Abstract
Purpose
This study investigated 18F-FDG PET/CT features of adenovirus-vectored vaccination against COVID-19 in healthy subjects.
Patients and Methods
Thirty-one health care workers had been vaccinated Vaxzevria and underwent FDG PET/CT as an optional test for a cancer screening program. Size and FDG uptake of the hypermetabolic lymph nodes were measured. Uptake value of spleen was also measured with liver for comparison.
Results
All examinees who underwent FDG PET/CT within 14 days’ interval showed hypermetabolic lymphadenopathies ipsilateral to vaccine injection. All examinees with hypermetabolic lymphadenopathy had simultaneous muscular uptakes until 23 days’ interval. Among 12 examinees who underwent FDG PET/CT more than 15 days after vaccination, only 3 male examinees did not show hypermetabolism in the axillary lymph nodes. There was no female examinee with negative hypermetabolic lymphadenopathy until 29 days after vaccination.
Conclusions
Hypermetabolic reactive lymphadenopathy in the ipsilateral axillary area with or without supraclavicular area is most likely to occur in a healthy person with recent adenovirus-vectored COVID-19 vaccination on FDG PET/CT.
Key Words: FDG PET/CT, COVID-19, Vaxzevria, vaccination, lymphadenopathy, axillary lymph node, AstraZeneca
PET/CT using 18F-FDG is a commonly used imaging modality for various malignant tumors but also for infection and inflammation. There have been series of articles showing FDG uptakes in the axillary lymph nodes on PET/CT after vaccination shots in the ipsilateral shoulder. Most of the articles presented cases from annual seasonal influenza vaccinations including adjuvant products. Besides seasonal influenza, many other vaccines also showed hypermetabolic lymphadenopathy including H1N1 influenza A, human papilloma virus, and so on.1–7 COVID-19 vaccines are no exceptions from other vaccines. Preliminary guidelines and medical articles including case reports on lymphadenopathy after COVID-19 vaccine on various imaging modalities are published.8–26
South Korean government imported 2 different vaccine products to fight against COVID-19: one from the Pfizer-BioNTech (Pfizer), which is mRNA biotechnology vaccine, and the other vaccine, Vaxzevria (previously COVID-19 Vaccine AstraZeneca) from Oxford-AstraZeneca, which is adenovirus-vectored vaccine. Vaxzevria is not approved by the US Food and Drug Administration at this moment. However, it is 1 of the most widely used vaccines against COVID-19 and has been approved for emergency use in health administrations of over 79 countries including European Medicines Agency, United Kingdom Medicines and Healthcare Products Regulatory Agency, and South Korea’s Ministry of Food and Drug Safety.
Several cases of deep vein thrombosis (DVT) and pulmonary embolism (PE) made the vaccine to be halted in some countries of Europe. The World Health Organization and European Medicines Agency concluded that there is no increased risk of DVT/PE and causal association with Vaxzevria. However, concerns about cerebral venous sinus thrombosis and thrombocytopenia are still going on especially in women.27,28
Previous but recent studies and case reports showed lymphadenopathy from the mRNA biotechnology vaccines from Pfizer-BioNTech and Moderna. One of the noticeable findings from other vaccine-related lymphadenopathy was the high spleen uptake in subjects with very short interval between vaccine and scan. It was suggested as an indirect sign of COVID-19 vaccination, especially when history of vaccination is missing or not accurate.
There is no investigational study about lymphadenopathy associated with Vaxzevria on FDG PET/CT at this moment except for a case report on Oxford-AstraZeneca COVID-19 vaccination–induced lymphadenopathy on 18F-choline PET/CT.20 We investigated FDG PET/CT features of adenovirus-vectored vaccination against COVID-19 in healthy subjects, more focused on distinctive features from other vaccines including mRNA COVID-19 vaccines.
