Abstract
The objective of this research was to evaluate the application of an electronic nose and chemometric analysis to discriminate volatile organic compounds between patients with COVID-19, post-COVID syndrome and controls in exhaled breath samples. A cross-sectional study was performed on 102 exhaled breath samples, 42 with COVID-19, 30 with the post-COVID syndrome and 30 control subjects. Breath-print analysis was performed by the Cyranose 320 electronic nose with 32 sensors. Group data were evaluated by Principal Component Analysis (PCA), Canonical Discriminant Analysis (CDA), and Support Vector Machine (SVM), and the test's diagnostic power was evaluated through a Receiver Operaring Characteristic curve(ROC curve). The results of the chemometric analysis indicate in the PCA a 97.6% (PC1 = 95.9%, PC2 = 1.0%, PC3 = 0.7%) of explanation of the variability between the groups by means of 3 PCs, the CDA presents a 100% of correct classification of the study groups, SVM a 99.4% of correct classification, finally the PLS-DA indicates an observable separation between the groups and the 12 sensors that were related. The sensitivity, specificity of post-COVID vs. controls value reached 97.6% (87.4%–99.9%) and 100% (88.4%–100%) respectively, according to the ROC curve. As a perspective, we consider that this technology, due to its simplicity, low cost and portability, can support strategies for the identification and follow-up of post-COVID patients. The proposed classification model provides the basis for evaluating post-COVID patients; therefore, further studies are required to enable the implementation of this technology to support clinical management and mitigation of effects.
Keywords: Post-COVID syndrome, COVID-19, Chemometric data analysis, Electronic nose system, Volatile organic compounds, Exhaled breath analysis
Graphical abstract
1. Introduction
The terms ‘long COVID’ or ‘post-COVID-19 syndrome’ refer to the implications and consequences of clinical manifestations reported in patients who have recovered from COVID-19. The most frequently occurring symptoms are shortness of breath, myalgia, headache and anxiety reported in 64–80% of patients in an interval ranging from 4 to 24 weeks after the onset of COVID-19 [1]. Although this disease has been shown to be multisystemic, there is substantial concern about the sequelae on the nervous, cardiac and respiratory systems [2].
The level of respiratory damage is closely related to the severity of SARS-CoV-2 infection, the virus can damage the lung by three main pathways: i) acute respiratory distress syndrome (ARDS) with diffuse alveolar damage, ii) diffuse thrombotic alveolar microvascular occlusion and iii) airway inflammation associated with inflammatory mediators. The results of these mechanisms include altered alveolar oxygenation, hypoxemia and acidosis resulting in patient death or sequelae of recovered patient lung injury mainly in the form of organizing pneumonia, diffuse alveolar damage and pulmonary fibrosis [3].
Following SARS-CoV-2 infection, similar to the reported effects of SARS-CoV-1 infection intra-alveolar thrombosis formation and inflammatory airway damage contribute to the development of pulmonary fibrosis. SARS-CoV-1 may induce this effect by promoting the upregulation of profibrotic signaling molecules, including transforming growth factor-beta (TGF-β) induced by the virus nucleocapsid [4]. Considering that the similarity of the nucleocapsid protein between SARS-CoV-2 and SARS-CoV-1 is up to 90% [5], it has been speculated that they have a similar molecular mechanism.
Thus, it is important to assess the post-COVID effects related to the gradual loss of lung function due to pulmonary interstitial fibrosis as it has negative effects on the life quality of people, who were initially thought to have recovered from COVID-19. Disturbingly, recent data indicate that one-third of people who have been infected with the SARS-CoV-2 virus may develop pulmonary fibrosis [6].
In the meantime, monitoring of recovered patients by a pneumologist or internist is advised when any clinical suspicion of residual lung damage is present, this should be assessed by pulmonary function and/or imaging tests. In terms of imaging, chest radiography is the acknowledged initial step. However, in patients with residual radiographic or functional damage, high-resolution chest computed tomography (CT) is indicated not only for further characterization of the anatomical regions of the lung parenchyma, but also to establish a baseline for follow-up and as the current method for the diagnosis of pulmonary fibrosis by classifying it by its anatomical pattern [4].
Some clinical management guidelines for post-COVID assessment have recently emerged with research findings on disease progression, e.g., the UK's National Institute for Health and Care Excellence recommends a chest X-ray for patients recovered after the 12th week and continuing respiratory symptoms [7]. The World Health Organization (WHO) developed a quick guide for the use of chest CT indicated for the evaluation of sequelae of COVID-19, particularly if the thrombotic or multisystemic disease is suspected. Although these images are key to the diagnosis, they do not reveal functionality, only anatomy. Therefore, adding respiratory function tests in these cases is indispensable, since one item is “how it looks”, and another one is “how it works” [8].
The WHO also states an important recommendation, especially for public policies in low- and middle-income countries where limited equipment is available for these patients, provisions should be considered to facilitate the transfer of these people to referral hospitals for these studies [8]. However, in addition to the limited availability of technology, it does not consider local scenarios such as particularly marginalized areas that are at great distances from these technologies and specialists who identify early the sequelae of COVID-19. For example, in Mexico according to the Breviary of Health Statistics of 2018 there were only 389 CT scanners. In addition, it is indicated that the equipment is mainly concentrated in hospitals in large urban centers. This social inequality violates the human right to health, particularly for those people who are unable to travel to hospitals or do not receive the necessary medical care [9]. Currently, efforts to control COVID-19 disease have focused on counting infected and “recovered” cases based on their infection capacity, transmission control, clinical severity, pharmacological treatment, vaccination schemes and social isolation. Although these measures are important, protocols for the care of post-COVID patients' sequelae are still in constant development. Monitoring of patients with this condition requires various tools to evaluate its progression, for example, in the NICE guideline on long COVID the application of several tests is recommended before and during pulmonary rehabilitation including a complete blood count, renal and liver function tests, a C-reactive protein test and an exercise tolerance test [10]. In this guide, it is also recommended that a chest X-ray be offered to all patients by 12 weeks after acute infection if they present ongoing respiratory symptoms. Key areas of research on post-COVID syndrome include risk factors for developing the syndrome (including its prevalence in different populations), clinically effective interventions, population screening, and the natural history of the disease.
The aforementioned techniques represent high costs for the patient and/or healthcare system and require highly qualified personnel for their execution and subsequent analysis of results, which is reflected in long periods to issue a reliable result. In this context, the development of advanced analytical platforms for monitoring patients with symptoms after the disease is of utmost importance in the present scenario. Providing simple, sensitive and specific tools to health personnel is vital for monitoring and treatment of the sequelae generated by COVID-19.
In this regard, our research group has demonstrated the application of exhalatory metabolomics during several studies as a viable screening method for chronic diseases such as chronic obstructive pulmonary disease, lung cancer, breast cancer, diabetes, preeclampsia, and others [[11], [12], [13], [14], [15], [16]]. Human exhaled breath is a complex composition of gases in which over 3000 compounds have been identified including small inorganic compounds such as NO, O2, CO2, volatile organic compounds (VOCs) and non-volatile organic compounds (NVCs) [17]. These mixtures of organic compounds are a product of cellular metabolism and exhibit low solubility in the blood. Hence, they are readily excreted during respiration and can be determined by appropriate analytical techniques.
