Abstract
Pancreatic cystic neoplasms (PCNs) are being detected increasingly frequently due to the widespread use of high-resolution abdominal imaging modalities. Some subtypes of PCNs have the potential for malignant transformation. Therefore, accurate diagnosis of PCNs is crucial to determine whether surgical resection or surveillance is the best management strategy. However, the current cross-section imaging modalities are not accurate enough to enable definite diagnoses. In the last decade, EUS-based techniques have emerged, aiming to overcome the limitations of standard cross-section imaging modalities. These novel EUS-based techniques were primarily designed to acquire distinct images to make radiological diagnoses, collect cyst fluid to undergo biochemical or molecular analyses, and obtain tissue to conclude the pathological diagnoses. In this article, we present a comprehensive and critical review of these emerging EUS techniques for the diagnosis of PCNs, with emphasis being placed on the advantages, feasibilities, diagnostic performances, and limitations of these novel techniques.
Keywords: pancreatic cystic neoplasm, contrast-enhanced harmonic, EUS, confocal laser endomicroscopy, cystoscopy, cyst fluid analysis, biopsy
INTRODUCTION
Pancreatic cystic neoplasms (PCNs) are being detected more frequently due to advances in radiological technologies. The prevalence of PCNs ranges from 1.9% to 49.1% in different races.[1,2,3] PCNs comprise a broad spectrum of tumors. In general, PCNs can be classified into four primary types: serous cystic neoplasms (SCNs), mucinous cystic neoplasms (MCNs), intraductal papillary mucinous neoplasms (IPMNs), and solid pseudopapillary neoplasms (SPNs). Nearly 90% of all PCNs consist of these four pathological types.[4] IPMNs are subcategorized into main duct IPMNs (MD-IPMNs), branch duct IPMNs (BD-IPMNs), and mixed IPMNs, according to the different types of pancreatic duct that is connected to the cysts. However, PCNs have varied biological behaviors. SCNs are benign tumors; only symptomatic SCNs require further management, and they are very rare.[5] SPNs are tumors with low potential for malignant, and surgical resection is required once this tumor is identified.[6] IPMNs and MCNs are mucinous neoplasms, which represent the tumors that have the potential for malignant transformation.[7]
If a PCN is identified, two characteristics should be verified. First, the biological nature of the cyst should be identified; in other words, we should know whether the cyst is malignant or benign. This characteristic is of crucial importance, as it influences the treatment strategy (surgical resection or surveillance). Second, if the cyst is benign, the malignant potential should be verified. In other words, we should know whether the cyst is mucinous or nonmucinous. This characteristic may influence the surveillance strategy. Conventionally, computerized tomography (CT) or magnetic resonance imaging (MRI) is applied to evaluate PCNs. Even with high-quality imaging, the correct classification of the cyst type can be challenging. Since no direct modality could predict the malignant transformation of a PCN, the imaging feature evaluation was adopted to predict the possibility of malignancy indirectly by the current guidelines. For example, the presence of mural nodules, dilated main pancreatic duct (MPD), and cyst size are important predictors of malignant PCNs. However, even a mural nodule measuring ≥5 mm on EUS has a sensitivity of 73%–85% and a specificity of 71%–100%.[8,9,10,11] As a result, the diagnostic accuracies of imaging modalities remain imperfect. The accuracy of CT for differentiating benign from malignant cysts was 71%–80%. CT was able to assess communication between the MPD and the cyst, with 80% sensitivity in distinguishing IPMNs vs. other cyst types. The accuracy of MRI for distinguishing a benign from a malignant cyst ranged from 55% to 76%, with 96% sensitivity for diagnosing an IPMN from other cyst types.[12] Thus, CT/MRI is imperfect in identifying the exact type of PCN. As a result, the management of MCNs and IPMNs represents the key and difficult points in the clinical practice of PCNs.[7]
EUS is recommended as an adjunct to other imaging modalities.[13] EUS was reported to have the ability to detect intracyst microstructures, for example, mural nodules, thickened septa, or mucous plugs.[5,14,15] However, EUS imaging alone was reported to have an accuracy of 65%–96% for differentiating a benign from a malignant cyst, which was similar to the accuracy of MRI and CT.[14] As a result, many techniques have been described to improve the ability of EUS to identify the true risk for a PCN. In this review, we describe the current novel EUS-based techniques in the evaluation of PCNs, primarily focusing on their advantages, feasibilities, diagnostic performances, and limitations. These techniques include imaging techniques (contrast-enhanced harmonic EUS [CE-EUS], through-the-needle confocal laser endomicroscopy, and through-the-needle cystoscopy), cyst fluid analyses (tumor biomarker analyses, biochemical analyses, and molecular and proteomic analyses), and through-the-needle tissue acquisition techniques.
CONTRAST-ENHANCED HARMONIC EUS
CE-EUS has a better ability to detect mural nodules.[16] The improved ability may be attributable to the injected second-generation ultrasound contrast agents (Sonazoid or SonoVue), which can detect microcirculation with better resolution and fewer artifacts than Doppler EUS images. Fujita et al. reported 21 patients with IPMNs who were suspected of having mural nodules and scheduled for surgical resection initially. The patients underwent CE-EUS, and four hypervascular lesions were identified. The patients with avascular lesions were diagnosed with mucous plugs and avoided undergoing unnecessary surgery.[17] However, the sample size for this study was small. Zhong et al.[18] divided the CE-EUS imaging mode into five types. These researchers concluded that the nonenhancement type, hypoenhancement type, and mixed type were associated with malignancy. The diagnostic accuracy was over 92%, which was significantly higher than CT/MRI/EUS. However, the classification mode is subjective, which limits its utilization. A total of 70 studies and 2297 resected IPMNs were included in a systematic review and meta-analysis conducted by Marchegiani et al.[19] These researchers concluded that mural nodule size, as measured by CE-EUS, had a considerable effect on predicting malignant IPMNs, with a pooled standardized mean difference of 0.79. In addition to the ability to detect mural nodules, a recent study conducted by Ohno et al.[20] concluded that CE-EUS could predict MPD involvement with a sensitivity, specificity, and accuracy of 83.5%, 87.0%, and 84.9%, respectively. However, only 71.6% of the malignant cases had MPD involvement.[20] Therefore, the value of this ability for CE-EUS needs to be further evaluated.
In general, due to its favorable ability in evaluating mural nodules, CE-EUS was recommended by the European evidence-based guidelines for the further evaluation of suspected mural nodules and vascularity within the cyst and septations.[13] Moreover, the impact of interobserver agreement should not be ignored. The interobserver agreement is favorable for Sonazoid and moderate for SonoVue.[21,22]
EUS-GUIDED FINE NEEDLE-BASED CONFOCAL LASER ENDOMICROSCOPY
EUS-guided needle-based confocal laser endomicroscopy enables visualization of the in vivo imaging of the epithelium of the cyst. The fluorescein is injected intravenously before EUS-nCLE, and an nCLE miniprobe is advanced into the cyst through a 19G needle. Intracystic epithelial and vascular image patterns are captured to identify the specific type of PCN. EUS-nCLE provides an optical biopsy, which can partially replace the cytological biopsy [Figure 1].
