Abstract
Background:
Pulmonary mycoses are important diseases of the respiratory tract caused by pulmonary fungal pathogens. These pathogens are responsible for significant morbidity and mortality rates worldwide; however, less attention has been paid to them. In this study we determined the prevalence of pulmonary fungal pathogens among individuals with clinical features of pulmonary tuberculosis at Mbarara Regional Referral Hospital.
Method:
This was a hospital based cross sectional survey. Sputum samples were collected from each study participant. For each sample, the following tests were performed: Sabouraud dextrose agar for fungal culture, GeneXpert for Mycobacteria tuberculosis (MTB) and potassium hydroxide for fungal screening. Filamentous fungal growth and yeasts were further examined with lactophenol cotton blue staining and germ tube respectively.
Results:
Out of 113 study participants, 80 (70.7%) had pulmonary fungal pathogens whilst those with pulmonary tuberculosis numbered five (4.4%). Candida albicans [21 (22.58%)] and Aspergillus species [16 (17.20%)] were the pathogens most identified among others. Two (1.7%) TB GeneXpert positive participants had fungal pathogens isolated from their sputum samples. We established a prevalence of 57 (71.3%) for pulmonary fungal pathogen (PFP) isolates, three (60.0%) for MTB in HIV positive patients and 18 (22.5%) for PFP, and zero (0.0%) for MTB in HIV negative patients. On the other hand, two (100%) HIV positive patients had both PFP isolates and MTB.
Conclusion:
Our findings highlight the diversity of neglected pulmonary fungal pathogens whose known medical importance in causing pulmonary mycoses cannot be overemphasised. Therefore this presents a need for routine diagnosis for pulmonary mycoses among TB suspects and set-up of antimicrobial profile for pulmonary fungal isolates to support clinical management of these cases.
Keywords: pulmonary fungal infections, pulmonary fungal pathogens, pulmonary mycoses, pulmonary tuberculosis
Introduction
Pulmonary tuberculosis (PTB) remains a fundamental cause of sub-acute and chronic pulmonary diseases and thus a major cause of mortality attributed mainly to Mycobacterium tuberculosis (MTB) complex. MTB claims over one million people worldwide with about 58% in Asia and 27% in Africa, where Uganda lies.1,2 In Africa and Asia, the annual incidence of TB is very high, reaching hundreds of cases per 100,000 people.3 In Uganda alone, TB is estimated at a prevalence of 200 per 100,000 people;4 in Mbarara, the prevalence of TB is estimated at 98 per 100,000 people.5 Certainly, MTB is still a major co-morbidity and a leading cause of death among individuals infected with human immunodeficiency virus (HIV/AIDS). Additionally, multidrug resistant TB and the emergence of extensively drug resistant TB have seriously complicated TB diagnosis, treatment and management. However, amidst all this, TB is not the only pulmonary infection of importance, especially with the expansion of at-risk populations worldwide. For instance, the role of pulmonary fungal infections caused by pulmonary fungal pathogens has been highlighted recently and the similarity in clinical and radiological characteristics with TB further complicates diagnosis and management of such pulmonary infections.6 Indeed, pulmonary mycoses can mimic and easily be misdiagnosed as TB and vice versa. In this context, fungi have over time gained attention for their recent emerging medical importance worldwide.7–9 Over the past 30 years now, fungi have transitioned into key aetiological agents for difficult to manage infections, killing at least one million people annually; and yet still remain among the neglected diseases globally. Serious invasive fungal infections occur in immune-compromised patients such as HIV/AIDS, cancer and transplantation patients among others, in many of whom they complicate and worsen the disease.10 For instance, in Africa, pulmonary fungal infections are reported at about 15–35%, mainly in HIV/TB co-infected cohorts. Additionally, the similarity in clinical and diagnostic manifestations between TB and pulmonary mycoses calls for equal attention if management of both infections is to be streamlined.11 This is posing enormous challenges to health care professionals, especially in resource limited settings where diagnosis is not precise. It is possible that in numerous cases missed fungal pulmonary mycoses due to lack of specific clinical manifestations cause a high rate of morbidity and mortality in patients initially suspected and treated for TB. The question at hand here is whether fungal pulmonary infection can be primary or secondary in TB infection. Although this is a challenging question to answer at the moment, through this study we sought to determine the prevalence and coexistence of pulmonary fungal pathogens, PTB and HIV among individuals with clinical signs of pulmonary tuberculosis at a TB referral clinic in south-western Uganda.
