Abstract
Non-invasive prenatal diagnosis (NIPD) of isolated cell-free DNA from maternal plasma has been applied to detect monogenic diseases in the fetus. Droplet digital PCR (ddPCR) is a sensitive and quantitative technique for NIPD. In the present study, the development and evaluation of ddPCR-based assays for common α and β-thalassemia variants amongst the Asian population was described; specifically, Southeast Asian (SEA) deletion, HbE, and 41/42 (-CTTT). SEA is caused by deletion of a 20 kb region surrounding the α-globin gene, whilst HbE and 41/42 (-CTTT) are caused by point mutations on the β-globin gene. Cell-free DNA samples from 46 singleton pregnant women who were carriers of these mutations were isolated and quantified using ddPCR with specially designed probes for each target allele. Allelic copy number calculation and likelihood ratio tests were used to classify fetal genotypes. Classification performances were evaluated against ground truth fetal genotypes obtained from conventional amniocentesis. Copy number variation analysis of SEA deletion accurately classified fetal genotypes in 20 out of 22 cases with an area under the receiver operating characteristic curve of 0.98 for detecting Hb Bart's hydrops fetalis. For HbE cases, 10 out of 16 samples were correctly classified, and three were inconclusive. For 41/42 (-CTTT) cases, 2 out of 8 were correctly classified, and four were inconclusive. The correct genotype was not rejected in any inconclusive case and may be resolved with additional ddPCR experiments. These results indicate that ddPCR-based analysis of maternal plasma can become an accurate and effective NIPD for SEA deletion α-(0) thalassemia. Although the performance of ddPCR on HbE and 41/42 (-CTTT) mutations were not sufficient for clinical application, these results may serve as a foundation for future works in this field.
Keywords: non-invasive prenatal test, cell-free fetal DNA, droplet digital PCR, α-thalassemia, β-thalassemia
Introduction
Thalassemia is an autosomal recessive disease that is caused by the reduction in hemoglobin chain synthesis. It is estimated that thalassemia affects 17% of over 330,000 newborns worldwide each year (1). The two major types of thalassemia are α- and β-thalassemia. α-thalassemia is caused by the loss of one or both of the α-globin genes (2). The Southeast Asian deletion (SEA) variant is a large deletion of the α-globin gene region, and is the most common cause of α-thalassemia in the Asian population. Couples carrying the SEA deletion do not show symptoms, but will have a 25% chance of conceiving a baby with Hb Bart's hydrops fetalis and experiencing toxemia during pregnancy (3). β-thalassemia is caused by mutations in β-globin genes, which lead to reduced β-globin chain synthesis (4). Children born with β-thalassemia may show symptoms of anemia and other complications throughout their lives (5). Thailand has a high (30-50%) prevalence of α- and β-thalassemia (6), particularly in the northern and northeastern regions of the country (7). As thalassemia treatments are costly and time-consuming, the most effective means of limiting thalassemia incidence is to screen for the mutant genes before birth. Towards this goal, prenatal screening for thalassemia is becoming a part of primary health services in the majority of developed countries, particularly in Asia (8). Nonetheless, traditional prenatal diagnoses, such as chorionic villi sampling and amniocentesis, are invasive and may cause infection and miscarriage (9,10).
The discovery of cell-free fetal DNA (11), which is released from placental tissue into the maternal bloodstream, has enabled the development of non-invasive prenatal diagnosis (NIPD) for detecting disease-causing mutations in the fetus (12,13). In recent years, there has been substantial interest in developing NIPD for thalassemia. Reverse transcription-quantitative PCR (RT-qPCR) using the gap-PCR technique with primers designed to bind at the deletion breakpoints have been used to diagnose common deletions such as -a3.7, -a4.2, -THAI, -MED and -SEA (14,15). Similarly, the PCR-based techniques were also applied to diagnose Hb Bart's hydrops fetalis (16) and other forms of α-thalassemia (17-19). Next-generation sequencing (NGS) is a powerful technique for NIPD, as it has high sensitivity and enables the detection of low abundance cell free-fetal DNA (20-23). Several studies have shown high accuracy of NGS-based genome-wide single-nucleotide polymorphism genotyping and targeted sequencing for diagnosing β-thalassemia (24-26). However, the high costs of NGS-based techniques make them inappropriate in routine clinical practice.
Droplet digital PCR (ddPCR) is a sensitive and quantitative PCR-based technique that can amplify a low initial amount of target DNA molecules and enumerate different PCR products using probe-specific fluorescent signals (27,28). ddPCR has been used to detect circulating tumor DNA and cell-free fetal DNA (29-31). A study on SEA deletion α-(0) thalassemia showed a promising prospect of ddPCR (32). However, it is unclear whether the fetal DNA analyzed came directly from fetal tissues or cell-free fetal DNA in the maternal bloodstream (32). Furthermore, digital PCR-based NIPD could be improved by utilizing a nucleic acid size selection technique to enrich fetal DNA molecules (33).
The present study developed ddPCR-based assays for detecting fetal α- and β-thalassemia mutations in cell-free DNA from maternal plasma. The assays were evaluated on 22 pregnant women carrying SEA deletions, 16 carrying HbE mutations, and 8 carrying 41/42 (-CTTT) mutations.
Materials and methods
Patient recruitment
In the present study 46 singleton pregnant women who were thalassemia carriers [22 cases with SEA deletion, 16 cases with HbE (G>A), and 8 cases with 41/42 (-CTTT)] were recruited at Radjavithi Hospital, Bangkok, Thailand between March and July 2018 with approval from the Institutional Review Board of the hospital. Peripheral blood samples (10 ml) were collected from each participant for subsequent cell-free DNA extraction and ddPCR analysis. The α- and β-thalassemia status of each parent and fetus was determined by Hb typing, complete blood count tests and genotyping by PCR from amniotic fluids. Samples were collected with the approval from the Institutional Review Board of Radjavithi Hospital, Bangkok (approval no. 59194; date of approval 17th November 2016). Written informed consent was obtained from all subjects prior to inclusion in the study. Written informed consent from the parent or legal guardian of all subjects under the age of 18 was also obtained.
Cell-free DNA extraction from blood samples
Maternal blood samples (10 ml) were placed in BCT tubes (Streck™) and centrifuged at 1,600 x g for 10 min at 4˚C. The supernatant was centrifuged again at 16,000 x g for 10 min at 4˚C. The pellet was discarded and the supernatant was stored kept at -20˚C until required for extraction. Cell-free DNA was extracted from plasma (3 ml) using a QIAamp Circulating Nucleic acid kit according to the manufacturer's protocol (Qiagen GmbH).
