Abstract
Background and Objectives:
Pancreatic cystic lesions (PCLs) are frequent incidental findings on cross-sectional imaging and represent a diagnostic challenge as different kinds of PCLs harbor a dissimilar risk of malignancy. Two diagnostic tools have recently been developed and introduced: through-the-needle biopsy (TTNB) and needle-based confocal laser endomicroscopy (nCLE). The aim of this meta-analysis was to compare the diagnostic yield and performance, as well as the safety profile of the two methods.
Methods:
This meta-analysis was performed in accordance with the PRISMA statement. Medline, Embase, Web of Science, and Cochrane Library databases were searched for studies with five or more patients undergoing either endoscopic ultrasound (EUS)-TTNB or EUS-nCLE for a PCL. Reviews, case reports, editorials, conference abstracts, and studies on exclusively solid pancreatic lesions were excluded. Outcomes of interest were diagnostic yield and performance, safety, and technical success.
Results:
Twenty studies with 1023 patients were included in the meta-analysis. Pooled diagnostic yield of EUS-nCLE was higher compared to EUS-TTNB (85% vs. 74%, P < 0.0001), while diagnostic performance was high and comparable for both methods (pooled sensitivity: 80% vs. 86% and pooled specificity: 80% vs. 83% for TTNB and nCLE, respectively, P > 0.05). Pooled estimate of total adverse event (AE) rate was 5% in the TTNB group and 3% in the nCLE group, P = 0.302. Technical success rates were high and comparable (94% and 99% for EUS-TTNB and nCLE, respectively; P = 0.07).
Conclusion:
EUS-TTNB and EUS-nCLE have a similar safety profile with a relatively low number of AEs. Technical success, sensitivity, and specificity are comparable; however, EUS-nCLE seems to have a slightly higher diagnostic yield.
Keywords: EUS-through-the-needle biopsy, intraductal papillary mucinous neoplasm, moray, needle-based confocal laser endomicroscopy, pancreatic cyst
INTRODUCTION
Pancreatic cystic lesions (PCLs) are frequent incidental findings on cross-sectional imaging performed for other reasons, and the prevalence increases with age.[1] There are many different types of PCLs, some of which have malignant potential. Distinction between mucinous (intraductal papillary mucinous neoplasm [IPMN] and mucinous cystic neoplasm [MCN]) and nonmucinous lesions (pseudocysts and serous cystic neoplasm [SCN]) is clinically relevant, as the former are considered premalignant lesions. Current guidelines recommend risk stratification based on a composite of variables, such as patient history, cross-sectional imaging, and in selected cases, EUS-FNA.[2] However, FNA cytology has only a moderate accuracy for distinguishing mucinous from nonmucinous lesions (pooled sensitivity 54%) mostly because of the low cellularity of the cyst fluid.[3] Carcinoembryonic antigen (CEA) in the cyst fluid is another diagnostic test but it performs rather modestly with a pooled sensitivity of 63% and a specificity of 88%.[3] In recent years, several other diagnostic tools have been introduced in an effort to improve the diagnostic process.
EUS-guided needle-based confocal laser endomicroscopy (EUS-nCLE) utilizes a laser beam to obtain in vivo microscopic images of the cyst wall epithelium, following injection of a contrast solution (2.5–5 mL 10% fluorescein sodium).[4] The probe (Cellvizio, Mauna Kea Technologies, Paris, France) is advanced into the cyst lumen following access by a 19G needle and is used for image acquisition of the cyst wall. Several different image features have been described and validated, such as papillary projections (consistent with IPMN) and a superficial vascular network (indicative of an SCN) to name a few.[5] The second novel method for the evaluation of PCLs is EUS-guided through-the-needle biopsy (EUS-TTNB) which uses a specifically developed microforceps (also inserted through a 19G needle) to obtain tissue samples from the cyst wall or suspected intracystic nodules/masses, overcoming the previously described issue of low cellularity.[6] Both methods are reported to have high technical success and a superior diagnostic yield compared to standard cytology,[7,8,9] but there is currently only a single, inconclusive comparison of the two methods.[10] Furthermore, concerns have been raised about the adverse event (AE) rate of EUS-TTNB, as several severe cases of pancreatitis have been reported.[11] The aim of this meta-analysis was to compare the diagnostic yield and performance, as well as the safety profile of EUS-TTNB and EUS-nCLE.
METHODS
Search strategy and study selection
A study protocol was developed and uploaded to PROSPERO (protocol no: 156867). Two authors (B.K. and G.A.) independently performed a systematic search of Medline, Embase, Web of Science, and Cochrane Library databases using a predefined search string (Supplementary Material). Inclusion criteria were studies with five or more patients undergoing either EUS-TTNB or EUS-nCLE for a PCL. Reviews, case reports, editorials, conference abstracts, and studies on exclusively solid pancreatic lesions were excluded. Additional papers were identified through backward snowballing. An online database (clinicaltrials.gov) was queried for any ongoing studies. In case of disagreement, a third author was consulted (J.G.K).