PATIENTS AND METHODS
Subjects
Vaxzevria vaccines were allocated to our medical center. During the 1-week period between March 8 and March 15, 2021, employees including health care workers received vaccination against COVID-19. Among more than 6000 employees who were vaccinated, 31 health care workers at out hospital underwent FDG PET/CT as an optional test for cancer screening program from March 12 to April 6, 2021. All the examinees were in good health condition with no history of malignancy.
Among 31 examinees, 20 were women and 11 were men. The age spectrum was from 39 to 60 years old with average of 45 (±5.8 SD) years old (Table 1). We obtained information about the date of vaccine injection and the location of vaccine injection site. Most of the examinees, 30 of 31, received vaccine shots on the left shoulder. Single examinee received vaccine shot on his right shoulder. This observational study was done retrospectively. This study was approved by our institutional review board, and the requirement to obtain written informed consent was waived.
TABLE 1.
Demographics and FDG PET/CT Methods of the Examinees
| No. Examinees | 31 |
|---|---|
| Sex | |
| Male | 11 (35%) |
| Female | 20 (65%) |
| Age, mean ± SD, y | 45 ± 5 |
| Interval between vaccination and FDG PET/CT, mean ± SD, d | 15 ± 7 |
| Site of vaccination | |
| Left | 30 (97%) |
| Right | 1 (3%) |
| Laterality of FDG administration to vaccination | |
| Ipsilateral | 12 (39%) |
| Contralateral | 19 (61%) |
SD, standard deviation.
FDG PET/CT
All examinees fasted for more than 6 hours and had blood glucose levels of less than 150 mg before the FDG administration. FDG administration was done intravenously on either side of the arms, irrespective of the vaccination injection site (5.0 MBq/kg body weight of FDG without IV or oral contrast). Most of the examinees had temporary IV catheters already placed for use in other medical tests before arrival. Fourteen examinees (52%) received FDG on the right and were all vaccinated on the left, contralateral to FDG administration. In other 13 examinees (48%), FDG was administered on the left. Eleven of the 13 examinees had been vaccinated on the same left. Only 1 of them had been vaccinated on the contralateral right.
Whole-body PET and CT images were acquired 60 minutes after injection of 5.0 MBq/kg FDG without IV or oral contrast on a Discovery STE PET/CT scanner (GE Healthcare, Milwaukee, WI). Continuous spiral CT was performed with 16-slice helical CT (140 keV, 30–170 mA; Discovery STE). An emission scan was then obtained from head to thigh for 2.5 minutes per frame in 3-dimensional mode. PET images were reconstructed using CT for the attenuation correction by ordered-subsets expectation maximization algorithm with 20 subsets and 2 iterations (matrix, 128 × 128; voxel size, 3.9 × 3.9 × 3.3 mm).
Image Interpretation and Analysis
Fiver nuclear medicine physicians visually interpreted the FDG PET/CT images and CT images and made initial interpretation reports (GE Advanced Workstation 4.7; GE Healthcare, Milwaukee, WI). Secondary thorough interpretations, measurements, and analyses were done by other 2 nuclear medicine physicians who have over 3000 PET/CT interpretation experience. Uptake intensity of lymph nodes was measured using SUVmax. SUVmax value was rounded up to first decimal. Size of the largest hypermetabolic lymph node was measured on CT images by millimeters in short-axis diameter. Spleen uptake value was also measured with liver. Femoral veins and pulmonary arteries were inspected in concerns of hypercoagulability and thrombosis adverse effects associated with Vaxzevria, which can be detected by FDG PET/CT if present.29,30 All statistical analysis and statistical figures were performed by using IBM SPSS Statistics V.27.0 (IBM, Armonk, NY).
