Abstract
Background
Immune checkpoint inhibitors (ICIs) are standard treatments for advanced non-small cell lung cancer and have expanded use in small cell lung cancer. Although generally better tolerated than traditional chemotherapy, immune-related adverse events, such as immune checkpoint inhibitor-related pneumonitis (ICI-P), remain poorly understood toxicities that limit ICI treatment and can result in considerable morbidity. In this retrospective case-control study, we assessed a lung cancer cohort to identify ICI-P risk factors.
Research Question
What are the risk factors, clinical presentations, radiographic findings, and outcomes for ICI-P in a real-world lung cancer cohort? Do chronic pulmonary diseases confer increased risk for ICI-P?
Study Design and Methods
Medical records from lung cancer patients receiving nivolumab, pembrolizumab, or combination ipilimumab and nivolumab at six centers in North Carolina were reviewed (January 2004-July 2017). Patients with ICI-P and control participants were characterized, and logistic regression was used to assess for ICI-P risk factors.
Results
Three hundred fifteen lung cancer patients who predominantly received nivolumab (76.5%) or pembrolizumab (22%) were included. The incidence of ICI-P was 9.5%, with a median time to diagnosis of 52.5 days. Most patients with ICI-P had cases of high severity, and eight patients (27%) died with ongoing ICI-P treatment. Development of ICI-P was independently associated with the presence of baseline fibrosis on chest CT scan (adjusted OR [aOR], 6.61; 95% CI, 2.48-17.7), a composite measure of obstructive lung disease (aOR, 2.79; 95% CI, 1.07-7.29), and treatment with pembrolizumab (aOR, 2.57; 95% CI, 1.08-6.11).
Interpretation
In this cohort, ICI-P was more common and severe than previously reported and carried an unexpectedly high mortality rate. Risk for ICI-P was shown to be independently associated with several chronic pulmonary diseases, which may account for the higher incidence of ICI-P in patients with lung cancer.
Key Words: immune checkpoint inhibitor, lung cancer, pneumonitis
Abbreviations: aOR, adjusted OR; GGO, ground-glass opacity; ICI, immune checkpoint inhibitor; ICI-P, immune checkpoint inhibitor-related pneumonitis; ILD, interstitial lung disease; IQR, interquartile range; irAE, immune-related adverse event; NSCLC, non-small cell lung cancer; PD-L1, programmed death-ligand 1
Since the Food and Drug Administration approval of ipilimumab for metastatic melanoma in 2011, the use of immune checkpoint inhibitors (ICIs) has grown dramatically, becoming standard treatments for locally advanced1 and advanced non-small cell lung cancer (NSCLC) without driver mutations and have expanded use in small cell lung cancer.2,3 Although clinical trials demonstrate improvements in cancer-related outcomes,1,4, 5, 6, 7, 8, 9 ICIs bring a unique class of toxicities termed immune-related adverse events (irAEs), which can affect multiple organ systems through poorly understood mechanisms. These irAEs range in severity from mild and self-limited to fulminant and life-threatening and often necessitate immunomodulatory treatments.10, 11, 12
Immune checkpoint inhibitor-related pneumonitis (ICI-P) is a morbid irAE13,14 that results in wide-ranging respiratory symptoms with pulmonary parenchymal abnormalities and can progress to respiratory failure and death.11,15 Although clinical trials suggest the incidence of ICI-P is rare (generally < 5%),16,17 restrictive enrollment criteria may underestimate the true incidence in clinical practice. Indeed, real-world experiences suggest the incidence of ICI-P ranges from 5% to 19% in lung cancer cohorts.18, 19, 20 Previous studies report that the incidence of ICI-P is greatest with program cell death protein 1 inhibitors in comparison to programmed death-ligand 1 [PD-L1] and cytotoxic T-lymphocyte-associated protein 4 inhibitors),16,21 the presence of autoantibodies,22 pre-existing pulmonary fibrosis,23 interstitial lung disease (ILD),19,24 tumor histologic subtype (squamous cell carcinoma > adenocarcinoma),18 radiation therapy,25 and advanced age.19
Best clinical practices regarding the diagnosis, management, and risk stratification of ICI-P remains a significant barrier to improved lung cancer treatment and outcomes.26 In this retrospective case-control study of ICI-P in lung cancer patients, we assessed the influence of underlying pulmonary diseases, demographics, cancer therapies, and comorbidities on ICI-P risk while also characterizing real-world ICI-P management practices and outcomes.
Methods
Patients
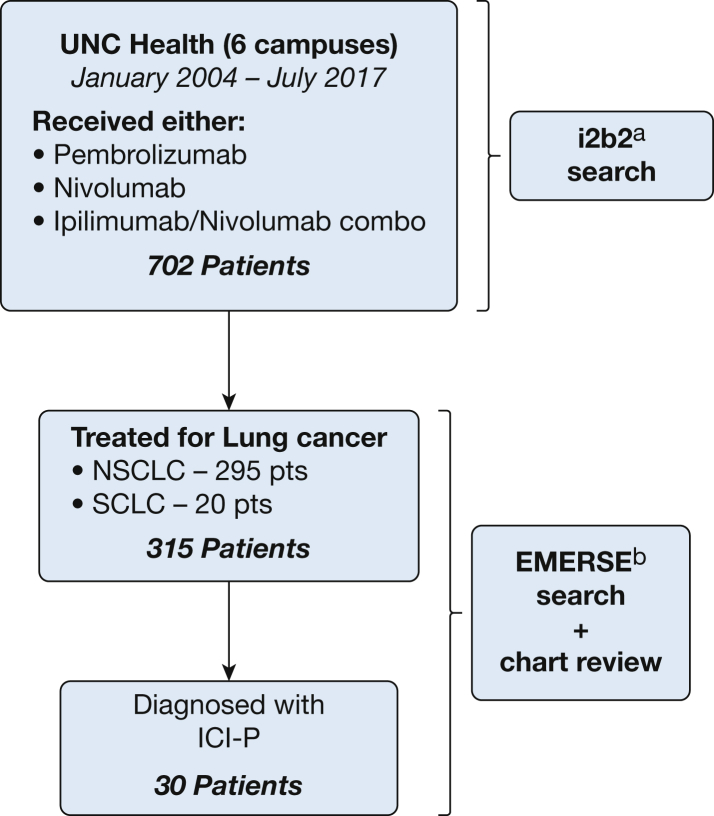
This study was conducted following the amended Declaration of Helsinki and approved by the University of North Carolina Institutional Review Board (Identifier: 17-1841). The i2b227 platform (Informatics for Integrating Biology and the Bedside Center) was used to query the electronic medical record for patients who received either nivolumab, pembrolizumab, or combination ipilimumab and nivolumab between January 2004 and July 2017. Six University of North Carolina health centers were included (one academic, one community referral center, and four community centers). All patients who received ICI for lung cancer treatment and those who developed ICI-P were identified through detailed chart reviews and the Electronic Medical Record Search Engine.28
Recorded Variables
A structured Research Electronic Data Capture29 database was developed for data collection. The Eastern Cooperative Oncology Group performance status, tumor histologic subtype, PD-L1 expression status, previous and concurrent cancer therapies, number of ICI cycles, occurrence of irAEs, and reasons for ICI cessation were recorded. Baseline pulmonary function testing and chest CT scan findings, including the presence of emphysema, fibrosis, pulmonary nodules, masses, and ground-glass opacities (GGOs), were recorded. The most recent chest CT scan obtained before the initiation of ICI treatment was recorded as baseline if both the radiology report and images were available for review. Pertinent radiographic abnormalities were recorded as present if the interpreting radiologist or reviewing study pulmonologist identified the presence of the referenced finding. Underlying fibrotic lung disease on baseline CT scan was recorded if fibrotic changes, including radiation fibrosis, fibrosis secondary to ILD, or fibrotic changes of unclear origin, were present. A composite measure of obstructive lung disease that included COPD by history, emphysema on baseline chest CT scan, or obstruction (FEV1 to FVC ratio less than lower limit of normal) on baseline spirometry was implemented to improve identification of obstructive lung disease in the cohort.