These chemical patterns - also called the ‘volatolome’ - are the result of normal physiological health conditions and specific pathophysiological conditions allowing its analysis to provide a low cost, rapid and non-invasive screening window into the physiological condition of a patient. In a recent study by our research group a global chemical pattern of VOCs in exhaled breath was identified capable of discriminating between patients with COVID-19 and controls using electrochemical nanosensors. According to our automated learning model, the methodology presented a sensitivity of 100% and a specificity of 97.6% in addition to identifying asymptomatic subjects [18]. This represents a practical screening approach that may rapidly identify suspected patients and provide useful epidemiological information to guide community health strategies in the context of COVID-19.
An outstanding feature of this methodology is that it has been demonstrated in several studies to assess lung damage associated with various pathologies such as COPD and lung cancer [[13], [14], [15]]. Thus, the present study aimed at evaluating the use of olfactory technology comprising functionalized nanosensors in an electronic nose approach augmented by chemometric analysis to discriminate volatile organic compound patterns characteristic for patients with COVID-19, post-COVID syndrome, and healthy controls.
2. Material and methods
2.1. Study design
The study was approved by the state health research ethics committee of San Luis Potosí, Mexico, with registration number SLP/08–2020, in compliance with national regulations for the execution of health research projects in humans.
The study design was cross-sectional and observational. Three study groups were included: i) COVID-19 positive patients; ii) post-COVID patients and iii) a healthy control group (negative to SARS-CoV-2 and without previous infection nor post-COVID syndrome). Data from COVID-19 positive patients were extracted from previous studies of our research group [18]. Inclusion criteria for the group of COVID-19 positive patients were: i) 18–70 years old, both sexes; ii) symptomatic (patients presenting, headache, sore throat, body aches, general discomfort, loss of taste and smell, among other typical COVID-19 symptoms), and asymptomatic (with positive SARS-CoV-2 RTq-PCR test); iii) Ct of the SARS-CoV-2 specific gene in RT-qPCR below 38 to be considered positive. The non-inclusion criteria were: i) pregnant patients; ii) patients with confirmed pulmonary infection other than COVID-19 (influenza, tuberculosis or other infectious diseases). Criteria for elimination included: i) subjects who withdrew informed consent and, ii) subjects who in the course of sampling acquired an infectious pathology.
For the post-COVID group, the inclusion criteria were the following, i) adult patients between 18 and 70 years of age, both sexes; ii) attending health care centers; iii) clinical history based on the Case Report Form (CRF) for Post COVID proposed by the World Health Organization [19]; iv) with the presence of persistent symptoms of COVID-19 after 4 weeks of disease onsetand, v) with negative RTq-PCR test for SARS-CoV-2 at the time of recruitment and evaluation.
The study elimination criteria for this group were: i) patients with suspected or confirmed diagnosis of previous pulmonary disease; ii) patients with signs and/or symptoms of infectious disease; iv) patients who did not present a negative confirmatory RTq-PCR test for SARS-CoV-2; iii) impossibility of collecting the exhaled breath sample; vi) impossibility of performing the pulmonary function test and, vii) subjects who withdrew informed consent.
For the control group (SARS-CoV-2 negative subjects), the inclusion criteria were: i) 18–70 years of age; ii) both sexes; iii) absence of respiratory diseases; iv) negative test for SARS-CoV-2 by RT-qPCR and, v) subjects having at least 7 days without apparent COVID-19 symptoms.
2.2. Respiratory function assessment to patients with the post-COVID syndrome
Assessment of respiratory function was performed only in the post-COVID group. Spirometry tests (pre-and post-bronchodilator) were performed on participants who met the inclusion criteria, following the guidelines of the American ATS/ERS standards employing an EasyOne® Plus Diagnostic portable spirometer, and normal values predicted were those established for the Mexican-American population in the NHANES III study [20].
2.3. Exhaled breath sampling
Breath sampling was based on previous studies of our research group. The participants were required to follow specific indications: i) under 8 h minimum fasting conditions; ii) no alcohol consumption 24 h prior to the study; iii) no smoking at least 8 h before the study and, iv) without oral hygiene. For exhaled breath sample collection, the participants were requested to be seated and relaxed. The sample collection consisted of three deep inhalations and afterwards exhalation into a 1.4-L metallized plastic bag previously purged with ultra-pure nitrogen. Subsequently, the bag was hermetically sealed for its transportation to the laboratory facilities. Every sample was obtained in duplicate, transported at 4 °C and analyzed on the same day [21].
2.4. Exhaled breath fingerprint assessment via electronic nose based on chemoresistive gas sensors
The Cyranose 320 (Sensigent®, California, USA) electronic nose was used to collect the exhaled breath fingerprint of the three study groups. This system incorporated 32 chemoresistive gas sensors for VOC detection. Once these sensors have contact with VOCs, polymers coated onto the individual sensor elements adsorb the vapor. During this process, the distances between conductive particles within the polymer material increase, and thus, an increase in resistance of the composite material is observed. This resistance change is evaluated as a pattern of changes for all sensors within the array, and is then used to identify the VOC fingerprint of the exhaled breath sample.
For sample processing, each sample was incubated at 37 °C for 5 min before analysis. The electronic nose was operated at a constant flow rate of 120 mL/min for 40 s of baseline recording with ultra-pure nitrogen, and then for a sample recording period of 90 s. Subsequently, the flow rate was increased to 180 mL/min of ultra-pure nitrogen for sample line purging and air inlet cleaning while the sensor substrate temperature was maintained at 32 °C through the analysis cycle. For internal quality control, the resistance of the 32 sensors was registered each day before and after measurements.
2.5. Chemometric data analysis
All data were normalized by using the following fractional difference model: ΔR/R0 = (Rmax-R0)/R0, where R is the response of the system to the sample gas, and R0 is the baseline recording, the reference gas being the ultra-pure nitrogen flow.
A self-scaling routine was performed to eliminate the effects of the magnitude of each sensor respons by subtracting the average of the samples from the individual response of each sample and dividing by the standard deviation of the samples.
To capture the maximum variability within the data, Principal Component Analysis (PCA) was performed. PCA is a multivariate data analysis technique that is used to reduce the dimensionality of the data while preserving its structure. PCA executes a singular value decomposition based on eigenvalues and eigenvectors to define a reduced data subspace utilizing a correlation matrix to enhance the influence of spectral features. Thereby, PCA can be used to study the contribution of each sensor to the separation of the studied patient groups [22]. Canonical Discriminant Analysis (CDA) was used to assess clustering within the data sets of all groups and to assign a new sample to a class considering the class that presents the shortest Mahalanobis distance between the centroid and the canonical space of the sample. For this purpose, cross-validation by the ‘leave-one-out-procedure’ was performed to predict the group belonging and to obtain overall classification success rates. The Mahalanobis distance between the group means is given in units of standard deviation.