Materials and methods
Study design
This was a hospital based cross sectional study. The study samples were processed from the Mycology Laboratory, Department of Microbiology, Mbarara University of Science and Technology (MUST), whose clinical Mycology Laboratory serves Mbarara Regional Referral Hospital (MRRH) patients.
Study duration
The study was carried out from November 2019 to June 2020.
Sample size
Sputum samples were collected from 113 clinically PTB suspected patients referred to the TB clinic of MRRH.
Inclusion criteria of study participants
Patients with signs and symptoms of PTB who visited the TB unit who required MTB GeneXpert diagnostic test were included.
The study included TB suspects with a negative TB diagnostic history and those who were not on antifungals.
Signed informed consent was sought from each study participant prior to sputum sample collection. As for children, written informed assent was obtained from their care givers.
Exclusion criteria
TB suspects who failed to provide sputum samples and whose request forms lacked demographics were excluded from this study.
Specimen collection
First morning sputum sample for MTB GeneXpert and fungal culture was collected aseptically from study participants in a sterile dry wide-necked, leak-proof universal container from each of study participant suspected for PTB.
Transport and storage of samples
Samples were transported in courier boxes from point of collection to the laboratory. Samples which were not processed within the same day of collection were stored in a refrigerator at 4–8°C awaiting processing
Specimen processing
Collected sputum samples were processed at the TB and Mycology Laboratory Unit at Microbiology Department, Faculty of Medicine, MUST. Demographic data and HIV status of each study participant were recorded.
GeneXpert MTB/ Rifampicin (RIF) assay
Sample reagent was added to sputum sample in a ratio of 2:1 ml and the sputum sample was allowed to liquefy for 15 min. A sterile dropper was used to transfer 2 ml of mixture into the GeneXpert cartridge, which was loaded into the GeneXpert machine for MTB detection for 2 h. The results were reported as ‘MTB detected’ or ‘MTB not detected’.
Potassium hydroxide (KOH) mounts
A drop of 10% KOH was placed on a clean glass slide using Pasteur pipette. A small portion of sputum was added into the KOH drop using a sterile wire loop and mixed well. The coverslip was placed on top of this mixture and the preparation was placed in a moist chamber and kept at room temperature for 30 min. The preparation was examined under low power microscope objectives for the presence or absence of fungal elements.
Gram stain
Gram stain smear was made from the sputum sample to test for the Gram reaction of fungi and also the size, shape and arrangement of fungal elements. For Gram positive yeast-like cells, mucopurulent absence or presence of pseudo hyphae was recorded.
Fungal culture
Sabouraud dextrose agar (SDA) containing antibiotic chloramphenicol and gentamicin was used to culture sputum samples. The specimens were streaked onto the medium in the Universal bottles with a sterile inoculating loop in order to obtain isolated colonies. The preparations were then incubated at 25–30°C in an inverted position (agar side up). Cultures were examined at least weekly for fungal growth and held for 4 weeks before being reported as negative. After sufficient incubation, considering colony morphology, texture, rate of growth, surface of the colony and pigmentation on the surface and reverse of the colony on SDA tubes were recorded. The significant fungal isolates on culture were identified to the species level, using standard mycological procedures.
Lactophenol cotton blue staining
Lactophenol cotton blue was used for microscopic identification and characterisation of fruiting bodies such AS conidia, sporangia, rhizoids and hypha or mycelia of cultivated fungi on SDA. A drop of lactophenol cotton blue stain was placed on a clean grease-free glass slide. A small fragment of cottony, woolly or powdery colony was picked from the midpoint of the culture using a sterile straight wire and placed on a clean glass slide for the staining process. A clean coverslip was applied avoiding air bubbles. Excess stain was removed with blotting paper and the preparation examined using ×10 and ×40 objectives of the microscope. Fungal element features such as microconidia, macroconidia, chlamydospores and hyphae with spiral, pertinate and antler-like structures were investigated. These features seen on the stained slide were compared with established characteristic fungal features using mycology atlases.
Germ tube test
Germ tube test is a simple, reliable procedure for the identification of Candida albicans. Human serum (0.5–1 ml) was put into a 12 mm × 75 mm test tube and the yeast colonies were suspended into the serum to obtain a faintly turbid suspension. The preparation was then incubated in the tubes at 37°C for 2–3 h in an incubator. Using a sterile Pasteur pipette, the suspension was removed and examined microscopically for the presence or absence of germ tubes. Positive test showed germ tubes arising directly from the yeast cell and had parallel walls without any constriction at their point of origin, which was diagnostic for C. albicans.
Safety and environment
All biological specimens, including used cartridges, capable of transmitting infectious agents were treated with universal precautions.