Because ICI-P is a diagnosis of exclusion, each case was reviewed to assess the diagnostic certainty of ICI-P and a classification scheme was implemented (Table 1). If ICI-P developed meeting definite, probable, or possible criteria, the patient was included in the ICI-P group for all analyses. Patients with indeterminant disease were excluded from the ICI-P group because of a lack of clinical or radiographic data supporting the diagnosis. The control group (no ICI-P) included all patients treated with ICI who did not meet the definite, probable, or possible ICI-P definitions from Table 1. Detailed ICI-P syndromic parameters were recorded including pulmonary signs and symptoms, concurrent irAEs, dominant CT scan radiographic patterns, confounding cardiopulmonary diagnoses, ICI-P severity, oxygen requirement, and treatment selection and duration. Time to death after ICI initiation and ICI-P diagnosis were recorded. For patients who received corticosteroids for ICI-P treatment, a cumulative prednisone-equivalent dose was calculated.30
Table 1.
ICI-P Diagnostic Certainty
| Disease Type | Description |
|---|---|
| Definite | Clinical and radiographic findings were consistent with ICI-P, appropriate diagnostic testing was performed, and low suspicion was present for competing cardiopulmonary diseases. |
| Probable | Clinical and radiographic findings were consistent with ICI-P, appropriate diagnostic testing was performed, but other concurrent cardiopulmonary diseases (eg, coexisting infection or radiation pneumonitis) could not be excluded entirely. |
| Possible | Clinical and radiographic presentation were consistent with ICI-P, but discordant data (eg, positive respiratory pathogen panel) or other cardiopulmonary disease (eg, volume overload, radiation pneumonitis) likely also contributing to the clinical presentation were present. |
| Indeterminant | The patient received a diagnosis of ICI-P and received treatment for it without supporting clinical, radiographic, or diagnostic testing. |
ICI-P = immune checkpoint inhibitor-related pneumonitis.
Statistical Analyses
Descriptive statistics were used to summarize the cohorts’ medical histories and clinical parameters. Counts and percentages were produced for categorical variables, whereas mean ± SD or median (interquartile range [IQR]) were computed for continuous variables. Vital status was assessed, and living patients were censored on the date of chart extraction. Kaplan-Meier estimates for median survival after ICI initiation were produced for ICI-P patients and control participants; the log-rank and Wilcoxon tests were conducted to assess for differences between these groups. A multivariate time-dependent Cox proportional hazards regression model for time to death after ICI initiation was performed, with ICI-P status managed as time varying using the counting process. This model also was adjusted for age, sex, smoking history, Eastern Cooperative Oncology Group performance status, and tumor type to produce adjusted hazard ratios and 95% CIs. Patients with unknown vital status because of loss to follow-up or incomplete records were excluded from the above survival analyses.
Univariate and multivariate logistic regression analysis was performed on prespecified risk factors for the odds of ICI-P development, including patient demographics (eg, age, sex), medical histories (eg, ILD, COPD), cancer-related factors (eg, radiation treatment, tumor histologic findings, ICI selection, PD-L1 expression), and radiographic patterns (eg, presence of baseline fibrosis, emphysema). Patients were excluded from multivariate logistic regression analyses if no baseline CT scan was available for review. ORs and asymptotic and exact 95% CIs were produced for univariate associations because of the small sample size. The multivariate analysis included predictors associated significantly with ICI-P risk at the .05 significance level from the univariate analysis and was parsimoniously reduced further to include only predictors independently significant (at the .05 level) to produce adjusted ORs (aORs) and 95% CIs. All analyses were performed with SAS version 9.4 software (SAS Institute Inc.). Statistical significance was determined at P = .05. Complete case analyses were performed during univariate and multivariate analyses, excluding patients with missing data items.
Results
Nivolumab (76.5%), pembrolizumab (22.2%), or combination ipilimumab and nivolumab (1.9%) were used for treatment of lung cancer in 315 patients. ICI-P meeting definite, probable, or possible criteria (Table 1) was diagnosed in 30 patients for an incidence of 9.5% (Fig 1). Two patients had indeterminant disease because of a lack of chest imaging at diagnosis. The cohort was predominantly White (79%) and current or former smokers (91.1%). A history of COPD or ILD was identified in 127 patients (40.3%) and five patients (1.6%), respectively. Ten patients (3.2%) had a history of connective tissue disease (Table 2). Baseline serologic results for connective tissue disease-associated antibodies were available in 10 patients. Antinuclear antibody was present in five patients and rheumatoid factor was present in one patient. A baseline chest CT scan was performed within the year before ICI initiation in 292 patients (92.7%). Baseline chest CT scans were notable for fibrotic changes in 24 patients (8.2%), GGOs in 66 patients (22.6%), emphysematous changes in 106 patients (36.3%), pulmonary mass or masses in 198 patients (67.8%), and pulmonary nodules in 219 patients (75%) subjects. Spirometry was performed in 76 patients (24.1%) within the year before ICI initiation. Airflow obstruction and restriction (normal FEV1 to FVC ratio and FVC less than lower limit of normal) were present in 61.8% and 23.7% of tested patients, respectively. Diffusion capacity for carbon monoxide, available for 69 patients, averaged 59.9 % predicted (SD, 17.1 % predicted) (Table 2).