Outcomes and data extraction
Primary outcomes were diagnostic yield, defined as a proportion of lesions where a diagnosis was obtained, and safety (AEs were classified as mild, moderate, and severe according to the American Society for Gastrointestinal Endoscopy lexicon).[12] Secondary outcomes included technical success, defined as successful acquirement of a macroscopically visible histological sample in case of EUS-TTNB or obtainment of recognizable images in case of EUS-nCLE, total procedural time, and diagnostic performance (concordance with final diagnosis in surgical subgroup and ability to discriminate between mucinous and nonmucinous lesions). Furthermore, following data were extracted: name of first author, year of publication, study characteristics (design, location, and study length), patient characteristics (age, gender, lesion size, and location), and procedural characteristics (indication, type of sedation, and use of antibiotics). Data extraction was performed independently by two authors (B.K. and G.A.), and any disagreements were solved by consensus or by consulting a third author (J.G.K.). Quality was similarly assessed using the first eight items of Methodological Index for Nonrandomized Studies tool.[13] Mean score was calculated, and the studies were graded as having low (≤8) or high quality (>8).
Statistical analysis
As primary and secondary outcomes were expected to deviate considerably from a proportion of 0.5, double arcsine transformation of the proportions was employed to obtain unbiased effect size estimates.[14] The effect sizes were weighted by the inverse of the study variance and forest plots were constructed. The results were pooled using DerSimonian-Laird method (random-effect model), as a high level of heterogeneity was expected a priori. Heterogeneity was assessed by visual inspection of the plots and corresponding statistics (Higgins I2, τ2, and Cochran's Q test) and was considered low, moderate, and substantial in case of I2 of <25%, 25%–75%, and >75%, respectively. A leave-one-out sensitivity analysis was performed to identify any influential studies.
In the surgical subgroup, the test diagnosis was compared to reference histology in an intention-to-diagnose approach. Discrimination between mucinous and nonmucinous lesions was evaluated by constructing 2 × 2 tables and calculating pooled sensitivity and specificity using the bivariate model. Furthermore, summary receiver operating characteristic (sROC) curves were plotted, and corresponding areas under the curve (AUC) were calculated for each method and compared. While an accuracy of a test is highly influenced by the distribution of classifiers and may be an inaccurate measure of test precision in case of an imbalanced classification (as often is the case with PCLs), AUC values give a more reliable estimate of a test performance.
Meta-regression was performed examining the effects of intervention, study design and quality, patient age, and lesion size on effect size estimates. Potential publication bias was evaluated by examining funnel plots, rank correlation test, and Egger's regression test for plot asymmetry. All statistical analyses were performed using R version 3.6.2 and R studio version 1.1.423 RStudio PBC, Boston, MA, US. Statistical significance was set at P < 0.05 for all calculations. All authors had access to the study data and had reviewed and approved the final manuscript.
RESULTS
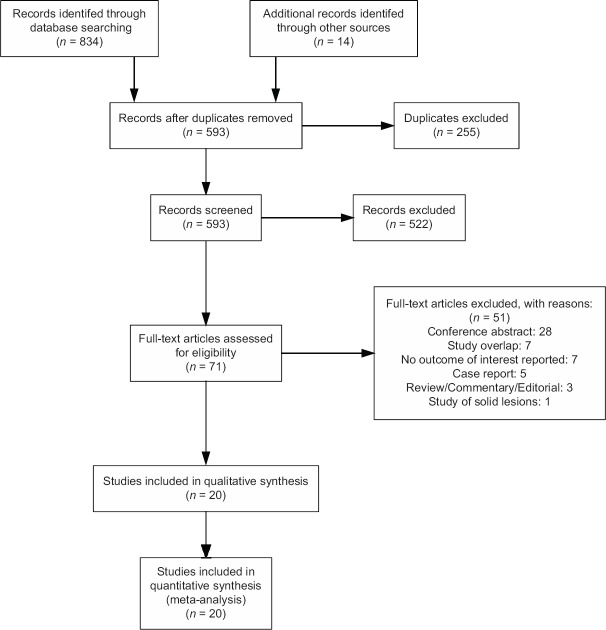
Literature search yielded 848 articles, of which 20 studies with 1023 patients were included in the meta-analysis [Figure 1]. The majority of the studies were single center (n = 12, 60%) and retrospective by design (n = 11, 55%). Mean lesion size ranged from 25.0 to 43.7 mm with an overweight of the lesions (61%) located in the body/tail portion of the pancreas [Table 1]. Only a few studies reported on procedural time without clear definitions: in case of EUS-TTNB, the total procedural time ranged from 23 to 34 min;[18,19] whereas only image acquisition time was reported (mean 5–7 min) for nCLE.[5,24,26,30] All but five studies reported on prophylactic use of antibiotics. A single dose of intravenous quinolones, cephalosporins, or a combination of penicillin and β-lactamase was usually administered periprocedurally. Some centers continued antibiotic therapy for 3–5 days either routinely[5,15,20,27] or at the discretion of an endoscopist.[10]
Figure 1.