Figure 1.
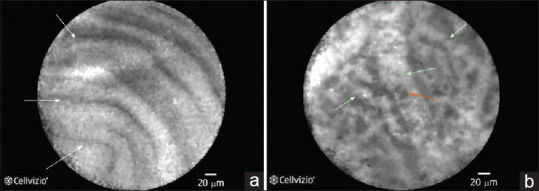
(a) EUS-guided fine nCLE image of an IPMN displaying multiple papillary projections. (b) EUS-nCLE image of the superficial vascular network pattern of a serous cystadenoma. Multiple interconnected vessels (white arrows). Red cells inside displayed as black structures (orange arrow). nCLE: Needle-based confocal laser endomicroscopy
The diagnostic criteria for EUS-nCLE were not established until 2015. A French multicenter study concluded that the “superficial vascular network” observed by nCLE was a unique feature of SCNs.[23] The sensitivity and specificity of the nCLE-based diagnosis of SCNs were 69% and 100%, respectively. In their phase 2 study, this group described nCLE patterns for MCNs, pseudocysts, and cystic neuroendocrine tumors (NETs).[24] The “finger-like papillae with outer epithelium (dark) and inner vascular core (white)” pattern was a feature of IPMNs. The “horizontal horizon-type epithelial bands” pattern was a feature of MCNs. The “dark background (no vasculature) with bright speckles (inflammatory cells)” pattern was a feature of pseudocysts. A trabecular pattern of cell clusters separated by the cyst stroma was a feature of cystic NETs. Recently, these researchers validated these patterns in a cohort of 78 patients. The sensitivity and specificity of EUS-nCLE to distinguish premalignant pancreatic cysts (SPNs, BD-IPMNs, cystic NETs, and MCNs) from benign lesions were 96% and 95%, respectively. To differentiate mucinous from nonmucinous lesions, the sensitivity and specificity were 95% and 100%, respectively.
In other centers, the diagnostic ability of EUS-nCLE was also favorable. In a Chinese center, Feng et al. adopted a pattern for malignant PCNs with “dark aggregates of neoplastic cells.”[25] The accuracy, sensitivity, and specificity of this feature for the diagnosis of malignant PCNs were 94%, 75%, and 100%, respectively. In a US center, Antonio et al. concluded that the diagnostic yield for nCLE was 84.1%, which was significantly higher than current “composite standard” (clinical, morphological, cyst fluid cytology, and chemical analyses).[26]
However, there are limitations to the widespread utilization of EUS-nCLE. First, due to the insufficient number of subjects included in studies and the lack of prospective research, the evidence to support the use of nCLE in the diagnostic algorithm of PCNs is limited.[27] Second, the interobserver agreement may also strongly influence the diagnostic accuracy.[28] Third, this novel technology is expensive, and EUS-nCLE adds to the cost of management of PCNs. Although total costs in both the public and private sectors will be decreased due to the reduction in the frequency of surgical interventions after EUS-nCLE,[23,29] individual patients may be reluctant to spend additional funds that are not covered by insurance. As a result, EUS-nCLE was not adopted by the current guidelines in the management of PCNs. Further prospective studies with large sample sizes are warranted to establish the indication and position of EUS-nCLE.
EUS-GUIDED THROUGH-THE-NEEDLE CYSTOSCOPY
The principle for cystoscopy is similar to that for nCLE. Cystoscopy is a procedure that enables direct visualization of the contents of cysts and the inner cyst wall by means of a single-operator cholangioscopy fiberoptic probe (Spyglass). The probe is introduced through a 19G needle into the cyst.
In a retrospective study, Chai et al. performed through-the-needle cystoscopy in 43 patients.[30] These researchers concluded that a tree-like branching pattern of blood vessels may suggest the diagnosis of an SCN (sensitivity, 69%; specificity, 91%) and that intracystic papilla-like structures may be characteristic of mucinous cysts (sensitivity, 22%; specificity, 92%). However, only 55.6% of the patients had a clear background. Adverse events were rare, no pancreatitis was observed, and only two patients presented with mild abdominal postprocedure pain.
In the prospective DETECT study, Nakai et al. performed cystoscopy followed by nCLE in 30 patients.[31] The quality of images was rated as fair or poor in 33% of patients, while the rate of fair or poor image quality for nCLE was 10%. Mucinous fluid was described as having a viscous, cloudy appearance. The sensitivity of cystoscopy in detecting mucinous cysts was 90%. When cystoscopy and nCLE were combined, the sensitivity increased from 90% to 100%. Postprocedure, pancreatitis was reported twice.
The images obtained by cystoscopy tend to be vague, and the sensitivity in diagnosing mucinous cysts is suboptimal. Moreover, the data of cystoscopy in diagnosing malignant cysts are scarce. Similar to nCLE, the cost of cystoscopy is also high. As a result, cystoscopy has a lower position than nCLE in the management of PCNs.
CYST FLUID TUMOR BIOMARKER AND CHEMICAL ANALYSIS
Cyst fluid cytology and tumor markers
The cyst fluid was obtained by EUS-fine needle aspiration (FNA). Conventionally, the evaluation of cyst fluid included cytological analysis to differentiate benign from malignant PCNs and tumor biomarker analysis to differentiate mucinous from nonmucinous PCNs. However, the diagnostic ability of EUS-FNA remains suboptimal.
The cytology in the cyst fluid is highly specific (83%–100%) in identifying malignant cysts, but it is relatively insensitive (27%–48%) in identifying malignant cysts, resulting in a low diagnostic accuracy (8%–59%).[32,33,34,35] Repeat EUS-FNA may improve the sensitivity up to 20%, though only in some specific subtypes (e.g., cystic NETs).[36]
CEA in the cyst fluid is considered to have the highest accuracy rate to differentiate mucinous cysts from nonmucinous cysts, but the cutoff level varies depending on reports. The most widely acknowledged cutoff level was 192 ng/mL, with a sensitivity of 52%–78% and specificity of 63%–91%.[33,34,37,38,39,40] In addition to the ability of identifying mucinous cysts, CEA >800 ng/mL was thought to be a marker for diagnosing malignant cysts with a suboptimal sensitivity of 48%.[41] Other tumor markers (CA19-9, CA724, CA125, and CA153) were also evaluated and were found to have a lower sensitivity in identifying mucinous cysts compared to that with CEA.[34,42,43] CA125 combined with CEA has the ability to differentiate MCNs from other cyst subtypes.[44] Cyst fluid amylase levels have been reported to be higher in IPMNs and pseudocysts compared to those in PCNs (<250 U/L).[41] Cyst fluid viscosity was considered to be a delineating marker for differentiating mucinous from nonmucinous PCNs. The diagnostic accuracy, sensitivity, and specificity for cyst fluid viscosity were 81.8%, 70%, and 91.7%, respectively.[45]
Cyst fluid glucose
Recently, the cyst fluid glucose level was expected to replace CEA in diagnosing mucinous cysts. Glucose measurement is simple, rapid, inexpensive, and requires only a little volume of the cyst fluid. Park et al.[46] first found that glucose levels were significantly lower in mucinous cysts (5 vs. 82 mg/dL, P = 0.002). The best performance for glucose level was observed by using a cutoff of 66 mg/dL, with a sensitivity, specificity, and accuracy of 94%, 64%, and 84%, respectively. The diagnostic accuracy was comparable to CEA (84% vs. 77%). In this study, kynurenine was also identified as another marker that can discriminate between mucinous and nonmucinous cysts with a sensitivity of 90%. These researchers validated these findings, with a larger cohort 2 years later.[47] The cutoff level for glucose level was set at 50 mg/mL in this study. The sensitivity and specificity reached 88% and 78%, respectively. Meanwhile, the CEA cutoff of 192 ng/mL had a sensitivity and specificity of 73% and 89%, respectively, in this study. Later, several studies demonstrated that cyst fluid glucose level performs better than CEA in differentiating mucinous from nonmucinous cysts.[48,49,50] However, similar to CEA, cyst fluid glucose level was unable to diagnose malignant PCNs. Therefore, cyst fluid glucose level is a promising biomarker in diagnosing mucinous cysts.