Figure 1.
Flow chart showing cohort selection. ai2b2 is a cohort identification tool developed by the Informatics for Integrating Biology and the Bedside Center,27 a National Institutes of Health-funded National Center for Biomedical Computing. bEMERSE allows users to search free text (unstructured) clinical notes from the electronic health record.28 EMERSE = Electronic Medical Record Search Engine; ICI-P = immune checkpoint inhibitor-related pneumonitis; NSCLC = non-small cell lung cancer; pts = patients; SCLC = small cell lung cancer; UNC = University of North Carolina.
Table 2.
Cohort Demographics, Medical and Social Histories, and Lung Function Test Results
| Variable | All Patients (N = 315) | ICI-P (n = 30) | No ICI-P (n = 285) |
|---|---|---|---|
| Age, yr | 66 (58-73) | 62 (58.3-70.5) | 66 (58-73) |
| Male sex | 158 (50.2) | 19 (63.3) | 139 (48.8) |
| Race | |||
| White | 249 (79.0) | 23 (76.7) | 226 (79.3) |
| Black | 58 (18.4) | 7 (23.3) | 51 (17.9) |
| Asian | 1 (0.3) | 0 (0.0) | 1 (0.4) |
| Other | 7 (2.2) | 0 (0.0) | 7 (2.5) |
| Smoking history | |||
| Ever | 287 (91.1) | 29 (96.7) | 258 (90.5) |
| Current | 55 (19.2) | 5 (17.2) | 50 (19.4) |
| Never | 28 (8.9) | 1 (3.3) | 27 (9.5) |
| Pack years | 36.5 (25-50) | 30 (25-47) | 40 (25-50) |
| Pulmonary disease history | |||
| Asthma | 21 (6.7) | 2 (6.7) | 19 (6.7) |
| COPD | 127 (40.3) | 18 (60.0) | 109 (38.2) |
| ILD | 5 (1.6) | 3 (10.0) | 2 (0.7) |
| Othera | 7 (2.2) | 3 (10.0) | 4 (1.4) |
| Autoimmune or CTD history | 10 (3.2) | 1 (3.3) | 9 (3.2) |
| History of chronic infection | |||
| HIV | 3 (1.0) | 2 (6.7) | 1 (0.4) |
| Hepatitis C | 4 (1.3) | 1 (3.3) | 3 (1.1) |
| Baseline spirometry | |||
| Normal results | 11 (3.5) | 0 (0.0) | 11 (3.9) |
| Obstructionb | 47 (14.9) | 6 (20.0) | 41 (14.4) |
| Restrictionc | 18 (5.7) | 3 (10.0) | 15 (5.3) |
| No spirometry performed | 239 (75.9) | 21 (70.0) | 218 (76.5) |
| Dlco % predictedd | 59.9 ± 17.1 | 54.9 ± 15.0 | 60.6 ± 17.4 |
All data are presented as No. (%), median (interquartile range), or mean ± SD. CTD = connective tissue disease, Dlco = diffusion capacity for carbon monoxide; ICI-P = immune checkpoint inhibitor-related pneumonitis; ILD = interstitial lung disease.
Sarcoidosis, pulmonary hypertension, and fungal or mycobacterial pulmonary infection.
FEV1 to FVC ratio less than lower limit of normal.
Normal FEV1 to FVC ratio and FVC less than lower limit of normal.
Obtained in 69 of 315 patients (21.9%).
Most of the cohort was treated for NSCLC (93.7% [295/315]) with adenocarcinoma (61.7%) and squamous cell carcinoma (28.5%) representing the dominant histologic subtypes (Table 3). ICI was used predominantly in the second-line setting, because most patients received chemotherapy (86.7%), thoracic radiotherapy (44.1%), or thoracic surgery (14.6%) before ICI initiation. PD-L1 expression was determined from histologic specimens in 96 (30.5%) of the cases and 68 (21.6%) showed positive PD-L1 results (PD-L1 ≥ 1%), with a median expression level for PD-L1 of 80% (IQR, 50%-90%) on commercial testing. Of the 68 patients with confirmed positive PD-L1 results, 54 patients (79.4%) received pembrolizumab. Of the 23 patients receiving ICI as a first-line treatment, 21 patients (91.3%) received pembrolizumab. The median number of ICI cycles received was six (IQR, 3-13), and 95.6% of patients received ICI as monotherapy. The most frequent reasons for ICI cessation were progression of disease (48.9%), hospice care or death (20.6%), and irAEs (17.5%) (Table 4).
Table 3.