All laboratory procedures were done in a level 2 TB laboratory.
Personal protective equipment such as disposable gloves, laboratory coats were used when handling specimens and reagents.
Washing of hands was done thoroughly after handling specimens and test reagents.
Disposing of used Xpert MTB/RIF cartridges was done according to the country’s safety guidelines for hazardous material.
Quality control measures
Well collected sputum samples were accepted for analysis.
Known standard fungal element morphologies and known fungal atlases were used to confirm established characteristic fungal features.12
Laboratory reference cultured fungal elements grown in the laboratory were also used.
Results
Demographic distribution, PTB, pulmonary fungal pathogens (PFPs) and PTB–fungal co-existence profiles
Of 113 sputum samples collected, 4.4% (n = 5) were positive for PTB, 20.4% (n = 23) were positive for PFP using KOH direct examination, while 70.7% (n = 80) yielded a positive fungal growth on culture, which was the diagnostic test for PFP. Two (0.02%) presented with a mixed infection of pulmonary TB–fungal co-existence (Table 1). Based on the findings of this study, the prevalence of TB was more in males [80.4% (n = 4)] than females (n = 1). While taking the average of KOH and culture results, the prevalence of PFP was more in females [62.0% (n = 31)] and the only two mixed infections detected were seen only in males (Table 1). The mean age of the participants was 41.9 ± 15 years and the minimum and maximum ages were 11 and 84 years respectively. The highest incidence of both PTB and PFP was found in patients aged 18–34 and 35–64 years respectively. Similarly, the only mixed infections detected were also in the same age category (Table 1).
Table 1.
Demographic distribution, pulmonary tuberculosis (PTB), pulmonary fungal pathogens and PTB–fungal co-existence.
| Variable | PTB detected | Mycological pathogens identified by KOH and culture | PTB-fungal co-existence | ||
|---|---|---|---|---|---|
| GeneXpert n = 5 |
KOH n = 23 |
Culture n = 80 |
PTB–PFP
co-existence n = 2 |
||
| Age group, years | Proportion (%) | Proportion (%) | Proportion (%) | Proportion (%) | Proportion (%) |
| Children, 1–13 | 3 (2.7) | 0 (0.0) | 1 (4.5) | 1 (1.2) | 0 (0.0) |
| Adolescents, 14–17 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Youth, 18–34 | 38 (33.6) | 2 (40.0) | 3 (13.0) | 29 (36.2) | 1 (50.0) |
| Middle aged, 35–64 | 61 (54.0) | 2 (40.0) | 16 (69.6) | 44 (55.0) | 1 (50.0) |
| Elderly, >64 | 11 (9.7) | 1 (20.0) | 3 (13.0) | 6 (7.5) | 0 (0.0) |
| Gender | |||||
| Male | 46 (40.7) | 4 (80.0) | 8 (34.8) | 33 (41.2) | 2 (100) |
| Female | 67 (59.3) | 1 (20.0) | 15 (65.2) | 47 (58.8) | 0 (0.0) |
KOH, potassium hydroxide; PFP, pulmonary fungal pathogen.
Prevalence of PTB and PFP by HIV status
Following the HIV status of our study participants obtained through their medical history, the majority of the participants were HIV positive [70.8% (n = 80)]. HIV negative participants were 23.0% (n = 26) whereas 6.2% (n = 7) had unknown HIV status. We further established that both PTB [60% (n = 3)] and pulmonary fungal pathogens, 56.5% by KOH and 71.3% by fungal culture, were more prevalent in the HIV positive cohort than in the HIV negative cohort, in which the prevalence was 0.0% PTB; and 21.7% by KOH and 22.5% by fungal culture for PFP. Prevalence in participants with unknown HIV status was 40.0% PTB; and 21.7% by KOH, and 6.2% by fungal culture for PFP (Table 2).
Table 2.
Prevalence of pulmonary tuberculosis and pulmonary fungal pathogens by HIV status.
| Variable | Number (%) | ||
|---|---|---|---|
| HIV status | |||
| Positive | 80 (70.8) | ||
| Negative | 26 (23.0) | ||
| Unknown | 7 (6.2) | ||
| Total | 113 (100) | ||
| Prevalence by HIV status | PFP | PTB | |
| KOH n = 23 (%) |
Culture n = 80 (%) |
GeneXpert n = 5 (%) |
|
| Positive, n = 80 | 13 (56.5) | 57 (71.3) | 3 (60.0) |
| Negative, n = 26 | 5 (21.7) | 18 (22.5) | 0 (0.00) |
| Unknown, n = 7 | 5 (21.7) | 5 (6.2) | 2 (40.0) |
| HIV and PTB–fungal co-infection, n = 2 | 1 (50.0) | 1 (50.0) | 2 (100) |
KOH, potassium hydroxide; PFP, pulmonary fungal pathogen; PTB, pulmonary tuberculosis.
