Abstract
Background and Objectives:
The clinical presentation of focal autoimmune pancreatitis (FAIP) and together with radiological overlap can mimic pancreatic cancer (PC). The aim of this study is to construct and validate a prediction model for differentiating FAIP from PC according to EUS characteristics.
Patients and Methods:
Ninety patients with FAIP and 196 patients with PC, who consecutively underwent EUS at our center from January 2013 to December 2018, were retrospectively included in the study. The enrolled patients were randomly divided into either a derivation sample or a validation sample. According to EUS characteristics, multivariate stepwise logistic regression and receiver operating characteristics (ROC) analyses were used to construct a prediction model in derivation sample, and then, the efficacy was assessed in validation sample.
Results:
EUS characteristics that were suggestive of FAIP included diffuse hypoechogenicity, hyperechoic foci/stands or lobularity (parenchymal heterogeneity), bile duct wall thickening and peripancreatic hypoechoic margin; and EUS features favoring PC included focal hypoechogenicity, absence of parenchymal heterogeneity, pancreatic duct dilation, and vessel involvement. The prediction model, with an area under the ROC curve of more than 0.95, had a good capability to distinguish FAIP from PC. By using the optimal cutoff value, the efficacy of model for diagnosing PC showed 83.7%–91.8% sensitivity and 93.3%–95.6% specificity.
Conclusions:
It is feasible to differentiate FAIP from PC based on EUS characteristics. The prediction model built in this study needs to be further confirmed by multicenter prospective researches.
Keywords: autoimmune pancreatitis, EUS, pancreatic cancer, prediction model
INTRODUCTION
Autoimmune pancreatitis (AIP) is an inflammatory process of the pancreas with a presumed autoimmune etiology, which is characterized by distinctive clinical, serological and histological features and by effectiveness of steroid therapy. It is now regarded as a separate type of chronic pancreatitis.[1,2] AIP occurs most commonly in elderly males and clinically presents with obstructive jaundice, abdominal pain and weight loss, which can mimic pancreatic cancer (PC). Typical AIP exhibits diffuse pancreatic enlargement, termed diffuse AIP (DAIP), but the focal form appears as mass-like enlargement, termed focal AIP (FAIP), which often involves the pancreatic head.[3,4] The common bile duct (CBD) is the most frequent extrapancreatic organ involved in AIP. In addition, AIP can also cause peripancreatic lymphadenopathy and vascular invasion.[5,6,7] Therefore, correctly diagnosing FAIP and differentiating it from PC is critical and challenging due to the overlap of clinical and imaging characteristics. EUS has become a routine modality for the evaluation of pancreatic disorders because it can display fine imaging of the pancreatic parenchyma and pancreaticobiliary system. EUS is superior to conventional imaging techniques in detecting pancreatic masses and assessing early changes in chronic pancreatitis,[8,9,10,11] and it has also been applied for revealing parenchymal and ductal changes of AIP.[12,13,14] However, the role of EUS characteristics in differentiating FAIP from PC has rarely been fully evaluated. The aim of the present study is by comparing the EUS features between FAIP and PC, to construct a prediction model for distinguishing FAIP from PC and further validate its efficacy.
PATIENTS AND METHODS
Patients
Two hundred and seventeen patients with AIP (90 FAIP patients and 127 DAIP patients) and 197 patients with pancreatic head cancer, who consecutively underwent EUS before the initiation of steroid therapy at our center from January 2013 to December 2018, were retrospectively included in the present study. The diagnosis of AIP met the revised Mayo clinic criteria (revised HISORt criteria) including features of histology, imaging, serology, other organs involvement, and response to steroid therapy.[15] The diagnosis of pancreatic duct adenocarcinoma was confirmed by surgical pathology or by cytology/histology after EUS-guided fine-needle aspiration or biopsy. The EUS examination was performed by experienced endosonographers with a radial or linear echoendoscope (GF-UM2000, GF-UCT260 or GF-UE260, Olympus, Tokyo, Japan) and ultrasonic processing system (EU-M2000, EU-ME1 or EU-ME2, Olympus, Tokyo, Japan; or ProSound α5, Aloka, Tokyo, Japan). EUS characteristics of the patients with AIP were compared with those of the PC patients. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was approved by the Ethics Committee of our institution.
EUS findings
The parenchymal and ductal changes of the pancreas were defined according to the Rosemont criteria.[9] The parenchymal characteristics included hyperechoic foci/strands and lobularity, and the ductal changes included main pancreatic duct (MPD) dilation. Other EUS characteristics not included in the conventional criteria were described based on the literature, including pancreatic diffuse hypoechogenicity, focal hypoechogenicity, pancreatic diffuse enlargement, focal enlargement, peripancreatic hypoechoic margin, CBD dilation, bile duct wall thickening, lymphadenopathy, and vessel involvement.[13,14] The definition criteria of the above EUS features are shown in Table 1.