Lung Cancer History and Treatment
| Variable | All Patients (N = 315) | ICI-P (n = 30) | No ICI-P (n = 285) |
|---|---|---|---|
| Treatment center | |||
| UNC Chapel Hill | 108 (34.4) | 9 (31.0) | 99 (34.7) |
| UNC Rex Healthcare | 108 (34.4) | 13 (44.8) | 95 (33.3) |
| UNC Johnston | 27 (8.6) | 1 (3.4) | 26 (9.1) |
| UNC Lenoir | 17 (5.4) | 2 (6.9) | 15 (5.3) |
| UNC High Point | 37 (11.8) | 2 (6.9) | 35 (12.3) |
| UNC Pardee | 17 (5.4) | 2 (6.9) | 15 (5.3) |
| Lung cancer histologic findings | |||
| Non-small cell lung cancer | 295 (93.7) | 29 (96.7) | 266 (93.3) |
| Adenocarcinoma | 182 (61.7) | 16 (55.2) | 166 (62.4) |
| Squamous cell carcinoma | 84 (28.5) | 10 (34.5) | 74 (27.8) |
| Large cell carcinoma | 8 (2.7) | 0 (0.0) | 8 (3.0) |
| Poorly differentiated | 18 (6.1) | 2 (6.9) | 16 (6.0) |
| Othera | 3 (1.0) | 1 (3.4) | 2 (0.7) |
| Small cell lung cancer | 20 (6.3) | 1 (3.3) | 19 (6.7) |
| Prior cancer treatment | |||
| Thoracic surgery | 46 (14.6) | 3 (10.0) | 43 (15.1) |
| Thoracic radiotherapy | 139 (44.1) | 18 (60.0) | 121 (42.5) |
| Targeted therapy | 49 (15.6) | 4 (13.3) | 45 (15.8) |
| Chemotherapy | 273 (86.7) | 23 (76.7) | 250 (87.7) |
| None | 23 (7.3) | 5 (16.7) | 18 (6.3) |
| ECOG performance status | |||
| 0 | 17 (5.4) | 1 (3.3) | 16 (5.6) |
| 1 | 228 (72.4) | 23 (76.7) | 205 (71.9) |
| 2 | 65 (20.6) | 6 (20.0) | 59 (20.7) |
| 3 | 3 (1.0) | 0 (0.0) | 3 (1.1) |
| 4 | 2 (0.6) | 0 (0.0) | 2 (0.7) |
All data are presented as No. (%). ECOG = Eastern Cooperative Oncology Group; ICI-P = immune checkpoint inhibitor-related pneumonitis; UNC = University of North Carolina.
Mucoepidermoid carcinoma, nonmucinous adenocarcinoma, not specified.
Table 4.
ICI Treatment Parameters and Outcomes
| Variable | All Patients (N = 315) | ICI-P (n = 30) | No ICI-P (n = 285) |
|---|---|---|---|
| ICI | |||
| Pembrolizumab | 70 (22.2) | 11 (36.7) | 59 (20.7) |
| Nivolumab | 241 (76.5) | 18 (60.0) | 223 (78.2) |
| Ipilimumab plus nivolumab, median (IQR) | 6 (1.9) | 1 (3.3) | 5 (1.8) |
| ICI cycles, median (IQR) | 6 (3-13) | 3.5 (2-6) | 6 (3-14) |
| PD-L1 expression status | |||
| Positivea | 68 (21.6) | 10 (33.3) | 58 (20.4) |
| Negative | 28 (8.9) | 3 (10.0) | 25 (8.8) |
| Not assessed | 219 (69.5) | 17 (56.7) | 202 (70.9) |
| Concurrent treatment with ICI | |||
| Chemotherapy | 7 (2.2) | 1 (3.3) | 6 (2.1) |
| Radiation | 6 (1.9) | 2 (6.7) | 4 (1.4) |
| Both | 1 (0.3) | 0 (0.0) | 1 (0.4) |
| None | 301 (95.6) | 27 (90) | 274 (96.1) |
| Development of an irAE | 80 (25.4) | 30 (100) | 50 (17.5) |
| Reason for ICI cessation | |||
| Progression of disease | 154 (48.9) | 2 (6.7) | 152 (53.3) |
| IrAE | 55 (17.5) | 26 (86.7) | 29 (10.2) |
| Treatment course completed | 8 (2.5) | 1 (3.3) | 7 (2.5) |
| Clinician/patient preference | 11 (3.5) | 0 (0.0) | 11 (3.9) |
| Shift in GOC, hospice, death | 65 (20.6) | 2 (6.7) | 63 (22.1) |
| ICI treatment ongoing | 8 (2.5) | 0 (0.0) | 8 (2.8) |
| Otherb | 25 (7.9) | 0 (0.0) | 25 (8.7) |
| Overall survival after ICI initiation, d | N = 285c | n = 29 | n = 256 |
| 90 | … | 103 (72-160), n = 21 | 296.5 (231-384), n = 264 |
| 180 | … | 145.5 (82-171), n = 26 | 278 (CI 229-362), n = 259 |
| 360 | … | 160 (84-231), n = 29 | 277.5 (225-362), n = 256 |
Data are presented as No. (%) or median (95% CI), unless otherwise indicated. Percentages may not sum to 100% because of rounding. GOC = goals of care; ICI = immune checkpoint inhibitor; ICI-P = immune checkpoint inhibitor-related pneumonitis; IQR = interquartile range; irAE = immune-related adverse event; PD-L1 = programmed cell death-ligand 1.
If PD-L1 expression was ≥ 1%.
Lost to follow-up (n = 23), anaphylaxis (n = 1), intolerance not attributed to irAE (n = 1).
Vital status unknown for 30 patients who either were lost to follow-up or transferred care to other centers.
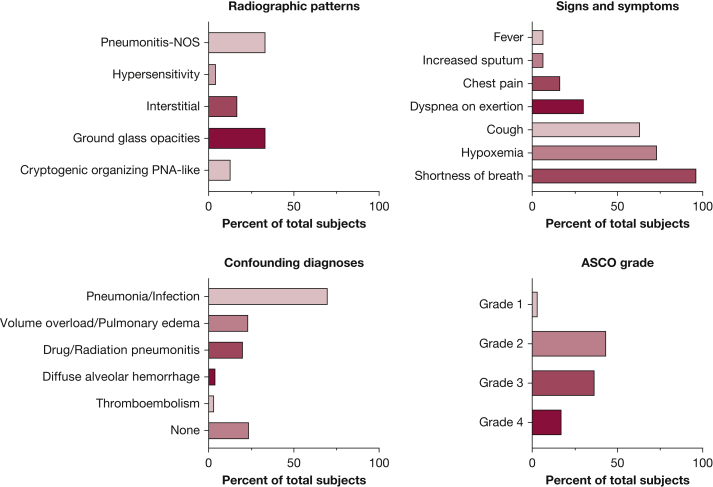
The median time to ICI-P diagnosis from initial ICI treatment was 52.5 days (IQR, 21-128 days). Shortness of breath (96.7%) and cough (63.3%) were the most frequent symptoms reported, and 73.3% of patients were hypoxemic with oxygen saturations of < 88% on room air at the time of diagnosis. More than half of patients with ICI-P had severe disease (American Society of Clinical Oncology grade ≥ 3)31 and 60% of patients were hospitalized for ICI-P management (Fig 2). Concurrent irAEs occurred in eight patients (26.7%), including hepatitis (three cases), dermatitis (two cases), and one case each of nephritis, inflammatory arthritis, myopericarditis, and adrenal insufficiency. Corticosteroids were used in 93.3% of patients with ICI-P, with a median duration of steroid treatment of 30 days (IQR, 11-78 days). The median cumulative prednisone-equivalent dose of corticosteroid was 1350 mg (IQR, 896-2,400 mg). In 21 patients (70%), ICI-P demonstrated clinical or radiographic improvement, or both, with corticosteroid treatment. In seven patients (23.3%), the response to treatment could not be determined either because of chronic steroid use (one patient), inadequate documentation of clinical follow-up (one patient), or time to death or hospice referral occurring within 1 week of steroid initiation (five patients). None of the patients received adjunct treatments to corticosteroids for ICI-P. Other cardiopulmonary diseases treated at the time of ICI-P diagnosis included pneumonia (70%), volume overload (23.3%), other drug or radiation pneumonitis (20%), alveolar hemorrhage (3.3%), and VTE (3.3%) (Fig 2). After ICI-P improvement, four patients (13.3%) demonstrated worsening disease with tapering of corticosteroids. Six patients, all with American Society of Clinical Oncology grade 2 ICI-P, were rechallenged with an ICI after treatment for ICI-P, and only one patient showed recurrence of ICI-P.