CYST FLUID MOLECULAR ANALYSIS
Molecular analysis of the cyst fluid obtained by EUS-FNA has become a promising modality for the differentiation of PCNs. The molecular analysis included DNA, RNA, protein, and metabolomic markers.
DNA markers
The cell-free supernatant in the cyst fluid contains DNA that can be analyzed.[51] Mutations in KRAS are thought to be early events in the biogenesis of IPMNs, as it is found in all IPMN subtypes.[52] Mutant KRAS in the cystic fluid was found to be highly specific (92%–96%) for mucinous cyst diagnosis but with low sensitivity (33%–45%).[53] Similarly, high amplitude KRAS mutations were able to detect malignancy with high specificity (96%) but low sensitivity (45%).[40] Cysts with high-grade dysplasia were found to have more KRAS mutations, as well as a higher risk of progression.[52] Thus, KRAS mutations may act as a marker for poor prognosis, rather than a cancer detection marker.
GNAS code is another important molecular marker. GNAS mutation is detected in 61% of IPMNs, but its presence does not correlate with clinical outcome.[54] When analyzed in combination with KRAS mutations, 96% of IPMNs were positive for at least one of the oncogenes.[55] In cyst fluid, either GNAS or KRAS mutation had a sensitivity and specificity of 65% and 100%, respectively, for mucinous differentiation.[56] The detection ability could be improved by the application of next-generation sequencing (NGS).[57,58,59]
Recently, the novel methylated DNA markers (TBX15 and BMP3) were described as accuracy markers in the diagnosis of malignant PCNs. The area under the receiver operating characteristic curve was 0.93, which was significantly higher than KRAS and CEA.[60] Moreover, the GNAS locus methylation change is associated with malignancy, and the sensitivity and specificity were 75% and 90%, respectively.[61] A study by Gaiser et al.[62] concluded that detection of oral bacteria DNA sequences (Fusobacterium nucleatum and Granulicatella adiacens) is an early marker for the progression of IPMNs. These researchers also concluded that a reduction in the pancreatic inflammatory microbiome may represent a therapeutic strategy for IPMNs.[62]
In addition to the DNA markers mentioned above, other DNA mutations (BRAF, CDKN2A, CTNNB1, NRAS, PIK3CA, RNF43, SMAD4, TP53, and VHL) were also reported to be able to achieve accurate classification of PCNs.[63] In contrast to detect malignant cases, the KLF4 mutations are more prevalent in low-grade IPMNs, either in tissue samples or in cyst fluid samples.[64]
RNA markers
Numerous RNA markers (micro-RNA, noncoding RNA, long noncoding RNA, and single-cell RNA sequencing) in resected specimens, pancreatic juice, and serum have been identified to differentiate low- and high-risk IPMNs,[65,66,67,68,69,70,71] but we will not discuss this in the present review. Notably, scarce research has focused on the value of these RNA markers in the tissue obtained by EUS-FNA. In cyst fluid, micro-RNA 21 (miR-21) was reported to have the ability to differentiate between mucinous and nonmucinous cystic lesions with 80% sensitivity and 76% specificity.[72] Another study concluded that the combination of miR-21 and miR-221 is indicative of malignancy in PCNs.[73] Matthaei et al.[74] found that nine microRNAs (miRNAs) in the cyst fluid were able to accurately identify cysts requiring resection vs. observation, obtaining a sensitivity and specificity of 89% and 100%, respectively. However, the conclusion was contradicted by Utomo et al., who achieved only 10% sensitivity.[75] The NGS from the cystic fluid differentiating low- and high-risk IPMNs yielded a panel of 13 miRNAs.[76]
Protein markers
Proteomic cystic fluid analysis for differentiating mucinous cysts and defining the risk of malignancy is of high accuracy.[77] However, proteomic analysis is even more complicated than molecular analysis. Ke et al. identified amylase isoenzymes, mucins (MUC1, MUC5AC, MUC5B, and MUC16), CEACAM family members (CEACAMs 5, 6, and 7), and S100 homologs that provide valuable information on the invasive potential of a pancreatic cyst.[78] A study by Jabbar et al. identified eight biomarker candidates for malignant potential and high-grade dysplasia/cancer by an explorative proteomic approach. Mucin-5AC and mucin-2 were identified as the optimal markers to discriminate premalignant/malignant lesions from benign lesion, with an accuracy of 97%.[79] A combination of mucin-5AC and prostate stem cell antigen could identify high-grade dysplasia/cancer with an accuracy of 96% (95% confidence interval, 90%–99%) and detected 95% of malignant/severely dysplastic lesions.[79] Another study identified olfactomedin-4 was associated with the presence of MCNs and IPMNs.[80] The mass to charge (m/z) ratio was observed to be different in malignant and benign IPMNs. Five protein peaks were identified that were highly accurate in discriminating malignant IPMNs.[81] The general inflammatory marker interleukin-1 was predictive for high-risk IPMNs, with a sensitivity and specificity of 79% and 95%, respectively.[82] The vascular endothelial growth factors (VEGF)-A and VEGF-C were found to be significantly increased in the cyst fluid from benign SCNs compared to those in MCNs.[83] Other protein markers (tissue polypeptide antigen, SPINK1, claudins, mAb Das-1, and plectin-1) in the cyst fluid were reported to have the ability to differentiate PCNs, but further validation is needed.[84,85,86,87,88] In conclusion, the proteomic analysis in the cyst fluid is useful in differentiating malignant from benign PCNs, as well as mucinous from nonmucinous cysts. However, due to high numbers and variability, the proteins tested display a lack of one definitive marker capable of accurate diagnoses.[89]
The limitation of molecular and proteomic analyses was similar to those of EUS-nCLE. The expensive molecular and proteomic analyses can only be performed in a small number of academic institutions. Moreover, although there are useful markers that have been evaluated for the differentiation of PCNs, as we mentioned above, some subtypes lack specific markers such as cystic NETs. Since it is impossible to do all molecular and proteomic tests at the same time for one single cyst, the diagnostic criteria should be established, and the indications for each molecular and proteomic marker should be verified.