Table 1.
The definition criteria of EUS characteristics
| EUS characteristics | Definitions |
|---|---|
| Diffuse hypoechogenicity | Reduced echogenicity involving>1/2 of pancreas (head/body, body/tail, or entire pancreas) |
| Focal hypoechogenicity | Reduced echogenicity involving≤1/2 of pancreas (head, body or tail) |
| Diffuse enlargement | Enlargement involving>1/2 of pancreas (head/body, body/tail or entire pancreas) |
| Focal enlargement | Enlargement involving≤1/2 of pancreas (head, body, or tail) |
| Peripancreatic hypoechoic margin | A capsule-like hypoechoic rim surrounding pancreas |
| Common bile duct dilation | Common bile duct diameter≥8 mm |
| Bile duct wall thickening | The hypoechoic intermediate layer of the bile duct is clearly thickened |
| Lymphadenopathy | Lymph node diameter≥8 mm with hypoechoic texture |
| Vessel involvement | Loss of interface between the pancreas and vessels of portal system |
Construction of the prediction model
Enrolled patients (90 FAIP patients and 197 PC patients) were randomly divided into the derivation and validation samples using 1:1 allocation [Figure 1]. In the derivation sample, the above 10 EUS characteristics were included in a multivariate stepwise logistic regression analysis to examine the correlation between EUS features and pancreatic diseases, and the correlation strength was assessed by odds ratios (OR) and 95% confidence intervals (CI). Points were assigned to each predictor based on the number of OR value minus 1. Individual risk estimates were based on the sum of weighted scores for each variable. Receiver operating characteristic (ROC) analysis was performed to evaluate the prediction power of the model based on area under the ROC curve (AUC), and the optimal cutoff points were obtained when Youden index reached maximum.
Figure 1.
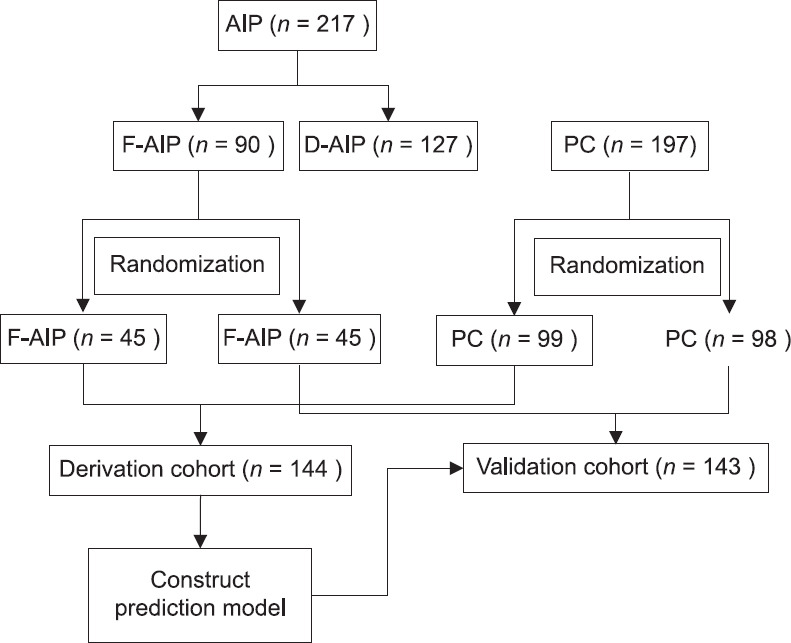
Flow diagram of the patient enrollment
Validation of the prediction model
Prediction model constructed in the derivation sample was applied to an independent validation sample to validate and assess the prediction efficacy. Sensitivity and specificity were used to assess the prediction efficacy.
Statistical analysis
Statistical analysis was performed using the SAS9.4 software package (SAS institute Inc., Cary, NC, USA). Continuous data were described by mean ± standard deviation and categorical data were described with number and percentage. Continuous data were compared using the t-test and categorical data were compared using the Chi-square test and Fisher's exact probability test. To control potential confounders, multivariate stepwise logistic regression analysis was conducted to identify the predictors and OR with a 95% CI excluding 1.00 was considered as statistically significant. A two-tailed P < 0.05 was defined as statistically significant.