Figure 2.
A-D, Bar graphs showing ICI-P clinical and radiographic features. A, Relative frequency of ICI-P radiographic patterns as classified by Naidoo et al.20 B, Relative frequency of cardiopulmonary signs and symptoms at the time of ICI-P diagnosis. C, Relative frequency of confirmed or clinically suspected comorbid and confounding cardiopulmonary diseases treated at the time of ICI-P diagnosis. D, Relative frequency of ASCO pneumonitis severity grade.31 ASCO = American Society of Clinical Oncology; ICI-P = immune checkpoint inhibitor-related pneumonitis; NOS = not otherwise specified; PNA = pneumonia.
During evaluation for ICI-P, 80% of patients (24/30) underwent chest CT imaging. The chest CT scan radiographic subtypes were classified as described previously,20 including cryptogenic organizing pneumonia-like, GGO, interstitial, hypersensitivity, and pneumonitis not otherwise specified. The subtypes were distributed as follows: GGO (8/24 [33.3%]), pneumonitis not otherwise specified (8/24 [33.3%]), interstitial (4/24 [16.7%]), cryptogenic organizing pneumonia-like (3/24 [12.5%]), and hypersensitivity (1/24 [4.2%]) (Fig 2). Radiographic disease involved two or more lobes in 91.7% of patients (22/24).
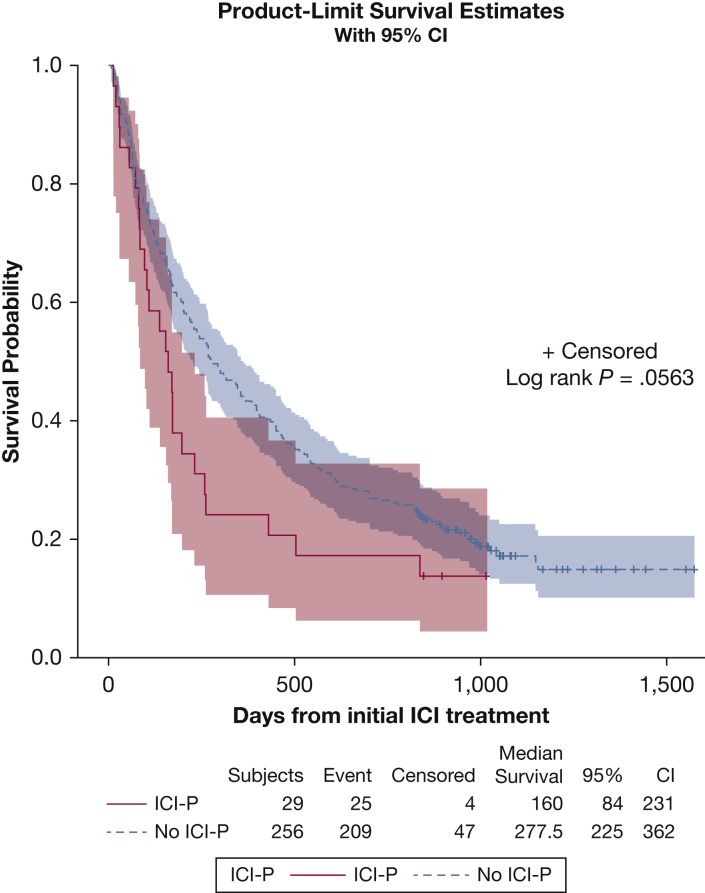
The survival analysis was performed on 285 patients (90%), because the remaining patients had unknown vital status resulting from loss to follow-up. At the time of chart extraction, 234 of these patients (74.3%) had died. Median survival after ICI initiation in the Kaplan-Meier analysis was 160 days (95% CI, 84-231 days) for patients with ICI-P and 277.5 days (95% CI, 225-362 days) for control participants (P = .056, log-rank statistic; P = .033, Wilcoxon statistic) (Fig 3). The multivariate time-dependent Cox regression model showed that patients who developed ICI-P had significantly increased risk of mortality (adjusted hazard ratio, 2.62; 95% CI, 1.71-4.01). Eight deaths (32% of patients with ICI-P) occurred while ICI-P treatment was ongoing, with a median time to death after ICI-P diagnosis of 8.5 days (IQR, 7-39 days). For each patient, progressive respiratory failure was the primary cause of death and uniformly resulted in transition to comfort care measures. Coexisting cardiopulmonary diseases also were considered as contributing factors in these deaths and included pneumonia (four patients) and one patient each with ILD, bronchial stenosis, thromboembolism, obstructive lung disease, and other irAEs (eg, myopericarditis). No autopsy results were available for review in deceased patients.
Figure 3.
Kaplan-Meier estimates for time to death after ICI initiation stratified by ICI-P status. The P value for the log-rank test for differences between ICI-P status is shown (middle right), along with median survival (days) for patients with ICI-P and control participants without ICI-P (bottom table). Of 285 patients, 30 were excluded because of unknown vital status (resulting from loss to follow-up). Censoring occurred on the day of chart extraction if patient was living. Median survival is reported in days. Event = death; ICI = immune checkpoint inhibitor; ICI-P = immune checkpoint inhibitor-related pneumonitis.