The cyst fluid markers and their ability to differentiate mucinous from nonmucinous cysts are summarized in Table 1. The other markers employed to differentiate benign from malignant cysts are summarized in Table 2.
Table 1.
Primary cystic fluids markers that identify mucinous and non-mucinous cysts
| Marker | Cyst type | Cut-off | Sensitivity/Specificity (%) |
|---|---|---|---|
| CEA | Mucinous | >192 ng/mL | 73/84 |
| CA125 | MCN | >10.0 U/ml | 94.4/81.3 |
| CA19-9 | Mucinous | >50,000 U/mL | 75/90 |
| Amylase | Pseudocyst | >250 U/mL | 44/98 |
| Cyst fluid viscosity | Mucinous | 1.3cP | 70/91.7 |
| Glucose | Mucinous | <50 mg/mL | 88/78 |
| KRAS combined with GNAS mutation | Mucinous | NA | 65/100* |
| GNAS mutation | IPMN | NA | 98/100* |
| miR-21 | Mucinous | NA | 80/76* |
| VEGF-A | Serous | >8,500 pg/mL | 100/97 |
| VEGF-C | Serous | >200 pg/mL | 100/90 |
| MUC5AC + endorepellin | Mucinous | NA | 92/94 |
| MUC5AC + CA19-9 | Mucinous | NA | 87/86 |
| Kynurenine | Mucinous | NA | 90/100 |
*The sensitivity and specificity can be improved by next generation sequencing. NA: Not available; CA: Cancer antigen; CEA: Carcinoembryonic antigen; KRAS: Kirsten rat sarcoma viral oncogene homolog; GNAS: Guanine nucleotide-binding protein; MUC: Mucin; VEGF: Vascular endothelial growth factor; IPMN: Intraductal papillary mucinous neoplasms; MCN: Mucinous cystic neoplasms
Table 2.
Primary cyst fluid markers for malignant pancreatic cystic lesions
| Maker | Cyst type | Cut-off | Sensitivity/Specificity (%) |
|---|---|---|---|
| Cytology | Malignant | NA | 27-48/83-100 |
| Cell block technique | Malignant | NA | 81/100 |
| CEA | Malignant | >800 ng/ml | 48/98 |
| miR-21 plus miR-221 | Malignant | NA | NA |
| miRNA panel (miR-24, 30a-3p, 18a, 92a, 342-3p, 106b, 142-3p, 532-3p) | Malignant | NA | 89/100 |
| MUC5AC plus MUC2 | Premalignant | 0.01 sum | 97/100 |
| MUC5AC plus PSCA | Malignant | 12 sum | 95/100 |
| IL-1 | Malignant | NA | 79/95 |
| mAb Das-1 | Malignant | Optical density 0.104 | 88/98 |
| Novel methylated DNA markers (TBX15, BMP3) | Malignant | NA | 90/92 |
| GNAS locus methylation change | Malignant | NA | 75/90 |
| KRAS mutation | Malignant | NA | 45/96 |
NA: Statistically significant but Sensitivity/Specificity not reported. NA: Not available; CEA: Carcinoembryonic antigen; MUC: Mucin; IL: Interleukin; KRAS: Kirsten rat sarcoma viral oncogene homolog; GNAS: Guanine nucleotide-binding protein; miR: microRNA; PSCA: Prostate stem cell antigen
NOVEL EUS-GUIDED TISSUE ACQUISITION TECHNIQUES
Cell block technique
In 2019, Newtown et al. described the cell block technique to process the specimen from the cyst fluid obtained by EUS-FNA. Cell block preparations are two times more likely to diagnose MCNs than are direct smears and fluid CEA biochemistry.[90] Cell block techniques may be a better specimen processing method than standard smear cytology. However, the data supporting the cell block technique as a routine process are scarce, which limits the wide utilization of the technique.
Brush cytology
To process brush cytology, the brushing device is introduced through a 19G needle under EUS guidance, and cells from the cyst wall were brushed and collected. In the study by Sendino et al.,[91] employing this technique, 50% of mucinous cells vs. 18% with standard EUS-FNA were identified. Al-Haddad et al.[92] reported that brush cytology is more likely to provide an adequate mucinous epithelium specimen (62%) than standard FNA (23%). The conclusion was similar to a study by Lozano et al.[93] Diagnostic material was obtained in 85.1% of the patients undergoing brush cytology compared with 66.3% of the patients undergoing EUS-FNA. However, in these studies, approximately 8%–10% of patients had postprocedural complications. One case died from retroperitoneal bleeding. As a result, the routine use of brush cytology is not permitted in clinical practice.
Targeted cyst wall puncture
This technique utilizes a standard FNA/FNB needle and introduces the needle inside the cyst followed by aspiration and decompression of the cyst. Next, the far wall of the cyst is punctured, and the needle is moved back and forth through the wall to collect epithelium cells.
In a study by Hong et al., a 22G standard FNA needle was applied.[94] Cellular material adequate for cytological evaluation was reported in 81% of cases. Four malignant cysts were independently diagnosed by cystic wall puncture cytology. In contrast to brush cytology, the percentage of adverse events reported with this technique in this study was relatively lower (1.45%).
In another study by Barresi et al.,[95] a 22G ProCore FNB needle was utilized. In this study, the diagnostic adequacy for cytological examination was approximately 65%, while the diagnostic adequacy for histological examination was 46.1%. The technique was especially useful in patients who had a solid component within a cyst, and malignant cysts with cytological adequacy rates increased to 94.4% and 100%, respectively. Mild complications were observed in 3.3% of the patients.
Despite the better results obtained with this technique compared with that of the cytology of cyst fluid, about one-third of the patients still had inclusive diagnoses, and less than half of the patients reached histological diagnoses. The adequacy rate was still not sufficient for clinical practice.
EUS-guided through-the-needle microforceps biopsy
EUS-guided through-the-needle microforceps biopsy (EUS-TTNB) was first described in 2016.[96] The forceps is introduced through a 19G FNA needle and has serrated jaws, enabling targeted biopsies of the cyst wall under EUS guidance [Figure 2]. This technique is the most promising technique in diagnosing PCNs, at present. Westerveld et al. conducted a meta-analysis to assess the diagnostic yield of EUS-TTNB.[97] Eight studies and 426 patients were included in this study.[98,99,100,101,102,103,104,105] EUS-TTNB was successfully performed in 418 of 426 cases for a pooled technical success of 98.2%. The pooled diagnostic yield for a specific cyst type was significantly higher with TTNB histology (72.5%) compared to that in FNA cytology (38.1%). The pooled concordance of TTNB and FNA with surgical pathology for a specific cyst type was 82.3% and 26.8%, respectively. The pooled concordance for mucinous cysts was also higher for TTNB (89%) vs. FNA (41%). Similarly, the pooled concordance with the histological grade of a mucinous cyst on surgical pathology was significantly higher with TTNB (75.6%) vs. FNA (26%). Moreover, two TTNB specimens at a procedure time reached 100% histological adequacy and a specific diagnosis in 74% of patients.[105] More than two specimen collection procedures for TTNB did not provide additional information.[105] However, the risk of adverse events should not be overlooked. The pooled rate of adverse events was 7.0% (5% with intracystic hemorrhage and 2.3% with acute pancreatitis). Fortunately, most of these cases required additional interventions. Only one case develosped a pseudocyst that required endoscopic drainage.[67] The data in this meta-analysis were similar to the data obtained in other meta-analyses.[106,107,108,109]
Figure 2.