RESULTS
Clinical characteristics of autoimmune pancreatitis and pancreatic cancer
The age distribution was slightly lower in patients with AIP than in those with PC and male sex was more frequent in AIP group than in PC group. Obstructive jaundice occurred in about half of patients in both groups and the frequencies did not differ significantly. On the basis of morphologic patterns in computed tomography and magnetic resonance imaging, patients with AIP can be divided into two types: diffuse enlargement (DAIP) and focal enlargement (FAIP). Among the 217 patients with AIP, 127 patients (58.5%) presented with diffuse enlargement with a typical “sausage-like” appearance (DAIP); 90 patients (41.5%) appeared as focal enlargement with a mass-like pancreatic head (FAIP). All 197 patients with PC presented with focal mass or focal enlargement [Table 2].
Table 2.
Clinical characteristics of autoimmune pancreatitis and pancreatic cancer
| Clinical characteristics | AIP (n=217) | PC (n=197) | P* |
|---|---|---|---|
| Age (years), median (range) | 59 (15–82) | 61.5 (36–88) | 0.0206 |
| Sex (male/female) | 174/43 | 107/90 | <0.0001 |
| Jaundice, n (%) | 133 (61.3) | 113 (57.4) | 0.4161 |
| Type of pancreatic form, n (%) | |||
| Diffuse enlargement | 127 (58.5) | 0 | - |
| Head/body | 0 | 0 | - |
| Body/tail | 12 (9.4) | 0 | - |
| Head/body/tail | 115 (90.6) | 0 | - |
| Focal enlargement or mass | 90 (41.5) | 197 (100) | - |
| Head | 90 (41.5) | 197 (100) | - |
| Body | 0 | 0 | - |
| Tail | 0 | 0 | — |
*t-test or Chi-square test. AIP: Autoimmune pancreatitis; PC: Pancreatic cancer
Comparison of characteristics between diffuse autoimmune pancreatitis and focal autoimmune pancreatitis
Patient age and gender ratio were similar between DAIP patents and FAIP patients, but obstructive jaundice was more common in FAIP patients compared to DAIP patients. For EUS characteristics, focal hypoechogenicity and MPD dilation were more frequent in FAIP patients, whereas diffuse hypoechogenicity was more common in DAIP patients. There was no significant difference noted in the frequency of other EUS features between two groups. It should be noted that among ninety patients with FAIP, 70 patients (77.8%) showed pancreatic diffuse hypoechogenicity, although the form of body and tail was not enlarged compared with a prominent enlargement of head; only 19 patients (21.1%) showed focal hypoechogenicity confined to head [Table 3].
Table 3.
Comparison of characteristics between diffuse autoimmune pancreatitis and focal autoimmune pancreatitis
| Clinical and EUS characteristics | DAIP (n=127), n (%) | FAIP (n=90), n (%) | P* |
|---|---|---|---|
| Age (years), median (range) | 59 (18–81) | 59 (15–82) | 0.8616 |
| Sex (male/female) | 100/27 | 74/16 | 0.5261 |
| Jaundice, n (%) | 68 (53.5) | 65 (72.2) | 0.0054 |
| Diffuse hypoechogenicity | 125 (98.4) | 70 (77.8) | <0.0001 |
| Focal hypoechogenicity | 0 | 19 (21.1) | <0.0001 |
| Hyperechoic foci/strands | 122 (96.1) | 85 (94.4) | 0.7445 |
| Lobularity | 35 (27.6) | 25 (27.8) | 0.9717 |
| Peripancreatic hypoechoic margin | 59 (46.5) | 30 (33.3) | 0.0528 |
| MPD dilation | 13 (10.2) | 23 (25.6) | 0.0028 |
| CBD dilation | 82 (64.6) | 69 (76.7) | 0.0563 |
| Bile duct wall thickening | 95 (74.8) | 73 (81.1) | 0.2735 |
| lymphadenopathy | 60 (47.2) | 47 (52.2) | 0.4699 |
| Vessel involvement | 24 (18.9) | 18 (20.0) | 0.8395 |
*t-test, Chi-square test or Fisher’s exact test. DAIP: Diffuse autoimmune pancreatitis; FAIP: Focal autoimmune pancreatitis; MPD: Main pancreatic duct; CBD: Common bile duct
Characteristics of focal autoimmune pancreatitis patients in the derivation and validation samples
The enrolled FAIP patients were randomly divided into either a derivation sample or a validation sample. The clinical and EUS characteristics were comparable between deviation sample and validation sample [Table 4].
Table 4.