RESULTS
All examinees who underwent FDG PET/CT within 14 days’ interval showed hypermetabolic lymphadenopathies ipsilateral to the injection site (Table 2). The range of axillary lymph node SUVmax was from 2.0 to 8.0 (Table 3). All examinees with hypermetabolic lymphadenopathy had simultaneous muscular uptakes up to 22 days’ interval (Fig. 1). In 1 examinee, his lateral portion of the injected arm was not covered. However, his intramuscular lymph node uptake has been helpful to assume the injection site (Fig. 2). From 23 days’ interval, examinee with hypermetabolic lymphadenopathy without muscular uptake has first appeared. No more muscle uptakes were visible afterward from 24 days postvaccination. Intramuscular lymph node uptake was visible in 3 examinees among 4 examinees with shortest interval period of 4 days after vaccination (Fig. 3).
TABLE 2.
FDG-Avid Lymphadenopathy in Regard to Interval Between Vaccination and Scan
| Interval | Positive (n = 28) | Negative (n = 3) | Total (n = 31) |
|---|---|---|---|
| 1–14 d | 19 (100%) | 0 (0%) | 19 |
| Male | 5 | 0 | 5 |
| Female | 14 | 0 | 14 |
| 15–29 d | 9 (63%) | 3 (37%) | 12 |
| Male | 3 (50%) | 3 (50%) | 6 |
| Female | 6 (100%) | 0 (0%) | 6 |
TABLE 3.
PET/CT Demonstration of Vaccination-Related Lymphadenopathy and Inflammation in 28 Examinees
| Examinee No. | Age, y | Sex | Interval, d | ALN Size, mm | ALN SUVmax | SCN SUVmax | Upper Arm LN SUVmax | Deltoid SUVmax |
|---|---|---|---|---|---|---|---|---|
| 1 | 40 | F | 4 | 6 | 3.8 | — | 2.7 | 2.1 |
| 2 | 40 | M | 4 | 8 | 5.0 | 2.9 | 3.8 | * |
| 3 | 40 | F | 4 | 8 | 7.6 | — | 2.4 | 2.6 |
| 4 | 50 | F | 4 | 9 | 6.6 | — | — | 3.7 |
| 5 | 44 | F | 6 | 9 | 3.2 | 2.4 | — | 5.4 |
| 6 | 44 | F | 9 | 6 | 5.4 | 3.4 | — | 2.6 |
| 7 | 39 | F | 9 | 6 | 3.8 | — | — | 2.2 |
| 8 | 44 | F | 11 | 8 | 4.1 | 3.4 | — | 3.0 |
| 9 | 40 | F | 11 | 5 | 2.2 | — | — | 2.6 |
| 10 | 44 | F | 12 | 7 | 2.9 | — | — | 3.6 |
| 11 | 55 | F | 12 | 9 | 8.0 | — | — | 5.2 |
| 12 | 50 | F | 12 | 6 | 2.6 | — | — | 2.7 |
| 13 | 44 | F | 12 | 6 | 3.9 | — | — | 2.2 |
| 14 | 40 | F | 12 | 7 | 3.2 | 2.5 | — | 3.4 |
| 15 | 60 | M | 14 | 7 | 2.4 | — | — | 2.6 |
| 16 | 49 | M | 14 | 6 | 4.1 | 1.5 | — | 3.0 |
| 17 | 49 | F | 14 | 8 | 6.3 | — | — | 2.6 |
| 18 | 49 | M | 14 | 7 | 3.9 | — | — | 2.7 |
| 19 | 49 | M | 14 | 7 | 2.0 | — | — | 1.6 |
| 20 | 40 | F | 15 | 8 | 3.6 | 2.4 | — | 2.4 |
| 21 | 40 | F | 21 | 7 | 5.3 | — | — | 1.6 |
| 22 | 49 | M | 22 | 4 | 2.0 | — | — | 1.2 |
| 23 | 41 | F | 23 | 6 | 5.4 | 1.6 | — | 2.0 |
| 24 | 40 | M | 23 | 8 | 2.4 | — | — | — |
| 25 | 40 | F | 26 | 4 | 2.5 | — | — | — |
| 26 | 45 | F | 27 | 5 | 2.8 | — | — | — |
| 27 | 44 | M | 28 | 4 | 3.2 | — | — | — |
| 28 | 49 | F | 29 | 6 | 2.3 | — | — | — |
ALN, axillary lymph node; SCN, supraclavicular lymph node; F, female; M, male.