PRISMA flowchart
Table 1.
Overview of the included studies
| First author | Year | Design | Single/multicenter | Country | Intervention | Number of patients | Female, n (%) | Mean age (range) | Mean cyst size, mm (range) | Mean quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| Barresi[15] | 2018 | Retrospective | Multicenter | IT, NL | TTNB | 56 | 39 (70) | 57.5 (27–80) | 38.6 (16–55) | 8.0 (low) |
| Basar[16] | 2018 | Retrospective | Multicenter | USA | TTNB | 42 | 23 (55) | 69.9 (27–91) | 28.2 (12–60) | 6.5 (low) |
| Cheesman[10] | 2020 | Retrospective | Single center | USA | TTNB | 44 | 28 (64) | 66.0 | 33.5 (19–90) | 7.0 (low) |
| Hashimoto[17] | 2019 | Retrospective | Single center | USA | TTNB | 56 | 30 (54) | 66.9 (SD 11.7) | 28.8 (12–85) | 7.5 (low) |
| Kovacevic[18] | 2018 | Retrospective | Multicenter | DK, NO, FR, IT, ES, IL | TTNB | 15* | 7 (47) | 65.0 (SD 10.3) | 34.0 | 7.0 (low) |
| Kovacevic[19] | 2018 | Retrospective | Single center | DK | TTNB | 31 | 15 (48) | 69.9 (40–87) | 33.6 (12–130) | 8.5 (high) |
| Kovacevic[11] | 2021 | Prospective | Single center | DK | TTNB | 101 | 54 (53) | 67.9 (37–85) | 25.0 (15–93) | 16.0 (high) |
| Mittal[20] | 2018 | Retrospective | Single center | USA | TTNB | 27 | 16 (59) | 65.0 (32–87) | 37.8 (SD 16.9) | 7.0 (low) |
| Yang[21] | 2018 | Retrospective | Multicenter | USA | TTNB | 47 | 26 (55) | 64.2 | 30.8 (12–110) | 10.0 (high) |
| Yang[22] | 2019 | Prospective | Multicenter | USA | TTNB | 114 | 64 (56) | 65.0 | 35.1 | 10.5 (high) |
| Zhang[23] | 2018 | Retrospective | Single center | USA | TTNB | 48 | 25 (52) | 69.6 (27–90) | 31.0 (12–60) | 6.0 (low) |
| Chin[24] | 2018 | Prospective | Single-center | SG | nCLE | 12 | 6 (50) | 66.5 | 33.9 (19–62) | 10.5 (high) |
| Kadayifci[25] | 2017 | Prospective | Single center | USA | nCLE | 20 | 10 (50) | 65.4 (SD 17.1) | 34.2 (SD 9.6) | 9.5 (high) |
| Keane[9] | 2019 | Prospective | Multicenter | UK | nCLE | 56 | 26 (46) | 68.0 (28–80) | 25.0 (10–70) | 14.0 (high) |
| Konda[26] | 2013 | Prospective | Multicenter | USA, FR, DE | nCLE | 66 | 30 (45) | 63.1 (27–89) | 28.0 (7–90) | 10.0 (high) |
| Krishna[5] | 2020 | Prospective | Single center | USA | nCLE | 144 | 76 (53) | 60.2 (SD 14.3) | 36.4 (SD 15.7) | 13.5 (high) |
| Nakai[27] | 2015 | Prospective | Single center | USA | nCLE | 30 | 21 (70) | 72.0 (37–86) | 31.0 (5–64) | 10.0 (high) |