Alternatively, a Support Vector Machine (SVM) algorithm was employed in the same way to evaluate clustering within the data sets of all studied patient groups. SVMs are kernel-based (i.e., radial Gaussian) supervised learning classification methods that determine the optimal boundaries (i.e., support vectors) that precisely separate groups [13]. SVMs construct a hyperplane or a set of hyperplanes in a high- or infinite-dimensional space, which can be used for classification. SVMs consider a set of input data and predict for each given input which of the possible classes constitutes the output. This procedure yields a non-probabilistic binary linear classifier. To cope with a large number of input data and the dimensionality of the data space, an optimal hyperplane is defined as the linear decision function with the maximum margin between the vectors of the classes. To construct optimal hyperplanes, support vectors reflecting the number of data are created, the support vectors then determine the optimal margins [21,22]. Chemometric analyses were performed by using the CDAnalysis (Sensigent®) statistical software package.
Following, Canonical Analysis of Principal Coordinates (CAP) was used to order the matrices and, to determine the level of misclassification between sampling regions, the leave-one-out method was applied to the variables in the canonical space (using a K-fold of n = 103) to predict the group associations and thereby obtain the overall classification success rates, using a value of m = 21. An external validation of the model was performed, randomly selecting 30% of the samples. This analysis was performed by the statistical software PRIMER v7 with PERMANOVA add-in.
Only the CAP1 axis was evaluated using the Receiver Operating Characteristic curve (ROC curve) because it represented 100% of the data. With a 95% confidence interval (CI) and the threshold value or cut-off point was selected with the highest specificity/sensitivity ratio [23].
Additionally, a Partial Least Squares - Discriminant Analysis (PLS-DA) and a Variable Importance in Projection (VIP) (Metaboanalyst.ca ® online free statistical software) were executed to identify differential sensors among groups and to rank the sensor response according to their importance in discriminating patient groups. Also, PLS-DA was used to differentiate the groups. PLS-DA is a supervised statistical method that uses multivariate regression techniques to extract information for predicting class belonging via a linear combination of independent variables.
3. Results
A total of 102 breath samples were collected; 42 from patients with COVID-19, 30 with the post-COVID syndrome and 30 from control subjects. The characteristics of the patients are described in Table 1 . For the control group, 23 women and 7 men participated with an average age of 42.4 ± 10.8 years and an average body mass index of 25.8 ± 3.9 kg/m2. For the COVID-19 group, 14 women and 28 men participated with an average age of 38 ± 14 years, symptom onset ranged from 4 to 8 days, with 100% of participants presenting anosmia, fever, arthralgia, and myalgia, and 19 patients reported being active smokers. Post-COVID patients presented an average of 129 days in a range of 45–250 days, 17 women and 13 men participated with an average age of 54.8 ± 12.8 years and a body mass index of 28.5 ± 6.3 kg/m2.
Table 1.
Evaluated parameters in the study groups.
| Parameters | Control | COVID-19 | Post-COVID |
|---|---|---|---|
| N | 30 | 42 | 30 |
| Female (N) | 23 | 14 | 17 |
| Male (N) | 7 | 28 | 13 |
| Age (Years) | 42.4 ± 10.8 | 38 ± 14 | 54.8 ± 12.8 |
| BMI (kg/m2) | 25.8 ± 3.9 | 26.7 ± 4.6 | 28.5 ± 6.3 |
| Dyspnoea (mMRC) | N/A | N/A | 3 (0–4) |
| Fatigue (VAS) | N/A | N/A | 2 (0–8) |
| Use of oxygen (days) | N/A | N/A | 28 (0–240) |
| Tobacco index | 0 (0–2) | N/A | 0 (0–40) |
| Functional grade | N/A | N/A | 1 (0–4) |
| Oximetry in COVID-19 (%) at sampling | 95 ± 2 | 81 ± 14 | 94.5 ± 2.1 |
| Restrictive spirometric pattern (N) | N/A | N/A | 21 |
| FVC (L) | N/A | N/A | 77.2 ± 20.5 |
| FEV1 (L) | N/A | N/A | 80.4 ± 21.3 |
| FEV1/FVC | N/A | N/A | 105.2 ± 6.4 |
| PEF (L/s) | N/A | N/A | 96.6 ± 17.5 |
| MEF- 25–75% (L) | N/A | N/A | 94.4 ± 30.4 |
BMI: Body Mass Index, mMRC: Modified Medical Research Council scale, VAS: Visual Analog Scale 0–10 points, Functional grade assessed by post functional status scale (B1), FVC: Forced Vital Capacity, FEV1: forced expiratory volume in 1 s, PEF: peak expiratory flow; MEF: mid-expiratory flow N/A: not assessed.
The most common symptoms reported by post-COVID group and during the acute phase of COVID-19 were anosmia, low oxygenation, arthralgia and myalgia. The average oxygenation was 80%, similar to the reported by patients in the COVID-19 group in acute phase.
According to the spirometric values, 21 patients presented a restrictive pattern and 60% required supplemental oxygen in a range of use from 0 to 240 days. The average oxygen saturation during the acute stage of the disease, measured by pulse oximetry, was 81%, 13% of the patients received hospital care due to COVID-19, likewise, 60% of the patients reported the use of supplemental oxygen required with average use of 53.7 days (Table 1). Seventy percent of the patients with post-COVID syndrome reported fatigue as the main persistent symptom, followed by 60% with dyspnea and 36% with cough. The male sex reported 30% greater persistence of symptoms than women (Supplementary Figure 1).
As the main risk factors for serious disease identified in post-COVID syndrome patients, smoking was reported by 40% of the population and hypertension was present in 26%. Moreover, the functional evaluation showed that 46.6% did not have functional limitations (Grade 0), 10% minimal functional limitations (Grade 1), 6.6% light functional limitations (Grade 2), 26.6% moderate functional limitations (Grade 3) and 6.6% severe functional limitations (Grade 4).
The lung function assessment in the post-COVID group revealed 30% of patients with normal criteria, 63.3% with pulmonary restriction criteria, and 6.6% with bronchial obstruction criteria without significant response to fast-acting β2 bronchodilator, the mean difference in the results of the pulmonary function was analyzed according to sex, where the FVC was significantly lower (p 0.02) in men vs. women (Supplementary material Table 1).
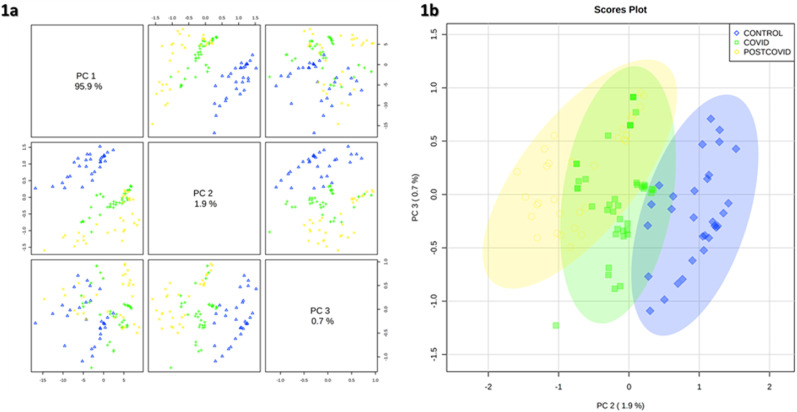
Regarding the chemometric analysis, Fig. 1 shows PCA results between the COVID-19 group, control group and the post-COVID group. The analysis achieves an explanation of 97.6% of the variability between the groups by 3 PCs (PC1 = 95.9%, PC2 = 1.9%, PC3 = 0.7%). In this graph, a separation between VOC patterns in the exhaled breath of the three groups is clearly observed.