Amniotic fluid DNA extraction
Amniotic fluids (5 ml) were collected during weeks 15-18 of gestation and centrifuged at 16,000 x g for 10 min at 4˚C. The supernatant was discarded, the pellet was washed twice with 1X PBS (1 ml) and stored at -80˚C in a sterile tube. The extraction step was performed according to the instruction manual of the QIAamp Blood Mini kit (Qiagen GmbH).
ddPCR
A ddPCR assay was developed for determining the copy number of the SEA allele. The HEX probe was designed to bind the genomic region inside the SEA deletion (NG_000006.1:g.26264_45564del19301) (34) whereas the FAM probe was designed to bind the genomic region just outside of the SEA locus (NG_000006.1:g.26140 to 26262), which is expected to be present in both a wild type fetus and a fetus with an SEA deletion (Table SI). For detecting HbE (G>A) and 41/42 (-CTTT), a rare mutation assay with specific probes designed to target the mutant and wild type amplicons was developed (Table SI).
ddPCR experiments were performed according to the manufacturer's protocol (Bio-Rad Laboratories, Inc.) with some modifications as described here. The master mix solution was prepared from 10 µl 2X ddPCR SuperMix for probes (Bio-Rad Laboratories, Inc.), 1 µl 20X prime PCR assay (Bio-Rad Laboratories, Inc.), and either 8 µl DNA (12 µg/ml) from plasma or 1 µl DNA (20 µg/ml) from amniotic fluid. Nuclease free water was added to adjust the final volume to 20 µl. A total of 13,500-15,000 droplets per reaction were created in a DG8 cartridge with 70 µl oil using a QX200 Droplet Generator (Bio-Rad Laboratories, Inc.). Droplets (40 µl) were transferred to a 96-well PCR plate and sealed with aluminium foil using PX1 (Bio-Rad Laboratories, Inc.) for 5 sec at 180˚C. Next the plate was placed in a T100 Thermal Cycler (Bio-Rad Laboratories, Inc.). PCR reactions began with a cycle of 10 min at 95˚C, followed by 40 cycles of 30 sec at 94˚C and 1 min at 54˚C, with an enzyme deactivation step for 10 min at 98˚C. Fluorescent signals from PCR products were measured using a QX200 Droplet Reader (Bio-Rad Laboratories, Inc.) and analyzed using QuantaSoft version 1.7.4 (Bio-Rad Laboratories, Inc.). ddPCR reactions for SEA cases were performed in duplicates. ddPCR reactions for HbE and 41/42 (-CTTT) cases were performed in triplicates.
Estimation of fetal fraction
Fetal fractions were estimated as previously described (27,35). Briefly, RASSF1A and ACTB alleles in plasma samples were digested with BstU I (Vivantis Technologies) at a ratio of 1 µg DNA to 1 U of enzyme for 1 h at 60˚C. The abundance of RASSF1A and ACTB alleles before and after digestion were measured using ddPCR analyses. ACTB locus, which is always unmethylated, acts as a control of BstU I activity (36). Conversely, as only the fetal RASSF1A locus is hypermethylated, the ratio of RASSF1A positive droplets before and after BstU I digestion approximate the fractional abundance of fetal DNA content of the sample.
Copy number variation (CNV) analysis to detect SEA deletion
QuantaSoft was used to determine the number of positive and negative droplets and analyze the relative CNV of SEA deletions. The average CNV value from triplicate wells was used to classify the fetal genotype using the following equation: CNV=(concentration of SEA allele/concentration of wild-type allele) x copy number of wild type allele.
Rare mutation assay to detect HbE and 41/42 (-CTTT) mutations
QuantaSoft was used to determine the number of positive droplets, negative droplets and double-positive droplets. A previously described sequential probability ratio test (SPRT) (33) was adopted to classify fetal HbE and 41/42 (-CTTT) genotypes. Briefly, the total number of DNA molecules in each sample was calculated under the assumption that the number of positive droplets (droplets with DNA materials) followed the Poisson distribution: The probability of observing k positive droplets is (λke-λ)/k!, where λ indicates the average number of DNA molecules per a droplet. Hence, by setting k=0, the number of DNA molecules can be calculated as: -ln(fraction of negative droplets) x number of droplets.
Next, the likelihood that the fetus has a specific genotype was calculated under the assumption that the numbers of negative and positive droplets for each allele followed the Binomial distribution. For example, if the fetal genotype is a homozygous mutant, then the fraction of mutant allele would be (1+fetal fraction)/2, whereas the fraction of wild type allele would be (1-fetal fraction)/2. Then, letting N be the total number of droplets, M be the total number of DNA molecules, mt be the number of positive droplets for the mutant allele, wt be the number of positive droplets for the wild type allele, and ff be the estimated fetal fraction, the likelihood of this observation can be expressed as:
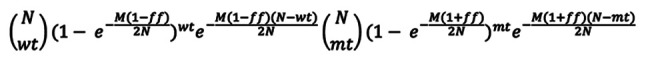 |
Two likelihood ratio tests were performed for each sample: One between the hypotheses that the fetal genotype is homozygous mutant and heterozygous, and another between the hypotheses that the fetal genotype is wild type and heterozygous. The threshold for accepting each test was set at type I and type II errors of 5% according to the SPRT criteria: ±log [(1-0.05)/0.05], which is ~±1.27 (33,37).
Real time PCR for melting curve analysis of SEA deletion from amniocentesis
The fetal genotypes were confirmed by high-resolution melting curve analysis in 10 samples were randomly selected from singleton parents for confirmation. Total reaction volume of 20 µl contained 10 µl Precision melt supermix (Bio-Rad Laboratories, Inc.), 1 µl of each primer and 5 µl of DNA sample. The sequences of the primers were NaI forward, 5'AGAAGCTGAGTGATGGGTCCG-3' and reverse, 5'ACAAACGCCCGTCCGACTCAA-3'; and NaIII reverse 5'-TGGACTTAAGTGATCCTCCTGCCC-3',. The amplification step began at 95˚C for 3 min followed by 40 PCR cycles at 95˚C for 20 sec, 60˚C for 20 sec, and 72˚C for 20 sec. Finally, a high-resolution melting cycle from 85-95˚C at a rate of 0.1˚C per 2 sec was performed (38). Dissociation curve analysis was performed using CFX96 Manager version 1.3 (Bio-Rad Laboratories, Inc.).
Statistical analyses
The sequential probability ratio test (SPRT) technique was used to analyze ddPCR results to identify the most likely fetal genotypes (39). For analysis, 5% type I and type II error thresholds were set. Numerical calculations were performed in Microsoft Excel according to the Poisson and Binomial distribution models described above.
Results
Patient characteristics
A total of 46 couples who were at high risk of having children with thalassemia were recruited in the present study. The median age (age range) of pregnant mothers was 27 (15-39) years old. The median gestational age (range) was 18 (17-27) weeks. The median BMI (range) of participants was 21.8 (15.7-32.8). The cohort consisted of 22 (48%) couples with SEA deletion, 16 (35%) couples with an HbE (G>A) mutation, and 8 (17%) couples with an 41/42 (-CTTT) mutation.