Microforceps
In general, these findings suggest that EUS-TTNB is feasible, the diagnostic yield is high, and the adverse event rate is moderate, but a serious adverse event is rarely observed. Future well-designed prospective studies with large sample sizes are warranted to enhance the role of EUS-TTNB in the management of PCNs.
CONCLUSION
PCNs are increasingly detected on imaging, but their characterization remains challenging due to limitations of the current imaging techniques. In this study, we presented a comprehensive review on emerging EUS tools for the diagnosis of PCNs. Among these tools, through-the-needle cystoscopy and targeted cyst wall puncture still have been utilized least frequently, with the most extensive experience being reported for CE-EUS, nCLE, and TTNB. Many novel cyst fluid markers have been described, but the diagnostic standard is lacking. Future studies should address the clinical impact of these markers on patient management, the optimal timing for their application in the diagnostic algorithm of PCNs, and their cost-effectiveness. Moreover, the combination of through-the-needle techniques and cyst fluid analyses may further improve the ability of clinicians to diagnose PCNs. The question of how these techniques may be best utilized warrants further research.
Financial support and sponsorship
Nil.
Conflicts of interest
Zhendong Jin is an Associate Editor of the journal Endoscopic Ultrasound. The article was subject to the journal's standard procedures, with peer review handled independently of this associate editor and their research groups.
REFERENCES
- 1.Sun L, Wang Y, Jiang F, et al. Prevalence of pancreatic cystic lesions detected by magnetic resonance imaging in the Chinese population. J Gastroenterol Hepatol. 2019;34:1656–62. doi: 10.1111/jgh.14658. [DOI] [PubMed] [Google Scholar]
- 2.Zerboni G, Signoretti M, Crippa S, et al. Systematic review and meta-analysis: Prevalence of incidentally detected pancreatic cystic lesions in asymptomatic individuals. Pancreatology. 2019;19:2–9. doi: 10.1016/j.pan.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Kromrey ML, Bülow R, Hübner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138–45. doi: 10.1136/gutjnl-2016-313127. [DOI] [PubMed] [Google Scholar]
- 4.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 5.Elta GH, Enestvedt BK, Sauer BG, et al. ACG clinical guideline: Diagnosis and management of pancreatic cysts. Am J Gastroenterol. 2018;113:464–79. doi: 10.1038/ajg.2018.14. [DOI] [PubMed] [Google Scholar]
- 6.Law JK, Ahmed A, Singh VK, et al. A systematic review of solid-pseudopapillary neoplasms: Are these rare lesions? Pancreas. 2014;43:331–7. doi: 10.1097/MPA.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–97. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Hirono S, Tani M, Kawai M, et al. The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy for branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:517–22. doi: 10.1097/SLA.0b013e3182444231. [DOI] [PubMed] [Google Scholar]
- 9.Kawada N, Uehara H, Nagata S, et al. Mural nodule of 10 mm or larger as predictor of malignancy for intraductal papillary mucinous neoplasm of the pancreas: Pathological and radiological evaluations. Pancreatology. 2016;16:441–8. doi: 10.1016/j.pan.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi N, Sugimori K, Shimamura T, et al. Endoscopic ultrasonographic findings predict the risk of carcinoma in branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology. 2012;12:141–5. doi: 10.1016/j.pan.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu Y, Yamaue H, Maguchi H, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: Analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas. 2013;42:883–8. doi: 10.1097/MPA.0b013e31827a7b84. [DOI] [PubMed] [Google Scholar]
- 12.Jones MJ, Buchanan AS, Neal CP, et al. Imaging of indeterminate pancreatic cystic lesions: A systematic review. Pancreatology. 2013;13:436–42. doi: 10.1016/j.pan.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi: 10.1136/gutjnl-2018-316027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tirkes T, Aisen AM, Cramer HM, et al. Cystic neoplasms of the pancreas; findings on magnetic resonance imaging with pathological, surgical, and clinical correlation. Abdom Imaging. 2014;39:1088–101. doi: 10.1007/s00261-014-0138-5. [DOI] [PubMed] [Google Scholar]
- 15.Khashab MA, Kim K, Lennon AM, et al. Should we do EUS/FNA on patients with pancreatic cysts.The incremental diagnostic yield of EUS over CT/MRI for prediction of cystic neoplasms? Pancreas. 2013;42:717–21. doi: 10.1097/MPA.0b013e3182883a91. [DOI] [PubMed] [Google Scholar]
- 16.Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: Contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–50. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M, Itoi T, Ikeuchi N, et al. Effectiveness of contrast-enhanced endoscopic ultrasound for detecting mural nodules in intraductal papillary mucinous neoplasm of the pancreas and for making therapeutic decisions. Endosc Ultrasound. 2016;5:377–83. doi: 10.4103/2303-9027.190927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong L, Chai N, Linghu E, et al. A prospective study on contrast-enhanced endoscopic ultrasound for differential diagnosis of pancreatic cystic neoplasms. Dig Dis Sci. 2019;64:3616–22. doi: 10.1007/s10620-019-05718-z. [DOI] [PubMed] [Google Scholar]
- 19.Marchegiani G, Andrianello S, Borin A, et al. Systematic review, meta-analysis, and a high-volume center experience supporting the new role of mural nodules proposed by the updated 2017 international guidelines on IPMN of the pancreas. Surgery. 2018;163:1272–9. doi: 10.1016/j.surg.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Ohno E, Kawashima H, Ishikawa T, et al. Can contrast-enhanced harmonic endoscopic ultrasonography accurately diagnose main pancreatic duct involvement in intraductal papillary mucinous neoplasms? Pancreatology. 2020;20:887–94. doi: 10.1016/j.pan.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Fusaroli P, Kypraios D, Mancino MG, et al. Interobserver agreement in contrast harmonic endoscopic ultrasound. J Gastroenterol Hepatol. 2012;27:1063–9. doi: 10.1111/j.1440-1746.2012.07115.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamata K, Kitano M, Omoto S, et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48:35–41. doi: 10.1055/s-0034-1393564. [DOI] [PubMed] [Google Scholar]
- 23.Napoléon B, Lemaistre AI, Pujol B, et al. A novel approach to the diagnosis of pancreatic serous cystadenoma: Needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26–32. doi: 10.1055/s-0034-1390693. [DOI] [PubMed] [Google Scholar]