*Presumed injection site of the upper arm not covered in the scan.
FIGURE 1.
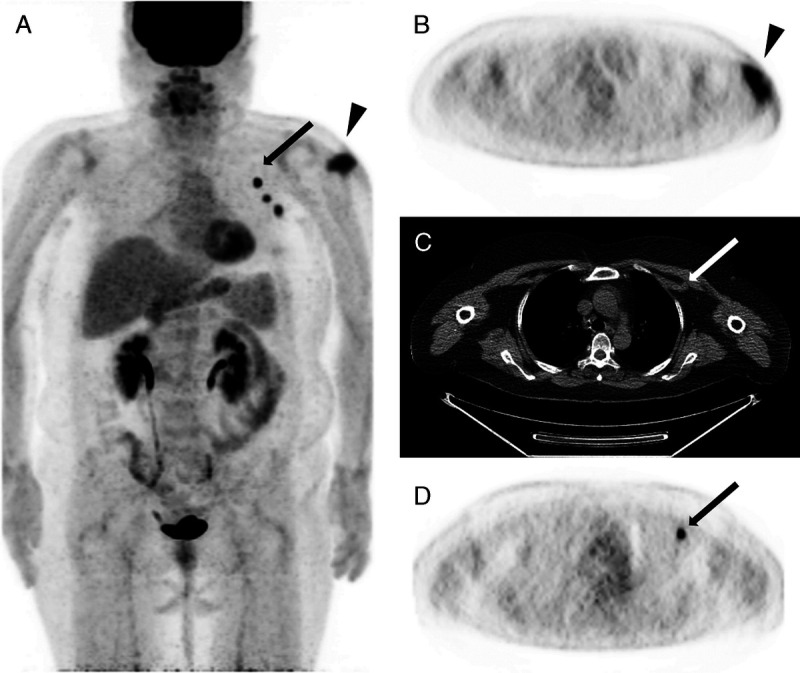
Case 11. FDG PET/CT scan of a 55-year-old woman: 12 days postvaccination. Multiple hypermetabolic axillary lymph nodes with highest uptake of SUVmax of 8.0, which is highest measured uptake value in the study (arrows). Not only the muscular uptake was visible, but also discrete subcutaneous local inflammation was shown (triangles). A, PET MIP image. B, Axial PET image. C, Axial nonenhanced CT. D, Axial PET image.
FIGURE 2.
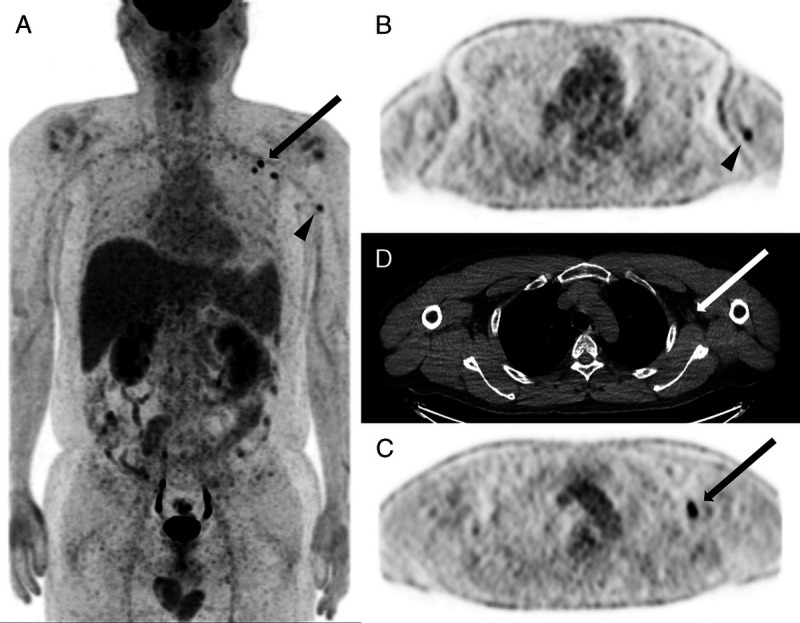
Case 2. FDG PET/CT scan of a 40-year-old man: 4 days postvaccination. Multiple hypermetabolic axillary lymph nodes with SUVmax of 5.0 (arrows) and supraclavicular lymph node of 2.9. Presumed injection site of the muscle is not covered by the scan. Adjacent intramuscular lymph node uptake implicates the site of injection (triangles). A, PET MIP image. B, Axial PET image. C, Axial nonenhanced CT. D, Axial PET image.