Aetiological profile of pulmonary fungal infections and PTB–PFP co-existence
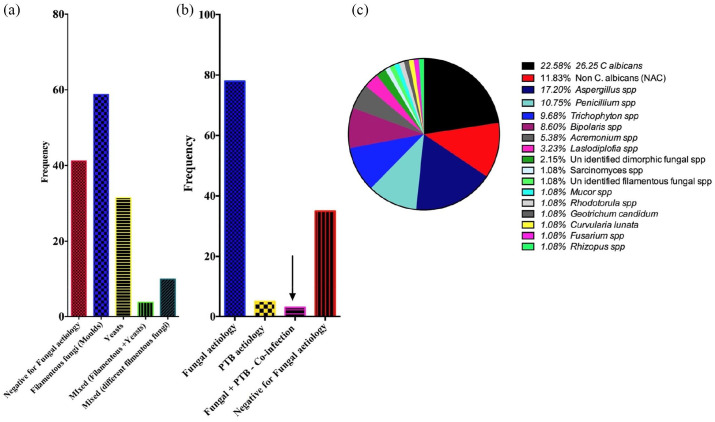
Categorically, fungal pathogens isolated were dominated by filamentous fungi [58.8% (n = 47)]. And in about 3.8% (n = 3) of the patients, mixed isolates of yeasts and filamentous fungi were also detected [Figure 1(a)]. According to this study fungal pathogens [70.8% (n = 80)] were by far the most prevalent when compared with PTB aetiology [4.4% (n = 5)]. However, 0.2% (n = 2) of the patients presented with PTB–PFP co-infection [Figure 1(b)]. This was an unlikely but interesting finding which needs to be investigated further. Of individual pathogen fungus isolated, C. albicans [22.58% (n = 21)] was the most prevalent isolate. This was followed closely by Aspergillus spp. [17.20% (n = 16)], non-albicans Candida [11.83% (n = 11)] and Penicillium spp. [10.75% (n = 10)]. Other fungal pathogens isolated also included Trichophyton spp. [9.68% (n = 9)], Bipolaris spp. [8.60% (n = 8)], Acremonium spp. [5.38% (n = 5)] and Lasiodiplodia spp. [3.23% (n = 3)]. The less encountered ones included Sarcinomyces spp., Rhodotorula spp., Mucor spp., Geotrichum candidium, Curvularia lunata, Fusarium spp. and Rhizopus spp., each of which had a 1.08% (n = 1) representation [Figure 1(c)]. As regards PTB–fungal co-existence, C. albicans was the only pathogenic fungus isolated from the two cases of a possible PTB–fungal co-infection.
Figure 1.
Aetiological profile of pulmonary fungal infections and pulmonary tuberculosis–pulmonary fungal pathogen co-infections.
C. albicans, Candida albicans; PTB, pulmonary tuberculosis.
Co-infection or co-existence by fungal pathogens
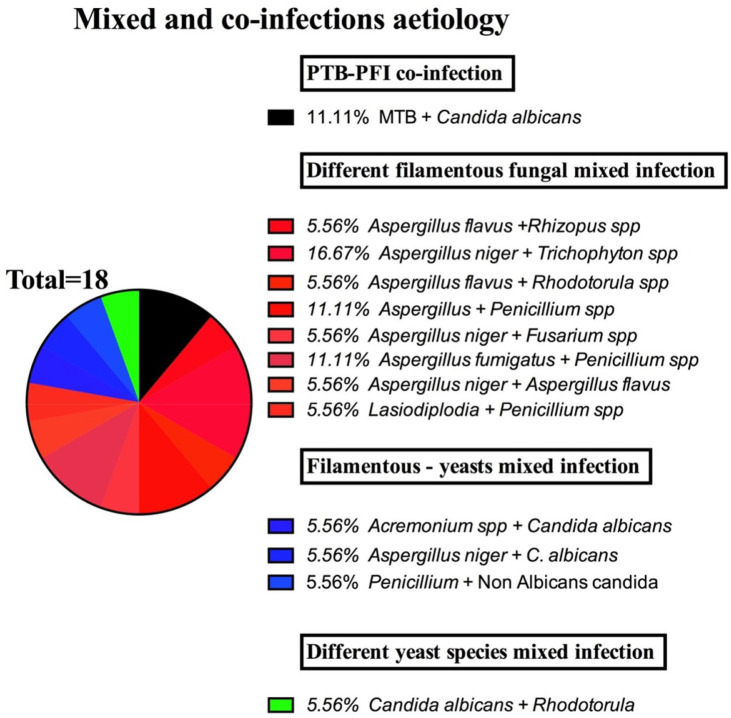
Through this study, we were able to establish what seemed like a co-infection or simply a co-existence by more than one organism in an infection niche in about 15.9% (n = 18) of the patients. We were able to determine a co-infection of PTB + PFP in 11.11% (n = 2), co-existence of yeasts and filamentous fungi in 16.7% (n = 3), of different yeasts’ pathogen in 5.56% (n = 1) and of different filamentous fungal pathogen in 44.4% (n = 8) (Table 3). In regard to age, gender and HIV status, the youth (18–34 years) and middle aged (35–64 years), females and the HIV positive cohorts were the most affected by co-infections. Individual pathogenic agents involved included mainly C. albicans predominantly among the PTB + PFP co-infections and yeast–filamentous fungal and different yeast aetiological mixed infections. On the other hand, Aspergillus spp. were predominant among the various filamentous fungal aetiological mixed infections (Figure 2).
