Abstract
Modern cancer immunotherapy techniques are aimed at enhancing the responses of the patients' immune systems to fight against the cancer. The main promising strategies include active vaccination of tumor antigens, passive vaccination with antibodies specific to cancer antigens, adoptive transfer of cancer-specific T cells and manipulation of the patient's immune response by inhibiting immune checkpoints. The application of immunogenic cell death (ICD) inducers has been proven to enhance the immunity of patients undergoing various types of immunotherapy. The dying, stressed or injured cells release or present molecules on the cell surface, which function as either adjuvants or danger signals for detection by the innate immune system. These molecules are now termed ‘damage-associated molecular patterns’. The term ‘ICD’ indicates a type of cell death that triggers an immune response against dead-cell antigens, particularly those derived from cancer cells, and it was initially proposed with regards to the effects of anticancer chemotherapy with conventional cytotoxic drugs. The aim of the present study was to review and discuss the role and mechanisms of ICD as a promising combined immunotherapy for gastrointestinal tumors.
Keywords: immunogenic cell death, gastrointestinal cancer, immunotherapy, immune response
1. Introduction
Modern cancer immunotherapy has been proposed to involve four primary promising strategies: i) Active vaccination of tumor antigens, ii) passive vaccination with antibodies specific to cancer antigens, iii) adoptive transfer of cancer-specific T cells, and iv) manipulation of the patient's immune response by inhibiting immune checkpoints. Additional emerging strategies include other antigen-non-specific interventions, including the applications of oncolytic or immune-enhancing viruses, innate immunity stimulators and immunogenic cell death (ICD) inducers (1). The focus of the present review was to describe the treatments that employ ICD to enhance the immunity of patients with gastrointestinal (GI) cancers.
The daily death of billions of ordinary cells from the human body goes essentially unrecognized by the immune system. This is crucial since the conservation of the entire bodies homeostasis includes the continuous turnover of various cell compartments. As such, the initiation of an immune response against dead-cell antigens would have detrimental outcomes. Conversely, the death of a few cells infected by a microorganism can trigger a potent antigen-specific immune reaction, which is associated with the clearance of the invading pathogen from the body and also enables the establishment of long-term immunological memory (2). The first proposal of the ‘danger theory’ in 1994 by Matzinger (3) was that the immune system can distinguish between dangerous and innocuous endogenous signaling. In her famous essay, Matzinger suggested that ‘unprogrammed cell death’ could give rise to the unusual release of internal molecules from the cytoplasm, nucleus or membrane to activate dendritic cells (DCs). The dying, stressed or injured cells release or present molecules on the cell surface, which can function as either adjuvants or danger signals for detection by the innate immune system. These molecules are now termed ‘damage-associated molecular patterns’ (DAMPs) (4). The term ‘ICD’ was introduced to indicate a type of cell death that triggers an immune response against dead-cell antigens, particularly those derived from cancer cells, including DAMPs. This model was initially proposed with regard to anticancer chemotherapy, in view of clinical proof demonstrating that tumor-specific immune responses reflect the efficacy of anticancer treatments using conventional cytotoxic drugs (5).
Currently, various routinely employed anticancer agents include doxorubicin, epirubicin, idarubicin, mitoxantrone, bleomycin, bortezomib, cyclophosphamide (CY) and oxaliplatin. The list also includes certain anticancer agents that are currently under preclinical or clinical development, such as some microtubular inhibitors of the epothilone family. Certain drugs, including digoxin, digitoxin and zoledronic acid, act to convert otherwise non-immunogenic events of cell death into bona fide ICD inducers, and may thus be used as adjuvants in combinatorial immunotherapy regimens (6). The known clinically applied or experimental anticancer agents that induce ICD act via one or several of the following mechanisms: Inducing apoptosis, causing a severe focused stress of the endoplasmic reticulum, overcoming loss-of-function mutations that hide danger signals during tumorigenesis and downregulating the cancer-based induction of pro-inflammatory transcription factors (4). In addition, one important consideration is the complex interactions with DAMPs and their receptors, known as the pattern recognition receptors. Attempts have been made to identify and detect multiple DAMPs in order to facilitate the development of next-generation anticancer regimens, which, in addition to killing cancer cells, can simultaneously convert them into a cancer-specific therapeutic vaccine (7).
2. GI cancers
GI cancers are amongst the malignancies most frequently diagnosed in European patients. These include gastric cancer (GC), colorectal cancer (CRC), as well as cancers affecting the liver, particularly hepatocellular carcinoma (HCC), the biliary tract, such as cholangiocarcinoma (CCA), and the pancreas (pancreatic cancer; PC). The frequency with which these conditions are diagnosed presents a significant challenge for public health systems in Europe and worldwide (8).
Different cancers of the GI tract
Most commonly reported in patients in Asia, GC is notable for its particularly poor survival rates. The condition is associated with certain bacterial infections, such as Helicobacter pylori (H. pylori) infection, and the effects of other pathogens, including Epstein-Barr virus (9).
The annual number of new CRC diagnoses falls in the range of 1-2 million cases, placing CRC third in terms of the most frequently occurring cancers, and fourth amongst the most common causes of cancer-related mortality. The main CRC risk factors include age and history of chronic disease, as well as various aspects of patient lifestyles. There are three different pathogenic mechanisms that can lead to the onset of CRC: Microsatellite instability, chromosomal instability and a CpG island methylator phenotype (10,11).
The most common type of liver cancer is HCC, which originates from hepatocytes and accounts for ~4 in 5 liver cancer diagnoses, with an increased prevalence in China and Eastern Africa (12). The onset of HCC is often a consequence of the interaction between genetic characteristics and environmental factors. In particular, patients diagnosed with liver cirrhosis, or infection with the hepatitis B virus (HBV) or the hepatitis C virus (HCV), are more likely to develop HCC, while other risk factors include alcohol abuse, the ingestion of aflatoxin B1 or non-alcoholic steatohepatitis (13,14).
CCA is the second most frequently occurring primary hepatic cancer after HCC. CCA has been most commonly reported across Asia, although in recent years it has become increasingly more widespread in North America and Europe (15,16). CCA originates in the biliary tract and is subdivided by location into three different subtypes, namely perihilar, intrahepatic and distal CCA. Several risk factors, both common and rare, have been associated with CCA, including hepatobiliary parasites, Caroli disease, HBV and HCV infection, and exposure to toxins (17-19).
PC is the 14th most prevalent type of cancer and the seventh largest cause of annual cancer-associated deaths globally. PC risk factors include obesity, alcohol consumption and smoking, as well as H. pylori infection (20).
Latest approaches to GI cancer treatment
Several potential treatments are currently being developed for GI cancers. A number of these are targeted approaches that make use of biological properties to achieve their objectives, and can be employed alone or as components of combination or adjuvant treatments. The most widely applied treatments at present include surgery, as a means of resecting solid tumors; radiation therapy, as a means of managing localized solid tumors; chemotherapy, which involves the use of cytotoxic agents to eliminate cancerous cells; and hormonal therapy, which serves as a systematic approach with the aim of targeting all cancerous cells found in the body (21-23).
3. Immunotherapy in GI cancers
Patients diagnosed with GI cancers are typically subjected to a combination of treatments, including surgery, chemotherapy and/or radiation therapy; however, the survival rates remain poor, particularly when the cancer has reached an advanced stage, or in the case of metastatic disease (24,25). For this reason, it is imperative to develop more effective, novel techniques to address the problem, and immunotherapy appears to hold promise for this purpose. To date, a number of cytokines, including IFN-γ or IL-2, have been used to limit the activity of certain types of cancer, such as renal cell carcinoma and melanoma, and a moderate level of inhibitory activity has been reported (26). However, the development of cancer vaccines has been less successful, with none generating statistically significant responses in test patients (23).
The progress in immunotherapy shows great potential in the context of GI cancers, whereas further therapies involving the administration of immunostimulatory monoclonal antibodies to treat GI malignancies are currently in their developmental phase. Immune checkpoint blockade is the primary type of immunotherapy currently used in GI cancer treatment. It can be anticipated that the vaccination approach will be streamlined with the lessons learned from initial successes, and the most suitable tumor-associated antigens will be identified for targeting. Adoptive cell therapies are now at an advanced stage of development and appear to hold significant promise for GI cancers. An improved understanding of the prevalent suppressive factors in patients with GI cancers may enable the development of superior strategies to limit immune suppression and promote endogenous immunity in patients. It is likely that deeper knowledge of the tumor microenvironment and the field of immunology will lead to the successful development of more efficacious treatments in the near future.