Univariate logistic regression analyses assessing hypothesized risk factors for ICI-P showed that the presence of ILD by history (OR, 15.7; 95% CI, 2.52-98.20), COPD by history (OR, 2.42; 95% CI, 1.12-5.22), the composite measure of obstructive lung disease (OR, 3.13; 95% CI, 1.24-7.88), the presence of fibrosis on baseline chest CT scan (OR, 7.06; 95% CI, 2.76-18.0), and the use of pembrolizumab (vs nivolumab; OR, 2.36; 95%, 1.06-5.30) were associated with increased risk of ICI-P developing (Table 5). A history of smoking, autoimmune disease, tumor histologic subtype, PD-L1 expression, and pertinent demographics did not significantly affect the risk of ICI-P developing (Table 5). Although not statistically significant, prior thoracic radiation therapy (OR, 2.03; 95% CI, 0.94-4.38) and baseline presence of GGO (OR, 2.18; 95% CI, 0.98-4.95) also demonstrated some degree of evidence for increased odds of ICI-P. The multivariate logistic regression analysis was performed on 290 patients (92%), because 23 patients were missing baseline chest CT imaging (missing baseline fibrosis status) and two patients received both nivolumab and pembrolizumab. Results showed that the composite measure of obstructive lung disease (aOR, 2.79; 95% CI, 1.07-7.29), baseline presence of fibrosis on chest CT scan (aOR, 6.61; 95% CI, 2.48-17.70), and treatment with pembrolizumab (vs nivolumab; aOR, 2.57; 95% CI, 1.08-6.11) were associated independently with increased odds for ICI-P developing.
Table 5.
Logistic Regression Analysis of Potential Risk Factors for ICI-P Development
| Variable | Univariate Analyses |
Multivariate Analyses (n = 290a) |
|||
|---|---|---|---|---|---|
| OR | 95% CI (Asymptotic) | 95% CI (Exact) | aOR | 95% CI (Asymptotic) | |
| Demographic risk factors | |||||
| Age, 10 y olderb | 0.90 | 0.62-1.30 | 0.62-1.30 | … | … |
| Female sex | 0.55 | 0.25-1.20 | 0.23-1.27 | … | … |
| Black racec | 1.40 | 0.57-3.43 | 0.48-3.59 | … | … |
| Medical history risk factors | |||||
| ILDd | 15.7 | 2.52-98.2 | 1.69-192.0 | … | … |
| COPDd | 2.42 | 1.12-5.22 | 1.05-5.73 | … | … |
| Composite obstructive lung diseasee | 3.13 | 1.24-7.88 | 1.13-9.08 | 2.79 | 1.07-7.29 |
| Autoimmune/connective tissue disease | 1.06 | 0.13-8.65 | 0.02-8.12 | … | … |
| Ever smoker | 3.03 | 0.40-23.2 | 0.46-128.0 | … | … |
| Current smokerf | 0.94 | 0.34-2.57 | 0.27-2.67 | … | … |
| Cancer-specific risk factors | … | … | |||
| Prior thoracic radiation therapy | 2.03 | 0.94-4.38 | 0.89-4.80 | … | … |
| Concurrent thoracic radiation therapyg | 4.00 | 0.74-21.6 | 0.36-25.7 | … | … |
| Squamous cell carcinoma (reference, adenocarcinoma) | 1.40 | 0.61-3.24 | 0.54-3.46 | … | … |
| SCLC or others (reference, adenocarcinoma) | 0.94 | 0.30-2.96 | 0.22-3.13 | … | … |
| Pembrolizumab (reference, nivolumab) | 2.36 | 1.06-5.30 | 0.95-5.63 | 2.57 | 1.08-6.11 |
| Ipilimumab and nivolumab (reference, nivolumab) | 2.44 | 0.27-22.2 | 0.05-23.6 | 3.48 | 0.36-33.2 |
| PD-L1 expression ≥ 1% | 1.44 | 0.36-5.67 | 0.33-8.78 | … | … |
| Radiographic risk factorsh | |||||
| Baseline fibrosisi | 7.06 | 2.76-18.0 | 2.39-19.60 | 6.61 | 2.48-17.7 |
| Baseline emphysema | 1.39 | 0.65-2.99 | 0.59-3.19 | … | … |
| Baseline ground-glass opacity | 2.18 | 0.98-4.85 | 0.88-5.14 | … | … |
| Baseline pulmonary mass(es) | 0.80 | 0.36-1.76 | 0.34-1.95 | … | … |
aOR = adjusted odds ratio; ICI = immune checkpoint inhibitor; ICI-P = immune checkpoint inhibitor-related pneumonitis; ILD = interstitial lung disease; PD-L1 = programed cell death-ligand 1; SCLC = small cell lung cancer. References for all categorical variables are the absence of the categorical variable unless otherwise noted.
Multivariate analysis modeled among those with complete case data on included covariables. Baseline CT scan was missing for 23 patients (hence missing baseline fibrosis status), and two patients sequentially received both pembrolizumab and nivolumab and were excluded.
Age is continuous. The effect of a 10-year (+1 SD) average increase is chosen as the unit of comparison.
Reference is all other races.
Recorded history in medical record of ILD or COPD.
Includes COPD by history, obstruction on spirometry, or emphysema on baseline chest CT scan.
Reference is never or prior smokers.
Concurrent with ICI therapy.
Radiographic findings were as reported by the radiologist or reviewing pulmonologist on CT scan performed before ICI initiation.
Includes radiation fibrosis, fibrosis secondary to ILD, or fibrotic changes from unclear causes.