- 24.Napoleon B, Lemaistre AI, Pujol B, et al. In vivo characterization of pancreatic cystic lesions by needle-based confocal laser endomicroscopy (nCLE): Proposition of a comprehensive nCLE classification confirmed by an external retrospective evaluation. Surg Endosc. 2016;30:2603–12. doi: 10.1007/s00464-015-4510-5. [DOI] [PubMed] [Google Scholar]
- 25.Feng Y, Chang X, Zhao Y, et al. A new needle-based confocal laser endomicroscopy pattern of malignant pancreatic mucinous cystic lesions (with video) Endosc Ultrasound. 2020 Jul 9; doi: 10.4103/eus.eus_35_20. Online ahead of printJul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheesman AR, Zhu H, Liao X, et al. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest Endosc. 2020;91:1095–104. doi: 10.1016/j.gie.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Facciorusso A, Buccino VR, Sacco R. Needle-based confocal laser endomicroscopy in pancreatic cysts: A meta-analysis. Eur J Gastroenterol Hepatol. 2020;32:1084–90. doi: 10.1097/MEG.0000000000001728. [DOI] [PubMed] [Google Scholar]
- 28.Krishna SG, Brugge WR, Dewitt JM, et al. Needle-based confocal laser endomicroscopy for the diagnosis of pancreatic cystic lesions: An international external interobserver and intraobserver study (with videos) Gastrointest Endosc. 2017;86:644–54. doi: 10.1016/j.gie.2017.03.002. e2. [DOI] [PubMed] [Google Scholar]
- 29.Jais B, Rebours V, Malleo G, et al. Serous cystic neoplasm of the pancreas: A multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas) Gut. 2016;65:305–12. doi: 10.1136/gutjnl-2015-309638. [DOI] [PubMed] [Google Scholar]
- 30.Chai N, Feng J, Guo Y, et al. Preliminary study of single-operator cholangioscopy for diagnosing pancreatic cystic lesions. Gastrointest Endosc. 2017;86:208–18. doi: 10.1016/j.gie.2017.01.038. [DOI] [PubMed] [Google Scholar]
- 31.Nakai Y, Iwashita T, Park DH, et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204–14. doi: 10.1016/j.gie.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 32.de Jong K, van Hooft JE, Nio CY, et al. Accuracy of preoperative workup in a prospective series of surgically resected cystic pancreatic lesions. Scand J Gastroenterol. 2012;47:1056–63. doi: 10.3109/00365521.2012.674970. [DOI] [PubMed] [Google Scholar]
- 33.Cizginer S, Turner BG, Bilge AR, et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 2011;40:1024–8. doi: 10.1097/MPA.0b013e31821bd62f. [DOI] [PubMed] [Google Scholar]
- 34.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Sedlack R, Affi A, Vazquez-Sequeiros E, et al. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc. 2002;56:543–7. doi: 10.1067/mge.2002.128106. [DOI] [PubMed] [Google Scholar]
- 36.Faias S, Pereira L, Fonseca R, et al. A second endoscopic ultrasound with fine-needle aspiration for cytology identifies high-risk pancreatic cysts overlooked by current guidelines. Diagn Cytopathol. 2021;49:109–18. doi: 10.1002/dc.24607. [DOI] [PubMed] [Google Scholar]
- 37.Al-Haddad M, DeWitt J, Sherman S, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc. 2014;79:79–87. doi: 10.1016/j.gie.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Gaddam S, Ge PS, Keach JW, et al. Suboptimal accuracy of carcinoembryonic antigen in differentiation of mucinous and nonmucinous pancreatic cysts: Results of a large multicenter study. Gastrointest Endosc. 2015;82:1060–9. doi: 10.1016/j.gie.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Kadayifci A, Al-Haddad M, Atar M, et al. The value of KRAS mutation testing with CEA for the diagnosis of pancreatic mucinous cysts. Endosc Int Open. 2016;4:E391–6. doi: 10.1055/s-0042-101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc. 2009;69:1095–102. doi: 10.1016/j.gie.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 41.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest Endosc. 2005;62:383–9. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 42.Frossard JL, Amouyal P, Amouyal G, et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol. 2003;98:1516–24. doi: 10.1111/j.1572-0241.2003.07530.x. [DOI] [PubMed] [Google Scholar]
- 43.Maire F, Voitot H, Aubert A, et al. Intraductal papillary mucinous neoplasms of the pancreas: Performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol. 2008;103:2871–7. doi: 10.1111/j.1572-0241.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 44.Nagashio Y, Hijioka S, Mizuno N, et al. Combination of cyst fluid CEA and CA 125 is an accurate diagnostic tool for differentiating mucinous cystic neoplasms from intraductal papillary mucinous neoplasms. Pancreatology. 2014;14:503–9. doi: 10.1016/j.pan.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Khamaysi I, Abu Ammar A, Vasilyev G, et al. Differentiation of pancreatic cyst types by analysis of rheological behavior of pancreatic cyst fluid. Sci Rep. 2017;7:45589. doi: 10.1038/srep45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park WG, Wu M, Bowen R, et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: Glucose and kynurenine. Gastrointest Endosc. 2013;78:295–302. doi: 10.1016/j.gie.2013.02.037. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zikos T, Pham K, Bowen R, et al. Cyst fluid glucose is rapidly feasible and accurate in diagnosing mucinous pancreatic cysts. Am J Gastroenterol. 2015;110:909–14. doi: 10.1038/ajg.2015.148. [DOI] [PubMed] [Google Scholar]
- 48.Carr RA, Yip-Schneider MT, Simpson RE, et al. Pancreatic cyst fluid glucose: Rapid, inexpensive, and accurate diagnosis of mucinous pancreatic cysts. Surgery. 2018;163:600–5. doi: 10.1016/j.surg.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 49.Faias S, Pereira L, Roque R, et al. Excellent accuracy of glucose level in cystic fluid for diagnosis of pancreatic mucinous cysts. Dig Dis Sci. 2020;65:2071–8. doi: 10.1007/s10620-019-05936-5. [DOI] [PubMed] [Google Scholar]
- 50.Ribaldone DG, Bruno M, Gaia S, et al. Differential diagnosis of pancreatic cysts: A prospective study on the role of intra-cystic glucose concentration. Dig Liver Dis. 2020;52:1026–32. doi: 10.1016/j.dld.2020.06.038. [DOI] [PubMed] [Google Scholar]
- 51.Deftereos G, Finkelstein SD, Jackson SA, et al. The value of mutational profiling of the cytocentrifugation supernatant fluid from fine-needle aspiration of pancreatic solid mass lesions. Mod Pathol. 2014;27:594–601. doi: 10.1038/modpathol.2013.147. [DOI] [PubMed] [Google Scholar]
- 52.Tan MC, Basturk O, Brannon AR, et al. GNAS and KRAS mutations define separate progression pathways in intraductal papillary mucinous neoplasm-associated carcinoma. J Am Coll Surg. 2015;220:845–540. doi: 10.1016/j.jamcollsurg.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rockacy M, Khalid A. Update on pancreatic cyst fluid analysis. Ann Gastroenterol. 2013;26:122–7. [PMC free article] [PubMed] [Google Scholar]