4. Vaccine therapy in GI cancers
Various cancer vaccines have been developed to date by using different technologies, including recombinant microorganisms, recombinant antigen cocktails, oncolytic viruses, DNA and gene therapy-based treatments, and anti-idiotypic antibodies. In addition, personalized vaccines have also been devised, including those based on adoptive cell transfer, or autologous cells and antigens. These may be more complex, and require specialized manufacturing approaches and expertise (27). However, although several trials have been conducted, approval has only been granted for one vaccine, which acts against metastatic castration-resistant prostate cancer. This vaccine is Dendreon's Provenge® (Sipuleucel-T), which was approved by the Food and Drug Administration in 2010 (28,29). Furthermore, two additional cancer vaccines, Vitespen® (30) and Melacine® (31), were approved in Russia and Canada, but not in the United States. These cancer vaccines rely upon the activation and strengthening of antitumor responses that target cancer. The dendritic cell-based cancer vaccine presents antigens and serves a critical role in formulating the immune response. They can also activate B cells, natural killer (NK) cells, as well as naïve and memory T cells, through the presentation of tumor antigens associated with major histocompatibility complex (MHC) molecules. When patients with cancer have higher numbers of DCs penetrating the tumors, this is a sign of reduced lymph node metastases and improved chances of survival (32). A number of approaches have been employed to create vaccines by loading DCs with tumor antigens. These include the use of synthetic peptides pulsed on DCs (33), DCs engineered with plasmid DNA (34), RNA (35) or viruses (36), tumor cell lysates combined with immature DCs (37) and, finally, DCs combined with whole tumor cells through polyethylene glycol or electroporation (38). The technique that uses DCs pulsed with MHC-restricted peptides, which are obtained from antigens known to be associated with tumors, is the most common type of vaccine approach (39-41). However, it can be challenging to use DCs for clinical purposes, as these cells have a relatively short life span (42). To date, vaccines have shown little effectiveness in preventing GI cancers. The vaccines developed thus far have targeted melanoma-associated antigen (MAGE)-A3(43), HER2 p369 peptide (44), gastrin-17 diphtheria toxoid (45), URLC10 or VEGFR1 epitopes (46) and heat shock protein (HSP) gp96(47) in patients with GI cancers. Furthermore, chemotherapy has been tested alongside adjuvant Bacillus Calmette-Guérin (48). In cases of PC, a number of specific antigens serve as targets, including carcinoembryonic antigen (CEA), EGFR, Wilms' tumor 1 and VEGF, whereas GVAX® is a whole-cell vaccine that has been tested in trials in the metastatic, neoadjuvant and adjuvant settings. Positive outcomes have been reported in the metastatic setting when combined with ipilimumab, with a reported survival rate of 27% after 1 year (49).
In the case of HCC, vaccines have shown no efficacy thus far. However, immune responses have been reported in phase I trials involving peptide vaccines (50), DC vaccines and tumor-associated antigens targeting oncolytic viruses, such as AFP (51), GPC-3(52) and human telomerase reverse transcriptase (53). DC-based vaccines have been included in trials on CCA, whereas a phase II study was conducted in the adjuvant setting in order to target mucin 1 (MUC1) in the biliary tract and in PC via DC vaccination, and the tolerability was reported to be good, although it was not possible to draw definitive conclusions regarding the immune response (54). Further phase II clinical trials involving CRC-specific peptide vaccines were performed with patients diagnosed with HLA-A*2402-positive stage III CRC, with the findings suggesting that an immune response would be generated by the vaccine, leading to higher survival rates (55). For patients with PC, 13-mer mutant ras peptide vaccines were not only shown to be safe, but to also generate the appropriate immune response (56). Additionally, the p53 synthetic long peptide vaccine has been shown to be safe for patients with metastatic CRC in whom specific T-cell responses were induced (57). However, several issues must be addressed if a novel cancer vaccine is to be approved, including commercial, clinical, manufacturing, operational and regulatory concerns. Risk evaluation must also be conducted to make full use of the expertise available. Moreover, a number of clinical studies revealed that some patients may not benefit from the cancer vaccine treatment (primary resistance), and some responders may relapse after a period of response (acquired resistance). For example, PC is associated with the presence of a highly immunosuppressive microenvironment, which is characterized by a dense desmoplastic stroma that impedes blood flow to the area, inhibits drug delivery and suppresses antitumor immune response (58). In CRC and GC, it has been shown that only patients with the subset of mismatch-repair-deficient or microsatellite instability-high tumors are likely to respond to immunotherapy (59,60). Once these issues have been addressed, ICD may prove to be the answer for vaccine development.
5. Cancer vaccination is the target of ICD
DC maturation is a crucial step for immune activation. Once in the lymph nodes, DCs activate T cells via three canonical signals: Binding of T-cell receptors, co-stimulatory receptor engagement, and the provision of cytokines and chemokines to facilitate T-cell polarization and differentiation. The ICD complementarily activates DCs. High mobility group box 1 (HMGB1) and HSP activate pro-inflammatory DCs through the Toll-like receptor (TLR)2/4-MyD88-NF-κB signaling pathway (61). Moreover, dying cancer cells also express calreticulin (CRT) on their plasma membrane, which signals to facilitate engulfment by DCs. The exposed CRT on the cell membrane can bind to CD91, the low-density lipoprotein-receptor-related protein 1, which promotes the engulfment of cellular compartments and debris by a mechanism that depends on the Rac family small GTPase 1(61). Hence, the original concept for using the combination treatment of ICD stimulation and DC-based anticancer vaccines originated from significant evidence that cancer treatments, such as chemotherapy, radiation, phototherapy, phytotherapy and immunotherapy, could elicit danger signals from dying cancer cells. Notably, chemoradiotherapy is known to elevate serum HMGB1 in patients with esophageal squamous cell carcinoma, and the levels of HMGB1 were found to be positively correlated with patient survival (62). Moreover, oxaliplatin and mitoxantrone induced cancer cell death accompanied by an exposure of CRT and a release of HMGB1, HSP70 and ATP, thereby strongly inducing in vitro immune responses of DCs (63,64). Interestingly, it has been demonstrated that pretreatment with the oxaliplatin nanoparticle followed by a rechallenge by tumor inoculation in PC-bearing mice can enhance therapeutic efficacy by increasing the numbers of tumor-infiltrating activated cytotoxic T cells (64). In addition to the use of chemotherapy, botanical cancer therapy has also been introduced. Treatment with Hemidesmus indicus was shown to induce CRC cell apoptosis characterized by surface expression of CRT, increased HSP70 expression, and a release of ATP and HMGB1. This immunogenic agent is promising when combined with a DC-based anticancer vaccine (Table I) (65). Recently, our group demonstrated that a potent bioactive compound, honokiol, can induce CCA cell apoptosis, which is associated with the release of HMGB1 and HSP90. Incubation of DCs with CCA cells that were pretreated with honokiol induced DC maturation, and thus enhanced the priming of cytotoxic T cells to kill cancer cells (37). Therefore, the priming of DCs with immunogenic agents may maximize antitumor responses though DC stimulation.
Table I.
List of studies on immunotherapies associated with ICD in gastrointestinal cancers.
