functional impairment, pain, and fatigue with their return to work (RTW) status 12 months after ARDS?
Results: Functional impairment phenotypes and symptoms were negatively associated with RTW after ARDS, and pre-ARDS occupational workload phenotype was not.
Interpretation: These findings are important for designing and implementing vocational rehabilitation interventions to help address a crisis of joblessness among ARDS survivors.
Acknowledgments
Author contributions: H. S. conceived the work. H. S. performed the statistical analyses and data interpretation and wrote the initial draft of the manuscript. H. J. T. and S. M. supervised the analysis. H. J. T., S. M., C. T. H., V. D. D., M. M. H., R. O. H., B. B. K., and D. M. N. contributed substantially to the study design and data interpretation and critically revised the manuscript for important intellectual content. All authors gave final approval of the submitted version of the manuscript and agreed to be accountable for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. H. S., H. J. T., and D. M. N. are the guarantors of the content of the manuscript.
Financial/nonfinancial disclosures: None declared.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Drs Kamdar and Needham contributed equally to this manuscript.
FUNDING/SUPPORT: H. S. is supported by The Hester McLaws Dissertation Research Award from University of Washington. This research was supported by the National Heart, Lung, and Blood Institute grants N01HR56170, R01HL091760, and 3R01HL091760-02S1 along with funding for the ALTA, EDEN, OMEGA and SAILS trials (NHLBI contracts HHSN268200536165C to HHSN268200536176C and HHSN268200536179C) and the Hester McLaws Dissertation Research Award.
Supplementary Data
References
- 1.Hodgson C.L., Haines K.J., Bailey M. Predictors of return to work in survivors of critical illness. J Crit Care. 2018;48:21–25. doi: 10.1016/j.jcrc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Kamdar B.B., Minxuan H., Dinglas V.D. Joblessness and lost earnings after acute respiratory distress syndrome in a 1-year national multicenter study. Am J Resp Crit Care Med. 2017;196(8):1012–1020. doi: 10.1164/rccm.201611-2327OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamdar B.B., Suri R., Suchyta M.R. Return to work after critical illness: a systematic review and meta-analysis. Thorax. 2020;75(1):17. doi: 10.1136/thoraxjnl-2019-213803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su H., Dreesmann N.J., Hough C.L., Bridges E., Thompson H.J. Factors associated with employment outcome after critical illness: systematic review, meta-analysis, and meta-regression. J Adv Nurs. 2021 Feb;77(2):653–663. doi: 10.1111/jan.14631. [DOI] [PubMed] [Google Scholar]
- 5.Kamdar B.B., Sepulveda K.A., Chong A. Return to work and lost earnings after acute respiratory distress syndrome: A 5-year prospective, longitudinal study of long-term survivors. Thorax. 2018;73(2):125–133. doi: 10.1136/thoraxjnl-2017-210217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herridge M.S., Cheung A.M., Tansey C.M. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 7.Rothenhäusler H.B., Ehrentraut S., Stoll C., Schelling G., Kapfhammer H.P. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23(2):90–96. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 8.Heerkens Y., Engels J., Kuiper C., Van der Gulden J., Oostendorp R. The use of the ICF to describe work related factors influencing the health of employees. Disabil Rehabil. 2004;26(17):1060–1066. doi: 10.1080/09638280410001703530. [DOI] [PubMed] [Google Scholar]
- 9.Fraser R., Machamer J., Temkin N., Dikmen S., Doctor J. Return to work in traumatic brain injury (TBI): A perspective on capacity for job complexity. J Vocat Rehabil. 2006;25:141–148. [Google Scholar]
- 10.Cancelliere C., Donovan J., Stochkendahl M.J. Factors affecting return to work after injury or illness: best evidence synthesis of systematic reviews. Chiropr Man Therap. 2016;24(1) doi: 10.1186/s12998-016-0113-z. 32-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feuerstein M., Todd B.L., Moskowitz M.C. Work in cancer survivors: a model for practice and research. J Cancer Surviv. 2010;4(4):415–437. doi: 10.1007/s11764-010-0154-6. [DOI] [PubMed] [Google Scholar]
- 12.Schultz I.Z., Stowell A.W., Feuerstein M., Gatchel R.J. Models of return to work for musculoskeletal disorders. J Occup Rehabil. 2007;17(2):327–352. doi: 10.1007/s10926-007-9071-6. [DOI] [PubMed] [Google Scholar]