Table 3.
Aetiology of co-infections or co-existence by age, gender and HIV status.
| Variable | Co-infections or co-existence by
fungal pathogens n = 18 |
|||
|---|---|---|---|---|
| PTB + PFP n = 2 (11.11%) |
Yeast + filamentous fungal
pathogens n = 3 (16.7%) |
Different yeasts
pathogens n = 1 (5.56%) |
Different filamentous fungal
pathogen n = 8 (44.4%) |
|
| Proportion (%) | Proportion (%) | Proportion (%) | Proportion (%) | |
| Age group, years | ||||
| Children, 1–13 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Adolescents, 14–17 | 0 (0.00) | 1 (5.56) | 0 (0.00) | 0 (0.00) |
| Youth, 18–34 | 1 (5.56) | 1 (5.56) | 0 (0.00) | 5 (27.8) |
| Middle aged, 35–64 | 1 (5.56) | 1 (5.56) | 1 (5.56) | 3 (16.7) |
| Elderly, >64 | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Gender | ||||
| Male | 2 (11.1) | 0 (0.00) | 0 (0.00) | 2 (11.1) |
| Female | 0 (0.00) | 3 (16.7) | 1 (5.56) | 6 (33.3) |
| HIV status | ||||
| Positive | 1 (5.56) | 3 (16.7) | 1 (5.56) | 7 (38.9) |
| Negative | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (5.56) |
| Unknown | 1 (5.56) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
Figure 2.
Aetiology of mixed and co-infections.
MTB, Mycobacterium tuberculosis; PFI, pulmonary fungal infection; PTB, pulmonary tuberculosis.
Discussion
For some time now PTB has been established as a major opportunistic pulmonary disease, especially in the HIV/AIDS infected cohort, with about two million people dying and one million infected worldwide each year.13 However, in many parts of the world endemic pulmonary mycoses, particularly the deep seated ones, are also prevalent and, just like TB, are also responsible for high rates of morbidity and mortality in an array of patient populations’.14,15–17 The challenge has always been that in addition to the fact that both entities present with similar symptoms, patients with a history of suffering from TB have proven prone to certain opportunistic fungal infections such as Aspergillus. For instance, chronic aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation.18 However, in many parts of the world the medical importance of pulmonary fungal infections continues to be ignored in the at-risk individuals.19
This brief background highlights the medical importance of both PTB and pulmonary mycoses. Yet the role of opportunistic fungi as secondary invaders of lungs, kidneys and other organs of patients having underlying conditions such as HIV/AIDS and cancer is documented in the literature with a considerable variation of rate of incidence of 9–80%.19 In Uganda, though, there is a general paucity of the burden of pulmonary mycoses. As a result, these infections have remained a silent challenge to public health due to the fact that they have been either neglected, ignored, missed or misdiagnosed.10,20,21 The spectrum of pulmonary fungal aetiology has been evolving over the years from the commonly isolated Candida species and primary dimorphic fungi to now several saprophytic moulds and dematiaceous fungi. Although C. albicans is still the most common, infections due to other members of the genus and other fungal categories are now on the increase. However, fungal diagnosis still remains a challenge in most health centres with limited diagnostic tools and very few trained mycologists to counter this challenge.