| Napoleon[28] | 2019 | Prospective | Multicenter | FR | nCLE | 78† | 52 (67) | 57.0 (28–81) | 40.0 (20–110) | 8.0 (high) |
| Cheesman[10] | 2020 | Retrospective | Single center | USA | nCLE | 44 | 28 (64) | 66.0 | 33.5 (16–90) | 6.5 (low) |
| Haghighi[29] | 2019 | Retrospective | Single center | USA | nCLE | 32 | 20 (63) | 65.6 (26–83) | 43.7 (9–136) | 7.0 (low) |
*After exclusion of overlapping patients; †A part of a larger cohort; technical success and diagnostic yield is hence reported on 209 patients. TTNB: Through-the-needle biopsy; nCLE: Needle-based confocal laser endomicroscopy; SD: Standard deviation
Diagnostic yield and safety
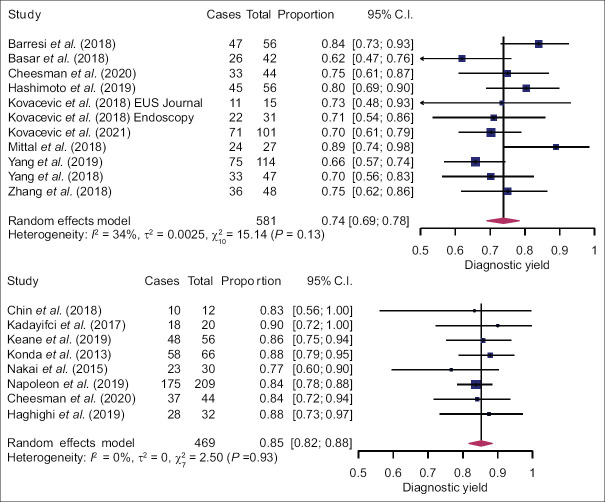
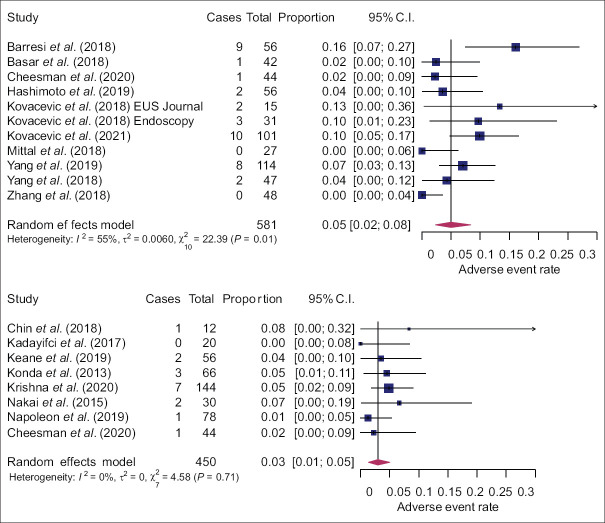
Pooled diagnostic yield of EUS-nCLE was higher compared to EUS-TTNB (85%, 95% confidence interval [CI]: 82%–88% and 74%, 95% CI: 69%–78%; P < 0.0001). Heterogeneity was low in the nCLE group and moderate in the TTNB group [Figure 2]. Meta-regression did not reveal any other variables associated with diagnostic yield [Supplementary Table S1]. Pooled estimate of total AE rate was 5% (95% CI: 2%–8%) in the TTNB group and 3% (95% CI: 1%–5%) in the nCLE group, P = 0.302 [Figure 3]. AEs were mainly mild; but moderate (nCLE: 1.5%, TTNB: 1.3%) and severe AEs (nCLE: 0.0%, TTNB: 0.7%) were also reported. Most common AE was pancreatitis (nCLE: 2.1%, TTNB: 3.9%), followed by intracystic hemorrhage (nCLE: 0.4%, TTNB: 2.4%) and infection (nCLE: 0.4%, TTNB: 0.4%). Meta-regression did not show any association between study design and quality, patient age or lesion size, and AE rate [Supplementary Table S1]. Funnel plots and statistical tests did not reveal any publication bias [Supplementary Figure S1 (2.7MB, tif) ].
Figure 2.
Forest plots of diagnostic yield of EUS-through-the-needle biopsy (top) and EUS-needle-based confocal laser endomicroscopy (bottom)
Figure 3.
Forest plots of adverse event rate of EUS-through-the-needle biopsy (top) and EUS-needle-based confocal laser endomicroscopy (bottom)
Table S1.
Meta-regression of intervention, study design and quality, patient age and lesion size on effect size estimates
| Effect size estimate | Lower 95% CI | Upper 95% CI | P | |
|---|---|---|---|---|
| Diagnostic yield | ||||
| Intervention | 0.1393 | 0.0740 | 0.2047 | <0.0001 |