Fig. 1.
Principal Component Analysis (PCA) plot of the study groups. Yellow circle post-COVID group, green square COVID-19 group and blue rhombus control group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
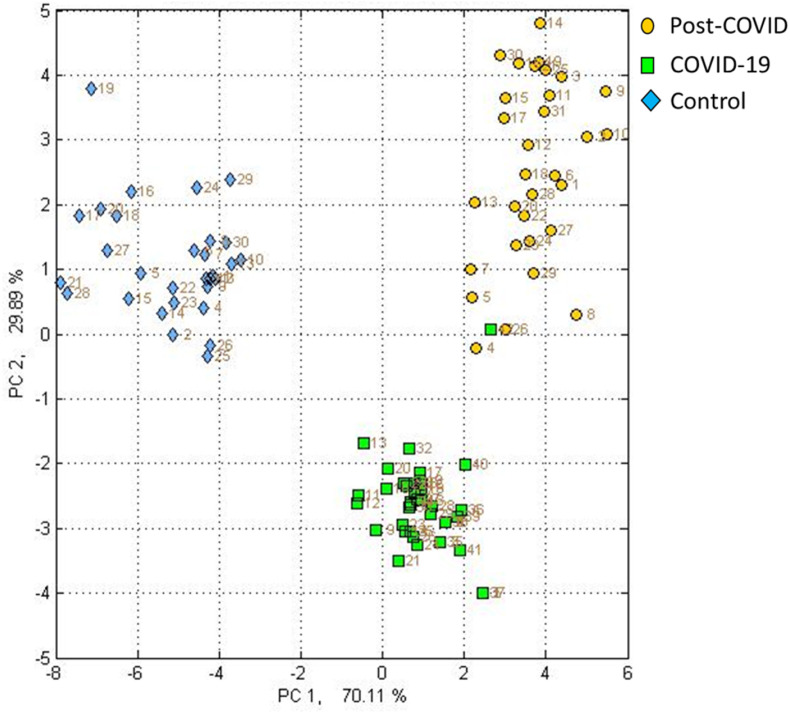
In Fig. 2 , the CDA model is presented, whereby the discrimination between the three study groups is achieved using only two PC axes with a correct classification of 100%. It is immediately evident that via the PC1 axis the separation between the control group concerning the patients with COVID-19 and post-COVID-19 is achieved, which represents 70.11% of the variability within the data. Likewise, it was found that through the PC2 axis provides a separation between patients with COVID-19 and post-COVID representing 29.89% of the variability within the data. In addition, in the SVM model (Fig. 3 ) the discrimination between the 3 groups at 99.4% is shown indicating that there is indeed a significant difference between the global VOC fingerprints of the groups.
Fig. 2.
Canonical Discriminant Analysis (CDA) of the study groups. Yellow circle post-COVID group, green square COVID-19 group and blue rhombus control group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Support Vector Machine Model (SVM). Yellow circle post-COVID group, green square COVID-19 group and blue rhombus control group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
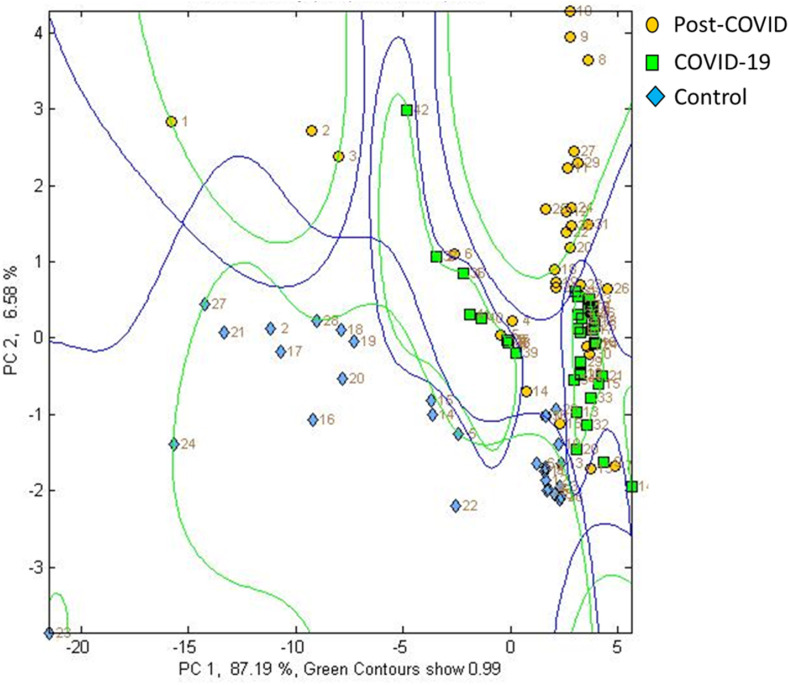
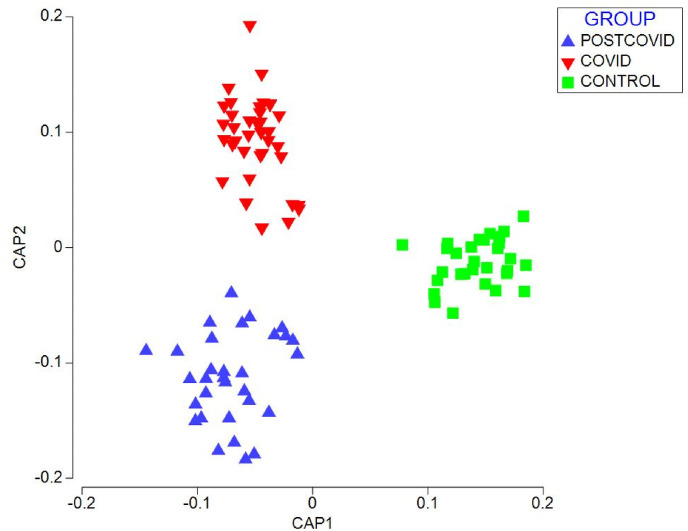
The CAP of post-COVID and controls showed a separation between the chemical fingerprints of each group through two canonical axes CAP1 (r2 = 0.9661) and CAP 2 (r2 = 0.8669), with a 100% of correct total classification (Fig. 4 ). Also, the values of the external validation of the CAP model obtained a percentage of correct prediction of 100%.
Fig. 4.
Canonical Analysis of Principal Coordinates (CAP) of chemical prints of patients with COVID, post-COVID and controls.