In this study, the prevalence of fungal pathogens using culture on SDA as our diagnostic test was 80 (70.7%) which dominated the prevalence of MTB in patients only suspected for MTB [5 (4.4%)]. The low TB prevalence here is interesting to know and it would have been important to establish whether some of these patients that presented with fungal pathogens had a history of TB treatment. The prevalence of pulmonary fungal pathogens according to gender was higher in females at 62.0% (n = 31). The highest incidence of both PTB and PFP was found in patients aged 18–34 and 35–64 years respectively. Similarly, the only co-infection or co-existing pathogens detected were also in the same age category (Table 1). These findings are in agreement with a study by Aluyi et al.22 and higher than in a study by Ekenna et al.23 This difference in prevalence can be attributed to geographical location, sample size and diagnostic approaches used. On the other hand the prevalence of TB was 5 (4.4%) (Table 1), higher than findings of the study by Buthia and Adhikari24 but lower than findings of Njunda et al.25 and Chadeganipour et al.26
In this study, C. albicans was the predominant isolate with a prevalence of 22.58% followed by Aspergillus 17.20%, non C. albicans 11.83%, Penicillium species 10.73%, Trichophyton species 9.68%, Bipolaris species 8.60%, Acremonium species 5.38%, Lasiodiplodia species 3.23% (Figure 2). These findings are similar to those of several studies including Nawange and Kavishwar27 that have named C. albicans and Aspergillus among the dominating aetiological agents of pulmonary mycosis. However, the aetiological profile determined is interestingly different from in some studies.23 For instance, the next most common aetiological agents other than yeasts and Aspergillus species were Penicillium species, Trichophyton species and Bipolaris species. These are dematiaceous Ascomycotina fungi that are often associated with soil and plant debris. Other rare opportunistic pathogens that were isolated included Sarcinomyces mucor, Fusarium, Rhodotorula, Curvularia lunata, Rhizopus, Geotrichum candidum, each accounting for 1.08%, and the dermatophytes are isolates in this infection niche. However, their isolation here could be linked to patients’ underlying conditions, exposure, geographical location and perhaps evolution in microbial adaption mechanisms. Indeed, the aetiological profile established in this study should send out a strong message regarding fungal aetiological evolution over the past few decades.
On the other hand it is not uncommon for fungi to co-exist with other microbes in the same infection niche. In this study, we also sought to establish the prevalence of PTB, PFP and fungal–PTB co-infections among the PTB suspects by HIV status. We established a prevalence of 57 (71.3%) for PFP, three (60.0%) for MTB in HIV positive patients and 18 (22.5%) for PFP in HIV negative patients and zero (0.0%) for MTB in HIV negative patients. On the other hand, two (100%) HIV positive patients were co-infected with both PFP and MTB (Table 2). These results prove the fact that HIV/AIDs is a major predisposing factor to both MTB and many of the saprophytic and environmental fungal opportunists. There is also existing evidence that individuals treated for PTB are prone to fungal infection.18
In the wider environment, fungi co-exist and interact with similar or other microbes to form different kinds of relationships including the highly addictive mutualistic or endosymbiotic interactions, whilst others can be of antagonistic nature. However, the impact of such interactions in an infection niche on clinical outcomes remains unclear. Through this study, we were able to establish what seemed like a mixed, a co-infection or simply a co-existence by more than one organism in an infection niche in about 15.9% (n = 18) of the patients. We were able to determine a co-infection of PTB + PFP in 11.11% (n = 2), mixed infection or co-existence of yeasts and filamentous fungi in 16.7% (n = 3), of different yeasts’ aetiology in 5.56% (n = 1) and of different filamentous fungal aetiology in 44.4% (n = 8) (Table 3). In regard to age, gender and HIV status, the youth (18–34) and middle aged (35–64), females and the HIV positive cohorts were the most affected by mixed and co-infections. Individual aetiological agents involved mainly C. albicans predominantly among the PTB + Pulmonary fungal infections (PFI) co-infections and yeast–filamentous fungal and different yeast aetiological mixed infections. On the other hand, Aspergillus spp. were predominant among the different filamentous fungal aetiological mixed pathogens (Figure 2): PFP and PTB co-infection prevalence of 11.11% in this study was apparently higher than the one found in a study done by Yahaya et al.28 In addition, we established C. albicans as the common fungus associated with TB–fungal co-infection similar to findings by Astekar et al.,19 Mathavi et al.,29 Hadadi-Fishani et al.,30 Amiri et al.31 but different from studies by Osman et al.32 and Bansod and Rai33; however, in our study no Aspergillus species were found to co-infect with PTB.