Classification performance of fetal SEA genotype
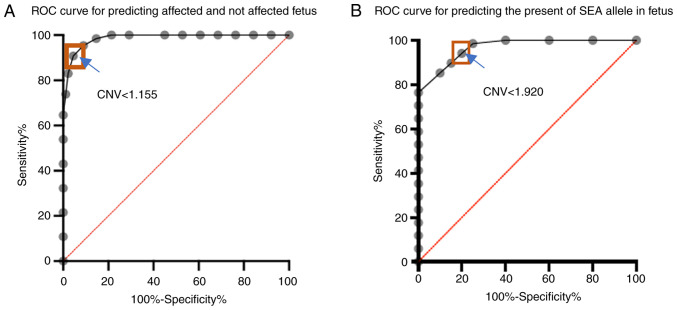
Analysis of ddPCR fluorescent signals showed clear separations between negative and positive droplets as well as lower abundances of the SEA locus in Bart's hydrops fetalis case as would be expected (Fig. 1A and B). The complete absence of positive droplets for the SEA locus in the amniocentesis sample of the Bart's hydrops fetalis case confirmed that the designed probe was highly specific to the SEA locus (Fig. 1C and D). SEA locus copy numbers estimated from cell-free DNA samples significantly differ across wild type, heterozygous and Bart's hydrops fetalis samples (Mann Whitney U test P=0.0124 for the comparison between the wild type and heterozygous cases; P=0.000311 for the comparison between heterozygous and Bart's hydrops fetalis cases). The average copy numbers for the wild type, heterozygous and Bart's hydrops fetalis groups were 2.27, 1.38 and 0.76, respectively (Fig. 2). To ensure the high quality of ground truth fetal genotypes, high resolution melting curve analyses on selected samples was also performed and 100% concordance was obtained (Fig. S1). Most samples exhibited the expected copy numbers of the SEA locus, except for one wild type case that may have been affected by a very low (3%) fetal fraction (Table I). The optimal copy number cut-off for distinguishing affected (Bart's hydrops fetalis) from unaffected (wild type and heterozygous) fetuses was ~1.155, which yielded 95.38% sensitivity [95% confidence interval (CI): 87.29-98.74] and 91.01% specificity (95% CI: 83.25-95.37) (Fig. 3A). The area under the receiver operating characteristic (AUROC) curve was 0.98. The optimal copy number cut-off for distinguishing the presence of SEA deletion in the fetus (Bart's hydrops fetalis and heterozygous) was 1.920, which yielded 98.53% sensitivity (95% CI: 92.13-99.92) and 75% specificity (95% CI: 53.13-88.81) (Fig. 3B). The AUROC curve in this scenario was 0.96.
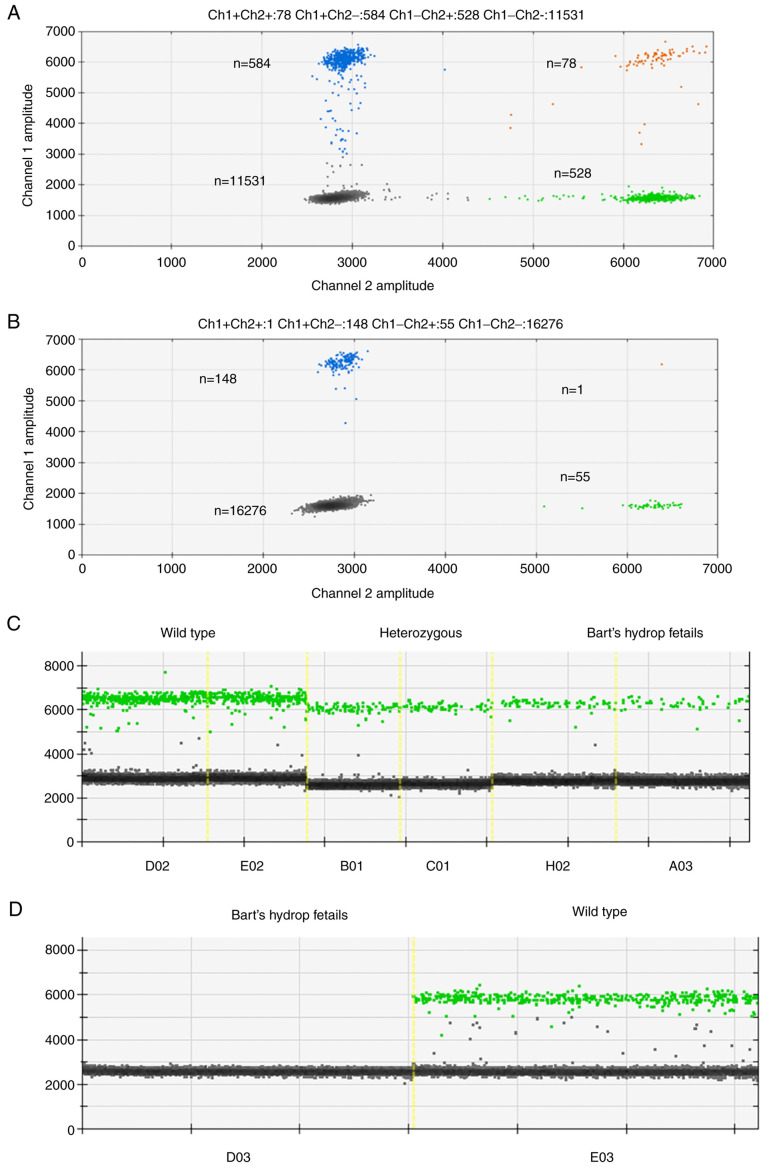
Figure 1.
Representative droplet digital PCR results for detecting the SEA deletion. (A and B) show the scatter plots of two fluorescent channels: FAM (blue) which detects the wild type allele and HEX (green) which detects the SEA locus. Negative droplets are shown in black and double-positive droplets are shown in orange. The number of droplets in each group is indicated. (A) Shows a case of a heterozygous SEA deletion and (B) shows a Bart's hydrops fetalis case. The ratio of SEA droplets (HEX) to wild type droplets (FAM) decreased from 1:1 to 1:3 in the Bart's hydrops fetalis case. (C and D) One-dimensional visualizations of HEX (green) which detects the SEA locus. (C) Results from maternal cell-free DNA samples and (D) from amniocentesis samples. No SEA droplets (HEX) were observed in the amniocentesis sample from a Bart's hydrops fetalis case as would be expected. SEA, Southeast Asian; Ch, fluorescent channels; A03, B01, C01, D02, D03, E02, E03 and H02 refer to the position in the 96-well plate.
Figure 2.