Discussion
We present a real-world assessment of ICI-P incidence, management, and outcomes in a lung cancer cohort and report important risk factors for ICI-P development. The ICI-P diagnostic certainty criteria (Table 1) reduced the impact of diagnostic errors on ICI-P risk assessments while highlighting the varied clinical practices in a real-world setting. The incidence of ICI-P in this cohort (9.5%) is appreciably higher than that reported in clinical trials,21,32 but is consistent with prior real-world reports18, 19, 20 and likely reflects a higher-risk population of patients who ultimately receive these important therapies. In addition, we found only one patient with asymptomatic ICI-P, in contrast to most reports in which up to 50% of patients with ICI-P have American Society of Clinical Oncology grade 1 disease (asymptomatic).20,33,34 Although the possibility of missed subclinical disease may explain this discrepancy, the high frequency of baseline chest CT scans available and the regular use of quarterly CT scans to monitor response to treatment significantly lowers this possibility. Importantly, any new or evolving changes identified on serial chest CT scans were evaluated for the possibility of ICI-P through detailed chart reviews, but missed mild or subclinical ICI-P remains possible.
Notably, most patients with ICI-P required hospitalization and 5.1% of all patients treated with ICI developed high-grade pneumonitis (grade ≥ 3) developed with hypoxemic respiratory failure. During the diagnosis and management of ICI-P, empiric antimicrobials were administered for possible pneumonia in 70% of patients. This likely reflects the high burden of acute respiratory failure (73%) and hospitalization (60%) during the evaluation and management of ICI-P in this cohort. Pneumonia is a common cause of acute respiratory failure in patients with cancer, and the use of empiric antimicrobials is not unexpected in the setting of new radiographic opacities and hypoxemia. However, despite the common use of antibiotics, in only one patient was a pathogen identified (coronavirus), confirming coinfection at the time of an ICI-P diagnosis. Progressive respiratory failure leading to death was unexpectedly common, with eight patients dying while receiving treatment for ICI-P. This should be interpreted in the context of how the ICIs were used, which often was in the palliative setting with limited treatment options during cancer progression. However, after adjusting for other factors associated with mortality, the development of ICI-P remained associated independently with increased risk of death, highlighting the profound impact ICI-P has on lung cancer outcomes and the importance of risk assessment when considering ICI therapies.
We hypothesized that the presence of chronic pulmonary diseases may be key to the higher incidence of ICI-P in the lung cancer population and assessed objective measures of pulmonary disease in this cohort. We also used a composite measure of obstructive lung disease to improve the identification of such patients from a retrospective high-risk cohort. Both COPD by history and the composite measure of obstructive lung disease were associated with increased risk for ICI-P in the univariate analysis. In addition, a history of ILD was associated strongly with ICI-P risk. Baseline chest CT scans were used to identify signs of chronic parenchymal lung diseases, and congruent with at least one prior report,23 baseline fibrosis was associated with increased risk of ICI-P and was the strongest independent predictor of ICI-P (Table 5). In summary, multiple markers of chronic pulmonary disease seem to increase the risk for ICI-P, which may account for the increased burden of ICI-P in the lung cancer population35,36 because risk factors for the development of lung cancer overlap considerably with those of chronic pulmonary diseases such as COPD and ILD. This also raises the question of the need for pretreatment pulmonary function testing or collaborative management with pulmonary specialists when risk factors for lung disease are present.
In addition, pembrolizumab (vs nivolumab) was found to be an independent risk factor for ICI-P developing. Although most patients who received pembrolizumab showed positive results for PD-L1 (77%), this cannot explain the risk fully because PD-L1 expression was not itself associated with ICI-P risk. Prior reports have found increased risk for higher-grade ICI-P with pembrolizumab16 and in treatment-naïve patients with NSCLC.21 In this cohort, most patients who were treatment-naïve received pembrolizumab (91.3%), which may account partially for the increased risk we observed. Finally, contrary to prior reports, we found no clear association with ICI-P risk and advancing age, thoracic radiation therapy, or tumor histologic type.
This study has several limitations. The period of this review reflects the early use of ICI in lung cancer and does not incorporate PD-L1 inhibitors, particularly durvalumab, which are now commonly used in the treatment of stage III NSCLC. Additionally, clinical familiarity and practice patterns surrounding ICI-P likely have evolved since the initial use of these agents, which may influence patient outcomes. The power to detect differences in risk factors for ICI-P developing was limited by the relatively low number of cases, and some CIs were correspondingly wide. The retrospective nature of this study is prone to biases from missing data and reliance on documentation available for review. As such, the ICI-P risk assessments must be repeated and ideally confirmed in prospective studies and registries with larger cohorts.
Interpretation
These data show that ICI-P in a real-world lung cancer cohort is more common and severe than reported previously and highlights the clinical practices and confounding diagnoses commonly encountered when patients receiving ICI treatment show worsening dyspnea or respiratory failure. Chronic pulmonary diseases such as ILD, COPD, and fibrosis on chest CT imaging seem to confer increased risk of ICI-P and may be key contributors to the higher burden of ICI-P in lung cancer patients. These findings warrant further study in prospective trials and suggest a need for additional pretreatment testing or testing during-treatment for patients with chronic lung disease receiving ICI therapy.
Take-home Points.
Study Question: What are the risk factors, clinical presentations, and outcomes for immune checkpoint inhibitor-related pneumonitis (ICI-P) in a real-world lung cancer cohort?
Results: ICI-P was relatively common (9.5% incidence), severe (> 25% of patients died with ongoing ICI-P treatment), and independently associated with the presence of baseline fibrosis on chest CT imaging (adjusted OR [aOR], 6.61; 95% CI, 2.48-17.7), a composite measure of obstructive lung disease (aOR, 2.79; 95% CI, 1.07-7.29), and treatment with pembrolizumab (aOR, 2.57; 95% CI, 1.08-6.11).
Interpretation: In a real-world lung cancer cohort, ICI-P is more common and severe than reported previously, and risk for ICI-P was found to be associated with several chronic pulmonary diseases, which may account for the higher burden of ICI-P in lung cancer patients.