Already shown by prior studies, our findings continue to highlight the medical importance of pulmonary fungal pathogens among patients suspected for TB. Most importantly, the aetiological diversity determined in this study reveals other neglected pulmonary fungal pathogens. Therefore this presents a need for routine diagnosis for pulmonary mycoses among TB suspects and set-up of antimicrobial profile for pulmonary fungal isolates to support clinical management of these cases.
Acknowledgments
We are grateful to the study participants and staff Mbarara University Microbiology department for all the support given to us during data collection.
Footnotes
Author contributions: IKN, MP and AM contributed in study conception and design, KK and JM collected data and participated in laboratory analysis, JT and EN carried out data cleaning, LA and BM carried out data analysis, IKN, BA and HI wrote the first draft of the manuscript while JK and TK reviewed the manuscript and JB supervised the whole research process.
Availability of data and materials: Data and materials are readily available from the corresponding author upon request.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: Ethical approval to conduct the study was obtained from Mbarara University of Science and Technology Research and Ethics Committee (REC) (Ref. MUREC 1/7). Upon ethical clearance, administrative clearance was sought from Mbarara Regional Referral Hospital to conduct the study from the hospital and written informed consent was sought from each participant. As for children, their written informed assent was obtained through their care givers. All participants who were found with fungal infections and/or pulmonary tuberculosis were referred to clinicians for further management.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Benson Musinguzi  https://orcid.org/0000-0002-1211-4617
https://orcid.org/0000-0002-1211-4617
Contributor Information
Israel Kiiza Njovu, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Benson Musinguzi, Department of Medical Laboratory Science, Faculty of Health Sciences, Muni University, Arua, Uganda; Department of Microbiology and Immunology College of Health, Medicine and Life Sciences King Ceasor University, Kampala, Uganda.
James Mwesigye, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Kennedy Kassaza, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Joseph Turigurwa, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Edwin Nuwagira, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Joel Bazira, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Taseera Kabanda, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Moses Mpeirwe, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Lucas Ampaire, Department of Medical Laboratory Sciences, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
Andrew Mutekanga, Department of Internal Medicine, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda.
James Kiguli, Department of Microbiology and Immunology, School of Health Sciences, Soroti University, Soroti, Uganda.
Beatrice Achan, Department of Microbiology, School of Biomedical Sciences, Makerere University, Uganda.
Herbert Itabangi, Department of Microbiology, Mycology Unit, Faculty of Medicine, Mbarara University of Science and Technology, Mbarara, Uganda; Department of Microbiology and Immunology, Faculty of Health Sciences, Busitema University, Mbale, Uganda.
References
- 1.Kyu HH, Maddison ER, Henry NJ, et al. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis 2018; 18: 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buynevich IV, Goponyako SV. Tuberculosis 2019. [Google Scholar]
- 3.MacNeil A, Glaziou P, Sismanidis C, et al. Global epidemiology of tuberculosis and progress toward achieving global targets - 2017. MMWR Morb Mortal Wkly Rep 2019; 68: 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Tuberculosis Report. Geneva, Switzerland: World Health Organization, 2020. [Google Scholar]
- 5.Ministry of Health, Republic of Uganda. National Tuberculosis and Leprosy Division July 2017–June 2018 Report December 2018. Uganda: Ministry of Health, Republic of Uganda, 2018. [Google Scholar]
- 6.Holt MR, Chan ED. Chronic cavitary infections other than tuberculosis. J Thorac Imaging 2018; 33: 322–333. [DOI] [PubMed] [Google Scholar]
- 7.Warnock DW.Trends in the epidemiology of invasive fungal infections. Nihon Ishinkin Gakkai Zasshi 2007; 48: 1–12. [DOI] [PubMed] [Google Scholar]
- 8.Chrdle A, Mallátová Na, Vašáková M, et al. Burden of serious fungal infections in the Czech Republic. Mycoses 2015; 58: 6–14. [DOI] [PubMed] [Google Scholar]