- 54.Molin MD, Matthaei H, Wu J, et al. Clinicopathological correlates of activating GNAS mutations in intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg Oncol. 2013;20:3802–8. doi: 10.1245/s10434-013-3096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66. doi: 10.1126/scitranslmed.3002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singhi AD, Nikiforova MN, Fasanella KE, et al. Preoperative GNAS and KRAS testing in the diagnosis of pancreatic mucinous cysts. Clin Cancer Res. 2014;20:4381–9. doi: 10.1158/1078-0432.CCR-14-0513. [DOI] [PubMed] [Google Scholar]
- 57.Singhi AD, Wood LD, Parks E, et al. Recurrent rearrangements in PRKACA and PRKACB in intraductal oncocytic papillary neoplasms of the pancreas and bile duct. Gastroenterology. 2020;158:573–82. doi: 10.1053/j.gastro.2019.10.028. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131–41. doi: 10.1136/gutjnl-2016-313586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones M, Zheng Z, Wang J, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest Endosc. 2016;83:140–8. doi: 10.1016/j.gie.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 60.Majumder S, Taylor WR, Yab TC, et al. Novel methylated DNA markers discriminate advanced neoplasia in pancreatic cysts: Marker discovery, tissue validation, and cyst fluid testing. Am J Gastroenterol. 2019;114:1539–49. doi: 10.14309/ajg.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faias S, Duarte M, Pereira L, et al. Methylation changes at the GNAS imprinted locus in pancreatic cystic neoplasms are important for the diagnosis of malignant cysts. World J Gastrointest Oncol. 2020;12:1056–64. doi: 10.4251/wjgo.v12.i9.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaiser RA, Halimi A, Alkharaan H, et al. Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut. 2019;68:2186–94. doi: 10.1136/gutjnl-2018-317458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 2015;149:1501–10. doi: 10.1053/j.gastro.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujikura K, Hosoda W, Felsenstein M, et al. Multiregion whole-exome sequencing of intraductal papillary mucinous neoplasms reveals frequent somatic KLF4 mutations predominantly in low-grade regions. Gut. 2021 May;70:928–39. doi: 10.1136/gutjnl-2020-321217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hernandez YG, Lucas AL. MicroRNA in pancreatic ductal adenocarcinoma and its precursor lesions. World J Gastrointest Oncol. 2016;8:18–29. doi: 10.4251/wjgo.v8.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang L, Zheng J, Sun C, et al. MicroRNA expression levels as diagnostic biomarkers for intraductal papillary mucinous neoplasm. Oncotarget. 2017;8:58765–70. doi: 10.18632/oncotarget.17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Permuth-Wey J, Chen YA, Fisher K, et al. A genome-wide investigation of microRNA expression identifies biologically-meaningful microRNAs that distinguish between high-risk and low-risk intraductal papillary mucinous neoplasms of the pancreas. PLoS One. 2015;10:e0116869. doi: 10.1371/journal.pone.0116869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee LS, Szafranska-Schwarzbach AE, Wylie D, et al. Investigating MicroRNA expression profiles in pancreatic cystic neoplasms. Clin Transl Gastroenterol. 2014;5:e47. doi: 10.1038/ctg.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Permuth JB, Chen DT, Yoder SJ, et al. Linc-ing circulating long non-coding RNAs to the diagnosis and malignant prediction of intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2017;7:10484. doi: 10.1038/s41598-017-09754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henry JC, Bassi C, Giovinazzo F, et al. MicroRNA from pancreatic duct aspirate differentiates cystic lesions of the pancreas. Ann Surg Oncol. 2013;20(Suppl 3):S661–6. doi: 10.1245/s10434-013-3138-8. [DOI] [PubMed] [Google Scholar]
- 71.Bernard V, Semaan A, Huang J, et al. Single-Cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin Cancer Res. 2019;25:2194–205. doi: 10.1158/1078-0432.CCR-18-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryu JK, Matthaei H, Dal Molin M, et al. Elevated microRNA miR-21 levels in pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology. 2011;11:343–50. doi: 10.1159/000329183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farrell JJ, Toste P, Wu N, et al. Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer. Am J Gastroenterol. 2013;108:1352–9. doi: 10.1038/ajg.2013.167. [DOI] [PubMed] [Google Scholar]
- 74.Matthaei H, Wylie D, Lloyd MB, et al. miRNA biomarkers in cyst fluid augment the diagnosis and management of pancreatic cysts. Clin Cancer Res. 2012;18:4713–24. doi: 10.1158/1078-0432.CCR-12-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Utomo WK, Looijenga LH, Bruno MJ, et al. A MicroRNA panel in pancreatic cyst fluid for the risk stratification of pancreatic cysts in a prospective cohort. Mol Ther Nucleic Acids. 2016;5:e350. doi: 10.1038/mtna.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J, Paris PL, Chen J, et al. Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions. Cancer Lett. 2015;356:404–9. doi: 10.1016/j.canlet.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwon RS, Simeone DM. The use of protein-based biomarkers for the diagnosis of cystic tumors of the pancreas. Int J Proteomics. 2011;2011:413646. doi: 10.1155/2011/413646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ke E, Patel BB, Liu T, et al. Proteomic analyses of pancreatic cyst fluids. Pancreas. 2009;38:e33–42. doi: 10.1097/MPA.0b013e318193a08f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jabbar KS, Arike L, Verbeke CS, et al. Highly accurate identification of cystic precursor lesions of pancreatic cancer through targeted mass spectrometry: A phase IIc diagnostic study. J Clin Oncol. 2018;36:367–75. doi: 10.1200/JCO.2017.73.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cuoghi A, Farina A, Z'graggen K, et al. Role of proteomics to differentiate between benign and potentially malignant pancreatic cysts. J Proteome Res. 2011;10:2664–70. doi: 10.1021/pr2000557. [DOI] [PubMed] [Google Scholar]
- 81.Corcos O, Couvelard A, Dargère D, et al. Proteomic assessment of markers for malignancy in the mucus of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2012;41:169–74. doi: 10.1097/MPA.0b013e3182289356. [DOI] [PubMed] [Google Scholar]
- 82.Maker AV, Katabi N, Qin LX, et al. Cyst fluid interleukin-1beta (IL1beta) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2011;17:1502–8. doi: 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yip-Schneider MT, Wu H, Dumas RP, et al. Vascular endothelial growth factor, a novel and highly accurate pancreatic fluid biomarker for serous pancreatic cysts. J Am Coll Surg. 2014;218:608–17. doi: 10.1016/j.jamcollsurg.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 84.Yang JM, Southern JF, Warshaw AL, et al. Proliferation tissue polypeptide antigen distinguishes malignant mucinous cystadenocarcinomas from benign cystic tumors and pseudocysts. Am J Surg. 1996;171:126–9. doi: 10.1016/S0002-9610(99)80086-5. [DOI] [PubMed] [Google Scholar]