| Study design | −0.0249 | −0.1209 | 0.0711 | 0.6110 |
| Quality | −0.0117 | −0.1078 | 0.0845 | 0.8119 |
| Lesion size | 0.0059 | −0.0032 | 0.0150 | 0.2053 |
| Safety | ||||
| Intervention | −0.0426 | −0.1235 | 0.0383 | 0.3022 |
| Study design | −0.0131 | −0.0969 | 0.0707 | 0.7586 |
| Quality | 0.0310 | −0.0542 | 0.1162 | 0.4756 |
| Patient age | −0.0019 | −0.0122 | 0.0084 | 0.7221 |
| Lesion size | −0.0009 | −0.0103 | 0.0084 | 0.8462 |
| Concordance with surgical histology | ||||
| Intervention | −0.1721 | −0.4406 | 0.0963 | 0.2089 |
| Study design | −0.0518 | −0.2438 | 0.1403 | 0.5973 |
| Quality | 0.1224 | −0.0641 | 0.3088 | 0.1983 |
| Patient age | 0.0152 | −0.0102 | 0.0406 | 0.2409 |
| Lesion size | −0.0150 | −0.0391 | 0.0091 | 0.2229 |
| Technical success | ||||
| Intervention | 0.1173 | −0.0079 | 0.2424 | 0.0664 |
| Study design | −0.0120 | −0.1527 | 0.1287 | 0.8675 |
| Quality | −0.0628 | −0.2021 | 0.0765 | 0.3769 |
| Lesion size | 0.0084 | −0.0060 | 0.0228 | 0.2518 |
CI: Confidence interval
Diagnostic performance and technical success
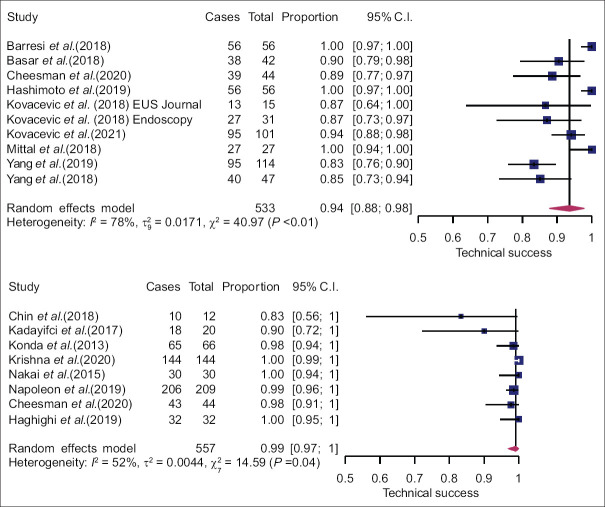
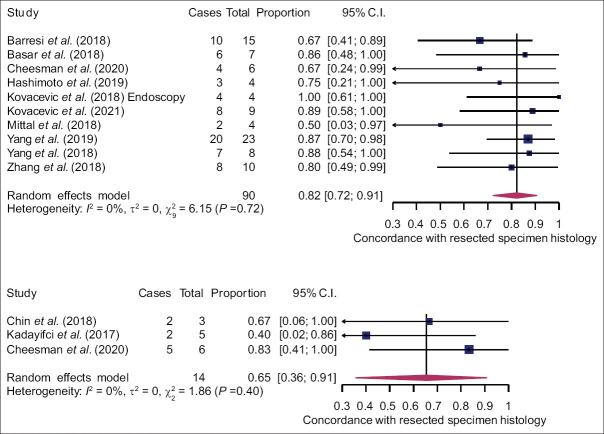
In case of classification into mucinous and nonmucinous lesions, the pooled sensitivity was 80% (95% CI: 65%–89%) and 86% (95% CI: 69%–94%) for EUS-TTNB and nCLE, respectively. Pooled specificity was 80% (95% CI: 53%–94%) for EUS-TTNB and 83% (95% CI: 62%–94%) for nCLE [Figure 4]. There was no difference in diagnostic performance between the two modalities (P > 0.05). A leave-one-out sensitivity analysis failed to identify any influential studies. Estimated AUC of sROC was comparable: 0.86 for EUS-TTNB and 0.91 for EUS-nCLE (P > 0.05). Pooled estimate for concordance with final diagnosis in the surgical subgroup was 82% (95% CI: 72%–91%) and 65% (95% CI: 36%–91%) for the TTNB and nCLE groups, respectively. However, small sample size in the latter resulted in wide CIs, and the difference was statistically insignificant (P = 0.21). Technical success was high and comparable for both methods [Figure 5], but the heterogeneity was substantial (I2 = 78% and 52%).
Figure 4.
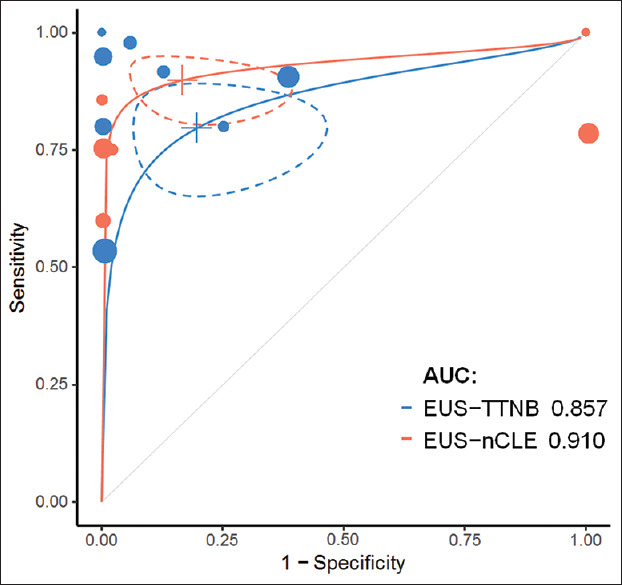
Receiver operating characteristics curves of the two methods with 95% confidence intervals (dashed lines) and individual studies plotted
Figure 5.