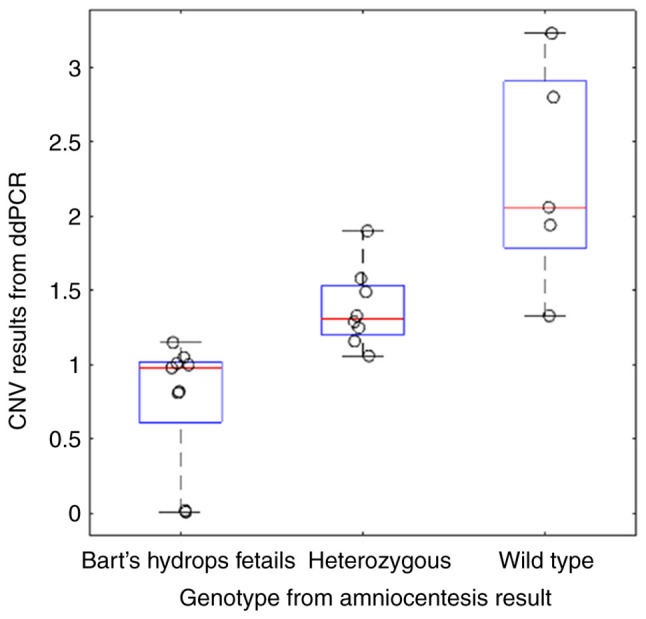
Plasma-derived CNVs distinguish fetal SEA deletion genotype groups. Boxplots show the distribution of CNV results from ddPCR for each fetal genotype group. The genotypes from amniocentesis results were determined from analyses of amniotic fluids at the same gestation age blood sampling. Red bars indicate the medians. Blue boxes indicate the 25-75th percentile. Black whiskers indicate the minimum and maximum values. Black circles indicate CNVs for individual cases. CNV, copy number variations; ddPCR, droplet digital PCR.
Table I.
Detailed parental genotypes and ddPCR test results for α (0) thalassemia (SEA).
| Sample ID | Paternal genotype | Maternal genotype | Fetal fraction,% | SEA deletion conc. | Wild type conc. | CNV by ddPCR | Interpretation | Fetal Genotype from conventional amniocentesis |
|---|---|---|---|---|---|---|---|---|
| 1 | (αα/αα) | (—SEA/αα) | 10 | 141.5 | 87.5 | 3.23 | Wild type | Wild type |
| 2 | (—SEA/αα) | (—SEA/αα) | 15 | 15.7 | 11.2 | 2.8 | Wild type | Wild type |
| 3 | (—SEA/αα) | (—SEA/αα) | 16 | 11.2 | 10.9 | 2.06 | Wild type | Wild type |
| 4 | (—SEA/αα) | (—SEA/αα) | 7 | 23.55 | 24.3 | 1.94 | Wild type | Wild type |
| 5 | (—SEA/αα) | (—SEA/αα) | 17 | 57.95 | 60.95 | 1.9 | Heterozygous | Heterozygous |
| 6 | (—SEA/αα) | (—SEA/αα) | 8 | 8.45 | 10.7 | 1.58 | Heterozygous | Heterozygous |
| 7 | (—SEA/αα) | (—SEA/αα) | 9 | 44.45 | 59.8 | 1.49 | Heterozygous | Heterozygous |
| 8 | (αα/αα) | (—SEA/αα) | 10 | 53.3 | 80.3 | 1.33 | Heterozygous | Heterozygous |
| 9 | (—SEA/αα) | (—SEA/αα) | 3 | 3.15 | 4.8 | 1.33 | Heterozygousa | Wild type |
| 10 | (—SEA/αα) | (—SEA/αα) | 11 | 7.05 | 10.9 | 1.29 | Heterozygous | Heterozygous |
| 11 | (—SEA/αα) | (—SEA/αα) | 9 | 11.5 | 18.45 | 1.25 | Heterozygous | Heterozygous |
| 12 | (—SEA/αα) | (—SEA/αα) | 8 | 11.8 | 20.35 | 1.16 | Heterozygous | Heterozygous |
| 13 | (—SEA/αα) | (—SEA/αα) | 10 | 8.05 | 14.05 | 1.15 | Heterozygousa | Bart |
| 14 | (—SEA/αα) | (—SEA/αα) | 15 | 10.75 | 20.35 | 1.06 | Heterozygous | Heterozygous |
| 15 | (—SEA/αα) | (—SEA/αα) | 11 | 15.3 | 29.15 | 1.05 | Bart | Bart |
| 16 | (—SEA/αα) | (—SEA/αα) | 11 | 173.5 | 344 | 1.01 | Bart | Bart |
| 17 | (—SEA/αα) | (—SEA/αα) | 10 | 5.05 | 10.15 | 1 | Bart | Bart |
| 18 | (—SEA/αα) | (—SEA/αα) | 15 | 4.85 | 9.85 | 0.98 | Bart | Bart |
| 19 | (—SEA/αα) | (—SEA/αα) | 15 | 4.4 | 10.7 | 0.82 | Bart | Bart |
| 20 | (—SEA/αα) | (—SEA/αα) | 10 | 8.1 | 20.1 | 0.81 | Bart | Bart |
| 21 | (—SEA/αα) | (—SEA/αα) | 15 | 0.22 | 21.3 | 0.02 | Bart | Bart |
| 22 | (—SEA/αα) | (—SEA/αα) | 10 | 0.14 | 27.15 | 0.01 | Bart | Bart |
aMisclassified cases. SEA, Southeast Asian; CNV, copy number variation; ddPCR, droplet digital PCR; αα, α-globin genes.
Figure 3.
ROC curves of the performance of the droplet digital PCR assays on SEA detection. Orange boxes indicate the best CNV cutoff. (A) ROC curve for distinguishing between affected (Bart's hydrops fetalis) vs. unaffected (wild type and heterozygous). The AUROC curve was 0.98. The optimal CNV cutoff was 1.155. (B) ROC curve for detecting the presence of the SEA allele in the fetus (Bart's hydrops fetalis and heterozygous). The AUROC curve was 0.96. The optimal CNV cutoff was 1.920. ROC, receiver operating characteristic; AUROC, area under the ROC; CNV, copy number variations; SEA, Southeast Asian.
Amniocentesis results from high resolution melting curve analysis
Amniocentesis DNA samples were collected from 10 randomly selected singleton parents to confirm the SEA genotypes. Amongst the 10 samples, 4 were diagnosed as Bart's hydrops fetalis, 5 were from normal fetuses and 1 was from a heterozygous fetus. The median maternal age was 27 (15-36) years old. The median gestational age was 18 (17-19) weeks. Dissociation curve analysis showed that the melting temperature for the wild-type fetuses was 91.5±0.15˚C, whereas the melting temperature for Bart's hydrops fetalis was 87.8±0.02˚C. Heterozygous fetuses showed peaks at the melting temperatures of both the wild-type and Bart's hydrops fetalis as expected.
Classification performance of fetal HbE (G>A) and 41/42 (-CTTT) genotype
Cell-free DNA from maternal plasma of 16 HbE carriers and 8 41/42(-CTTT) carriers was next investigated. ddPCR was performed using probes targeting either the wild type or mutant allele. The ground truth genotypes for several samples were confirmed with high-resolution melting curve analyses (Figs. S1 and 2). ddPCR results were then analyzed based on a published sequential probability ratio test method (33). At type I and type II errors of 5%, out of 16 HbE cases, the results for 3 patients were inconclusive, and 10 out of the remaining 13 cases were correctly classified. For 41/42 (-CTTT), the results for 4 out of 8 cases were inconclusive, and 2 out of the remaining 4 cases were correctly classified (Tables II and III). It should be noted that all inconclusive results still retain the correct genotypes and may be correctly classified if more ddPCR reactions are performed.