| Type of therapy | Type of cancer | ICD inducer | Treatment condition | Summary of results | Reference/NCT |
|---|---|---|---|---|---|
| DC-based anticancer vaccine | PC | Encapsulated oxaliplatin induced exposure of CRT and release of HMGB1 HSP70 and ATP | Vaccination with oxaliplatin-treated cancer cells and subsequent rechallenge with tumor inoculation | Enhanced the therapeutic efficacy by increasing tumor-infiltrating cytotoxic T cell numbers | (64) |
| HCC | Radiation | Combination treatment of radiation and IL-12 in an orthotopic tumor model | Inhibited tumor growth by activation of DCs, tumor-infiltrating CD8+ T cells and NK cells, and suppression of tumor-infiltrating MDSCs | (66) | |
| CCA | Honokiol induced release of HMGB1 and HSP90 | Culture of DCs with honokiol-treated CCA cells, and subsequently priming with T lymphocytes for cancer cell killing in vitro | Honokiol-treated CCA cells induced DC maturation and type I IFN production and enhanced cancer cell killing by effector T lymphocytes | (37) | |
| CRC | Phototherapy induced CRT exposure and release of ATP | Combination treatment of phototherapy and oxygenation booster in tumor-bearing mice | Facilitated DC maturation and inhibited tumor growth and recurrence | (67) | |
| CRC | S-1/CPT-11 chemotherapy | Phase I trial to evaluate the safety and immune response of RNF43-721 peptide vaccine combined with chemotherapy | No results yet | NCT00641615 | |
| HCC | CY | Phase I/II vaccination of multi-peptide-based HCC vaccine plus adjuvant by a single pre-vaccination of low-dose (CY) in patients with very early, early, intermediate stage HCC | No results yet | NCT03203005 | |
| CRC | mFOLFOX6 | Phase II trial of perioperative CV301 vaccination in combination with nivolumab and systemic chemotherapy for metastatic CRC | No results yet | NCT03547999 | |
| GC or gastroesophageal cancer | Cisplatin and 5-FU | Phase III trial of G17T immunogen in combination with chemotherapy in patients with metastatic or locally recurrent cancer | No results yet | NCT00020787 | |
| PC | CY | Phase II study by pretreatment with CY before injecting with GVAX peptide cancer vaccine | Overall survival of 34.2, 15.4 and 16.5 months, and disease-free survival of 18.92, 8.54 and 5.56 months for GVAX without CY, GVAX with intravenous CY and GVAX with p.o. CY, respectively | NCT00727441 | |
| Immune checkpoint blockade Immune | CRC | Cisplatin and CBP501 induced cell surface exposure of CRT and release of HMGB1 | Combination treatment with cisplatin, CBP and anti PD-1 or PD-L1 | CBP501 enhanced cisplatin-induced ICD and increased the efficacy of immune checkpoint inhibitors against tumors in mice | (80) |
| CRC | PARP and PI3K inhibitors induced exposure of CRT on cell membrane | Nano-formulation of PARP and PI3K inhibitors combine with X-ray irradiation and anti-CTLA-4 immunotherapy | PARP and PI3K inhibitors sensitized efficacy of anti-CTLA-4 by improved tumor control and increased tumor infiltrating lymphocytes | (81) | |
| CRC | Lurbinectedin induced exposure of CRT, release of ATP, release of HMGB1 and type I IFN responses | Combination treatment of lurbinectedin and immune checkpoint blockade | Strong antitumor response as both prophylaxis and therapy in a cancer-bearing mouse model | (123) | |
| CRC | NIR-PIT induced release of cellular ATP and HMGB1 | CD44-targeted NIR-PIT combined with CTLA4 or PD-1 blockade | Combination of NIR-PIT with antibody blockade showed greater tumor inhibition with prolong survival | (83) | |
| CRC | Trifluridine/tipiracil plus oxaliplatin induced surface CRT exposure, eIF2a- dependent endoplasmic reticulum stress, HMGB1 and ATP release | Trifluridine/tipiracil plus Oxaliplatin combined anti PD-1 antibody | Combination treatment enhanced the antitumor efficacy via elimination of type-2 tumor-associated macrophages | (124) | |
| HCC | Oxaliplatin elicited cell surface exposure of CRT and release of HMGB1 | Combination treatment with oxaliplatin and anti-PD-1 | Combination therapy with oxaliplatin and anti-PD-1 inhibited tumor growth, activated CD8+ T-cell expansion and DC maturation | (82) | |
| CRC | FOLFOXIRI | Phase II, FOLFOXIRI plus bevacizumab with atezolizumab treatment for patients with unresectable metastatic CRC | No results yet | NCT03721653 | |
| CRC | FOLFOX | Phase I/II, FOLFOX combination treatment with durvalumab and tremelimumab in patients with metastatic CRC | No results yet | NCT03202758 | |
| GC | 5-FU and cisplatin | Phase II, 5-FU and cisplatin combination treatment with pembrolizumab in patients with advanced GC or gastroesophageal junction cancer | Pembrolizumab + 5-FU + cisplatin showed manageable safety and promising antitumor activity | NCT02335411 | |
| T-cell adoptive transfer | CRC | Mitochondria-targeted small molecule, IR-780 induced cell surface exposure of CRT and release of HMGB1 and ATP | Combination treatment of cytotoxic T-cell adoptive transfer with IR-780 | Improves the inhibitory effect on tumor growth due to DC maturation and synergistic effector T-cell priming and tumor infiltration | (119) |
PC, pancreatic cancer; HCC, hepatocellular carcinoma; CRC, colorectal cancer; GC, gastric cancer; ICD, immunogenic cell death; HMGB1, high mobility group box 1; CRT, calreticulin; MDSC, myeloid-derived suppressor cell; DC, dendritic cell; NK cells, natural killer cells; Treg, regulatory T cell; TLR, Toll-like receptor; PD1, programmed death 1; PD-L1, programmed death ligand 1; CY, cyclophosphamide; FOLFOX, oxaliplatin, 5-FU and leucovorin; FOLFOXIRI, FOLFOX and irinotecan; 5-FU, 5-fluorouracil; HSP, heat shock protein; CBP, calmodulin-binding peptide; NIR-PIT, near-infrared photoimmunotherapy; CTLA-4, cytotoxic T lymphocyte-associated protein 4; eIF2a, eukaryotic translation initiation factor 2A; PARP, poly adenosine diphosphate-ribose polymerase.
Radiation as a cancer treatment also induces ICD, which may pave the way for anticancer vaccines in such patients. For example, in large orthotopic HCC, synergistic antitumor effects may be obtained when radiation is combined with the administration of IL-12. This has been shown to be associated with the activation of tumor-infiltrating DCs, CD8+ T cells and NK cells, as well as the suppression of tumor-infiltrating myeloid-derived suppressor cells (MDSCs) (66). Similar to radiation, phototherapy has been suggested to be a new platform for enhancing the immunogenicity of cancer. Photodynamic and photothermal therapies proficiently promote immunotherapy via the induction of ICD. In addition, phototherapy combined with oxygenation boosters can promote CRC cell apoptosis and induce ICD, thus facilitating DC maturation and inhibiting tumor growth and recurrence in animal models (67). Plasma-treated PBS, which is physical cold atmospheric plasma consisting of reactive oxygen and nitrogen species, has demonstrated cytotoxic activity against PC cells with immunogenic features. This increases the potential of phagocytosing DCs and DC maturation, which may hold promise for combinations with DC cancer vaccines (68).
Several clinical studies have corroborated the concept that ICD-inducing pretreatment may act as an immunomodulator (Table I). There are ~50 trials currently investigating the benefit of ICD in vaccines against cancer, mostly PC and CRC. A phase I/II trial study, in which 22 patients with HCC have been enrolled, is investigating treatment interventions comprising pre-infusion of CY and vaccination using a multi-peptide-based HCC vaccine (IMA970A) plus CV8102 adjuvant (RNAdjuvant®). This trial is investigating whether IMA970A and CV8102 are safe and whether they can trigger an immune response against the tumor under pre-conditioning by induction of ICD (NCT03203005). Moreover, mFOLFOX6, which is a formulation of 5-fluorouracil (5-FU), leucovorin and oxaliplatin, has been used in combination with nivolumab and vaccination with a CV301 peptide vaccine in patients with advanced CRC (NCT03547999). Another clinical trial including patients with PC used CY followed by vaccination with a GVAX peptide cancer vaccine. However, pretreatment with CY did not improve overall survival or disease-free survival when compared with the uncombined intervention (NCT00727441). Collectively, the majority of preclinical and clinical data have demonstrated that combining the immunogenic potential of ICD with cancer vaccination is a promising approach that could achieve future translational success.
6. Checkpoint inhibitor therapies in GI cancers
The field of immuno-oncology has witnessed significant advances with regard to immune checkpoint inhibitors (ICIs). Immune checkpoints are the mechanisms through which T-cell immune responses are regulated (69). Tumor cells are considered to be able to avoid host immune clearance when T-cell immune responses are downregulated. If immune checkpoints can be targeted, the endogenous response of the immune system to tumors may be used as a means of addressing the risk of disease. A number of antibodies have been shown to be effective against immune checkpoints, and more are under examination (69). These antibodies most commonly serve to target cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1). CTLA-4 acts as a receptor capable of inhibiting the activation of T cells (70). Accordingly, when CTLA-4 is blocked, the T cells will proliferate and become activated. PD-1 is expressed on T cells and other immune cells, and PD-L1 serves as one of its ligands. PD-1 and PD-L1 binding creates an inhibitory signal affecting T cells; by contrast, if the binding is inhibited, the T cells will become activated, leading to a heightened response from cold tumor (non-inflammatory T-cell) to hot tumor (inflammatory T-cell) immune response (71-73). Another targeted checkpoint receptor protein is the lymphocyte activation gene-3, which may control the activity of T cells through its binding with MHC class II-molecules (74). However, earlier studies examining such checkpoint inhibitors in CRC were not very successful, phase II trials involving the CTLA-4 inhibitor, tremelimumab, in patients diagnosed with metastatic CRC have shown no significant levels of efficacy (75,76).
As a consequence of tumor heterogeneity, along with the complexity of immunosuppression, treatment of GC, as has been the case with CRC, has shown little success in the area of immunotherapy, for reasons possibly related to mechanisms that are incompletely understood. Previous findings have revealed that a certain level of checkpoint inhibition can be achieved, often in correlation with enhanced classification and characterization of GC and an improved understanding of its histopathology (48). PC presents the greatest challenge amongst all types of GI cancer in the context of immunotherapy, most likely as a result of inadequate immunogenicity, along with a low mutational burden and a unique vascular and stromal microenvironment. These conditions make it very difficult for the immune cells and molecules to penetrate into the tumors, particularly compared with the conditions in other types of cancer (77). If the microenvironment exhibits immunosuppressive qualities, checkpoint inhibition may represent a suitable goal for HCC immunotherapy, although success has been limited to date in the case of single-agent checkpoint blockade (78).
7. Induction of ICD sensitizes blockade inhibitor therapies
In this decade, antibodies directed against immune checkpoints have been intensely investigated in patients with cancer following the discovery by the Nobel prize winners James P. Allison and Tasuku Honjo, who demonstrated that cancer therapy can be enhanced by the inhibition of negative immune regulation (79). CTLA-4 and PD-1 are the receptors that are commonly found on the surface of activated T cells. The interaction of CTLA-4 and PD-1 with their ligands inhibits activated T cells and converts them into exhausted T cells, which abrogates the antitumor response. The targeting of CTLA-4 and PD-1 molecules has demonstrated durable response rates, increased survival time of responders and a manageable safety profile. Recently, checkpoint inhibition plus chemotherapy has been considered for use in the first-line setting for the treatment of CRC (11). The potential clinical responses may be associated with the induction therapy with ICD inducers and cancer immunotherapy.