- 13.Dinglas V.D., Hopkins R.O., Wozniak A.W. One-year outcomes of rosuvastatin versus placebo in sepsis-associated acute respiratory distress syndrome: prospective follow-up of SAILS randomised trial. Thorax. 2016;71(5):401–410. doi: 10.1136/thoraxjnl-2015-208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Needham D.M., Dinglas V.D., Bienvenu O.J. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532. doi: 10.1136/bmj.f1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Needham D.M., Colantuoni E., Dinglas V.D. Rosuvastatin versus placebo for delirium in intensive care and subsequent cognitive impairment in patients with sepsis-associated acute respiratory distress syndrome: an ancillary study to a randomised controlled trial. Lancet Respir Med. 2016;4(3):203–212. doi: 10.1016/S2213-2600(16)00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Needham D.M., Dinglas V.D., Morris P.E. Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding: EDEN trial follow-up. Am J Respir Crit Care Med. 2013;188(5):567–576. doi: 10.1164/rccm.201304-0651OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay M.A., Brower R.G., Carson S. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rice T.W., Wheeler A.P., Thompson B.T. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice T.W., Wheeler A.P., Thompson B.T., deBoisblanc B.P., Steingrub J., Rock P. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truwit J.D., Bernard G.R., Steingrub J. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Equator Network The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. https://www.equator-network.org/reporting-guidelines/strobe/ Accessed August 30, 2020. [DOI] [PMC free article] [PubMed]
- 22.Knaus W.A., Wagner D.P., Draper E.A. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 23.Ruhl A.P., Huang M., Colantuoni E. Healthcare utilization and costs in ARDS survivors: a 1-year longitudinal national US multicenter study. Intensive Care Med. 2017;43(7):980–991. doi: 10.1007/s00134-017-4827-8. [DOI] [PubMed] [Google Scholar]
- 24.National Center for O∗NET Development. O∗NET Resource Center. www.onetcenter.org Accessed May, 24, 2019.
- 25.National Center for O∗NET Development. O∗NET OnLine. www.onetonline.org/help/online/scales Accessed May, 24, 2019.
- 26.Hopkins R.O., Brett S. Chronic neurocognitive effects of critical illness. Curr Opin Crit Care. 2005;11(4):369–375. doi: 10.1097/01.ccx.0000166399.88635.a5. [DOI] [PubMed] [Google Scholar]
- 27.Newkirk L.A., Kim J.M., Thompson J.M., Tinklenberg J.R., Yesavage J.A., Taylor J.L. Validation of a 26-point telephone version of the Mini-Mental State Examination. J Geriatr Psychiatry Neurol. 2004;17(2):81–87. doi: 10.1177/0891988704264534. [DOI] [PubMed] [Google Scholar]
- 28.Pfoh E.R., Chan K.S., Dinglas V.D. Cognitive screening among acute respiratory failure survivors: a cross-sectional evaluation of the Mini-Mental State Examination. Crit Care. 2015;19(1):220. doi: 10.1186/s13054-015-0934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware J., Kosinski M.A., Dewey J. QualityMetric Incorporated; Lincoln: 2000. How to score version 2 of the SF-36® Health Survey. [Google Scholar]
- 30.Butt Z., Lai J.-S., Rao D., Heinemann A.W., Bill A., Cella D. Measurement of fatigue in cancer, stroke, and HIV using the Functional Assessment of Chronic Illness Therapy—Fatigue (FACIT-F) scale. J Psychosom Res. 2013;74(1):64–68. doi: 10.1016/j.jpsychores.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearney J., Dyson K., Andrew E., Bernard S., Smith K. Factors associated with return to work among survivors of out-of-hospital cardiac arrest. Resuscitation. 2020;146:203–212. doi: 10.1016/j.resuscitation.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Walker W.C., Marwitz J.H., Kreutzer J.S., Hart T., Novack T.A. Occupational categories and return to work after traumatic brain injury: a multicenter study. Arch Phys Med Rehabil. 2006;87(12):1576–1582. doi: 10.1016/j.apmr.2006.08.335. [DOI] [PubMed] [Google Scholar]
- 33.Haveraaen L.A., Skarpaas L.S., Aas R.W. Job demands and decision control predicted return to work: the rapid-RTW cohort study. BMC Public Health. 2017;17(1):154. doi: 10.1186/s12889-016-3942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lombardi A.V., Jr., Nunley R.M., Berend K.R. Do patients return to work after total knee arthroplasty? Clin Orthop Relat Res. 2014;472(1):138–146. doi: 10.1007/s11999-013-3099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haveraaen L., Skarpaas L., Berg J., Aas R.W. Do psychological job demands, decision control and social support predict return to work three months after the end of a return-to-work (RTW) programme? The rapid-RTW cohort study. Work. 2015;53 doi: 10.3233/WOR-152216. [DOI] [PubMed] [Google Scholar]