Table II.
Detailed parental genotypes and droplet digital PCR test results for HbE β-thalassemia.
| Sample ID | Total droplet | Wild type droplet | Mutant droplet | Double- positive droplet | Average concentration | Fetal fraction, % | Mutant/mutant to mutant/wild type LLR | P<0.05 | Wild type/wild type to mutant/wild type LLR | P<0.05 | Interpretation | Fetal genotype from conventional amniocentesis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38,941 | 396 | 234 | 205 | 10.9 | 10 | -8.5 | Yes | 5.7 | Yes | Wild type | Wild type |
| 2 | 41,227 | 315 | 321 | 117 | 9.1 | 24.5 | -7.9 | Yes | -9.2 | Yes | Heterozygousa | Wild type |
| 3 | 44,109 | 243 | 141 | 56 | 14.6 | 26.1 | -17.8 | Yes | 6 | Yes | Wild type | Wild type |
| 4 | 38,124 | 130 | 89 | 143 | 7.3 | 12.7 | -3 | Yes | 1.5 | Yes | Wild type | Wild type |
| 5 | 37,237 | 510 | 389 | 134 | 10.6 | 11.1 | -8.3 | Yes | 3.5 | Yes | Wild type | Wild type |
| 6 | 42,706 | 548 | 102 | 84 | 9.23 | 15.3 | -33.4 | Yes | 26.7 | Yes | Wild type | Wild type |
| 7 | 43,713 | 75 | 66 | 61 | 12.3 | 11.5 | -0.9 | No | 0 | No | Inconclusive | Heterozygous |
| 8 | 42,939 | 407 | 405 | 401 | 11.6 | 26.6 | -13.2 | Yes | -12.7 | Yes | Heterozygous | Heterozygous |
| 9 | 37,535 | 332 | 348 | 338 | 11 | 26 | -8.5 | Yes | -12.2 | Yes | Heterozygous | Heterozygous |
| 10 | 37,001 | 175 | 181 | 178 | 6.46 | 12.6 | -0.9 | No | -1.6 | Yes | Mutant or Heterozygous | Heterozygous |
| 11 | 37,322 | 234 | 243 | 225 | 8.64 | 27.6 | -7.1 | Yes | -9.3 | Yes | Heterozygous | Heterozygous |
| 12 | 42,845 | 863 | 191 | 186 | 14.62 | 19.2 | -65.8 | Yes | 48.6 | Yes | Wild typea | Heterozygous |
| 13 | 37,355 | 145 | 150 | 144 | 5.1 | 24.2 | -3.3 | Yes | -4.4 | Yes | Heterozygous | Heterozygous |
| 14 | 39,795 | 713 | 761 | 713 | 18.4 | 17.6 | -6.3 | Yes | -13.8 | Yes | Heterozygous | Heterozygous |
| 15 | 37,309 | 94 | 15 | 91 | 11.32 | 18.8 | -7.4 | Yes | 5.7 | Yes | Wild typea | Heterozygous |
| 16 | 38,696 | 113 | 91 | 76 | 5.97 | 13.5 | -2.1 | Yes | 0.5 | No | Wild type or heterozygous | Heterozygous |
aMisclassified cases. LLR, Likelihood ratio.
Table III.
Detailed parental genotypes and droplet digital PCR test results for 41/42 (-CTTT) β thalassemia.
| Sample ID | Total droplet | Wild type droplet | Mutant droplet | Double- positive droplet | Average concentration | Fetal fraction, % | Mutant/mutant to mutant/wild type LLR | P<0.05 | Wild type/wild type to mutant/wild type LLR | P<0.05 | Interpretation | Fetal genotype from conventional amniocentesis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38,196 | 139 | 205 | 137 | 16.6 | 24 | 2.6 | Yes | -11.5 | Yes | Mutanta | Wild type |
| 2 | 33,835 | 357 | 17 | 15 | 8.5 | 14 | -22.5 | Yes | 19.3 | Yes | Wild type | Wild type |
| 3 | 29,144 | 505 | 440 | 108 | 22.1 | 13.5 | -7.7 | Yes | 0.1 | No | Wild type or heterozygous | Heterozygous |
| 4 | 31,830 | 197 | 159 | 7 | 9.5 | 13.9 | -3.8 | Yes | 0.8 | No | Wild type or heterozygous | Heterozygous |
| 5 | 33,596 | 549 | 631 | 37 | 25.2 | 12.7 | 0.4 | No | -8.8 | Yes | Mutant or heterozygous | Heterozygous |
| 6 | 32,369 | 254 | 277 | 0 | 12.2 | 22.8 | -3.8 | Yes | -8.5 | Yes | Heterozygous | Heterozygous |
| 7 | 33,437 | 157 | 123 | 12 | 6.02 | 10 | -2.1 | Yes | 0.9 | No | Wild type or heterozygous | Heterozygous |
| 8 | 37,846 | 571 | 35 | 23 | 12.2 | 11.5 | -28.8 | Yes | 25.3 | Yes | Wild typea | Heterozygous |
aMisclassified cases. LLR, Likelihood ratio.
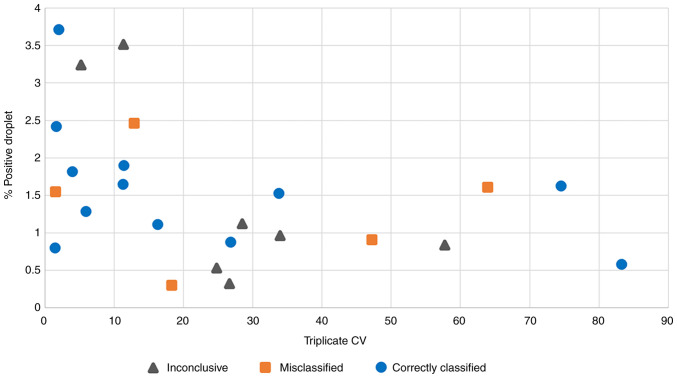
Typical causes of poor ddPCR assay performances include a low fetal fraction, low amount of amplifiable DNA, and poor binding between ddPCR probes and target alleles, all of which would hinder confident determination of the fetal genotype. Although a low fetal fraction is linked to inconclusive results (Fig. 4A, lower fetal fractions among inconclusive cases; Mann-Whitney U test P=0.0173), it cannot explain misclassifications. The 12-25% fetal fractions amongst misclassified samples are high and should be adequate for NIPD applications. There is also no apparent difference in the amount of amplified DNA between correctly classified and misclassified cases (Fig. 4B). Finally, to test whether poor probe binding could explain the observed results, the coefficient of variation (CV) of the mutant-to-wild type droplet ratios from the triplicate ddPCR experiments and the percentage of positive droplets of each patient sample to the classification result were compared. The rationale here was that poor probe binding should result in a low percentage of positive droplets and a high variability across replicate experiments. This revealed a clear association between the variability of triplicate ddPCR experiments and the genotype classification accuracy (Fig. 5). Amongst the 11 samples with <15% CV, seven were correctly classified, two were misclassified, and two were inconclusive. Conversely, amongst the remaining 13 samples with 16-83% CV, only five were correctly classified, three were misclassified and five were inconclusive. The analysis also showed a borderline significant correlation between the CV of triplicate ddPCR results and the fraction of total positive droplets (Spearman Rank correlation=-0.4417 with permutation test P=0.0306).