It has been reported that calmodulin-binding peptide (CBP)501, a CBP that can induce ICD when combined with cisplatin treatment, can induce cell membrane exposure of CRT and the release of HMGB1. Treatment of CRC-bearing mice with CBP501 and cisplatin, and subsequently with anti PD-1 or PD-L1 antibodies, significantly enhanced the antitumor activity of immune blockade via upregulating the percentage of tumor-infiltrating CD8+ T cells (80). Similarly, in CRC-bearing mouse models, the combination treatment of PARP and PI3K inhibitors induced radio-sensitization and the induction of ICD. Thus, subsequent anti-CTLA-4 treatment strongly inhibited tumor growth and increased the numbers of tumor-infiltrating lymphocytes (TILs) (81). The induction treatment of not only CRC, but also HCC, has been investigated using immunogenic chemotherapy, through oxaliplatin promoting the exposure of CRT and the release of HMGB1. In HCC, oxaliplatin combined with anti PD-1 antibodies achieved marked tumor suppression, activation of CD8+ T cells and stimulation of DCs (82). Moreover, radiation and phototherapy are additional examples of immunogenic cancer therapies that sensitize the immune checkpoint blockade. CD44-targeted near-infrared photoimmunotherapy combined with anti CTLA-4 and PD-1 antibodies was shown to inhibit tumor growth and prolong the survival of CRC-bearing mice via a mechanism associated with the induction of ATP and the release of HMGB1(83).
The ICD inducer-enhancing immune blockade has been proven in several clinical reports (Table I), but has not been well established in GI cancers. Current phase II trials have been investigating the ICD-inducing effect of 5-FU, leucovorin and oxaliplatin (FOLFOX) and FOLFOX plus irinotecan (FOLFOXIRI) to enhance the efficacy of ICIs. Patients with unresectable metastatic CRC have been treated with either FOLFOX/FOLFOXIRI or anti-PD-L1/CTLA-4 antibodies. After each treatment cycle, the safety, disease progression, death and intolerable toxicity will be continuously recorded (84,85). Furthermore, the use of 5-FU and cisplatin is encouraged owing to their safety and strong enhancement of the antitumor response in advanced GC when combined with pembrolizumab (86).
The insight into the mechanism of ICD-sensitized immune blockade inhibitors is under investigation. Two possible effects have been proposed, depending on the type of ICD inducer. The first effect is the direct consequence of the chemotherapeutic agents proficiently upregulating PD-L1 expression. Those that exert this effect include 5-FU, gemcitabine, cisplatin, oxaliplatin, doxorubicin and paclitaxel (87). This is an important method for increasing the sensitivity to blockade inhibitors. Treatment with FOLFOX can activate the secretion of IFN-γ from PD-1+ CD8+ T cells, which is associated with the overexpression of PD-L1 on tumor cells. Hence, the combination treatment of FOLFOX with anti PD-1/PD-L1 antibodies has achieved complete cure in CRC-bearing mice, while monotherapy was unsuccessful (88). Similarly, the anthracycline drug, epirubicin, could upregulate PD-L1 expression in HCC, which causes sensitization to immune blockade therapy (89). The other effect would be due to the indirect function of ICD inducer agents via modulating the tumor microenvironment. This may be associated with the depletion of regulatory T cells and MDSCs that potentiates stronger antitumor responses (66). Taken together, these results from preclinical and clinical studies have indicated that the concept of priming treatment with ICD inducer agents prior to checkpoint blockade treatment could elicit a stronger antitumor response.
8. Adoptive therapies in GI cancer
A potential approach to cancer treatment is harnessing the properties of T cells and NK cells to attack tumor cells. NK cells and TILs are of both predictive and prognostic value in GI cancers (90). These properties can be applied through the various modalities of adoptive cell therapies, typically involving the isolation of immune cells obtained from patients diagnosed with cancer, whereupon they can be genetically modified in order to strengthen their capacity to identify and kill cancerous cells. By expanding these isolated cells ex vivo, they can then be re-introduced to the patient, in a form of treatment which can theoretically be effective for all patients with cancer who do not demonstrate adequate cancer immunity without assistance, and would therefore be incapable of responding to ICIs. Adoptive cell therapy as a cancer treatment can involve various strategies, which have either been previously examined in clinical settings, or are undergoing trials to assess their suitability against GI cancers (91). One particular approach of adoptive cell therapy makes use of the immunotherapeutic properties of cytokine-induced killer cells (CIK), which can be obtained through the treatment of peripheral blood lymphocytes using IFN-γ. This is a type of monoclonal antibody that counteracts the CD3 molecule, along with IL-2. CIK cells are predominantly expansions of CD3+/CD8+/CD56- cells to become terminally differentiated CD56-positive NK cells. These are uniquely able to identify the tumor cells regardless of the presence or absence of antibodies and MHC molecules and, accordingly, have the ability to identify any tumor cells lacking MHC molecules on their surface (92). In addition to T-cell adoptive transfer, NK cell-based immunotherapy has shown promising antitumor effects in a number of studies (93-95). Adoptive transfer is being used to increase the infiltration of NK cells in the tumor site by using NK cells from different origins, such as autologous cells, allogeneic peripheral blood mononuclear cells, umbilical cord blood, human embryonic stem cells and induced pluripotent stem cells (90). However, although preclinical data indicate high efficacy of NK cell adoptive transfer in in vitro and in vivo studies, there is little information on the clinical efficiency of this method. A phase I clinical trial demonstrated that HSP70-induced activation of autologous NK cells was achieved in patients without any treatment-related negative side effects, but no significant clinical response was observed due to the high tumor burden and limited sample size (96). Moreover, adoptive macrophage transfer has recently become a hot research field (97,98). Due to their innate immune function and more prominent penetrating ability, macrophages may kill tumor cells when T-cell therapy fails (99). However, sufficient clinical evidence is urgently needed to support pre-clinical data, particularly in the field of GI cancer treatment.
In order to make adoptive cell therapy more readily applicable and more effective in destroying tumor cells, relatively more recent approaches have sought to introduce antitumor antigen receptors to regular T cells, which may have promising therapeutic applications. It is possible to eliminate the requirement for MHC interaction and co-stimulatory molecules when T cells are engineered to include chimeric antigen receptors (CAR), in which the B-cell receptor-derived and T-cell receptor domains are combined (100,101). To date, the antitumor qualities from the adoptive transfer of CAR-T cells has been evident in the case of advanced hematological malignancies, but in the case of solid tumors the results have been less promising (102,103). It may be possible to explain this as a consequence of the expression of heterogeneous tumor antigens, the immunosuppressive network activity within the microenvironment of the tumor, the T-cell trafficking into solid tumors, which is less than optimal, and the absence of the necessary co-stimulatory signals to achieve CAR-T cell persistence following infusion (100,101). The affinity of HER2-directed CAR-T cells for cancer cells of the GI tract is high, even for cells exhibiting low levels of HER2 expression. Moreover, CAR-T-HER2 cells may offer the potential to inhibit disease recurrence of metastasis. Specific CAR-T-HER2 cells have been developed, which are consistently active within the cardiovascular system, and which can accumulate within and inhibit tumors (104). In the context of PC, CAR-T-cell therapy has been the subject of a number of studies. The tumor-specific antigens of PC can be targeted by CAR-T-cell therapy. In particular, antigens that are overexpressed, such as CEA (NCT02349724), mesothelin (NCT02159716), HER2 (NCT02713984) and MUC1 (NCT02587689), are promising targets. In HCC, there have been no complete CAR-T-cell tests, but there is some evidence that the antigens CEA, MUC1 and glypican-3 could be effectively targeted (105,106). For GC, only a few studies have examined CAR-T-cell therapy to date (107,108), but the results are cautiously optimistic when developing CAR-T cells to target 3H11(109), despite an inability to overcome the biological obstacles presented by solid tumors. A further study based on the overexpression of the folate 1 receptor (FOLR1) in GC compared with healthy tissue showed that FOLR1 CAR-T cells display antitumor properties by recognizing FOLR1-positive GC cells (110). In the case of hepatobiliary cancer, small-scale trials have reported positive outcomes of immunotherapy, and recent work has assessed the potential of CAR-T cells when treating cancers of the biliary tract (111). The suggested targets include EGFR, mesothelin and HER2, due to their propensity to be overexpressed in the aforementioned malignancies (112-114).
Although adoptive transfer therapy is promising and intensely under review, the major obstacle of this approach in GI cancer treatment is the heterogeneity of solid tumor, particularly in colon and GC. The heterogenicity is caused by genomic instability, which contributes to clonal evolution and immune evasion resulting in immune-resistance and tumor recurrence. Interestingly, combination treatment of cellular adoptive transfer and chemotherapy can improve clinical outcomes and may prevent recurrence in patients with advance GC that may be attributed to the synergistic effect of ICD (115). Moreover, multiclonal elimination of tumor cells could be improved by using multi-peptide vaccine or broad-array tumor antigen (116,117). Overall, these would be the solution for immune exhaustion in clinical settings of adoptive therapies.