FIGURE 3.
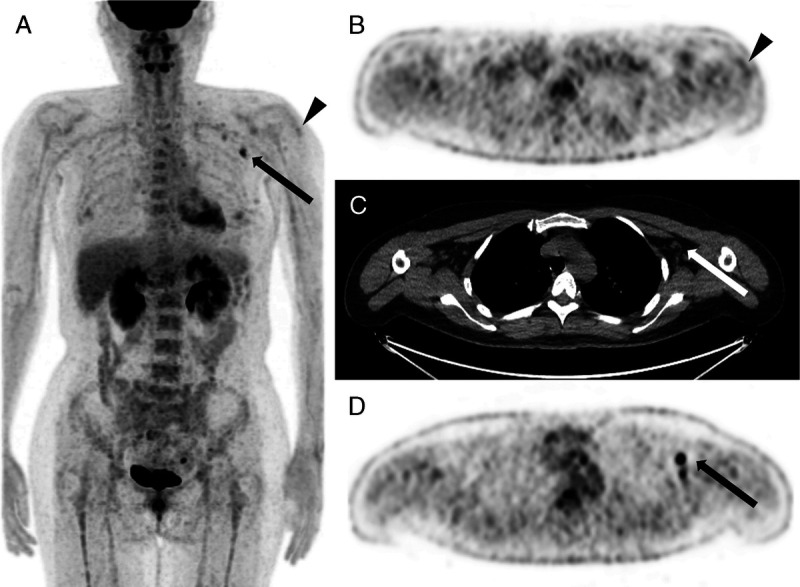
Case 23. FDG PET/CT scan of a 41-year-old woman: 23 days postvaccination. Multiple areas of axillary lymph node accumulation with SUVmax of 5.4 (arrows) and still showing peri-injectional muscle uptake (triangles). A, PET MIP image. B, Axial PET image. C, Axial nonenhanced CT. D, Axial PET image.
Three male examinees in the 18, 19, and 23 days’ postvaccination intervals showed no glucose hypermetabolism in the axillary lymph nodes. Male examinee with 22 days’ interval showed discriminable but very mild uptake value of axillary lymph node (SUVmax, 2.0). On the contrary, there was no female examinee with negative lymphadenopathy up to 29 days after vaccination, which is the longest interval included in this study. In examinees without hypermetabolic lymphadenopathy, muscular activity was also not visible (Fig. 4).
FIGURE 4.