Characteristics of focal autoimmune pancreatitis patients in the derivation and validation samples
| Clinical and EUS characteristics of FAIP | Derivation sample (n=45), n (%) | Validation sample (n=45), n (%) | P* |
|---|---|---|---|
| Age (years), median (range) | 59 (27–82) | 60 (15–79) | 0.5405 |
| Sex (male/female) | 38/7 | 36/9 | 0.5814 |
| Jaundice, n (%) | 35 (77.8) | 30 (66.7) | 0.2393 |
| Diffuse hypoechogenicity | 37 (82.2) | 33 (73.3) | 0.3105 |
| Focal hypoechogenicity | 8 (17.8) | 11 (24.4) | 0.4384 |
| Hyperechoic foci/strands | 41 (91.1) | 44 (97.8) | 0.3607 |
| Lobularity | 13 (28.9) | 12 (26.7) | 0.8139 |
| Peripancreatic hypoechoic margin | 17 (37.8) | 13 (28.9) | 0.3711 |
| MPD dilation | 8 (17.8) | 15 (33.3) | 0.0907 |
| CBD dilation | 31 (68.9) | 38 (84.4) | 0.0811 |
| Bile duct wall thickening | 38 (84.4) | 35 (77.8) | 0.4191 |
| lymphadenopathy | 24 (53.3) | 23 (51.1) | 0.8329 |
| Vessel involvement | 11 (24.4) | 7 (15.6) | 0.2918 |
*t-test, Chi-square test or Fisher’s exact test. FAIP: Focal autoimmune pancreatitis; MPD: Main pancreatic duct; CBD: Common bile duct
Characteristics of pancreatic cancer patients in the derivation and validation samples
The enrolled PC patients were randomized into either the derivation sample or the validation sample. No significant differences were seen in the clinical and EUS characteristics between derivation sample and validation sample [Table 5].
Table 5.
Characteristics of pancreatic cancer patients in the derivation and validation samples
| Clinical and EUS characteristics of PC | Derivation sample (n=99), n (%) | Validation sample (n=98), n (%) | P* |
|---|---|---|---|
| Age (years), median (range) | 63 (36–88) | 59.5 (36–80) | 0.1851 |
| Sex (male/female) | 55/44 | 52/46 | 0.7253 |
| Jaundice, n (%) | 60 (60.6) | 53 (54.1) | 0.3545 |
| Diffuse hypoechogenicity | 2 (2.0) | 6 (6.1) | 0.1696 |
| Focal hypoechogenicity | 97 (98.0) | 92 (93.9) | 0.1696 |
| Hyperechoic foci/strands | 23 (23.2) | 13 (13.3) | 0.0703 |
| Lobularity | 1 (1.0) | 0 | 1.0000 |
| Peripancreatic hypoechoic margin | 0 | 0 | — |
| MPD dilation | 78 (78.8) | 86 (87.8) | 0.0920 |
| CBD dilation | 68 (68.7) | 61 (62.2) | 0.3417 |
| Bile duct wall thickening | 19 (19.2) | 14 (14.3) | 0.3565 |
| lymphadenopathy | 43 (43.4) | 35 (35.7) | 0.2679 |
| Vessel involvement | 58 (58.6) | 56 (57.1) | 0.8375 |
*t-test, Chi-square test or Fisher’s exact test. PC: Pancreatic cancer; MPD: Main pancreatic duct; CBD: Common bile duct
Comparison of EUS characteristics between focal autoimmune pancreatitis and pancreatic cancer in the derivation sample
Pancreatic diffuse hypoechogenicity, hyperechoic foci/strands, lobularity, peripancreatic hypoechoic margin and thickening of bile duct wall were more common in FAIP than in PC. On the contrary, focal hypoechogenicity, MPD dilation and vessel involvement were more common in PC than in FAIP. Frequencies of CBD dilation and lymphadenopathy were equivalent in two groups [Table 6].
Table 6.