9. Implications of ICD and adoptive cell transfer
The implications of adoptive transfer of cytotoxic T cells or CAR-T cells with ICD has not been extensively studied in the context of GI cancer treatment. However, it has been shown that the antitumor immune response of cytotoxic T cells may lead to immunogenic tumor cell death, improving their own tumor cell-killing capacity (118). Interestingly, treatment with mitochondria-targeted small molecules, including ATP, CRT and HMGB1, can induce upregulation of ICD in both in vitro and in vivo models. This is surprising, as priming by an ICD inducer effectively suppressed tumor growth and lung metastasis by enhancing the adoptive T-cell therapy against colon cancer in a mouse model (119). Moreover, it has been demonstrated that the mitochondrial DAMPs could play a role as immunomodulators through activation of γδ-T cells. Mitochondrial DAMPs induced expression of TLR2 and TLR4, which may positively regulate the antitumor response (120,121). TLR/type I IFN/CXCL10 has been proposed as the signaling pathway implicated in the recruitment of CXCR3+ T cells and the activation of γδ-T cells (122). Hence, not only the direct impact from activated antigen-presenting cells, but also T-cell-based anticancer therapy may be targeted when considering ICD (Table I). However, although the implications of T-cell therapy and ICD appear to be very promising, sufficient evidence is currently lacking.
10. Conclusions
ICD plays a key role in enhancing the efficacy of immunotherapy. DC-based anticancer vaccines directly activate and strengthen the co-stimulatory signal through DAMPs to further stimulate the immune response. The treatment outcomes from both the immune checkpoint blockade and adoptive transfer cell therapy may be enhanced by using ICD inducer agents. The mechanisms involving ICD-enhanced immunotherapy in GI cancer treatment are demonstrated in Fig. 1. These findings confirm the effectiveness of induction therapies combined with immunotherapy. Intervention may be implemented at only one or a few cycles at effectively low doses to avoid adverse effects and to obtain optimal ICD induction. Neoadjuvant chemotherapy can induce either cancer cell apoptosis or necrosis, which effectively promotes ICD induction, particularly HMGB1 release. In addition to the HSP family, CRT and S100 protein expression is elicited by chemotherapy and may be the primary DAMP molecules strengthening the efficacy of immunotherapy. However, the use of ICD inducers in immunotherapy for GI cancers has been limited due to a lack of research evidence, accounting for only 5-10% of all clinical trials. Further investigation should yield more positive outcomes regarding the induction of ICD in immunotherapy for GI cancer.
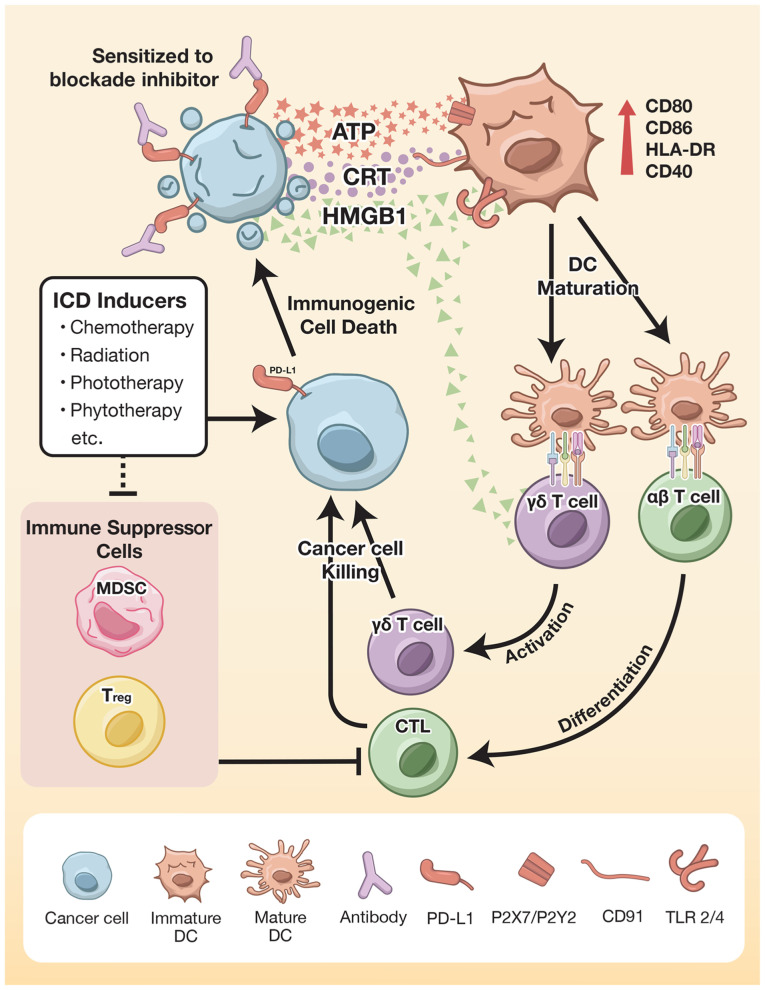
Figure 1.
ICD enhances the antitumor immune response. Anticancer therapies, such as chemotherapeutic agents, radiation and phytotherapy, elicit the exposure of CRT on cell membranes and the extracellular release of ATP, HMGB1 and HSP. Interestingly, some types of ICD inducers increase PD-L1 expression, which increases the sensitivity to blockade inhibitor treatment. ICD potentiates DCs though upregulation of costimulatory signals, thereby strengthening adaptive immune system activation. Activation of αβ T cells results in differentiation to cytotoxic T cells, whereas activation of γδ T cells is facilitated by either mature antigen-presenting cells or as a direct effect of ICD. These modulate antitumor activity by cytotoxic granules and proinflammatory cytokine production. Moreover, the ICD inducer plays a role in the inhibition of immune suppressor cells. Therefore, ICD may be considered as priming therapy that may be suitably combined with cancer immunotherapy. ICD, immunogenic cell death; HMGB1, high mobility group box 1; CRT, calreticulin; MDSC, myeloid-derived suppressor cell; DC, dendritic cell; Treg, regulatory T cell; CTL, cytotoxic T lymphocyte; TLR, Toll-like receptor; PD-L1, programmed death ligand 1; HSP, heat shock protein.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was supported by the National Science Research and Innovation Fund (NSRF) (grant nos. R2564B011, R2564B012, R2563C029 and R2564B015) and the Thailand Science Research and Innovations (TSRI) (grant no. BRG6180010).
Availability of data and materials
Not applicable.