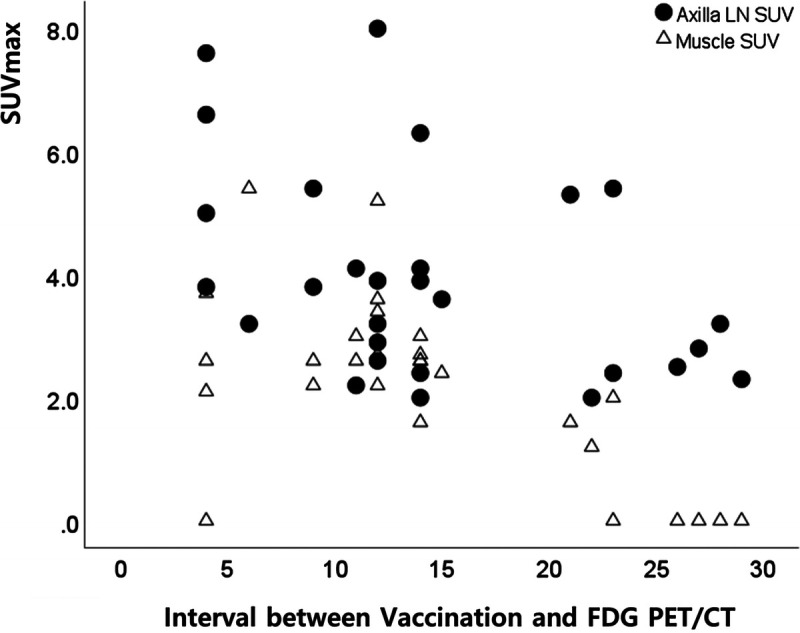
Distribution of axillary lymph node and muscular uptakes according to interval period between vaccination and FDG PET/CT.
Mean SUVmax of spleen was 2.9 (±0.5 SD). Mean SUVmax of liver was 3.8 (±0.6 SD). No examinee showed splenomegaly or higher uptake of the spleen compared with the liver. No examinee showed any uptake suggesting DVT or PE. Platelet counts were within range from lowest of 172 × 103/μL to highest with clinically nonsignificant borderline thrombocytosis of 384 × 103/μL (average, 269 × 103/μL).
Malignancy was not detected in all 31 examinees who went through FDG PET/CT scans for cancer screening.
DISCUSSION
In our study, 31 examinees underwent FDG PET/CT and had vaccination within a month before the scan. Except for 3 male examinees, they all showed abnormal FDG accumulations in the axilla, supraclavicular, or intramuscular lymph nodes of upper arm. Lymphadenopathy by adenovirus-vectored COVID-19 vaccine seems to last longer and are more prominent than other vaccines. Shirone et al2 showed 4 of 5 positive vaccine-related lymphadenopathy in examinees with interval less than 7 days for influenza vaccine in health examination setting similar as ours. There was no positive lymphadenopathy by influenza vaccine after 7 days. Cohen et al10 showed 26.3% of vaccine-associated hypermetabolic lymphadenopathy with mRNA vaccine against COVID-19 in group with initial shot without second booster. Bernstine et al22 showed much less proportion of 14.5% in cancer patients with only first shot. Because the examinees in our study are generally healthy and have no history of malignancy, we can presume they are more immunocompetent than the most patients enrolled in mRNA COVID-19 studies with history of malignancy. Immune competency may have played roll in higher positivity of lymphadenopathy.
Vaccine-associated lymphadenopathies in females were more prominent and lasted longer than males. In the fact that some particular adverse effects including CSVT and thrombocytopenia from Vaxzevria tend to occur more in the women, this difference might have some implication to the immune reactions.
Questionnaire about vaccination history including details of the vaccination site can be helpful and is essential data to be obtained. Muscular uptake on the deltoid muscle and subcutaneous uptake in adjacent area can be helpful sign to presume the reactive axillary lymph nodes to be from vaccination. If the injection site is not covered in the field of view, intramuscular lymph node can also be a useful alternative clue for recent vaccine injection. However, thorough searching for causes of origin other than vaccination, especially malignancy, should be performed.
In administration of FDG, highest priority should be injecting contralateral side of the tumor regardless of the vaccination. If laterality of the FDG administration does not interfere with tumor, we suggest FDG injected to the ipsilateral side of the vaccination. Because it is highly likely for the hypermetabolic lymphadenopathy to occur after Vaxzevria vaccine, sparing the contralateral side of axillary and neck lymph nodes would be a reasonable choice.
LIMITATIONS
Regarding the demographics, sex ratio is skewed toward female probably from unbalanced sex ratio of hospital employees. Age spectrum is relatively narrow from 39 to 60 years old. There are no examinees or subjects with other COVID-19 vaccinations including mRNA-mediated vaccines at our medical center. Direct comparison with mRNA vaccines was not capable.