- 85.Shirai Y, Sogawa K, Yamaguchi T, et al. Protein profiling in pancreatic juice for detection of intraductal papillary mucinous neoplasm of the pancreas. Hepatogastroenterology. 2008;55:1824–9. [PubMed] [Google Scholar]
- 86.Tanaka M, Shibahara J, Fukushima N, et al. Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J Histochem Cytochem. 2011;59:942–52. doi: 10.1369/0022155411420569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Das KK, Xiao H, Geng X, et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN) Gut. 2014;63:1626–34. doi: 10.1136/gutjnl-2013-306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bausch D, Mino-Kenudson M, Fernández-Del Castillo C, et al. Plectin-1 is a biomarker of malignant pancreatic intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2009;13:1948–54. doi: 10.1007/s11605-009-1001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carmicheal J, Patel A, Dalal V, et al. Elevating pancreatic cystic lesion stratification: Current and future pancreatic cancer biomarker(s) Biochim Biophys Acta Rev Cancer. 2020;1873:188318. doi: 10.1016/j.bbcan.2019.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong NA, Gwiti P, Murigu T, et al. Cell block processing is optimal for assessing endoscopic ultrasound fine needle aspiration specimens of pancreatic mucinous cysts. J Clin Pathol. 2020;73:102–6. doi: 10.1136/jclinpath-2019-206079. [DOI] [PubMed] [Google Scholar]
- 91.Sendino O, Fernández-Esparrach G, Solé M, et al. Endoscopic ultrasonography-guided brushing increases cellular diagnosis of pancreatic cysts: A prospective study. Dig Liver Dis. 2010;42:877–81. doi: 10.1016/j.dld.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 92.Al-Haddad M, Gill KR, Raimondo M, et al. Safety and efficacy of cytology brushings versus standard fine-needle aspiration in evaluating cystic pancreatic lesions: A controlled study. Endoscopy. 2010;42:127–32. doi: 10.1055/s-0029-1215351. [DOI] [PubMed] [Google Scholar]
- 93.Lozano MD, Subtil JC, Miravalles TL, et al. EchoBrush may be superior to standard EUS-guided FNA in the evaluation of cystic lesions of the pancreas: Preliminary experience. Cancer Cytopathol. 2011;119:209–14. doi: 10.1002/cncy.20133. [DOI] [PubMed] [Google Scholar]
- 94.Hong SK, Loren DE, Rogart JN, et al. Targeted cyst wall puncture and aspiration during EUS-FNA increases the diagnostic yield of premalignant and malignant pancreatic cysts. Gastrointest Endosc. 2012;75:775–82. doi: 10.1016/j.gie.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 95.Barresi L, Tarantino I, Traina M, et al. Endoscopic ultrasound-guided fine needle aspiration and biopsy using a 22-gauge needle with side fenestration in pancreatic cystic lesions. Dig Liver Dis. 2014;46:45–50. doi: 10.1016/j.dld.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 96.Pham KD, Engjom T, Gjelberg Kollesete H, et al. Diagnosis of a mucinous pancreatic cyst and resection of an intracystic nodule using a novel through-the-needle micro forceps. Endoscopy. 2016;48(Suppl 1):E125–6. doi: 10.1055/s-0042-105437. [DOI] [PubMed] [Google Scholar]
- 97.Westerveld DR, Ponniah SA, Draganov PV, et al. Diagnostic yield of EUS-guided through-the-needle microforceps biopsy versus EUS-FNA of pancreatic cystic lesions: A systematic review and meta-analysis. Endosc Int Open. 2020;8:E656–67. doi: 10.1055/a-1119-6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mittal C, Obuch JC, Hammad H, et al. Technical feasibility, diagnostic yield, and safety of microforceps biopsies during EUS evaluation of pancreatic cystic lesions (with video) Gastrointest Endosc. 2018;87:1263–9. doi: 10.1016/j.gie.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 99.Barresi L, Crinò SF, Fabbri C, et al. Endoscopic ultrasound-through-the-needle biopsy in pancreatic cystic lesions: A multicenter study. Dig Endosc. 2018;30:760–70. doi: 10.1111/den.13197. [DOI] [PubMed] [Google Scholar]
- 100.Kovacevic B, Klausen P, Hasselby JP, et al. A novel endoscopic ultrasound-guided through-the-needle microbiopsy procedure improves diagnosis of pancreatic cystic lesions. Endoscopy. 2018;50:1105–11. doi: 10.1055/a-0625-6440. [DOI] [PubMed] [Google Scholar]
- 101.Basar O, Yuksel O, Yang DJ, et al. Feasibility and safety of microforceps biopsy in the diagnosis of pancreatic cysts. Gastrointest Endosc. 2018;88:79–86. doi: 10.1016/j.gie.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 102.Yang D, Samarasena JB, Jamil LH, et al. Endoscopic ultrasound-guided through-the-needle microforceps biopsy in the evaluation of pancreatic cystic lesions: A multicenter study. Endosc Int Open. 2018;6:E1423–30. doi: 10.1055/a-0770-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang ML, Arpin RN, Brugge WR, et al. Moray micro forceps biopsy improves the diagnosis of specific pancreatic cysts. Cancer Cytopathol. 2018;126:414–20. doi: 10.1002/cncy.21988. [DOI] [PubMed] [Google Scholar]
- 104.Yang D, Trindade AJ, Yachimski P, et al. Histologic analysis of endoscopic ultrasound-guided through the needle microforceps biopsies accurately identifies mucinous pancreas cysts. Clin Gastroenterol Hepatol. 2019;17:1587–96. doi: 10.1016/j.cgh.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 105.Crinò SF, Bernardoni L, Brozzi L, et al. Association between macroscopically visible tissue samples and diagnostic accuracy of EUS-guided through-the-needle microforceps biopsy sampling of pancreatic cystic lesions. Gastrointest Endosc. 2019;90:933–43. doi: 10.1016/j.gie.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 106.Facciorusso A, Del Prete V, Antonino M, et al. Diagnostic yield of EUS-guided through-the-needle biopsy in pancreatic cysts: A meta-analysis. Gastrointest Endosc. 2020;92:1–8. doi: 10.1016/j.gie.2020.01.038. e3. [DOI] [PubMed] [Google Scholar]
- 107.Balaban VD, Cazacu IM, Pinte L, et al. EUS-through-the-needle microbiopsy forceps in pancreatic cystic lesions: A systematic review. Endosc Ultrasound. 2021;10:19–24. doi: 10.4103/eus.eus_23_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tacelli M, Celsa C, Magro B, et al. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig Endosc. 2020;32:1018–30. doi: 10.1111/den.13626. [DOI] [PubMed] [Google Scholar]
- 109.Kohoutova D, Zar S, Repak R, et al. Pancreatic cysts: Diagnostic role of EUS-guided microforceps biopsy and confocal laser endomicroscopy. Gastroenterol Res Pract. 2019;2019:3431048. doi: 10.1155/2019/3431048. [DOI] [PMC free article] [PubMed] [Google Scholar]