Acknowledgments
Author contributions: W. T. A. is the guarantor of the content in the manuscript. W. T. A., R. C. I., S. S.-B., K. P. P., and M. P. R. contributed to the conception and design of the study. W. T. A., S. S.-B., and R. C. I. contributed to the acquisition of the data. W. T. A., C. A., S. S.-B., and R. C. I. (principal investigator of institutional review board study) had full access to all of the data in the study and take responsibility for data integrity and accuracy. W. T. A., T. A. S., and C. A. contributed substantially to statistical analyses. W. T. A., K. P. P., and M. P. R. contributed to drafting of the manuscript. All authors contributed to revisions of the manuscript for critically important content and approved this version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. P. R. receives funding from the National Institutes of Health and has served on the advisory boards of Biodesix, bioAffinities Technologies, and Johnson & Johnson. None declared (W. T. A., C. A., S. S.-B., T. A. S., R. C. I., K. P. P.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: i2b2 software, Electronic Medical Record Search Engine (EMERSE), and the Carolina Data Warehouse for Health (CDW-H) were used in conducting this study. i2b2 is the flagship tool developed by the i2b2 (Informatics for Integrating Biology and the Bedside) Center, a National Institutes of Health-funded National Center for Biomedical Computing based at Partners HealthCare System. EMERSE allows users to search free-text (unstructured) clinical notes from the electronic health record. The CDW-H is a central data repository containing clinical, research, and administrative data sourced from the University of North Carolina Health system. Both current and legacy hospital systems are represented, with the ability to query most data elements. These research tools at the University of North Carolina are supported by the National Center for Advancing Translational Sciences, National Institutes of Health [Grant UL1TR002489]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study also was supported by the University of North Carolina Thurston Arthritis Research Center Dean’s Fund, the National Center for Advancing Translational Sciences, National Institutes of Health [Grants UL1 TR002489 and UL1 TR001111], the National Heart, Blood and Lung Institute, National Institutes of Health [Grant 5T32 HL007106-43], and the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health [Grant P30 AR072520-01].
References
- 1.Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 2.Hanna N.H., Schneider B.J., Temin S. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol. 2020;38(14):1608–1632. doi: 10.1200/JCO.19.03022. [DOI] [PubMed] [Google Scholar]
- 3.Calles A., Aguado G., Sandoval C., Álvarez R. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol. 2019;21(8):961–976. doi: 10.1007/s12094-018-02011-9. [DOI] [PubMed] [Google Scholar]
- 4.Hellmann M.D., Paz-Ares L., Bernabe Caro R. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 5.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishijima T.F., Shachar S.S., Nyrop K.A., Muss H.B. Safety and tolerability of PD-1/PD-L1 inhibitors compared with chemotherapy in patients with advanced cancer: a meta-analysis. Oncologist. 2017;22(4):470–479. doi: 10.1634/theoncologist.2016-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reck M., Rodríguez-Abreu D., Robinson A.G. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Hellmann M.D., Ciuleanu T.-E., Pluzanski A. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoja L., Day D., Wei-Wu Chen T., Siu L.L., Hansen A.R. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–2385. doi: 10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 11.Wang D.Y., Salem J.-E., Cohen J.V. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 13.Cadranel J., Canellas A., Matton L. Pulmonary complications of immune checkpoint inhibitors in patients with nonsmall cell lung cancer. Eur Respir Rev. 2019;28(153):190058. doi: 10.1183/16000617.0058-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rashdan S., Minna J.D., Gerber D.E. Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir Med. 2018;6(6):472–478. doi: 10.1016/S2213-2600(18)30172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martins F., Sofiya L., Sykiotis G.P. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 16.Su Q., Zhu E.C., Wu J.-B. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta-analysis. Front Immunol. 2019;10:108. doi: 10.3389/fimmu.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Osta B., Hu F., Sadek R., Chintalapally R., Tang S.C. Not all immune-checkpoint inhibitors are created equal: meta-analysis and systematic review of immune-related adverse events in cancer trials. Crit Rev Oncol Hematol. 2017;119:1–12. doi: 10.1016/j.critrevonc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Suresh K., Naidoo J., Lin C.T., Danoff S. Immune checkpoint immunotherapy for non-small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154(6):1416–1423. doi: 10.1016/j.chest.2018.08.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho J.Y., Kim J., Lee J.S. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150–156. doi: 10.1016/j.lungcan.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Naidoo J., Wang X., Woo K.M. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–717. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khunger M., Rakshit S., Pasupuleti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 22.Toi Y., Sugawara S., Sugisaka J. Profiling preexisting antibodies in patients treated with anti–PD-1 therapy for advanced non–small cell lung cancer. JAMA Oncol. 2019;5(3):376–383. doi: 10.1001/jamaoncol.2018.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi T., Shimizu J., Hasegawa T. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: a retrospective analysis. Lung Cancer. 2018;125:212–217. doi: 10.1016/j.lungcan.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Kanai O., Kim Y.H., Demura Y. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer. 2018;9(7):847–855. doi: 10.1111/1759-7714.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui P., Liu Z., Wang G. Risk factors for pneumonitis in patients treated with anti-programmed death-1 therapy: a case-control study. Cancer Med. 2018;7(8):4115–4120. doi: 10.1002/cam4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sears C.R., Peikert T., Possick J.D. Knowledge gaps and research priorities in immune checkpoint inhibitor-related pneumonitis. An Official American Thoracic Society research statement. Am J Respir Crit Care Med. 2019;200(6):e31–e43. doi: 10.1164/rccm.201906-1202ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy S.N., Weber G., Mendis M. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2) J Am Med Inform Assoc. 2010;17(2):124–130. doi: 10.1136/jamia.2009.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanauer D.A., Mei Q., Law J., Khanna R., Zheng K. Supporting information retrieval from electronic health records: a report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE) J Biomed Inform. 2015;55:290–300. doi: 10.1016/j.jbi.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D., Ahmet A., Ward L. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1) doi: 10.1186/1710-1492-9-30. 30-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Velasco G., Je Y., Bossé D. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312–318. doi: 10.1158/2326-6066.CIR-16-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishino M., Ramaiya N.H., Awad M.M. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051–6060. doi: 10.1158/1078-0432.CCR-16-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delaunay M., Cadranel J., Lusque A. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50(2):1700050. doi: 10.1183/13993003.00050-2017. [DOI] [PubMed] [Google Scholar]
- 35.Nishino M., Giobbie-Hurder A., Hatabu H., Ramaiya N.H., Hodi F.S. Incidence of programmed cell death 1 inhibitor–related pneumonitis in patients with advanced cancer. JAMA Oncol. 2016;2(12):1607–1610. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 36.Ma K., Lu Y., Jiang S., Tang J., Li X., Zhang Y. The relative risk and incidence of immune checkpoint inhibitors related pneumonitis in patients with advanced cancer: a meta-analysis. Front Pharmacol. 2018;9:1430. doi: 10.3389/fphar.2018.01430. [DOI] [PMC free article] [PubMed] [Google Scholar]