CONCLUSIONS
We investigated the influence of adenovirus-vectored COVID-19 vaccination, Vaxzevria, on FDG PET/CT imaging. If adenovirus-vectored COVID-19 vaccination was administered recently, reactive lymphadenopathy in the ipsilateral axillary and/or supraclavicular area is most likely to occur on FDG PET/CT, especially within 14 days. Muscular inflammatory uptake and intramuscular lymph node uptake of the upper arm can be used as additional signs for verification of the vaccination.
Footnotes
Conflicts of interest and sources of funding: none declared.
Contributor Information
Muheon Shin, Email: muheon.shin@samsung.com.
Chae Young Hyun, Email: chaeyoung.hyun@samsung.com.
Yoon Ho Choi, Email: yh38.choi@samsung.com.
Joon Young Choi, Email: jynm.choi@samsung.com.
Kyung-Han Lee, Email: khnm.lee@samsung.com.
REFERENCES
- 1.Focosi D Caracciolo F Galimberti S, et al. False positive PET scanning caused by inactivated influenza virus vaccination during complete remission from anaplastic T-cell lymphoma. Ann Hematol. 2008;87:343–344. [DOI] [PubMed] [Google Scholar]
- 2.Shirone N Shinkai T Yamane T, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26:248–252. [DOI] [PubMed] [Google Scholar]
- 3.Thomassen A Lerberg Nielsen A Gerke O, et al. Duration of 18F-FDG avidity in lymph nodes after pandemic H1N1v and seasonal influenza vaccination. Eur J Nucl Med Mol Imaging. 2011;38:894–898. [DOI] [PubMed] [Google Scholar]
- 4.Burger IA Husmann L Hany TF, et al. Incidence and intensity of F-18 FDG uptake after vaccination with H1N1 vaccine. Clin Nucl Med. 2011;36:848–853. [DOI] [PubMed] [Google Scholar]
- 5.Panagiotidis E Exarhos D Housianakou I, et al. FDG uptake in axillary lymph nodes after vaccination against pandemic (H1N1). Eur Radiol. 2010;20:1251–1253. [DOI] [PubMed] [Google Scholar]
- 6.Coates EE Costner PJ Nason MC, et al. Lymph node activation by PET/CT following vaccination with licensed vaccines for human papillomaviruses. Clin Nucl Med. 2017;42:329–334. [DOI] [PubMed] [Google Scholar]
- 7.Williams G, Joyce RM, Parker JA. False-positive axillary lymph node on FDG-PET/CT scan resulting from immunization. Clin Nucl Med. 2006;31:731–732. [DOI] [PubMed] [Google Scholar]
- 8.Ahn RW Mootz AR Brewington CC, et al. Axillary lymphadenopathy after mRNA COVID-19 vaccination. Radiol Cardiothorac Imaging. 2021;3:e210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker AS Perez-Johnston R Chikarmane SA, et al. Multidisciplinary recommendations regarding post-vaccine adenopathy and radiologic imaging: radiology scientific expert panel. Radiology. 2021;210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen D Krauthammer SH Wolf I, et al. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [(18)F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imaging. 2021;48:1854–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edmonds CE, Zuckerman SP, Conant EF. Management of unilateral axillary lymphadenopathy detected on breast MRI in the era of coronavirus disease (COVID-19) vaccination. AJR Am J Roentgenol. 2021. [DOI] [PubMed] [Google Scholar]