Figure 4.
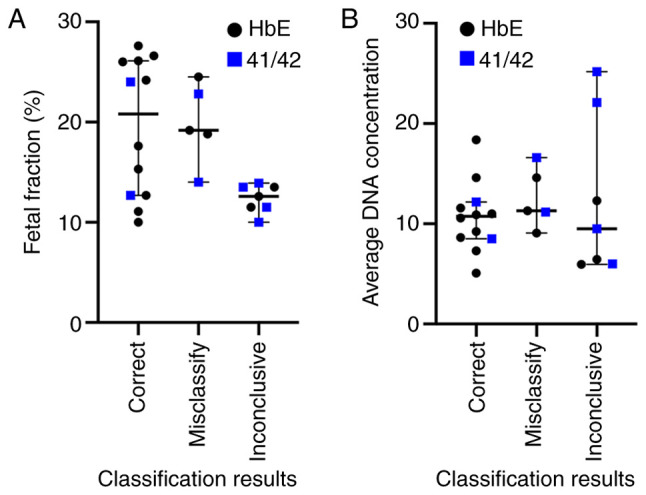
Low fetal fractions leads to inconclusive HbE and 41/42 (-CTTT) detections. Scatter plots showing the distribution of (A) fetal fractions and (B) average DNA concentration across 24 triplicate droplet digital PCR experiments for HbE and 41/42 (-CTTT) cases. The distributions for correctly classified, misclassified and inconclusive cases are shown. Black dots and blue boxes indicate data from HbE and 41/42 (-CTTT), respectively.
Figure 5.
Coefficients of variation and percentage of positive droplets are good indicators of genotyping classification quality. Scatter plot of the coefficient of variations and the average percentages of positive droplets calculated from triplicate droplet digital PCR experiments of 24 HbE and 41/42 (-CTTT) cases.
Discussion
The growing interest in non-invasive prenatal testing has driven the development of various assays for diagnosing monogenic diseases from cell-free fetal DNA (24-26,30,31,33). One of the main challenges is the interference from high maternal DNA background that can overwhelm the signal from circulating fetal DNA fragments (40). Prior studies have focused on autosomal dominant monogenic diseases, such as achondroplasia, myotonic dystrophy and some forms of β-thalassemia, where the mutated fetal allele could be clearly distinguished from wild type maternal DNA (41-44). However, this consideration does not apply to autosomal recessive diseases and several other situations that involve maternal inherited alleles (44). Notably, the digital relative mutation dosage and SPRT methods have improved the confidence of assessment of the fetal genotype in the presence of the background maternal DNA (33,45). These techniques enabled interpretation of fetal status and detection of small allelic imbalances when the mother carries the pathologic allele.
ddPCR is a high-sensitivity and high-specificity technique that improves upon conventional PCR and has been utilized in prenatal diagnoses of thalassemia (29,46). Early studies of SEA deletion α-(0) thalassemia using RT-qPCR demonstrated high sensitivities for Bart's hydrops fetalis detection. However, these methods could not clearly distinguish between heterozygous and wild type fetuses (37,46,47). A study using the ddPCR technique on fetal genomic DNA samples provided the first accurate classification of fetal SEA genotype, suggesting that ddPCR is suitable for non-invasive prenatal diagnosis of SEA deletion (32). Nonetheless, no prior study had applied ddPCR to cell-free DNA without sample preprocessing, such as enrichment of fetal DNA by size selection, to the best of our knowledge.
The present study is the first to showcase the application of ddPCR to identify the copy number of SEA deletion in unprocessed cell-free DNA obtained from maternal plasma, thereby eliminating the need for invasive fetal DNA collection and sample preprocessing. The SEA CNV assay accurately classified 20 out of 22 samples, and detected Hb Bart's hydrops fetalis with 95% sensitivity and 91% specificity. A possible explanation for the misclassified cases may be a low fetal fraction (3% in one case) and instability of the cell-free fetal DNA in the SEA region (16). These findings demonstrate the readiness of ddPCR-based assay for NIPD of SEA deletion α-(0) thalassemia.
Although the current performances of ddPCR for HbE and 41/42 (-CTTT) point mutations still do not meet the requirements for clinical use, the results for HbE are promising. Out of 16 samples, 10 were correctly classified, and three may be correctly classified with additional ddPCR experiments, whilst three misclassifications were made. The association between inaccurate classifications and high variability amongst triplicate ddPCR experiments and a low percentage of positive droplets suggests that these errors were likely caused by poor binding between ddPCR probes and target alleles. Some remedies for this issue include designing additional probes for targeting mutated DNA molecules, pre-amplifying the sample, and performing size selection to enrich fetal DNA fragments (48,49).