Comparison of characteristics between focal autoimmune pancreatitis and pancreatic cancer in the derivation sample
| Clinical and EUS characteristics | FAIP (n=45), n (%) | PC (n=99), n (%) | P* |
|---|---|---|---|
| Age (years), median (range) | 59 (27–82) | 63 (36–88) | 0.0310 |
| Sex (male/female) | 38/7 | 55/44 | 0.0008 |
| Jaundice, n (%) | 35 (77.8) | 60 (60.6) | 0.0438 |
| Diffuse hypoechogenicity | 37 (82.2) | 2 (2.0) | <0.0001 |
| Focal hypoechogenicity | 8 (17.8) | 97 (98.0) | <0.0001 |
| Hyperechoic foci/strands | 41 (91.1) | 23 (23.2) | <0.0001 |
| Lobularity | 13 (28.9) | 1 (1.0) | <0.0001 |
| Peripancreatic hypoechoic margin | 17 (37.8) | 0 | <0.0001 |
| MPD dilation | 8 (17.8) | 78 (78.8) | <0.0001 |
| CBD dilation | 31 (68.9) | 68 (68.7) | 0.9807 |
| Bile duct wall thickening | 38 (84.4) | 19 (19.2) | <0.0001 |
| lymphadenopathy | 24 (53.3) | 43 (43.4) | 0.2697 |
| Vessel involvement | 11 (24.4) | 58 (58.6) | 0.0001 |
*t-test, Chi-square test or Fisher’s exact test. FAIP: Focal autoimmune pancreatitis; PC: Pancreatic cancer; MPD: Main pancreatic duct; CBD: Common bile duct
Construction of prediction model for differentiating focal autoimmune pancreatitis from pancreatic cancer
In the derivation sample, all EUS characteristics were included in a multivariate stepwise logistic regression analysis. The results indicated that diffuse hypoechogenicity, bile duct wall thickening, and hyperechoic foci/strands were three statistically significant predictors (95%CI for OR not including 1.00). A score was assigned to each predictor according to the number of OR value minus 1. For example, the OR value of the predictor “diffuse hypoechogenicity” was 347.0, then the weighted score was 347.0 − 1 = 346.0 if one patient presented without diffuse hypoechogenicity; otherwise the score was 0 if one patient with diffuse hypoechogenicity. The weighted score for bile duct wall thickening and hyperechoic foci/stands were 24.6 (with = 0, without = 24.6) and 8.9 (with = 0, without = 8.9) respectively. The prediction model (model 1) was based on the sum of weighted score for each of above three predictors. The predictive power of this model was assessed using ROC analysis and AUC was calculated to be 0.975 (95%CI, 0.959–0.990). The optimal cutoff value was obtained to be 350.5 based on Youden index, then the patient with a score ≥350.5 was diagnosed as PC, otherwise the patient with a score <350.5 was diagnosed as FAIP [Table 7].
Table 7.
Multivariate stepwise logistic regression and receiver operating characteristic analysis in derivation sample
| Predictors | OR | 95% CI | Weighted score (OR-1) | Area under ROC curve (95% CI) | Cutoff value | |
|---|---|---|---|---|---|---|
|
| ||||||
| With | Without | |||||
| Prediction model 1 | ||||||
| Diffuse hypoechogenicity | 347.0 | 36.8–>999.9 | 0 | 346.0 | 0.975 (0.959–0.990) | 350.5 |
| Bile duct wall thickening | 25.6 | 4.1–501.1 | 0 | 24.6 | ||
| Hyperechoic foci/strands | 9.9 | 1.9–80.1 | 0 | 8.9 | ||
| Prediction model 2† | ||||||
| MPD dilation | 41.3 | 7.2–450.1 | 40.3 | 0 | 0.951 (0.929–0.974) | 543.3 |
| CBD dilation | 16.6 | 1.9–314.0 | 15.6 | 0 | ||
| Bile duct wall thickening | 516.3 | 39.6–>999.9 | 0 | 515.3 | ||
| Hyperechoic foci/strands | 44.6 | 7.9–456.2 | 0 | 43.6 | ||
†Exclude the variables of diffuse/focal hypoechogenicity. OR: Odds ratio; CI: Confidence interval; ROC: Receiver operating characteristic; MPD: Main pancreatic duct; CBD: Common bile duct
Considering that the determination of diffuse or focal hypoechogenecity may be subjective, we additionally designed a prediction model (model 2), in which the above two EUS characteristics were excluded before conducting multivariate stepwise logistic regression analysis. The results showed that MPD dilation, CBD dilation, bile duct wall thickening, and hyperechoic foci/strands were the four independent predictors. The weighted score for MPD dilation, CBD dilation, bile duct wall thickening, and hyperechoic foci/stands were 40.3 (with = 40.3, without = 0), 15.6 (with = 15.6, without = 0), 515.3 (with = 0, without = 515.3) and 43.6 (with = 0, without = 43.6), respectively. The predictive performance of model 2 was also satisfactory with an AUC of 0.951 (95%CI, 0.929–0.974) and the optimal diagnostic threshold based on Youden index was 543.3, then the patient with a score ≥543.3 was diagnosed as PC, otherwise the patient with a score <543.3 was diagnosed with FAIP.