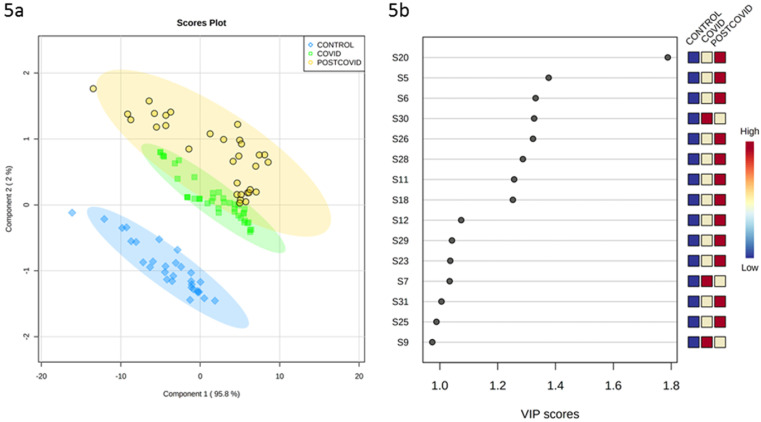
Fig. 5a shows the PLS-DA plot for the three groups. The graph indicates the group each sample belongs to and the discrimination between the three evaluated patient groups. A separation between the post-COVID group, COVID-19 and the control group is clearly observed. The variable importance in projection (Fig. 5b) indicates that for the post-COVID group the sensors S20, S5, S6, S26, S28, S11, S18, S12, S29, S23, S31 and S25 contribute more significantly to the separation of the groups, while for the COVID-19 group the sensors S30, S7 and S9 present the dominating contribution to the separation of the group.
Fig. 5.
Partial Least Squares - Discriminant Analysis (PLS-DA). 5a) Plot of component 1 vs component 2; 5 b) plot of variable importance in projection.
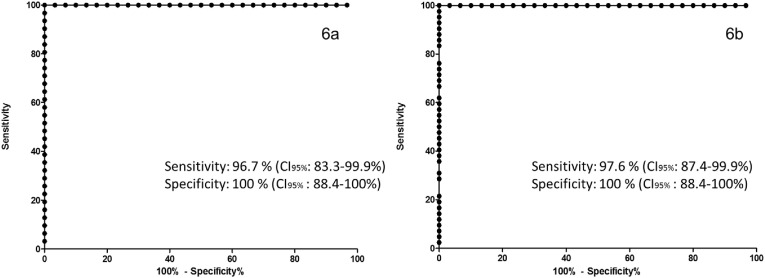
Furthermore, with the values generated in the CAP 1 score, the cut-off point of −0.015 was established for COVID-19 vs controls, which provided 96.7% sensitivity (confidence intervals at 95%: 83.3–99.9%) and 100% specificity (confidence intervals at 95%: 88.4–100%) (Fig. 6 a). The cut-off point of −0.012 was established for COVID-19 vs controls, which provided 97.6% sensitivity (confidence intervals at 95%: 87.4–99.9%) and 100% specificity (confidence intervals at 95%: 88.4–100%) (Fig. 6b).
Fig. 6.
ROC curve for the screening of COVID-19 (6a) and post-COVID (6 b) when using the CAP1 axis. An AUC of 1.0 was obtained with a cut-off point of −0.015 for COVID-19 vs controls and −0.012 for post-COVID vs controls.
4. Discussion
The present study demonstrates the potential of using electronic nose systems augmented by automated learning techniques for the discrimination of post-COVID patients. The main contribution of this work is the determination and differentiation of characteristic global VOC fingerprint patterns from the exhaled breath of COVID-19 patients, post-COVID patients and healthy controls.
A systematic review that recruited 380 patients from 19 studies for the evaluation of pulmonary function in post-COVID patients through spirometry, reported a prevalence of 15% of patterns suggestive of restriction and 7% with an obstructive pattern, reporting that between 6 and 18% of the patients reported a history of smoking [24], even though the population of this study had greater socio-demographic homogeneity as well as a larger sample size, the behavior of the variables of lung function and smoking was similar to the results obtained in our study, in contrast to the fact that in this sample those patients with any type of previous lung disease were excluded.
On the other hand, a cohort of COVID-19 survivors, who were evaluated for functional capacity and lung function in 3 and 6 months after infection, showed a predominance and persistence over time of a pattern suggestive of pulmonary restriction in combination with a decreased pulmonary diffusion of carbon monoxide (DLco) [25].
The alteration of pulmonary gas diffusion has been widely reported as one of the main mechanisms that contribute to residual respiratory failure and hypoxia after infection by SARS-CoV-2 [26]. In this sense, an interesting variable in the post-COVID group is the prolonged use of oxygen after obtaining a negative result for SARS CoV-2, showing a positive relationship with age, a decrease in oxygen saturation as well as a higher fatigue score and dyspnoea. According to the above, a study on prolonged oxygen therapy after COVID-19 infection, reported age older than 50 years as one of the main risk factors for a poor result in hospital discharge in addition to the presence of 3 or more co-morbidities [27]. Another study found that being 70 years of age or older and having dyspnea symptoms was associated with a prolonged duration of need for supplemental oxygen therapy [28].
Likewise, persistent dyspnea as one of the main symptoms referred to in the post-COVID group, has been associated with a significant decrease in forced vital capacity, pulmonary gas exchange and oxygen saturation in a study carried out in 186 surviving patients of varying degrees of severity of COVID-19 [29]. On the other hand, fatigue as the most common post-COVID symptom in this study group, is reported in a similar way in other studies with an incidence that ranges between 17.5% and 72% among hospitalized patients, extending in several occasions beyond seven months after disease onset [30].
Considering the persistence of symptoms in 70% and abnormalities in lung function in 63% at 4 months as the average time of evolution of the post-COVID group, a cohort study of clinical, functional and imaging evaluation 4 months after hospital discharge due to COVID-19, reported that 61% still had symptoms, 39% showed abnormalities in pulmonary gas diffusion, decreased oxygen saturation in a 6-min walk, as well as pulmonary restriction and 41% with radiological abnormalities [31].
Even though there is wide clinical and socio-demographic heterogeneity in the various scientific reports, it is possible to appreciate a similar trend of symptoms and persistent factors reported in this study and the mentioned scientific references, which could be analyzed in subsequent scientific studies.
Regarding our metabolomic results, clinical research collaborators of our research team on COVID-19 have identified transient and persistent systemic changes in molecular signatures in blood samples from patients three months after the acute phase of the disease. These biochemical abnormalities, which were identified by a label-free quantitative assay platform that integrated nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) are related to ongoing symptoms of post-COVID. Markers that change significantly during disease progression and arise from cytokines, lipoprotein interactions, and markers of inflammation have been identified. The damage caused by SARS-CoV-2 in different organs has been explained by the increase in oxidative stress caused by the cytokine storm and the hyper-reactivity of the immune system [32], this process has been proven to modify the overall cellular metabolism of the host [33]. Several investigations support the statement that this metabolic phenotype appears to be an effective tool for assessing systemic functional recovery of patients after COVID-19 [[34], [35], [36], [37]]. Holmes et al. reported the sequelae of metabolic changes in serum of 27 post-COVID patients within 3 months of evolution, they found that biomarkers related to liver damage, muscle, tissue regeneration and immune functions (taurine and glutamine/glutamate) were present with significant differences with respect to controls and that some molecules such as apolipoprotein B100/A1 were similar, reflecting the reversion to the metabolic phenotype of healthy subjects [37].