Forest plots of technical success of EUS-through-the-needle biopsy (top) and EUS-needle-based confocal laser endomicroscopy (bottom)
DISCUSSION
This meta-analysis provides a first comparison between EUS-TTNB and EUS-nCLE in the evaluation of PCLs. The study included 20 studies with 1023 patients and employed meticulous assessment and high methodological standards. However, no randomized controlled studies were identified, and half of the included studies were retrospective by design, which is a lower level of evidence. Conversely, we did not find any evidence of publication bias. The major limitation of this meta-analysis is an indirect comparison of the two methods due to the lack of comparative studies, which is why the results must be interpreted with caution. Study population in the EUS-TTNB and EUS-nCLE studies may be heterogeneous, as well as the technical setup and overall workflow, which must also be taken into consideration when interpreting the results. Furthermore, gold standard reference test (surgical histology) was only available in a small proportion of cases (n = 104, 10.2%), why estimates of diagnostic performance may be inaccurate [Figure 6].
Figure 6.
Forest plots of concordance rates of EUS-through-the-needle biopsy (top) and EUS-needle-based confocal laser endomicroscopy (bottom) with surgical histology
The results of this study show that EUS-TTNB and EUS-nCLE seem to have a similar technical success rate, diagnostic performance, and safety profile, but the diagnostic yield of nCLE appears significantly higher than that of TTNB. Confocal endomicroscopy is an older technique with the first human pilot study for pancreatic pathology published in 2011,[31] whereas TTNB was first introduced in 2016.[6] This difference can explain not only the lower heterogeneity in nCLE studies but possibly also the higher diagnostic yield observed, as it may well be the result of the high level of experience in image acquisition and interpretation in the high-volume centers performing EUS-nCLE. Several studies have confirmed this learning curve effect and showed an increased accuracy and levels of agreement following training period.[32,33,34] Similarly, EUS-TTNB yields very small tissue samples that require not only delicate processing but also an experienced pathologist to ensure a correct interpretation and diagnosis. Same learning curve effects seem to be present, hence the larger heterogeneity observed in EUS-TTNB studies.[11,35] Economical consideration must also be taken into account as the price of nCLE system is higher compared to TTNB (approximately $100,000 as the initial cost for the CLE system followed by $400 per examination compared to $500 for the TTNB forceps).[36]
AEs following EUS-FNA of PCLs are well known and include hemorrhage, pancreatitis, abdominal pain, and infection. A meta-analysis by Zhu et al. with >5000 patients estimated AE rate of EUS-FNA to be 2.7% (95% CI: 1.8%–3.6%), which does not seem to deviate significantly the results of this meta-analysis.[37] However, although mild and moderate AEs were similarly distributed in both groups, severe AEs due to pancreatitis were only observed in the EUS-TTNB group (n = 4, 0.7%). A single study reported a reduction in AEs following the use of high-volume perioperative resuscitation and prophylactic nonsteroid anti-inflammatory drugs; however, the difference was not statistically significant.[11] To determine whether this approach will reduce the occurrence of acute pancreatitis, a large number of patients is needed due to the low rate of this AE. However, it would be most relevant to identify high-risk patients who would benefit the most from this treatment.
Both diagnostic methods have high sensitivity and specificity, and the diagnostic performance is comparable. Moderate heterogeneity and low sample size when determining specificity are inherent to a naturally unbalanced distribution of PCLs, explaining why we decided to additionally calculate sROC curves with corresponding AUC values. Both methods had high and comparable AUC values. However, as surgical confirmation was only available in a small proportion of patients and the reference diagnosis was in most cases based on a combination of cytology, cyst fluid CEA, and cross-sectional imaging, the results must be interpreted carefully. Furthermore, when determining concordance with the histological diagnosis in the surgical subgroup, a very small sample of 14 patients was encountered in the nCLE group. Although TTNB seemed to have a higher overall concordance rate (82% vs. 65%), any conclusions regarding nCLE may be unreliable due to previously mentioned limitations.
Extracting tissue samples provides the possibility of performing additional analyses, such as immunohistochemistry or even molecular sequencing, which is not feasible in case of EUS-nCLE. Specifically, IPMNs can be subdivided into three subtypes: gastric, intestinal, and pancreatobiliary, and the subtype seems to be associated with risk of malignancy and recurrence in case of resected lesions. Current guidelines recommend different follow-up based on the subtype of resected IPMNs, but the role of preoperative subtyping is currently unknown.[2] Furthermore, discerning IPMNs from MCNs is difficult and requires detection of an ovarian-type stroma, which is pathognomonic for MCN. Immunohistochemistry is useful in these cases, as different subtypes of IPMN show dissimilar expression of different MUC-proteins, whereas ovarian-type stroma stains positive for estrogen and progesterone receptor. Molecular analysis of the cyst fluid has shown promising results, as detection of certain mutations can reliably discern mucinous from nonmucinous lesions but also predict the grade of dysplasia.[38] Molecular sequencing of TTNBs may play a similar role in the future, but the current data are scarce.
CONCLUSION
This is the first systematic review and meta-analysis comparing EUS-TTNB and EUS-nCLE. Both methods have a similar safety profile with a relatively low number of AEs. Diagnostic performance is comparable although EUS-nCLE seems to have a significantly higher diagnostic yield. While additional diagnostic possibilities of EUS-TTNB such as molecular analyses and IPMN subclassification will outweigh EUS-nCLE, remains to be seen.