The present study developed ddPCR-based assays for identifying the fetal thalassemia status from unprocessed cell-free DNA extracted from maternal plasma. Although the 20 kb deletions of the SEA region can be reliably detected, the detection accuracies for HbE and 41/42 (-CTTT) point mutations were too low for recommendation of this technique for clinical use. The critical limitation of this work is the small number of HbE and 41/42 (-CTTT) samples, especially the complete lack of cases with a homozygous mutant allele. Further studies with a larger sample size and additional ddPCR probe designs are required to improve and validate the utility of these assays.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
Funding: This study was supported by funding from Radjavithi Hospital (grant no. 59194) and the Research Fund of Chulabhorn International College of Medicine, Thammasat University.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
SPO, SJ managed the project. SPO, WQ, SC and KS conceived and designed the study. SJ recruited patients and collected data. WN, SPR and KT collected samples and extracted cell-free DNA. KS performed the experiments. SS and KS analyzed the data and interpreted the results. KS, SPO and SS wrote and revised the manuscript. SS, SJ and SPO supervised the project. All authors provided critical comments and contributed to the revision of the manuscript. KS and SPO confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Samples were collected with the approval from the Institutional Review Board of Radjavithi Hospital, Bangkok (approval no. 59194 date of approval, 17th November 2016). Written informed consent was obtained from all subjects prior to inclusion in the study. Written informed consent from the parents or legal guardians of all subjects under the age of 18 was also obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480–487. doi: 10.2471/blt.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galanello R, Cao A. Gene test review. Alpha-thalassemia. Genet Med. 2011;13:83–88. doi: 10.1097/GIM.0b013e3181fcb468. [DOI] [PubMed] [Google Scholar]
- 3.Fucharoen S, Winichagoon P, Siritanaratkul N, Chowthaworn J, Pootrakul P. Alpha- and beta-thalassemia in Thailand. Ann N Y Acad Sci. 1998;850:412–414. doi: 10.1111/j.1749-6632.1998.tb10507.x. [DOI] [PubMed] [Google Scholar]
- 4.Cao A, Galanello R. Beta-thalassemia. Genet Med. 2010;12:61–76. doi: 10.1097/GIM.0b013e3181cd68ed. [DOI] [PubMed] [Google Scholar]
- 5.Origa R. β-thalassemia. Genet Med. 2017;19:609–619. doi: 10.1038/gim.2016.173. [DOI] [PubMed] [Google Scholar]
- 6.Chaibunruang A, Sornkayasit K, Chewasateanchai M, Sanugul P, Fucharoen G, Fucharoen S. Prevalence of thalassemia among newborns: A re-visited after 20 years of a prevention and control program in Northeast Thailand. Mediterr J Hematol Infect Dis. 2018;10(e2018054) doi: 10.4084/MJHID.2018.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fucharoen S, Winichagoon P. Thalassemia in SouthEast Asia: Problems and strategy for prevention and control. Southeast Asian J Trop Med Public Health. 1992;23:647–655. [PubMed] [Google Scholar]
- 8.Cao A, Kan YW. The prevention of thalassemia. Cold Spring Harb Perspect Med. 2013;3(a011775) doi: 10.1101/cshperspect.a011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Old J. Chapter 71 - Hemoglobinopathies and thalassemias. In: Emery and rimoin's principles and practice of medical genetics. 6th edition. Academic Press, Cambridge, MA, pp1-44, 2013. [Google Scholar]
- 10.Evans MI, Wapner RJ. Invasive prenatal diagnostic procedures 2005. Semin Perinatol. 2005;29:215–218. doi: 10.1053/j.semperi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–487. doi: 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins LA, Deans ZC, Lewis C, Allen S. Delivering an accredited non-invasive prenatal diagnosis service for monogenic disorders and recommendations for best practice. Prenat Diagn. 2018;38:44–51. doi: 10.1002/pd.5197. [DOI] [PubMed] [Google Scholar]
- 13.Rabinowitz T, Shomron N. Genome-wide noninvasive prenatal diagnosis of monogenic disorders: Current and future trends. Comput Struct Biotechnol J. 2020;18:2463–2470. doi: 10.1016/j.csbj.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baysal E, Huisman TH. Detection of common deletional alpha-thalassemia-2 determinants by PCR. Am J Hematol. 1994;46:208–213. doi: 10.1002/ajh.2830460309. [DOI] [PubMed] [Google Scholar]
- 15.Bowden DK, Vickers MA, Higgs DR. A PCR-based strategy to detect the common severe determinants of alpha thalassaemia. Br J Haematol. 1992;81:104–108. doi: 10.1111/j.1365-2141.1992.tb08180.x. [DOI] [PubMed] [Google Scholar]
- 16.Sirichotiyakul S, Charoenkwan P, Sanguansermsri T. Prenatal diagnosis of homozygous alpha-thalassemia-1 by cell-free fetal DNA in maternal plasma. Prenat Diagn. 2012;32:45–49. doi: 10.1002/pd.2892. [DOI] [PubMed] [Google Scholar]
- 17.Tungwiwat W, Fucharoen S, Fucharoen G, Ratanasiri T, Sanchaisuriya K. Development and application of a real-time quantitative PCR for prenatal detection of fetal alpha(0)-thalassemia from maternal plasma. Ann N Y Acad Sci. 2006;1075:103–107. doi: 10.1196/annals.1368.013. [DOI] [PubMed] [Google Scholar]
- 18.Yan TZ, Mo QH, Cai R, Chen X, Zhang CM, Liu YH, Chen YJ, Zhou WJ, Xiong F, Xu XM. Reliable detection of paternal SNPs within deletion breakpoints for non-invasive prenatal exclusion of homozygous α-thalassemia in maternal plasma. PLoS One. 2011;6(e24779) doi: 10.1371/journal.pone.0024779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pornprasert S, Sukunthamala K, Kunyanone N, Sittiprasert S, Thungkham K, Junorse S, Pongsawatkul K, Pattanaporn W, Jitwong C, Sanguansermsri T. Analysis of real-time PCR cycle threshold of alpha-thalassemia-1 Southeast Asian type deletion using fetal cell-free DNA in maternal plasma for noninvasive prenatal diagnosis of bart's hydrops fetalis. J Med Assoc Thai. 2010;93:1243–1248. [PubMed] [Google Scholar]
- 20.Norton ME, Brar H, Weiss J, Karimi A, Laurent LC, Caughey AB, Rodriguez MH, Williams J III, Mitchell ME, Adair CD, et al. Non-invasive chromosomal evaluation (NICE) study: Results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207(137) doi: 10.1016/j.ajog.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119:890–901. doi: 10.1097/AOG.0b013e31824fb482. MatErnal BLood IS Source to Accurately diagnose fetal aneuploidy (MELISSA) Study Group. [DOI] [PubMed] [Google Scholar]
- 22.Rava RP, Srinivasan A, Sehnert AJ, Bianchi DW. Circulating fetal cell-free DNA fractions differ in autosomal aneuploidies and monosomy X. Clin Chem. 2014;60:243–250. doi: 10.1373/clinchem.2013.207951. [DOI] [PubMed] [Google Scholar]
- 23.Chiu RW, Chan KC, Gao Y, Lau VY, Zheng W, Leung TY, Foo CH, Xie B, Tsui NB, Lun FM, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci USA. 2008;105:20458–20463. doi: 10.1073/pnas.0810641105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong L, Barrett AN, Hua R, Ho S, Jun L, Chan K, Mei Z, Choolani M. Non-invasive prenatal testing for fetal inheritance of maternal β-thalassaemia mutations using targeted sequencing and relative mutation dosage: A feasibility study. BJOG. 2018;125:461–468. doi: 10.1111/1471-0528.15045. [DOI] [PubMed] [Google Scholar]