In this context, Lamote et al. have proposed that changes in the profile of volatile compounds in the breath during the acute phase of COVID-19 can be used to assess the evolution of the disease [38].
VOCs are generally the end products of carbohydrate and lipid metabolism, as well as oxidative stress and cytochrome p450 liver enzymes in human cells, they are also endogenous gaseous transmitters involved in the regulation of many biological processes [39]. VOCs signatures have already been used as descriptive patterns in the diagnosis and monitoring of various pulmonary diseases such as COPD, lung cancer, cystic fibrosis, and other chronic and infectious lung diseases [13,14,40]. In this regard, several VOCs have been described in exhaled breath in the acute phase of COVID-19 disease, among which the presence of several biomarkers has been described with the most outstanding ones being identified as 2,3-butandione, aldehyde, 2,8-dimethyl-undecane, n-propyl acetate [41]; ethanal, acetone, 2-butanone, methanol, octanal, isoprene, heptanal, propanal, propane [42]; methylpent-2-enal, 2,4-octadiene 1-chloroheptane, nonanal [43]; butanoate, butyraldehyde, isopropanol [44]; alcohol, acetone, carbon monoxide [45] and, octanal, nonanal, heptanal, decane, tridecane, and 2-pentyl furan [46].
Regarding VOCs in exhaled breath of post-COVID patients, no reports were found in the literature at the time of the study; however, VOCs have been reported in conditions similar to post-COVID, for example, in a study by Ruskiewicz et al., they report that the identity of the marker compounds identified are consistent with a combination of extrapulmonary metabolic, and gastrointestinal manifestations of COVID- 19 within the body as well as airway inflammatory responses [42]. Many of these metabolites have also been associated with lung damage caused by different etiologies like cystic fibrosis, COPD, asthma and lung cancer [40,47,48]. These studies indicate that VOC patterns may indeed be used in disease progression monitoring, this has been proven in liver diseases, diabetes, and asthma [[49], [50], [51]]. Thus, based on our results this technique could more ubiquitously be used to evaluate post-COVID patients and determine their progression vs. the chemical VOC fingerprint phenotype of healthy controls. However, to test this hypothesis extended studies will be required to follow-up on patients and their progression.
While our preliminary study lays a solid basis for extending the evaluation of electronic nose systems in this context, several limitations should be mentioned. Among the most important ones are the numbers of the studied populations, the lack of a group where people fully recovered from COVID-19 would be analyzed, the inability to perform tests to evaluate lung capacity in patients with ongoing SARS-CoV-2 infection due to biosafety considerations, and the lack of identification of specific metabolites by methods such as mass spectrometry. The latter would help to improve the fundamental understanding of the metabolic processes that are altered in post-COVID. However, the test concept we present herein is perfectly capable of discriminating between patients with infection and patients with sequelae at a 100% correct classification rate, at a complete analysis time of 5 min, at very low cost compared to high-resolution tomography, and in a compact and portable system format that renders its application at the point-of-need (e.g., in rehabilitation centers) on a massive scale entirely feasible. Hence, medical personnel even in less favored regions may be provided with a real-time assessment tool on patient progression. Last but not least, this will aid physicians and researchers to establish rapid mitigation strategies to face future pandemic scenarios and to develop preventive and therapeutic strategies for similar hyperinflammatory conditions.
5. Conclusion
In the present study it was possible to identify global profiles of volatile organic compounds in patients with COVID-19, post-COVID syndrome and controls by using an electronic nose system augmented by chemometric data analysis. Hundred percent correct classification was achieved between the studied patient groups using supervised learning algorithms.
As a perspective, we consider that this technology, due to its simplicity, low cost and portability, can support strategies for the identification and follow-up of post-COVID patients. The proposed classification model provides the basis for evaluating post-COVID patients; therefore, further studies are required to enable the implementation of this technology to support clinical management and mitigation of long-term effects.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Omar Ornelas Rebolledo who is director of the company LABINNOVA which financed the study and the Cyranose 320 equipment.
Acknowledgement
COPOCYT. Fideicomiso 23871 Multas Electorales Convocatoria 2021–01, Desarrollo y evaluación de un Sistema de tamizaje basado en la metabolómica exhalatoria para la clasificación y predicción de COVID largo mediante métodos de aprendizaje automatizado. LABINNOVA, Center of Investigation in Breath for early detection diseases. BM thanks the Ministerium für Wissenschaft, Forschung und Kunst (MKW) Baden-Württemberg, Germany under the Program “Special Measures against the SARS-CoV-2 Pandemic”. BM and LDLM than the German Academic Exchange Service (DAAD) for a stipend granting a research stay at Ulm University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.talanta.2021.122832.
Author contribution
BNZM: Conceptualization, Sampling; LDLM: Conceptualization, Analytical Methods, Writing, Editing; MRA: Conceptualization, Analytical Methods, Writing; BM: Supervision, Editing; RFR: Conceptualization, Analytical Methods, Writing, Editing, Funding.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sykes D.L., Holdsworth L., Jawad N., Gunasekera P., Morice A.H., Crooks M.G. 2021. Post-COVID-19 Symptom Burden: what Is Long-COVID and How Should We Manage it? Lung. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahanic S., Sonnweber T., Pizzini A., Widmann G., Luger A., Aichner M., Böhm A., Weiss G., Tschurtschenthaler C., Petzer V., Haschka D., Theurl M., Lener D., Wildner S., Bellmann-Weiler R., Wöll E., Löffler-Ragg J., Tancevski I. Late Breaking Abstract - persisting pulmonary impairment following severe SARS-CoV-2 infection, preliminary results from the CovILD study. Eur. Respir. J. 2020;56(suppl 64):4143. [Google Scholar]
- 3.Wang F., Kream R.M., Stefano G.B. Long-term respiratory and neurological sequelae of COVID-19. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2020;26 doi: 10.12659/MSM.928996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao X., Nicholls J.M., Chen Y.G. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J. Biol. Chem. 2008;283(6):3272–3280. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik Y.A. Properties of coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020;42(1):3–11. [PubMed] [Google Scholar]