Efficacy of the prediction model in validation sample
Efficacy of the prediction model was evaluated in the validation sample. For the prediction model 1, if one patient presented with bile duct wall thickening and hyperechoic foci/strands, while diffuse hypoechogenicity was absent, the weighted score for each of three predictors was as follows: without diffuse hypoechogenicity = 346.0, with bile duct wall thickening = 0, with hyperechoic foci/strands = 0, calculating the sum of above three scores reaching a total point of 346.0 (346.0 + 0 + 0 = 346.0). Given that the accumulated score (346.0) was less than the optimal cutoff value of model 1 (350.5), then the patient was diagnosed with FAIP. For the prediction model 2, if one patient presented with MPD dilation, CBD dilation, and hyperechoic foci/strands, whereas bile duct wall thickening was absent, the weighted score for each of four predictors was as follows: with MPD dilation = 40.3, with CBD dilation = 15.6, with hyperechoic foci/strands = 0, without bile duct wall thickening = 515.3, calculating the sum of above four scores reaching a total point of 571.2 (40.3 + 15.6 + 0 + 515.3 = 571.2). Given that the accumulated score (571.2) was more than the optimal cutoff value of model 2 (543.3), then the patient was diagnosed with PC [Table 8].
Table 8.
Efficacy of prediction model in validation sample
| Patients | Prediction model 1 | Prediction model 2† | ||
|---|---|---|---|---|
|
|
|
|||
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| FAIP vs. PC (%) | 91.8 (0.864–0.973) | 95.6 (0.849–0.995) | 83.7 (0.764–0.910) | 93.3 (0.817–0.986) |
| FAIP with jaundice vs. PC with jaundice (%) | 87.5 (0.788–0.962) | 97.1 (0.851–0.999) | 78.6 (0.678–0.893) | 91.4 (0.769–0.982) |
| FAIP without jaundice vs. PC without jaundice (%) | 97.6 (0.874–0.999) | 90.0 (0.555–0.998) | 90.5 (0.774–0.973) | 100 (0.692–1.000) |
†Exclude the variables of diffuse/focal hypoechogenicity. FAIP: Focal autoimmune pancreatitis; PC: Pancreatic cancer, CI: Confidence interval
The results showed that the sensitivity and specificity of the prediction model 1 for diagnosing PC were 91.8% and 95.6%, respectively, and the prediction model 2 showed 83.7% sensitivity and 93.3% specificity. Further validation analysis of subgroups showed that the power of prediction model for patients with jaundice may be not as good as that for patients without jaundice.
DISCUSSION
AIP is regarded as a distinctive type of chronic pancreatitis. To date, no consensus about the diagnosis of AIP on EUS has been reached.[9,11,13] In this retrospective study, we found that 58.5% of AIP patients characteristically presented with DAIP, whereas 41.5% appeared as FAIP. For both, hyperechoic foci/strands or lobularity, termed parenchymal heterogeneity, on the background of diffuse hypoechogenicity was the most frequent EUS feature, while focal hypoechogenicity and MPD dilation were more common in FAIP compared to DAIP. In general, DAIP can be very different from PC based on its characteristic EUS findings, but distinguishing FAIP from PC is always challenging as EUS features can overlap.
By comparing EUS characteristics between FAIP [Figure 2] and PC [Figure 3], we found that diffuse hypoechogenicity, hyperechoic foci/strands, lobularity, peripancreatic hypoechoic margin, and bile duct wall thickening were more indicative of FAIP. On the contrary, focal hypoechogenictiy, MPD dilation, and vascular invasion were more characteristic of PC. In our study, 77.8% (70/90) of FAIP showed diffuse hypoechogenicity, which was characterized by hypoechotexture involving nonenlarged body and tail [Figure 2c]. It is presumed that besides head, pancreatic body and tail are also infiltrated by inflammation, rather than be really exempted. However, this hypothesis needs to be confirmed by histopathology. On the other hand, FAIP showed uniformly enlarged head with parenchymal heterogeneity on the background of hypoechogenicity [Figure 2a and b]. This differed from what was typically seen with PC, in which a solitary, irregular hypoechoic mass, lack of heterogeneous changes, could be observed at pancreatic head [Figure 3a and b], and the demarcation between the mass and surrounding parenchyma may be discerned [Figure 3c].[16] In addition, the involvement of bile duct in AIP was diffuse, homogeneous or profound [Figure 2a, b and d], while that in PC was focal, irregular or interrupted [Figure 3a and b].[17,18,19] Peripancreatic hypoechoic margin, with a high specificity but an insufficient sensitivity, was a characteristic EUS feature favoring FAIP, which was consistent with the literature reports.[13,20]
Figure 2.