- 25.Lam KW, Jiang P, Liao GJ, Chan KC, Leung TY, Chiu RW, Lo YM. Noninvasive prenatal diagnosis of monogenic diseases by targeted massively parallel sequencing of maternal plasma: Application to β-thalassemia. Clin Chem. 2012;58:1467–1475. doi: 10.1373/clinchem.2012.189589. [DOI] [PubMed] [Google Scholar]
- 26.Lo YM, Chan KC, Sun H, Chen EZ, Jiang P, Lun FM, Zheng YW, Leung TY, Lau TK, Cantor CR, Chiu RWK. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci Transl Med. 2010;2(61ra91) doi: 10.1126/scitranslmed.3001720. [DOI] [PubMed] [Google Scholar]
- 27.Orhant L, Anselem O, Fradin M, Becker PH, Beugnet C, Deburgrave N, Tafuri G, Letourneur F, Goffinet F, El Khattabi LA, et al. Droplet digital PCR combined with minisequencing, a new approach to analyze fetal DNA from maternal blood: Application to the non-invasive prenatal diagnosis of achondroplasia. Prenat Diagn. 2016;36:397–406. doi: 10.1002/pd.4790. [DOI] [PubMed] [Google Scholar]
- 28.Hudecova I. Digital PCR analysis of circulating nucleic acids. Clin Biochem. 2015;48:948–956. doi: 10.1016/j.clinbiochem.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 29.van Ginkel JH, Huibers MMH, van Es RJJ, de Bree R, Willems SM. Droplet digital PCR for detection and quantification of circulating tumor DNA in plasma of head and neck cancer patients. BMC Cancer. 2017;17(428) doi: 10.1186/s12885-017-3424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruber A, Pacault M, El Khattabi LA, Vaucouleur N, Orhant L, Bienvenu T, Girodon E, Vidaud D, Leturcq F, Costa C, et al. Non-invasive prenatal diagnosis of paternally inherited disorders from maternal plasma: Detection of NF1 and CFTR mutations using droplet digital PCR. Clin Chem Lab Med. 2018;56:728–738. doi: 10.1515/cclm-2017-0689. [DOI] [PubMed] [Google Scholar]
- 31.Hudecova I, Chiu RWK. Non-invasive prenatal diagnosis of thalassemias using maternal plasma cell free DNA. Best Pract Res Clin Obstet Gynaecol. 2017;39:63–73. doi: 10.1016/j.bpobgyn.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Pornprasert S, Prasing W. Detection of alpha(0)-thalassemia South-East Asian-type deletion by droplet digital PCR. Eur J Haematol. 2014;92:244–248. doi: 10.1111/ejh.12246. [DOI] [PubMed] [Google Scholar]
- 33.Lun FM, Tsui NB, Chan KC, Leung TY, Lau TK, Charoenkwan P, Chow KCK, Lo WYW, Wanapirak C, Sanguansermsri T, et al. Noninvasive prenatal diagnosis of monogenic diseases by digital size selection and relative mutation dosage on DNA in maternal plasma. Proc Natl Acad Sci USA. 2008;105:19920–19925. doi: 10.1073/pnas.0810373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kho SL, Chua KH, George E, Tan JA. A novel gap-PCR with high resolution melting analysis for the detection of alpha-thalassaemia Southeast Asian and Filipino β˚-thalassaemia deletion. Sci Rep. 2015;5(13937) doi: 10.1038/srep13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan KC, Ding C, Gerovassili A, Yeung SW, Chiu RW, Leung TN, Lau TK, Chim SS, Chung GT, Nicolaides KH, Lo YM. Hypermethylated RASSF1A in maternal plasma: A universal fetal DNA marker that improves the reliability of noninvasive prenatal diagnosis. Clin Chem. 2006;52:2211–2218. doi: 10.1373/clinchem.2006.074997. [DOI] [PubMed] [Google Scholar]
- 36.Manokhina I, Singh TK, Peñaherrera MS, Robinson WP. Quantification of cell-free DNA in normal and complicated pregnancies: Overcoming biological and technical issues. PLoS One. 2014;9(e101500) doi: 10.1371/journal.pone.0101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Karoui N, Zhou W, Whittemore AS. Getting more from digital SNP data. Stat Med. 2006;25:3124–3133. doi: 10.1002/sim.2379. [DOI] [PubMed] [Google Scholar]
- 38.Pornprasert S, Phusua A, Suanta S, Saetung R, Sanguansermsri T. Detection of alpha-thalassemia-1 Southeast Asian type using real-time gap-PCR with SYBR Green1 and high resolution melting analysis. Eur J Haematol. 2008;80:510–514. doi: 10.1111/j.1600-0609.2008.01055.x. [DOI] [PubMed] [Google Scholar]
- 39.Tsui NB, Kadir RA, Chan KC, Chi C, Mellars G, Tuddenham EG, Leung TY, Lau TK, Chiu RW, Lo YM. Noninvasive prenatal diagnosis of hemophilia by microfluidics digital PCR analysis of maternal plasma DNA. Blood. 2011;117:3684–3691. doi: 10.1182/blood-2010-10-310789. [DOI] [PubMed] [Google Scholar]
- 40.Lun FM, Chiu RW, Chan KC, Leung TY, Lau TK, Lo YM. Microfluidics digital PCR reveals a higher than expected fraction of fetal DNA in maternal plasma. Clin Chem. 2008;54:1664–1672. doi: 10.1373/clinchem.2008.111385. [DOI] [PubMed] [Google Scholar]
- 41.Amicucci P, Gennarelli M, Novelli G, Dallapiccola B. Prenatal diagnosis of myotonic dystrophy using fetal DNA obtained from maternal plasma. Clin Chem. 2000;46:301–302. [PubMed] [Google Scholar]
- 42.Saito H, Sekizawa A, Morimoto T, Suzuki M, Yanaihara T. Prenatal DNA diagnosis of a single-gene disorder from maternal plasma. Lancet. 2000;356(1170) doi: 10.1016/S0140-6736(00)02767-7. [DOI] [PubMed] [Google Scholar]
- 43.Chiu RW, Lau TK, Leung TN, Chow KC, Chui DH, Lo YM. Prenatal exclusion of beta thalassaemia major by examination of maternal plasma. Lancet. 2002;360:998–1000. doi: 10.1016/s0140-6736(02)11086-5. [DOI] [PubMed] [Google Scholar]
- 44.Ding C, Chiu RW, Lau TK, Leung TN, Chan LC, Chan AY, Charoenkwan P, Ng IS, Law HY, Ma ES, et al. MS analysis of single-nucleotide differences in circulating nucleic acids: Application to noninvasive prenatal diagnosis. Proc Natl Acad Sci USA. 2004;101:10762–10767. doi: 10.1073/pnas.0403962101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hudecova I, Jiang P, Davies J, Lo YMD, Kadir RA, Chiu RWK. Noninvasive detection of F8 int22h-related inversions and sequence variants in maternal plasma of hemophilia carriers. Blood. 2017;130:340–347. doi: 10.1182/blood-2016-12-755017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor SC, Carbonneau J, Shelton DN, Boivin G. Optimization of droplet digital PCR from RNA and DNA extracts with direct comparison to RT-qPCR: Clinical implications for quantification of Oseltamivir-resistant subpopulations. J Virol Methods. 2015;224:58–66. doi: 10.1016/j.jviromet.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Sirichotiyakul S, Charoenkwan P, Sanguansermsri T. Prenatal diagnosis of homozygous alpha-thalassemia-1 by cell-free fetal DNA in maternal plasma. Prenat Diagn. 2012;32:45–49. doi: 10.1002/pd.2892. [DOI] [PubMed] [Google Scholar]
- 48.Whale AS, Cowen S, Foy CA, Huggett JF. Methods for applying accurate digital PCR analysis on low copy DNA samples. PLoS One. 2013;8(e58177) doi: 10.1371/journal.pone.0058177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vermeulen J, Derveaux S, Lefever S, De Smet E, De Preter K, Yigit N, De Paepe A, Pattyn F, Speleman F, Vandesompele J. RNA pre-amplification enables large-scale RT-qPCR gene-expression studies on limiting sample amounts. BMC Res Notes. 2009;2(235) doi: 10.1186/1756-0500-2-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.