- 6.Han X., Fan Y., Alwalid O., Li N., Jia X., Yuan M., Li Y., Cao Y., Gu J., Wu H., Shi H. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. 2021;9(2):129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akl E.A., Blažić I., Yaacoub S., Frija G., Chou R., Appiah J.A., Fatehi M., Flor N., Hitti E., Jafri H., Jin Z.-Y., Kauczor H.U., Kawooya M., Kazerooni E.A., Ko J.P., Mahfouz R., Muglia V., Nyabanda R., Sanchez M., Shete P.B., Ulla M., Zheng C., Deventer E.v., Perez M.d.R. Use of chest imaging in the diagnosis and management of COVID-19: a WHO rapid advice guide. Radiology. 2021;298(2):E63–E69. doi: 10.1148/radiol.2020203173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz de Leon-Martinez L., de la Sierra-de la Vega L., Palacios-Ramirez A., Rodriguez-Aguilar M., Flores-Ramirez R. Critical review of social, environmental and health risk factors in the Mexican indigenous population and their capacity to respond to the COVID-19. Sci. Total Environ. 2020;733:139357. doi: 10.1016/j.scitotenv.2020.139357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesan P. NICE guideline on long COVID. The Lancet Respiratory Medicine. 2021;9(2):129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz de Leon-Martinez L., Rodriguez-Aguilar M., Gorocica-Rosete P., Dominguez-Reyes C.A., Martinez-Bustos V., Tenorio-Torres J.A., Ornelas-Rebolledo O., Cruz-Ramos J.A., Balderas-Segura B., Flores-Ramirez R. Identification of profiles of volatile organic compounds in exhaled breath by means of an electronic nose as a proposal for a screening method for breast cancer: a case-control study. J. Breath Res. 2020;14(4) doi: 10.1088/1752-7163/aba83f. [DOI] [PubMed] [Google Scholar]
- 12.Mendez-Rodriguez K.B., Figueroa-Vega N., Ilizaliturri Hernandez C.A., Cardona-Alvarado M., Borjas Garcia J.A., Kornhauser C., Malacara J.M., Flores-Ramirez R., Perez-Vazquez F.J. Identification of metabolic markers in patients with type 2 Diabetes Mellitus by Ultrafast gas chromatography coupled to electronic nose. A pilot study, Biomed Chromatogr. 2020 doi: 10.1002/bmc.4956. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Aguilar M., Diaz de Leon-Martinez L., Gorocica-Rosete P., Padilla R.P., Thirion-Romero I., Ornelas-Rebolledo O., Flores-Ramirez R. Identification of breath-prints for the COPD detection associated with smoking and household air pollution by electronic nose. Respir. Med. 2020;163:105901. doi: 10.1016/j.rmed.2020.105901. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Aguilar M., Díaz de León-Martínez L., Gorocica-Rosete P., Pérez-Padilla R., Domínguez-Reyes C.A., Tenorio-Torres J.A., Ornelas-Rebolledo O., Mehta G., Zamora-Mendoza B.N., Flores-Ramírez R. Application of chemoresistive gas sensors and chemometric analysis to differentiate the fingerprints of global volatile organic compounds from diseases. Preliminary results of COPD, lung cancer and breast cancer. Clin. Chim. Acta. 2021;518:83–92. doi: 10.1016/j.cca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Aguilar M., Ramirez-Garcia S., Ilizaliturri-Hernandez C., Gomez-Gomez A., Van-Brussel E., Diaz-Barriga F., Medellin-Garibay S., Flores-Ramirez R. Ultrafast gas chromatography coupled to electronic nose to identify volatile biomarkers in exhaled breath from chronic obstructive pulmonary disease patients: a pilot study. Biomed. Chromatogr. 2019;33(12) doi: 10.1002/bmc.4684. [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez K.B.M., Gómez L.M.R., Yáñez L.C., Ramírez R.F., Ornelas-Rebolledo O., Borjas-García J.A., Pérez-Vázquez F., Aguilar M.R. Archives of Medical Research; 2021. Application of the Electronic Nose in Predicting Preeclampsia in High-Risk Pregnancies. Pilot Study. [DOI] [PubMed] [Google Scholar]
- 17.Phillips M., Herrera J., Krishnan S., Zain M., Greenberg J., Cataneo R.N. Variation in volatile organic compounds in the breath of normal humans. J. Chromatogr. B Biomed. Sci. Appl. 1999;729(1–2):75–88. doi: 10.1016/s0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Aguilar M., Díaz de León-Martínez L., Nohemí Zamora-Mendoza B., Comas-García A., Elizabeth Guerra Palomares S., Alberto García-Sepúlveda C., Eugenia Alcántara-Quintana L., Díaz-Barriga F., Flores-Ramírez R. Clinica Chimica Acta; 2021. Comparative Analysis of Chemical Breath-Prints through Olfactory Technology for the Discrimination between SARS-CoV-2 Infected Patients and Controls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO . World Health Organization. World Health Organization; 2021. Global COVID-19 clinical platform case report form (CRF) for post COVID condition (post COVID-19 CRF) [Google Scholar]
- 20.CDC N., III . Spirometry Training Program; 2011. Reference Values.https://www.cdc.gov/niosh/topics/spirometry/nhanes.html Accessed July 15th 2021. [Google Scholar]
- 21.Koo I., Shi X., Kim S., Zhang X. iMatch2: compound identification using retention index for analysis of gas chromatography-mass spectrometry data. J. Chromatogr. A. 2014;1337:202–210. doi: 10.1016/j.chroma.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X.-z., Lan Y.-b., Zhu J.-m., Westbrook J., Hoffmann W.C., Lacey R.E. Rapid identification of rice samples using an electronic nose. JBE. 2009;6(3):290–297. [Google Scholar]
- 23.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4(2):627–635. [PMC free article] [PubMed] [Google Scholar]
- 24.Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., Solis-Navarro L., Burgos F., Puppo H., Vilaró J. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27(4):328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerardo A., Almeida T., Maduro S., Carvalho M., Boléo-Tomé J., Liberato H. Función pulmonar, capacidad funcional y estado de salud en una cohorte de sobrevivientes de COVID-19 a los 3 y 6 meses después del alta hospitalaria. Revista de Medicina Clínica. 2021;5(2) [Google Scholar]