Authors' contributions
WS wrote the paper. CN designed and created the graphic figure. SP and AJ wrote and edited the paper. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rammensee HG. From basic immunology to new therapies for cancer patients. In: Cancer Immunotherapy Meets. Oncology. In Honor of Christoph Huber. Britten CM, Kreiter S, Diken M and Rammensee HG (eds). Springer International Publishing, Cham, pp3-11, 2014. [Google Scholar]
- 2.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 3.Matzinger P. Tolerance, danger, and the extended family. Annual Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 4.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 5.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 6.Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, Sautès-Fridman C, Fucikova J, Galon J, Spisek R, et al. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4(e1008866) doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3(e955691) doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toomey PG, Vohra NA, Ghansah T, Sarnaik AA, Pilon-Thomas SA. Immunotherapy for gastrointestinal malignancies. Cancer Control. 2013;20:32–42. doi: 10.1177/107327481302000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawla P, Barsouk A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Gastroenterol Rev. 2019;14:26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse MA, Hochster H, Benson A. Perspectives on treatment of metastatic colorectal cancer with immune checkpoint inhibitor therapy. Oncologist. 2020;25:33–45. doi: 10.1634/theoncologist.2019-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 13.Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol. 2013;47 (Suppl):S2–S6. doi: 10.1097/MCG.0b013e3182872f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: Present and future. Clin Liver Dis. 2011;15:223–243. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamsa-Ard S, Luvira V, Suwanrungruang K, Kamsa-Ard S, Luvira V, Santong C, Srisuk T, Pugkhem A, Bhudhisawasdi V, Pairojkul C. Cholangiocarcinoma trends, incidence, and relative survival in Khon Kaen, Thailand from 1989 through 2013: A population-based cancer registry study. J Epidemiol. 2019;29:197–204. doi: 10.2188/jea.JE20180007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Blechacz B. Cholangiocarcinoma: Current knowledge and new developments. Gut Liver. 2017;11:13–26. doi: 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sripa B, Pairojkul C. Cholangiocarcinoma: Lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira NP, Corrêa JR. Pancreatic cancer: Treatment approaches and trends. J Cancer Metastasis Treat. 2018;4(18) [Google Scholar]
- 21.Matsuoka T, Yashiro M. Precision medicine for gastrointestinal cancer: Recent progress and future perspective. World J Gastrointest Oncol. 2020;12:1–20. doi: 10.4251/wjgo.v12.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdul-Latif M, Townsend K, Dearman C, Shiu KK, Khan K. Immunotherapy in gastrointestinal cancer: The current scenario and future perspectives. Cancer Treat Rev. 2020;88(102030) doi: 10.1016/j.ctrv.2020.102030. [DOI] [PubMed] [Google Scholar]
- 23.Tannapfel A, Reinacher-Schick A. Immunotherapy in gastrointestinal cancer: Where Do We Stand? Visc Med. 2019;35:1–2. doi: 10.1159/000497294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suntharalingam M, Winter K, Ilson DH, Dicker A, Kachnic LA, Chakravarthy AAK, Gaffney DK, Thakrar HV, Horiba MN, Deutsch M, et al. The initial report of RTOG 0436: A phase III trial evaluating the addition of cetuximab to paclitaxel, cisplatin, and radiation for patients with esophageal cancer treated without surgery. J Clin Oncol. 2014;32:LBA6–LBA6. [Google Scholar]
- 25.O'Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S, Pitot HC, Shields AF, Landry JC, Ryan DP, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: Surgical end points from national surgical adjuvant breast and bowel project trial R-04. J Clin Oncol. 2014;32:1927–1934. doi: 10.1200/JCO.2013.53.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortara L, Balza E, Bruno A, Poggi A, Orecchia P, Carnemolla B. Anti-cancer therapies employing il-2 cytokine tumor targeting: Contribution of innate, adaptive and immunosuppressive cells in the anti-tumor efficacy. Front Immunol. 2018;9(2905) doi: 10.3389/fimmu.2018.02905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4(7) doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 29.Kudrin A. Overview of cancer vaccines: Considerations for development. Hum Vaccin Immunother. 2012;8:1335–1353. doi: 10.4161/hv.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reitsma DJ, Combest AJ. Challenges in the development of an autologous heat shock protein based anti-tumor vaccine. Hum Vaccin Immunother. 2012;8:1152–1155. doi: 10.4161/hv.21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ozao-Choy J, Lee DJ, Faries MB. Melanoma vaccines: Mixed past, promising future. Surg Clin North Am. 2014;94:1017–1030. doi: 10.1016/j.suc.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niccolai E, Taddei A, Prisco D, Amedei A. Gastric cancer and the epoch of immunotherapy approaches. World J Gastroenterol. 2015;21:5778–5793. doi: 10.3748/wjg.v21.i19.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morse MA, Deng Y, Coleman D, Hull S, Kitrell-Fisher E, Nair S, Schlom J, Ryback ME, Lyerly HK. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res. 1999;5:1331–1338. [PubMed] [Google Scholar]
- 34.Li J, Valentin A, Beach RK, Alicea C, Felber BK, Pavlakis GN. DNA is an efficient booster of dendritic cell-based vaccine. Hum Vaccin Immunother. 2015;11:1927–1935. doi: 10.1080/21645515.2015.1020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda Y, Yoshimura K, Matsui H, Shindo Y, Tamesa T, Tokumitsu Y, Hashimoto N, Tokuhisa Y, Sakamoto K, Sakai K, et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: A phase 1 dose escalation clinical trial. Cancer Immunol Immunother. 2015;64:1047–1056. doi: 10.1007/s00262-015-1709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song W, Kong HL, Carpenter H, Torii H, Granstein R, Rafii S, Moore MA, Crystal RG. Dendritic cells genetically modified with an adenovirus vector encoding the cDNA for a model antigen induce protective and therapeutic antitumor immunity. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiraviriyakul A, Songjang W, Kaewthet P, Tanawatkitichai P, Bayan P, Pongcharoen S. Honokiol-enhanced cytotoxic T lymphocyte activity against cholangiocarcinoma cells mediated by dendritic cells pulsed with damage-associated molecular patterns. World J Gastroenterol. 2019;25:3941–3955. doi: 10.3748/wjg.v25.i29.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottfried E, Krieg R, Eichelberg C, Andreesen R, Mackensen A, Krause SW. Characterization of cells prepared by dendritic cell-tumor cell fusion. Cancer Immun. 2002;2(15) [PubMed] [Google Scholar]
- 39.Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M, Schillinger B, Liu W, Lu Y, Mitsky P, et al. Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother. 2007;30:762–772. doi: 10.1097/CJI.0b013e318133451c. [DOI] [PubMed] [Google Scholar]
- 40.Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, Stephans KF, Masutomi K, Loda M, Xia Z, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828–839. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]
- 41.Rosalia RA, Quakkelaar ED, Redeker A, Khan S, Camps M, Drijfhout JW, Silva AL, Jiskoot W, van Hall T, van Veelen PA, et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol. 2013;43:2554–2565. doi: 10.1002/eji.201343324. [DOI] [PubMed] [Google Scholar]
- 42.Medema JP, Schuurhuis DH, Rea D, van Tongeren J, de Jong J, Bres SA, Laban S, Toes RE, Toebes M, Schumacher TN, et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: Differential modulation by T helper type 1 and type 2 cells. J Exp Med. 2001;194:657–667. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang QM, He SJ, Shen N, Luo B, Fan R, Fu J, Luo GR, Zhou SF, Xiao SW, Xie XX. Overexpression of MAGE-D4 in colorectal cancer is a potentially prognostic biomarker and immunotherapy target. Int J Clin Exp Pathol. 2014;7:3918–3927. [PMC free article] [PubMed] [Google Scholar]
- 44.Kono K, Takahashi A, Sugai H, Fujii H, Choudhury AR, Kiessling R, Matsumoto Y. Dendritic cells pulsed with HER-2/neu-derived peptides can induce specific T-cell responses in patients with gastric cancer. Clin Cancer Res. 2002;8:3394–3400. [PubMed] [Google Scholar]
- 45.Smith AM, Justin T, Michaeli D, Watson SA. Phase I/II study of G17-DT, an Anti-gastrin immunogen, in advanced colorectal cancer. Clin Cancer Res. 2000;6:4719–4724. [PubMed] [Google Scholar]
- 46.Higashihara Y, Kato J, Nagahara A, Izumi K, Konishi M, Kodani T, Serizawa N, Osada T, Watanabe S. Phase I clinical trial of peptide vaccination with URLC10 and VEGFR1 epitope peptides in patients with advanced gastric cancer Int J. Oncol. 2014;44:662–668. doi: 10.3892/ijo.2013.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchianò A, Andreola S, et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9:3235–3245. [PubMed] [Google Scholar]
- 48.Dolcetti R, De Re V, Canzonieri V. Immunotherapy for gastric cancer: Time for a Personalized Approach? Int J Mol Sci. 2018;19(1602) doi: 10.3390/ijms19061602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA Jr, Donehower RC, Jaffee EM, Laheru DA. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikeda M, Okusaka T, Ohno I, Mitsunaga S, Kondo S, Ueno H, Morizane C, Gemmoto K, Suna H, Ushida Y, Furuse J. Phase I studies of peptide vaccine cocktails derived from GPC3, WDRPUH and NEIL3 for advanced hepatocellular carcinoma. Immunotherapy. 2021;13:371–385. doi: 10.2217/imt-2020-0278. [DOI] [PubMed] [Google Scholar]