Financial support and sponsorship
The authors wish to thank Danish Cancer Society, Arvid Nilsson Foundation, Ingrid Munkholm Foundation, Toyota Foundation, Novo Nordisk Foundation, Harboefonden, Tømrermester Jørgen Holm og hustru Elisa f. Hansens Mindelegat, and Herlev Hospital for their financial support.
Conflicts of interest
There are no conflicts of interest.
Supplementary Materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Funnel plots of effect size estimates for diagnostic yield, adverse event rate, sensitivity for mucinous lesions, specificity for mucinous lesions, and concordance with surgical histology respectively for each row. Left column corresponds to EUS-through-the-needle biopsy, whereas the right column depicts EUS-needle-based confocal laser endomicroscopy
SUPPLEMENTARY MATERIAL
Search string
For EUS-TTNB: (“Moray” OR “microbiops*” OR “TTNF” OR “TTNB” OR “microforceps” OR “micro-biops*” OR “micro-forceps” OR “*through-the-needle*”) AND (“pancrea*” OR “IPMN” OR “cyst*”)
For EUS-nCLE: (“*CLE” OR “confocal laser endomicroscop*”) AND (“pancrea*” OR “IPMN” OR “cyst*”).
REFERENCES
- 1.Kromrey ML, Bülow R, Hübner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138–45. doi: 10.1136/gutjnl-2016-313127. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–53. doi: 10.1016/j.pan.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 4.Anand K, Kahaleh M, Tyberg A. Use of needle-based confocal laser endomicroscopy in the diagnosis and management of pancreatic cyst lesions. Endosc Ultrasound. 2018;7:306–9. doi: 10.4103/eus.eus_46_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishna SG, Hart PA, Malli A, et al. Endoscopic ultrasound-guided confocal laser endomicroscopy increases accuracy of differentiation of pancreatic cystic lesions. Clin Gastroenterol Hepatol. 2020;18:432–40. doi: 10.1016/j.cgh.2019.06.010. e436. [DOI] [PubMed] [Google Scholar]
- 6.Pham KD, Engjom T, Gjelberg Kollesete H, et al. Diagnosis of a mucinous pancreatic cyst and resection of an intracystic nodule using a novel through-the-needle micro forceps. Endoscopy. 2016;48(Suppl 1):E125–6. doi: 10.1055/s-0042-105437. [DOI] [PubMed] [Google Scholar]
- 7.Tacelli M, Celsa C, Magro B, et al. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig Endosc. 2020;32:1018–30. doi: 10.1111/den.13626. [DOI] [PubMed] [Google Scholar]
- 8.Facciorusso A, Del Prete V, Antonino M, et al. Diagnostic yield of EUS-guided through-the-needle biopsy in pancreatic cysts: A meta-analysis. Gastrointest Endosc. 2020;92:1–8. doi: 10.1016/j.gie.2020.01.038. e3. [DOI] [PubMed] [Google Scholar]
- 9.Keane MG, Wehnert N, Perez-Machado M, et al. A prospective trial of CONfocal endomicroscopy in CYSTic lesions of the pancreas: CONCYST-01. Endosc Int Open. 2019;7:E1117–22. doi: 10.1055/a-0957-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheesman AR, Zhu H, Liao X, et al. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest Endosc. 2020;91:1095–104. doi: 10.1016/j.gie.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Kovacevic B, Klausen P, Rift CV, et al. Clinical impact of endoscopic ultrasound-guided through-the-needle microbiopsy in patients with pancreatic cysts. Endoscopy. 2021;53:44–52. doi: 10.1055/a-1214-6043. [DOI] [PubMed] [Google Scholar]
- 12.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J Surg. 2003;73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 14.Freeman M, Tukey J. Transformations related to the angular and the square root. Ann Math Statist. 1950;21:607–11. [Google Scholar]
- 15.Barresi L, Crinò SF, Fabbri C, et al. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A multicenter study. Dig Endosc. 2018;30:760–70. doi: 10.1111/den.13197. [DOI] [PubMed] [Google Scholar]
- 16.Basar O, Yuksel O, Yang DJ, et al. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest Endosc. 2018;88:79–86. doi: 10.1016/j.gie.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto R, Lee JG, Chang KJ, et al. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A large single center experience. World J Gastrointest Endosc. 2019;11:531–40. doi: 10.4253/wjge.v11.i11.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovacevic B, Karstensen JG, Havre RF, et al. Initial experience with EUS-guided microbiopsy forceps in diagnosing pancreatic cystic lesions: A multicenter feasibility study (with video) Endosc Ultrasound. 2018;7:383–8. doi: 10.4103/eus.eus_16_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacevic B, Klausen P, Hasselby JP, et al. A novel endoscopic ultrasound-guided through-the-needle microbiopsy procedure improves diagnosis of pancreatic cystic lesions. Endoscopy. 2018;50:1105–11. doi: 10.1055/a-0625-6440. [DOI] [PubMed] [Google Scholar]