- 26.Lombardi F., Calabrese A., Iovene B., Pierandrei C., Lerede M., Varone F., Richeldi L., Sgalla G. 2021. Residual Respiratory Impairment after COVID-19 Pneumonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray A., Chaudhry R., Rai S., Mitra S., Pradhan S., Sunder A., Nag D.S. Prolonged oxygen therapy post COVID-19 infection: factors leading to the risk of poor outcome. Cureus. 2021;13(2) doi: 10.7759/cureus.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daher A., Balfanz P., Aetou M., Hartmann B., Müller-Wieland D., Müller T., Marx N., Dreher M., Cornelissen C.G. Clinical course of COVID-19 patients needing supplemental oxygen outside the intensive care unit. Sci. Rep. 2021;11(1):1–7. doi: 10.1038/s41598-021-81444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortés-Telles A., López-Romero S., Figueroa-Hurtado E., Pou-Aguilar Y.N., Wong A.W., Milne K.M., Ryerson C.J., Guenette J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2021;288:103644. doi: 10.1016/j.resp.2021.103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maltezou H.C., Pavli A., Tsakris A. Post-COVID syndrome: an insight on its pathogenesis. Vaccines. 2021;9(5):497. doi: 10.3390/vaccines9050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noel-Savina E., Viatgé T., Faviez G., Lepage B., Mhanna L.T., Pontier S., Dupuis M., Collot S., Thomas P., Idoate Lacasia J., Crognier L., Bouharaoua S., Silva Sifontes S., Mazieres J., Prévot G., Didier A. Severe SARS-CoV-2 pneumonia: clinical, functional and imaging outcomes at 4 months. Respir Med Res. 2021;80 doi: 10.1016/j.resmer.2021.100822. 100822-100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Geng X., Tan Y., Li Q., Xu C., Xu J., Hao L., Zeng Z., Luo X., Liu F., Wang H. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed. Pharmacother. 2020;127:110195. doi: 10.1016/j.biopha.2020.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doğan H.O., Şenol O., Bolat S., Yıldız Ş.N., Büyüktuna S.A., Sarıismailoğlu R., Doğan K., Hasbek M., Hekim S.N. Understanding the pathophysiological changes via untargeted metabolomics in COVID-19 patients. J. Med. Virol. 2021;93(4):2340–2349. doi: 10.1002/jmv.26716. [DOI] [PubMed] [Google Scholar]
- 34.Kimhofer T., Lodge S., Whiley L., Gray N., Loo R.L., Lawler N.G., Nitschke P., Bong S.H., Morrison D.L., Begum S., Richards T., Yeap B.B., Smith C., Smith K.G.C., Holmes E., Nicholson J.K. Integrative modeling of quantitative plasma lipoprotein, metabolic, and amino acid data reveals a multiorgan pathological signature of SARS-CoV-2 infection. J. Proteome Res. 2020;19(11):4442–4454. doi: 10.1021/acs.jproteome.0c00519. [DOI] [PubMed] [Google Scholar]
- 35.Lodge S., Nitschke P., Kimhofer T., Coudert J.D., Begum S., Bong S.H., Richards T., Edgar D., Raby E., Spraul M. NMR spectroscopic windows on the systemic effects of SARS-CoV-2 infection on plasma lipoproteins and metabolites in relation to circulating cytokines. J. Proteome Res. 2021;20(2):1382–1396. doi: 10.1021/acs.jproteome.0c00876. [DOI] [PubMed] [Google Scholar]
- 36.Lawler N.G., Gray N., Kimhofer T., Boughton B., Gay M., Yang R., Morillon A.-C., Chin S.-T., Ryan M., Begum S. Systemic perturbations in amine and kynurenine metabolism associated with acute SARS-CoV-2 infection and inflammatory cytokine responses. J. Proteome Res. 2021;20(5):2796–2811. doi: 10.1021/acs.jproteome.1c00052. [DOI] [PubMed] [Google Scholar]
- 37.Holmes E., Wist J., Masuda R., Lodge S., Nitschke P., Kimhofer T., Loo R.L., Begum S., Boughton B., Yang R. Incomplete systemic recovery and metabolic phenoreversion in Post-Acute-Phase nonhospitalized COVID-19 Patients: implications for assessment of Post-Acute COVID-19 syndrome. J. Proteome Res. 2021;20:3315–3329. doi: 10.1021/acs.jproteome.1c00224. [DOI] [PubMed] [Google Scholar]
- 38.Lamote K., Janssens E., Schillebeeckx E., Lapperre T.S., De Winter B.Y., van Meerbeeck J.P. The scent of COVID-19: viral (semi-)volatiles as fast diagnostic biomarkers? J. Breath Res. 2020;14(4) doi: 10.1088/1752-7163/aba105. [DOI] [PubMed] [Google Scholar]
- 39.Belizário J.E., Faintuch J., Malpartida M.G. Breath biopsy and discovery of exclusive volatile organic compounds for diagnosis of infectious diseases. Front Cell Infect Microbiol. 2021;10 doi: 10.3389/fcimb.2020.564194. 564194-564194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barker M., Hengst M., Schmid J., Buers H.J., Mittermaier B., Klemp D., Koppmann R. Volatile organic compounds in the exhaled breath of young patients with cystic fibrosis. Eur. Respir. J. 2006;27(5):929. doi: 10.1183/09031936.06.00085105. [DOI] [PubMed] [Google Scholar]
- 41.Gould O., Ratcliffe N., Król E., de Lacy Costello B. Breath analysis for detection of viral infection, the current position of the field. J. Breath Res. 2020;14(4) doi: 10.1088/1752-7163/ab9c32. [DOI] [PubMed] [Google Scholar]
- 42.Ruszkiewicz D.M., Sanders D., O'Brien R., Hempel F., Reed M.J., Riepe A.C., Bailie K., Brodrick E., Darnley K., Ellerkmann R. Diagnosis of COVID-19 by analysis of breath with gas chromatography-ion mobility spectrometry-a feasibility study. EClinicalMedicine. 2020;29:100609. doi: 10.1016/j.eclinm.2020.100609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grassin-Delyle S., Roquencourt C., Moine P., Saffroy G., Carn S., Heming N., Fleuriet J., Salvator H., Naline E., Couderc L.-J., Devillier P., Thévenot E.A., Annane D. Metabolomics of exhaled breath in critically ill COVID-19 patients: a pilot study. EBioMedicine. 2021;63:103154. doi: 10.1016/j.ebiom.2020.103154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H., Qi X., Ma J., Zhang C., Feng H., Yao M. MedRxiv; 2020. Breath-borne VOC Biomarkers for COVID-19. [Google Scholar]
- 45.Miller T.C., Morgera S.D., Saddow S.E., Takshi A., Palm M. Electronic nose with detection method for alcohol, acetone, and carbon monoxide in coronavirus disease 2019 breath simulation model. IEEE Sensor. J. 2021 doi: 10.1109/JSEN.2021.3076102. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berna A.Z., Akaho E.H., Harris R.M., Congdon M., Korn E., Neher S., M'Farrej M., Burns J., John A.R.O. 2020. Breath Biomarkers of Pediatric SARS-CoV-2 Infection: a Pilot Study, medRxiv : the Preprint Server for Health Sciences; p. 2020. 12.04.20230755. [Google Scholar]
- 47.Ratiu I.A., Ligor T., Bocos-Bintintan V., Mayhew C.A., Buszewski B. Volatile organic compounds in exhaled breath as fingerprints of lung cancer, asthma and COPD. J. Clin. Med. 2021;10(1):32. doi: 10.3390/jcm10010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Mastrigt E., Reyes-Reyes A., Brand K., Bhattacharya N., Urbach H.P., Stubbs A.P., de Jongste J.C., Pijnenburg M.W. Exhaled breath profiling using broadband quantum cascade laser-based spectroscopy in healthy children and children with asthma and cystic fibrosis. J. Breath Res. 2016;10(2) doi: 10.1088/1752-7155/10/2/026003. [DOI] [PubMed] [Google Scholar]
- 49.De Vincentis A., Pennazza G., Santonico M., Vespasiani-Gentilucci U., Galati G., Gallo P., Vernile C., Pedone C., Antonelli Incalzi R., Picardi A. Breath-print analysis by e-nose for classifying and monitoring chronic liver disease: a proof-of-concept study. Sci. Rep. 2016;6(1):25337. doi: 10.1038/srep25337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montoya J.J.M., Penalva G.T., Navarro E.À., Chilo J. 2020 IEEE International Instrumentation and Measurement Technology Conference (I2MTC) 2020. Monitoring diabetic ketoacidosis by urine ketones tracing using an E-nose; pp. 1–5. [Google Scholar]
- 51.Tenero L., Sandri M., Piazza M., Paiola G., Zaffanello M., Piacentini G. Electronic nose in discrimination of children with uncontrolled asthma. J. Breath Res. 2020;14(4) doi: 10.1088/1752-7163/ab9ab0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.