- 36.Norman B.C., Jackson J.C., Graves J.A. Employment outcomes after critical illness: an analysis of the bringing to light the risk factors and incidence of neuropsychological dysfunction in ICU survivors cohort. Crit Care Med. 2016;44(11):2003–2009. doi: 10.1097/CCM.0000000000001849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfoh E.R., Chan K.S., Dinglas V.D. The SF-36 offers a strong measure of mental health symptoms in survivors of acute respiratory failure: a tri-national analysis. Ann Am Thorac Soc. 2016;13(8):1343–1350. doi: 10.1513/AnnalsATS.201510-705OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikayin S., Rabiee A., Hashem M.D. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabiee A., Nikayin S., Hashem M.D. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744–1753. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Righy C., Rosa R.G., da Silva R.T.A. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Crit Care. 2019;23(1):213. doi: 10.1186/s13054-019-2489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker A.M., Sricharoenchai T., Raparla S., Schneck K.W., Bienvenu O.J., Needham D.M. Posttraumatic stress disorder in critical illness survivors: a metaanalysis. Crit Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 42.Bienvenu O.J., Friedman L.A., Colantuoni E. Psychiatric symptoms after acute respiratory distress syndrome: a 5-year longitudinal study. Intensive Care Med. 2018;44(1):38–47. doi: 10.1007/s00134-017-5009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang M., Parker A.M., Bienvenu O.J. Psychiatric symptoms in acute respiratory distress syndrome survivors: a 1-year national multicenter study. Crit Care Med. 2016;44(5):954–965. doi: 10.1097/CCM.0000000000001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bienvenu O.J., Colantuoni E., Mendez-Tellez P.A. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury: a 2-year longitudinal study. Crit Care Med. 2015;43(3) doi: 10.1097/CCM.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steenstra I.A., Munhall C., Irvin E. Systematic review of prognostic factors for return to work in workers with sub acute and chronic low back pain. J Occup Rehabil. 2017;27(3):369–381. doi: 10.1007/s10926-016-9666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barsevick A.M., Whitmer K., Nail L.M., Beck S.L., Dudley W.N. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31(1):85–95. doi: 10.1016/j.jpainsymman.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Dorland H.F., Abma F.I., Van Zon S.K.R. Fatigue and depressive symptoms improve but remain negatively related to work functioning over 18 months after return to work in cancer patients. J Cancer Surviv. 2018;12(3):371–378. doi: 10.1007/s11764-018-0676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herr J.K., Salyer J., Lyon D.E., Goodloe L., Schubert C., Clement D.G. Heart failure symptom relationships: a systematic review. J Cardiovasc Nurs. 2014;29(5):416–422. doi: 10.1097/JCN.0b013e31829b675e. [DOI] [PubMed] [Google Scholar]
- 49.Wintermann G.B., Rosendahl J., Weidner K., Strauß B., Hinz A., Petrowski K. Self-reported fatigue following intensive care of chronically critically ill patients: a prospective cohort study. J Intensive Care. 2018;6:27. doi: 10.1186/s40560-018-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spadaro S., Capuzzo M., Valpiani G. Fatigue in intensive care survivors one year after discharge. Health Qual Life Outcomes. 2016;14(1):148. doi: 10.1186/s12955-016-0554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neufeld K.J., Leoutsakos J.S., Yan H. Fatigue symptoms during the first year after ARDS. Chest. 2020;158(3):999–1007. doi: 10.1016/j.chest.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan K.S., Aronson Friedman L., Dinglas V.D. Are physical measures related to patient-centred outcomes in ARDS survivors? Thorax. 2017;72(10):884–892. doi: 10.1136/thoraxjnl-2016-209400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Devlin J.W., Skrobik Y., Gélinas C. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.