- 51.Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, Seja E, Todd K, Glaspy JA, McBride WH, Economou JS. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9:5902–5908. [PubMed] [Google Scholar]
- 52.Tsuchiya N, Yoshikawa T, Fujinami N, Saito K, Mizuno S, Sawada Y, Endo I, Nakatsura T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology. 2017;6(e1346764) doi: 10.1080/2162402X.2017.1346764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q, Chen G, Peng L, Wang X, Yang Y, Liu C, Shi W, Su C, Wu H, Liu X, et al. Increased safety with preserved antitumoral efficacy on hepatocellular carcinoma with dual-regulated oncolytic adenovirus. Clin Cancer Res. 2006;12:6523–6531. doi: 10.1158/1078-0432.CCR-06-1491. [DOI] [PubMed] [Google Scholar]
- 54.Lepisto AJ, Moser AJ, Zeh H, Lee K, Bartlett D, McKolanis JR, Geller BA, Schmotzer A, Potter DP, Whiteside T, et al. A phase I/II study of a MUC1 peptide pulsed autologous dendritic cell vaccine as adjuvant therapy in patients with resected pancreatic and biliary tumors. Cancer Ther. 2008;6:955–964. [PMC free article] [PubMed] [Google Scholar]
- 55.Kawamura J, Sugiura F, Sukegawa Y, Yoshioka Y, Hida JI, Hazama S, Okuno K. Multicenter, phase II clinical trial of peptide vaccination with oral chemotherapy following curative resection for stage III colorectal cancer. Oncology Lett. 2018;15:4241–4247. doi: 10.3892/ol.2018.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahma OE, Hamilton JM, Wojtowicz M, Dakheel O, Bernstein S, Liewehr DJ, Steinberg SM, Khleif SN. The immunological and clinical effects of mutated ras peptide vaccine in combination with IL-2, GM-CSF, or both in patients with solid tumors. J Transl Med. 2014;12(55) doi: 10.1186/1479-5876-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quandt J, Schlude C, Bartoschek M, Will R, Cid-Arregui A, Schölch S, Reissfelder C, Weitz J, Schneider M, Wiemann S, et al. Long-peptide vaccination with driver gene mutations in p53 and Kras induces cancer mutation-specific effector as well as regulatory T cell responses. Oncoimmunology. 2018;7(e1500671) doi: 10.1080/2162402X.2018.1500671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hessmann E, Patzak MS, Klein L, Chen N, Kari V, Ramu I, Bapiro TE, Frese KK, Gopinathan A, Richards FM, et al. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut. 2018;67:497–507. doi: 10.1136/gutjnl-2016-311954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scarpa M, Ruffolo C, Canal F, Scarpa M, Basato S, Erroi F, Fiorot A, Dall'Agnese L, Pozza A, Porzionato A, et al. Mismatch repair gene defects in sporadic colorectal cancer enhance immune surveillance. Oncotarget. 2015;6:43472–43482. doi: 10.18632/oncotarget.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, et al. Efficacy of Pembrolizumab in patients with noncolorectal high microsatellite Instability/Mismatch Repair-deficient cancer: Results from the phase II KEYNOTE-158 Study. J Clin Oncol. 2019;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G, Galluzzi L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11(1013) doi: 10.1038/s41419-020-03221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72:3967–3976. doi: 10.1158/0008-5472.CAN-12-0851. [DOI] [PubMed] [Google Scholar]
- 63.Ratschker T, Egenberger L, Alev M, Zschiesche L, Band J, Schreiber E, Frey B, Derer A, Alexiou C, Janko C. Mitoxantrone-loaded nanoparticles for magnetically controlled tumor therapy-induction of tumor cell death, release of danger signals and activation of immune cells. Pharmaceutics. 2020;12(923) doi: 10.3390/pharmaceutics12100923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao X, Yang K, Zhao R, Ji T, Wang X, Yang X, Zhang Y, Cheng K, Liu S, Hao J, et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187–197. doi: 10.1016/j.biomaterials.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 65.Turrini E, Catanzaro E, Muraro MG, Governa V, Trella E, Mele V, Calcabrini C, Morroni F, Sita G, Hrelia P, et al. Hemidesmus indicus induces immunogenic death in human colorectal cancer cells. Oncotarget. 2018;9:24443–24456. doi: 10.18632/oncotarget.25325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu CJ, Tsai YT, Lee IJ, Wu PY, Lu LS, Tsao WS, Huang YJ, Chang CC, Ka SM, Tao MH. Combination of radiation and interleukin 12 eradicates large orthotopic hepatocellular carcinoma through immunomodulation of tumor microenvironment. Oncoimmunology. 2018;7(e1477459) doi: 10.1080/2162402X.2018.1477459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He H, Liu L, Liang R, Zhou H, Pan H, Zhang S, Cai L. Tumor-targeted nanoplatform for in situ oxygenation-boosted immunogenic phototherapy of colorectal cancer. Acta Biomaterialia. 2020;104:188–197. doi: 10.1016/j.actbio.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 68.Van Loenhout J, Flieswasser T, Freire Boullosa L, De Waele J, Van Audenaerde J, Marcq E, Jacobs J, Lin A, Lion E, Dewitte H, et al. Cold atmospheric plasma-treated PBS eliminates immunosuppressive pancreatic stellate cells and induces immunogenic cell death of pancreatic cancer cells. Cancers. 2019;11(1597) doi: 10.3390/cancers11101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 71.Borch TH, Donia M, Andersen MH, Svane IM. Reorienting the immune system in the treatment of cancer by using anti-PD-1 and anti-PD-L1 antibodies. Drug Discov Today. 2015;20:1127–1134. doi: 10.1016/j.drudis.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Schildberg FA, Klein SR, Freeman GJ, Sharpe AH. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity. 2016;44:955–972. doi: 10.1016/j.immuni.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee B, Hutchinson R, Wong HL, Tie J, Putoczki T, Tran B, Gibbs P, Christie M. Emerging biomarkers for immunomodulatory cancer treatment of upper gastrointestinal, pancreatic and hepatic cancers. Semin Cancer Biol. 2018;52:241–252. doi: 10.1016/j.semcancer.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 74.Zhou G, Noordam L, Sprengers D, Doukas M, Boor PPC, van Beek AA, Erkens R, Mancham S, Grünhagen D, Menon AG, et al. Blockade of LAG3 enhances responses of tumor-infiltrating T cells in mismatch repair-proficient liver metastases of colorectal cancer. Oncoimmunology. 2018;7(e1448332) doi: 10.1080/2162402X.2018.1448332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huyghe N, Baldin P, Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: What is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep (Oxf) 2020;8:11–24. doi: 10.1093/gastro/goz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chung KY, Gore I, Fong L, Venook A, Beck SB, Dorazio P, Criscitiello PJ, Healey DI, Huang B, Gomez-Navarro J, Saltz LB. Phase II study of the anti-cytotoxic T-Lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 77.Torphy RJ, Zhu Y, Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann Gastroenterol Surg. 2018;2:274–281. doi: 10.1002/ags3.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kudo M, Matilla A, Santoro A, Melero I, Gracian AC, Acosta-Rivera M, Choo SP, El-Khoueiry AB, Kuromatsu R, El-Rayes BF, et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status. J Clin Oncol. 2019;37(327) [Google Scholar]
- 79.Ledford H, Else H, Warren M. Cancer immunologists scoop medicine Nobel prize. Nature. 2018;562:20–21. doi: 10.1038/d41586-018-06751-0. [DOI] [PubMed] [Google Scholar]
- 80.Sakakibara K, Sato T, Kufe DW, VonHoff DD, Kawabe T. CBP501 induces immunogenic tumor cell death and CD8 T cell infiltration into tumors in combination with platinum, and increases the efficacy of immune checkpoint inhibitors against tumors in mice. Oncotarget. 2017;8:78277–78288. doi: 10.18632/oncotarget.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Landry MR, DuRoss AN, Neufeld MJ, Hahn L, Sahay G, Luxenhofer R, Sun C. Low dose novel PARP-PI3K inhibition via nanoformulation improves colorectal cancer immunoradiotherapy. Materials today Bio. 2020;8(100082) doi: 10.1016/j.mtbio.2020.100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu H, Shan Y, Ge K, Lu J, Kong W, Jia C. Oxaliplatin induces immunogenic cell death in hepatocellular carcinoma cells and synergizes with immune checkpoint blockade therapy. Cell Oncol (Dordr) 2020;43:1203–1214. doi: 10.1007/s13402-020-00552-2. [DOI] [PubMed] [Google Scholar]
- 83.Maruoka Y, Furusawa A, Okada R, Inagaki F, Fujimura D, Wakiyama H, Kato T, Nagaya T, Choyke PL, Kobayashi H. Near-infrared photoimmunotherapy combined with CTLA4 checkpoint blockade in syngeneic mouse cancer models. Vaccines (Basel) 2020;8(528) doi: 10.3390/vaccines8030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Antoniotti C, Borelli B, Rossini D, Pietrantonio F, Morano F, Salvatore L, Lonardi S, Marmorino F, Tamberi S, Corallo S, et al. AtezoTRIBE: A randomised phase II study of FOLFOXIRI plus bevacizumab alone or in combination with atezolizumab as initial therapy for patients with unresectable metastatic colorectal cancer. BMC Cancer. 2020;20(683) doi: 10.1186/s12885-020-07169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fumet JD, Isambert N, Hervieu A, Zanetta S, Guion JF, Hennequin A, Rederstorff E, Bertaut A, Ghiringhelli F. Phase Ib/II trial evaluating the safety, tolerability and immunological activity of durvalumab (MEDI4736) (anti-PD-L1) plus tremelimumab (anti-CTLA-4) combined with FOLFOX in patients with metastatic colorectal cancer. ESMO Open. 2018;3(e000375) doi: 10.1136/esmoopen-2018-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bang YJ, Muro K, Fuchs CS, Golan T, Geva R, Hara H, Jalal SI, Borg C, Doi T, Wainberg ZA, et al. KEYNOTE-059 cohort 2: Safety and efficacy of pembrolizumab (pembro) plus 5-fluorouracil (5-FU) and cisplatin for first-line (1L) treatment of advanced gastric cancer. J Clin Oncol. 2017;35(4012) [Google Scholar]