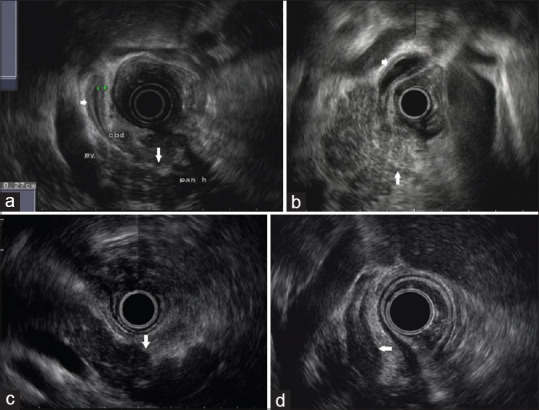
EUS characteristics of the patient with focal autoimmune pancreatitis. (a and b) EUS image showing a uniformly enlarged pancreatic head, which is characterized by hyperechoic foci/strands or lobularity (parenchymal heterogeneity) on the background of reduced echogenicity (long arrow) and a homogeneous, regular thickening of common bile duct wall (short arrow), which is characterized by a hyper-hypo-hyperechoic series of layers of the duct wall (sandwich type). (c) EUS image showing a nonenlarged pancreatic body and tail with parenchyma that presents with diffuse hypoechogenicity (arrow). (d) EUS image showing a profound thickening of common bile duct wall (arrow) that occupies the entire lumen with appearances of parenchymal echo (parenchymal-echo type)
Figure 3.
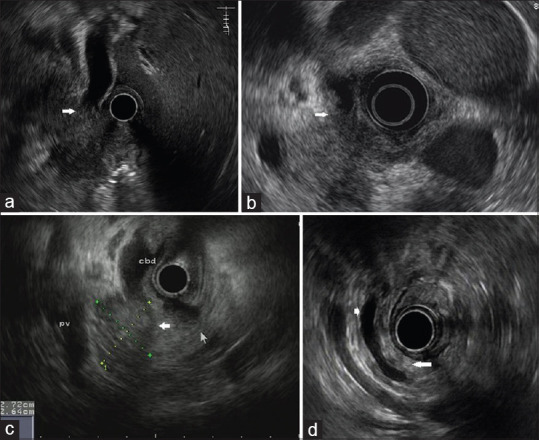
EUS characteristics of the patient with pancreatic cancer. (a) EUS image showing an irregular hypoechoic mass located in the pancreatic head and a dilated common bile duct which is suddenly interrupted by the mass (arrow). (b) EUS image showing an irregular hypoechoic lesion confined to pancreatic head and an asymmetric thickening of common bile duct wall (arrow). (c) EUS image showing a solitary hypoechoic mass in the pancreatic head with a discernable demarcation between the mass and surrounding parenchyma (wide arrow). (d) EUS image showing an isoechoic pancreatic body/tail (long arrow) and an ectatic main pancreatic duct (short arrow)
Hoki et al. reported a scoring system for differentiating EUS features between AIP and PC.[13] Small sample size and simple design were the limitations of this study, in which FAIP were not isolated from AIP and the weights of various EUS characteristics were not considered. To overcome the above shortcomings, a more objective scoring prediction model for differentiating FAIP from PC was constructed in our study. ROC analysis showed that AUC of the prediction model was more than 0.95. In validation sample, the model for diagnosing PC achieved a sensitivity ranging between 83.7% and 91.8% and a specificity ranging between 93.3% and 95.6%.
This study has some limitations. First, the role of biopsy was not considered throughout the model. EUS-guided biopsy is becoming the gold standard for the evaluation of any suspicious mass lesion in the pancreas, while it was not widely used in the early work. Moreover, given that our study focused on EUS characteristics, we worried that once biopsy was incorporated into the model, its heavier weight may obscure the role of other EUS features. Second, the determination of diffuse/focal hypoechogenecity is subjective and ambiguous. This is why we designed another prediction model, in which the two EUS characteristics were excluded. Third, our study is a retrospective single-center research, and more prospective multi-center studies are needed for external validation.
CONCLUSIONS
It is feasible to differentiate between FAIP and PC based on EUS characteristics. The prediction model constructed in this study needs to be confirmed by more prospective researches.
Financial support and sponsorship
Nil.
Conflicts of interest
Aiming Yang is an Editorial Board Member of Endoscopic Ultrasound. The article was subject to the journal's standard procedures, with peer review handled independently of this Member and his research groups. There are no other conflicts of interest.
Acknowledgments
The generous contribution and dedicated work of endoscopic staff for this study is gratefully acknowledged.
