Since its appearance in 2019, the SARS-CoV-2 virus and related pandemic has challenged healthcare systems all across the world.1,2 The immune response from vaccination in type 1 diabetes is well recognised. What is less clear is the effect of vaccination on glycaemic control. Evidence is increasing that some people with type 1 diabetes mellitus (T1DM) experience temporary instability of blood glucose (BG) levels post-vaccination which normally settles within a few days.
In a retrospective analysis, we examined the BG profile of 96 consecutive adults (age ≥ 18 years) with T1DM using the FreeStyle Libre® flash glucose monitor in the periods immediately before and after their first COVID-19 vaccination. All were on a basal bolus regime of long acting analogue insulin (Insulin Degludec/Glargine) and prandial short acting analogue insulin (Insulin Aspart/Insulin Lispro). Additional oral hypoglycaemic therapy was used by n = 26 individuals,
The primary outcome measure was percentage (%)BG readings within the designated target range 3.9 to 10 mmol/L as reported on the LibreView® portal3 for 7 days prior to the vaccination (week −1) and 7 days after the vaccination (week +1).
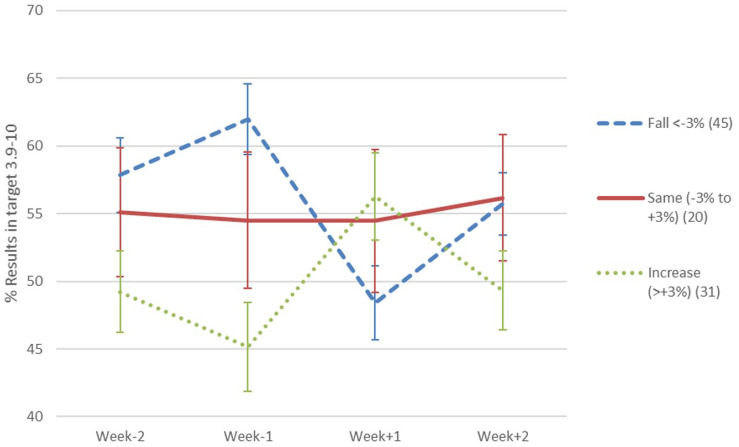
Fifty-nine percent of individuals experienced major perturbation of BG levels with 30% of individuals showing a decrease of time within range of over 10%, and one in ten individuals showing a decrease in time within range of over 20% (Figure 1 shows change in %BG on target for those whose control deteriorated by >3% vs the rest). There was a small but significant overall decrease in the %BG on target (3.9-10.0) for the 7 days following vaccination (mean 52.2% ± 2.0%) vs pre-COVID-19 vaccination (mean 55.0% ± 2.0%). Importantly there was no difference in vaccine effect between the AstraZeneca and Pfizer vaccines.
Figure 1.
Change in BG % in target range for 3 groups: (i) fall of <−3% vs pre-vaccination; (ii) same i.e. −3% to +3% vs pre-vaccination; (iii) increase of >3% vs pre-vaccination. Graph shows means and 95% confidence intervals.
The decrease in BG proportion on target in the week following vaccination was more at pronounced at −5.7% for people with when HbA1c was below the median. For the 49 patients with better HbA1c control (≤56 mmol/mol (7.3%)) 65% showed a fall in time in range, of whom 37% showed a fall of more than 10% in the % of readings on target. A multivariate linear regression analysis including age, BMI and type of vaccine indicated that estimated HbA1C (standardised beta 0.22, P = .02) and mode of treatment (insulin + oral hypoglycaemic agents (standardised beta −0.22, P = .02) were independently associated with a greater reduction in proportion of BG readings in the target range (r2 = 0.10)
Clinical data supports a robust neutralizing antibody response in COVID-19 patients with diabetes.4 Notably vaccination for influenza has also been noted to cause blood glucose levels to become unstable for a time, perhaps related not only to a reaction to the attenuated virus but also to the excipients within the administered vaccine.5 Our findings do indicate that patients with T1DM should be
counselled and prepared for possible transient hyperglycaemia following the COVID-19 vaccine.6
In conclusion, in T1DM, we have shown that first COVID-19 vaccination can cause temporary perturbation of BG in many individuals, with this effect more pronounced when HbA1c is lower. There was no difference in effect between the vaccines administered in the UK in early 2021.
Footnotes
Abbreviations: BG, blood glucose; T1DM, type 1 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Adrian H. Heald  https://orcid.org/0000-0002-9537-4050
https://orcid.org/0000-0002-9537-4050
References
- 1.Rawaf S, Allen LN, Stigler FL, et al. , Global Forum on Universal Health Coverage and Primary Health Care. Lessons on the COVID-19 pandemic, for and by primary care professionals worldwide. Eur J Gen Pract. 2020;26(1):129-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krist AH, DeVoe JE, Cheng A, Ehrlich T, Jones SM. Redesigning primary care to address the COVID-19 pandemic in the midst of the pandemic. Ann Fam Med. 2020;18(4):349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Accessed April 29, 2021. https://www.libreview.com/
- 4.Pal R, Bhadada SK, Misra A. COVID-19 vaccination in patients with diabetes mellitus: current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15(2):505-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glaess SS, Benitez RM, Cross BM, Urteaga EM. Acute hyperglycemia after influenza vaccination in a patient with type 2 diabetes. Diabetes Spectr. 2018;31(2):206-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mifsud S, Schembri EL, Gruppetta M. Stress-induced hyperglycaemia. Br J Hosp Med (Lond). 2018;79(11):634-663. [DOI] [PubMed] [Google Scholar]