- 87.Bailly C, Thuru X, Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: Modern times. NAR Cancer. 2020;2(zcaa002) doi: 10.1093/narcan/zcaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dosset M, Vargas TR, Lagrange A, Boidot R, Végran F, Roussey A, Chalmin F, Dondaine L, Paul C, Lauret Marie-Joseph E, et al. PD-1/PD-L1 pathway: An adaptive immune resistance mechanism to immunogenic chemotherapy in colorectal cancer. Oncoimmunology. 2018;7(e1433981) doi: 10.1080/2162402X.2018.1433981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chu TH, Chan HH, Hu TH, Wang EM, Ma YL, Huang SC, Wu JC, Chang YC, Weng WT, Wen ZH, et al. Celecoxib enhances the therapeutic efficacy of epirubicin for Novikoff hepatoma in rats. Cancer Med. 2018;7:2567–2580. doi: 10.1002/cam4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang F, Lau JKC, Yu J. The role of natural killer cell in gastrointestinal cancer: Killer or helper. Oncogene. 2021;40:717–730. doi: 10.1038/s41388-020-01561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amedei A, Niccolai E, D'Elios MM. T cells and adoptive immunotherapy: Recent developments and future prospects in gastrointestinal oncology. Clin Dev Immunol. 2011;2011(320571) doi: 10.1155/2011/320571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo Y, Han W. Cytokine-induced killer (CIK) cells: From basic research to clinical translation. Chin J Cancer. 2015;34:99–107. doi: 10.1186/s40880-015-0002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, Sakai F, Kato A, Tanabe M, Enoki T, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13(277) doi: 10.1186/s12967-015-0632-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shiozawa M, Chang CH, Huang YC, Chen YC, Chi MS, Hao HC, Chang YC, Takeda S, Chi KH, Wang YS. Pharmacologically upregulated carcinoembryonic antigen-expression enhances the cytolytic activity of genetically-modified chimeric antigen receptor NK-92MI against colorectal cancer cells. BMC Immunol. 2018;19(27) doi: 10.1186/s12865-018-0262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu B, Liu ZZ, Zhou ML, Lin JW, Chen XM, Li Z, Gao WB, Yu ZD, Liu T. Development of c-MET-specific chimeric antigen receptor-engineered natural killer cells with cytotoxic effects on human liver cancer HepG2 cells. Mol Med Rep. 2019;20:2823–2831. doi: 10.3892/mmr.2019.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krause SW, Gastpar R, Andreesen R, Gross C, Ullrich H, Thonigs G, Pfister K, Multhoff G. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70-peptide-activated, autologous natural killer cells: A clinical phase i trial. Clin Cancer Res. 2004;10:3699–3707. doi: 10.1158/1078-0432.CCR-03-0683. [DOI] [PubMed] [Google Scholar]
- 97.Andreesen R, Scheibenbogen C, Brugger W, Krause S, Meerpohl HG, Leser HG, Engler H, Löhr GW. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: A new approach to cancer immunotherapy. Cancer Res. 1990;50:7450–7456. [PubMed] [Google Scholar]
- 98.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–953. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y, Wang R. Immunotherapy targeting tumor-associated macrophages. Front Med (Lausanne) 2020;7(583708) doi: 10.3389/fmed.2020.583708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fesnak AD, June CH, Levine BL. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mirzaei HR, Rodriguez A, Shepphird J, Brown CE, Badie B. Chimeric antigen receptors T cell therapy in solid tumor: Challenges and clinical applications. Front Immunol. 2017;8(1850) doi: 10.3389/fimmu.2017.01850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hou B, Tang Y, Li W, Zeng Q, Chang D. Efficiency of CAR-T therapy for treatment of solid tumor in clinical trials: A meta-analysis. Disease Markers. 2019;2019(3425291) doi: 10.1155/2019/3425291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bebnowska D, Grywalska E, Niedźwiedzka-Rystwej P, Sosnowska-Pasiarska B, Smok-Kalwat J, Pasiarski M, Góźdź S, Roliński J, Polkowski W. CAR-T cell therapy-an overview of targets in gastric cancer. J Clin Med. 2020;9(1894) doi: 10.3390/jcm9061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alrifai D, Sarker D, Maher J. Prospects for adoptive immunotherapy of pancreatic cancer using chimeric antigen receptor-engineered T-cells. Immunopharm Immunot. 2016;38:50–60. doi: 10.3109/08923973.2015.1100204. [DOI] [PubMed] [Google Scholar]
- 106.Cheng X, Zhao G, Zhao Y. Combination immunotherapy approaches for pancreatic cancer treatment. Can J Gastroenterol Hepatol. 2018;2018(6240467) doi: 10.21037/jgo.2018.05.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo Y, Zhao X, Wang Y, Wang Z, Han W, Chen L. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. 2018;9:867–878. doi: 10.1007/s13238-017-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tao K, He M, Tao F, Xu G, Ye M, Zheng Y, Li Y. Development of NKG2D-based chimeric antigen receptor-T cells for gastric cancer treatment. Cancer Chemother Pharmacol. 2018;82:815–827. doi: 10.1007/s00280-018-3670-0. [DOI] [PubMed] [Google Scholar]
- 109.Han H, Wang S, Hu Y, Li Z, Yang W, Lv Y, Wang L, Zhang L, Ji J. Monoclonal antibody 3H11 chimeric antigen receptors enhance T cell effector function and exhibit efficacy against gastric cancer. Oncol Lett. 2018;15:6887–6894. doi: 10.3892/ol.2018.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim M, Pyo S, Kang CH, Lee CO, Lee HK, Choi SU, Park CH. Folate receptor 1 (FOLR1) targeted chimeric antigen receptor (CAR) T cells for the treatment of gastric cancer. PLoS One. 2018;13(e0198347) doi: 10.1371/journal.pone.0198347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DeLeon TT, Zhou YM, Nagalo BM, Yokoda RT, Ahn DH, Ramanathan RK, Salomao MA, Aqel BA, Mahipal A, Bekaii-Saab TS, Borad MJ. Novel immunotherapy strategies for hepatobiliary cancers. Immunotherapy. 2018;10:1077–1091. doi: 10.2217/imt-2018-0024. [DOI] [PubMed] [Google Scholar]
- 112.Xu JY, Ye ZL, Jiang DQ, He JC, Ding YM, Li LF, Lv SQ, Wang Y, Jin HJ, Qian QJ. Mesothelin-targeting chimeric antigen receptor-modified T cells by piggyBac transposon system suppress the growth of bile duct carcinoma. Tumor Biol. 2017;39(1010428317695949) doi: 10.1177/1010428317695949. [DOI] [PubMed] [Google Scholar]
- 113.Guo Y, Feng K, Liu Y, Wu Z, Dai H, Yang Q, Wang Y, Jia H, Han W. Phase I study of chimeric antigen receptor-modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin Cancer Res. 2018;24:1277–1286. doi: 10.1158/1078-0432.CCR-17-0432. [DOI] [PubMed] [Google Scholar]
- 114.Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: Review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–164. doi: 10.1007/s10555-015-9552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cui J, Li L, Wang C, Jin H, Yao C, Wang Y, Li D, Tian H, Niu C, Wang G, et al. Combined cellular immunotherapy and chemotherapy improves clinical outcome in patients with gastric carcinoma. Cytotherapy. 2015;17:979–988. doi: 10.1016/j.jcyt.2015.03.605. [DOI] [PubMed] [Google Scholar]
- 116.El-Sayes N, Vito A, Mossman K. Tumor heterogeneity: A great barrier in the age of cancer immunotherapy. Cancers. 2021;13(806) doi: 10.3390/cancers13040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 118.Minute L, Teijeira A, Sanchez-Paulete AR, Ochoa MC, Alvarez M, Otano I, Etxeberrria I, Bolaños E, Azpilikueta A, Garasa S, et al. Cellular cytotoxicity is a form of immunogenic cell death. J Immunother Cancer. 2020;8(e000325) doi: 10.1136/jitc-2019-000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang Q, Zhang C, Wang H, Peng T, Zhang L, Wang Y, Han W, Shi C. Mitochondria-targeting immunogenic cell death inducer improves the adoptive T-cell therapy against solid tumor. Front Oncol. 2019;9(1196) doi: 10.3389/fonc.2019.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwacha MG, Rani M, Nicholson SE, Lewis AM, Holloway TL, Sordo S, Cap AP. Dermal γδ T-cells can be activated by mitochondrial damage-associated molecular patterns. PLoS One. 2016;11(e0158993) doi: 10.1371/journal.pone.0158993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schwacha MG, Rani M, Zhang Q, Nunez-Cantu O, Cap AP. Mitochondrial damage-associated molecular patterns activate γδ T-cells. Innate immunity. 2014;20:261–268. doi: 10.1177/1753425913488969. [DOI] [PubMed] [Google Scholar]
- 122.Gebremeskel S, Johnston B. Concepts and mechanisms underlying chemotherapy induced immunogenic cell death: Impact on clinical studies and considerations for combined therapies. Oncotarget. 2015;6:41600–41619. doi: 10.18632/oncotarget.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xie W, Forveille S, Iribarren K, Sauvat A, Senovilla L, Wang Y, Humeau J, Perez-Lanzon M, Zhou H, Martínez-Leal JF, et al. Lurbinectedin synergizes with immune checkpoint blockade to generate anticancer immunity. Oncoimmunology. 2019;8(e1656502) doi: 10.1080/2162402X.2019.1656502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Limagne E, Thibaudin M, Nuttin L, Spill A, Derangère V, Fumet JD, Amellal N, Peranzoni E, Cattan V, Ghiringhelli F. Trifluridine/Tipiracil plus Oxaliplatin improves PD-1 blockade in colorectal cancer by inducing immunogenic cell death and depleting macrophages. Cancer Immunol Res. 2019;7:1958–1969. doi: 10.1158/2326-6066.CIR-19-0228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.