REFERENCES
- 1.Hart PA, Zen Y, Chari ST. Recent advances in autoimmune pancreatitis. Gastroenterology. 2015;149:39–51. doi: 10.1053/j.gastro.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Kanai K, Maruyama M, Kameko F, et al. Autoimmune pancreatitis can transform into chronic features similar to advanced chronic pancreatitis with functional insufficiency following severe calcification. Pancreas. 2016;45:1189–95. doi: 10.1097/MPA.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 3.Muhi A, Ichikawa T, Motosugi U, et al. Mass-forming autoimmune pancreatitis and pancreatic carcinoma: Differential diagnosis on the basis of computed tomography and magnetic resonance cholangiopancreatography, and diffusion-weighted imaging findings. J Magn Reson Imaging. 2012;35:827–36. doi: 10.1002/jmri.22881. [DOI] [PubMed] [Google Scholar]
- 4.Kamisawa T, Egawa N, Nakajima H, et al. Clinical difficulties in the differentiation of autoimmune pancreatitis and pancreatic carcinoma. Am J Gastroenterol. 2003;98:2694–9. doi: 10.1111/j.1572-0241.2003.08775.x. [DOI] [PubMed] [Google Scholar]
- 5.Miyabe K, Zen Y, Cornell LD, et al. Gastrointestinal and extra-intestinal manifestations of IgG4-related disease. Gastroenterology. 2018;155:990–1003. doi: 10.1053/j.gastro.2018.06.082. e1. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa T, Itoh A, Kawashima H, et al. Peripancreatic vascular involvements of autoimmune pancreatitis. J Gastroenterol Hepatol. 2012;27:1790–5. doi: 10.1111/j.1440-1746.2012.07248.x. [DOI] [PubMed] [Google Scholar]
- 7.Vlachou PA, Khalili K, Jang HJ, et al. IgG4-related sclerosing disease: Autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011;31:1379–402. doi: 10.1148/rg.315105735. [DOI] [PubMed] [Google Scholar]
- 8.Gardner TB, Levy MJ. EUS diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:1280–9. doi: 10.1016/j.gie.2010.02.038. [DOI] [PubMed] [Google Scholar]
- 9.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: The Rosemont classification. Gastrointest Endosc. 2009;69:1251–61. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Dewitt J, Devereaux BM, Lehman GA, et al. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: A systematic review. Clin Gastroenterol Hepatol. 2006;4:717–25. doi: 10.1016/j.cgh.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Sahai AV, Zimmerman M, Aabakken L, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- 12.Kubota K, Kato S, Akiyama T, et al. A proposal for differentiation between early- and advanced-stage autoimmune pancreatitis by endoscopic ultrasonography. Dig Endosc. 2009;21:162–9. doi: 10.1111/j.1443-1661.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoki N, Mizuno N, Sawaki A, et al. Diagnosis of autoimmune pancreatitis using endoscopic ultrasonography. J Gastroenterol. 2009;44:154–9. doi: 10.1007/s00535-008-2294-2. [DOI] [PubMed] [Google Scholar]
- 14.Farrell JJ, Garber J, Sahani D, et al. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60:927–36. doi: 10.1016/s0016-5107(04)02230-8. [DOI] [PubMed] [Google Scholar]
- 15.Chari ST, Takahashi N, Levy MJ, et al. A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clin Gastroenterol Hepatol. 2009;7:1097–103. doi: 10.1016/j.cgh.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Othman MO, Wallace MB. The role of endoscopic ultrasonography in the diagnosis and management of pancreatic cancer. Gastroenterol Clin North Am. 2012;41:179–88. doi: 10.1016/j.gtc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 17.De Lisi S, Buscarini E, Arcidiacono PG, et al. Endoscopic ultrasonography findings in autoimmune pancreatitis: Be aware of the ambiguous features and look for the pivotal ones. JOP. 2010;11:78–84. [PubMed] [Google Scholar]
- 18.Naitoh I, Nakazawa T, Ohara H, et al. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009;44:1147–55. doi: 10.1007/s00535-009-0108-9. [DOI] [PubMed] [Google Scholar]
- 19.Koyama R, Imamura T, Okuda C, et al. Ultrasonographic imaging of bile duct lesions in autoimmune pancreatitis. Pancreas. 2008;37:259–64. doi: 10.1097/MPA.0b013e31816b30e7. [DOI] [PubMed] [Google Scholar]
- 20.Palazzo M, Palazzo L, Aubert A, et al. Irregular narrowing of the main pancreatic duct in association with a wall thickening is a key sign at endoscopic ultrasonography for the diagnosis of autoimmune pancreatitis. Pancreas. 2015;44:211–5. doi: 10.1097/MPA.0000000000000242. [DOI] [PubMed] [Google Scholar]