- 20.Mittal C, Obuch JC, Hammad H, et al. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video) Gastrointest Endosc. 2018;87:1263–9. doi: 10.1016/j.gie.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Yang D, Samarasena JB, Jamil LH, et al. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: A multicenter study. Endosc Int Open. 2018;6:E1423–30. doi: 10.1055/a-0770-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D, Trindade AJ, Yachimski P, et al. Histologic analysis of endoscopic ultrasound-guided through the needle microforceps biopsies accurately identifies mucinous pancreas cysts. Clin Gastroenterol Hepatol. 2019;17:1587–96. doi: 10.1016/j.cgh.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ML, Arpin RN, Brugge WR, et al. Moray micro forceps biopsy improves the diagnosis of specific pancreatic cysts. Cancer Cytopathol. 2018;126:414–20. doi: 10.1002/cncy.21988. [DOI] [PubMed] [Google Scholar]
- 24.Chin YK, Khor CJ, Goh BK, et al. The clinical evaluation of needle-based confocal laser endomicroscopy in the assessment of pancreatic cystic lesion: A pilot study. Proc Singapore Healthc. 2018;27:96–102. [Google Scholar]
- 25.Kadayifci A, Atar M, Basar O, et al. Needle-based confocal laser endomicroscopy for evaluation of cystic neoplasms of the pancreas. Dig Dis Sci. 2017;62:1346–53. doi: 10.1007/s10620-017-4521-2. [DOI] [PubMed] [Google Scholar]
- 26.Konda VJ, Meining A, Jamil LH, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–13. doi: 10.1055/s-0033-1344714. [DOI] [PubMed] [Google Scholar]
- 27.Nakai Y, Iwashita T, Park DH, et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204–14. doi: 10.1016/j.gie.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Napoleon B, Palazzo M, Lemaistre AI, et al. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: A prospective multicenter validation study in patients with definite diagnosis. Endoscopy. 2019;51:825–35. doi: 10.1055/a-0732-5356. [DOI] [PubMed] [Google Scholar]
- 29.Haghighi M, Sethi A, Tavassoly I, et al. Diagnosis of pancreatic cystic lesions by virtual slicing: Comparison of diagnostic potential of needle-based confocal laser endomicroscopy versus endoscopic ultrasound-guided fine-needle aspiration. J Pathol Inform. 2019;10:34. doi: 10.4103/jpi.jpi_32_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keane MG, Wehnert N, Perez-Machado M, et al. A prospective trial of CONfocal endomicroscopy in CYSTic lesions of the pancreas: CONCYST-01. Endosc Int Open. 2019;7:E1117–22. doi: 10.1055/a-0957-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konda VJ, Aslanian HR, Wallace MB, et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos) Gastrointest Endosc. 2011;74:1049–60. doi: 10.1016/j.gie.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Chang J, Ip M, Yang M, et al. The learning curve, interobserver, and intraobserver agreement of endoscopic confocal laser endomicroscopy in the assessment of mucosal barrier defects. Gastrointest Endosc. 2016;83:785–910. doi: 10.1016/j.gie.2015.08.045. [DOI] [PubMed] [Google Scholar]
- 33.Krishna SG, Brugge WR, Dewitt JM, et al. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: An international external interobserver and intraobserver study (with videos) Gastrointest Endosc. 2017;86:644–54. doi: 10.1016/j.gie.2017.03.002. e642. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Li M, Li Z, et al. Learning curve and interobserver agreement of confocal laser endomicroscopy for detecting precancerous or early-stage esophageal squamous cancer. PLoS One. 2014;9:E99089. doi: 10.1371/journal.pone.0099089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larghi A, Manfrin E, Fabbri C, et al. Interobserver agreement among expert pathologists on through-the-needle microforceps biopsy samples for evaluation of pancreatic cystic lesions. Gastrointest Endosc. 2019;90:784–92. doi: 10.1016/j.gie.2019.07.011. e784. [DOI] [PubMed] [Google Scholar]
- 36.Karia K, Kahaleh M. A review of probe-based confocal laser endomicroscopy for pancreaticobiliary disease. Clin Endosc. 2016;49:462–6. doi: 10.5946/ce.2016.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu H, Jiang F, Zhu J, et al. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Dig Endosc. 2017;29:667–75. doi: 10.1111/den.12851. [DOI] [PubMed] [Google Scholar]
- 38.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131–41. doi: 10.1136/gutjnl-2016-313586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Funnel plots of effect size estimates for diagnostic yield, adverse event rate, sensitivity for mucinous lesions, specificity for mucinous lesions, and concordance with surgical histology respectively for each row. Left column corresponds to EUS-through-the-needle biopsy, whereas the right column depicts EUS-needle-based confocal laser endomicroscopy