- 12.Eifer M, Eshet Y. Imaging of COVID-19 vaccination at FDG PET/CT. Radiology. 2021;299:E248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanneman K, Iwanochko RM, Thavendiranathan P. Evolution of lymphadenopathy at PET/MRI after COVID-19 vaccination. Radiology. 2021;210386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehman CD D’Alessandro HA Mendoza DP, et al. Unilateral lymphadenopathy after COVID-19 vaccination: a practical management plan for radiologists across specialties. J Am Coll Radiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman CD, Lamb LR, D’Alessandro HA. Mitigating the impact of coronavirus disease (COVID-19) vaccinations on patients undergoing breast imaging examinations: a pragmatic approach. AJR Am J Roentgenol. 2021. [DOI] [PubMed] [Google Scholar]
- 16.McIntosh LJ Bankier AA Vijayaraghavan GR, et al. COVID-19 vaccination-related uptake on FDG PET/CT: an emerging dilemma and suggestions for management. AJR Am J Roentgenol. 2021. [DOI] [PubMed] [Google Scholar]
- 17.Mortazavi S. Coronavirus disease (COVID-19) vaccination associated axillary adenopathy: imaging findings and follow-up recommendations in 23 women. AJR Am J Roentgenol. 2021. [DOI] [PubMed] [Google Scholar]
- 18.Ozutemiz C Krystosek LA Church AL, et al. Lymphadenopathy in COVID-19 vaccine recipients: diagnostic dilemma in oncology patients. Radiology. 2021;210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu G, Lu Y. COVID-19 mRNA vaccination-induced lymphadenopathy mimics lymphoma progression on FDG PET/CT. Clin Nucl Med. 2021;46:353–354. [DOI] [PubMed] [Google Scholar]
- 20.Nawwar AA Searle J Singh R, et al. Oxford-AstraZeneca COVID-19 vaccination induced lymphadenopathy on [18F]Choline PET/CT-not only an FDG finding. Eur J Nucl Med Mol Imaging. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed N Muzaffar S Binns C, et al. COVID-19 vaccination manifesting as incidental lymph nodal uptake on 18F-FDG PET/CT. Clin Nucl Med. 2021;46:435–436. [DOI] [PubMed] [Google Scholar]
- 22.Bernstine H Priss M Anati T, et al. Axillary lymph nodes hypermetabolism after BNT162b2 mRNA COVID-19 vaccination in cancer patients undergoing 18F-FDG PET/CT: a cohort study. Clin Nucl Med. 2021;46:396–401. [DOI] [PubMed] [Google Scholar]
- 23.Brown AH Shah S Groves AM, et al. The challenge of staging breast cancer with PET/CT in the era of COVID vaccination. Clin Nucl Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doss M Nakhoda SK Li Y, et al. COVID-19 vaccine-related local FDG uptake. Clin Nucl Med. 2021;46:439–441. [DOI] [PubMed] [Google Scholar]
- 25.Moghimi S, Wilson D, Martineau P. FDG PET findings post-COVID vaccinations: signs of the times? Clin Nucl Med. 2021;46:437–438. [DOI] [PubMed] [Google Scholar]
- 26.Ulaner GA, Giuliano P. 18F-FDG-avid lymph nodes after COVID-19 vaccination on 18F-FDG PET/CT. Clin Nucl Med. 2021;46:433–434. [DOI] [PubMed] [Google Scholar]
- 27.WHO: Statement of the WHO Global Advisory Committee on Vaccine Safety (GACVS) COVID-19 subcommittee on safety signals related to the AstraZeneca COVID-19 vaccine . Available at: https://www.who.int/news/item/19-03-2021-statement-of-the-who-global-advisory-committee-on-vaccine-safety-(gacvs)-covid-19-subcommittee-on-safety-signals-related-to-the-astrazeneca-covid-19-vaccine. Accessed March 19, 2021.
- 28.EMA: COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets . Available at: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots. Accessed March 18, 2021.
- 29.Rondina MT Lam UT Pendleton RC, et al. (18)F-FDG PET in the evaluation of acuity of deep vein thrombosis. Clin Nucl Med. 2012;37:1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittram C, Scott JA. 18F-FDG PET of pulmonary embolism. AJR Am J Roentgenol. 2007;189:171–176. [DOI] [PubMed] [Google Scholar]