- 9.Khwakhali US, Denning DW.Burden of serious fungal infections in Nepal. Mycoses 2015; 58: 45–50. [DOI] [PubMed] [Google Scholar]
- 10.Bongomin F, Gago S, Oladele RO, et al. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi (Basel) 2017; 3: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalyani CS, Koripella RL, Madhu C.Fungal isolates in sputum samples of multidrug-resistant tuberculosis suspects. Int J Sci Stud 2016; 4: 164–166. [Google Scholar]
- 12.Campbell CK, Johnson EM.Identification of pathogenic fungi. Chichester, West Sussex: John Wiley & Sons, 2013. [Google Scholar]
- 13.De Backer AI, Mortelé KJ, De Keulenaer BL, et al. Tuberculosis: epidemiology, manifestations, and the value of medical imaging in diagnosis. JBR-BTR 2006; 89: 243–250. [PubMed] [Google Scholar]
- 14.Di Mango AL, Zanetti G, Penha D, et al. Endemic pulmonary fungal diseases in immunocompetent patients: an emphasis on thoracic imaging. Expert Rev Respir Med 2019; 13: 263–277. [DOI] [PubMed] [Google Scholar]
- 15.Wheat LJ, Goldman M, Sarosi G.State-of-the-art review of pulmonary fungal infections. Seminars in respiratory infections. Semin Respir Infect2002; 17: 158–181. [DOI] [PubMed] [Google Scholar]
- 16.Lass-Flörl C.The changing face of epidemiology of invasive fungal disease in Europe. Mycoses 2009; 52: 197–205. [DOI] [PubMed] [Google Scholar]
- 17.Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Sci Transl Med 2012; 4: 165rv13. [DOI] [PubMed] [Google Scholar]
- 18.Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J 2019; 53: 1801184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astekar M, Bhatiya PS, Sowmya GV.Prevalence and characterization of opportunistic candidal infections among patients with pulmonary tuberculosis. J Oral Maxillofac Pathol 2016; 20: 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parkes-Ratanshi R, Achan B, Kwizera R, et al. Cryptococcal disease and the burden of other fungal diseases in Uganda; where are the knowledge gaps and how can we fill them? Mycoses 2015; 58: 85–93. [DOI] [PubMed] [Google Scholar]
- 21.Tufa TB, Denning DW.The burden of fungal infections in Ethiopia. J Fungi (Basel) 2019; 5: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aluyi H, Otajevwo F, Iweriebor O.Incidence of pulmonary mycoses in patients with acquired immunodeficiency diseases. Niger J Clin Pract 2010; 13: 78–83. [Google Scholar]
- 23.Ekenna O, Uba A, Chikwem J, et al. Relevance of moldy fungi as agents of chronic lower respiratory tract infection in patients seen in Maiduguri, Nigeria. West Afr J Med 2007; 26: 117–120. [PubMed] [Google Scholar]
- 24.Buthia T, Adhikari L.Pulmonary mycoses among the clinically suspected cases of pulmonary tuberculosis. Int J Res Med Sci 2015; 3: 260–268. [Google Scholar]
- 25.Njunda AL, Ewang AA, Kamga L-HF, et al. Respiratory tract Aspergillosis in the sputum of patients suspected of tuberculosis in Fako division-Cameroon. J Microbiol Res 2012; 2: 68–72. [Google Scholar]
- 26.Chadeganipour M, Shadzi S, Dehghan P, et al. The incidence of opportunistic fungi in patients suspected of tuberculosis. Mycoses 2000; 43: 269–272. [DOI] [PubMed] [Google Scholar]
- 27.Nawange M, Kavishwar A.Prevalence of opportunistic fungal infection in patients with pulmonary tuberculosis in Madhya Pradesh, Central India. J Microbiol Biomed Res 2015; 1: 6. [Google Scholar]
- 28.Yahaya H, Taura D, Aliyu I, et al. Spectrum of opportunistic mould infections in suspected pulmonary tuberculosis (TB) patients. Int J Microbiol Appl 2015; 2: 6–11. [Google Scholar]
- 29.Mathavi S, Shankar R, Kavitha A, et al. A study on prevalence of pulmonary candidiasis among tuberculosis patients and use of chromagar in identification of Candida species. J Drug Deliv Ther 2014; 4: 118–121. [Google Scholar]
- 30.Hadadi-Fishani M, Shakerimoghaddam A, Khaledi A.Candida coinfection among patients with pulmonary tuberculosis in Asia and Africa; a systematic review and meta-analysis of cross-sectional studies. Microb Pathog 2020; 139: 103898. [DOI] [PubMed] [Google Scholar]
- 31.Amiri MRJ, Siami R, Khaledi A.Tuberculosis status and coinfection of pulmonary fungal infections in patients referred to reference laboratory of Health Centers Ghaemshahr City during 2007–2017. Ethiop J Health Sci 2018; 28: 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osman NM, Gomaa AA, Sayed NM.Microarray detection of fungal infection in pulmonary tuberculosis. Egypt J Chest Dis Tuberc 2013; 62: 151–157. [Google Scholar]
- 33.Bansod S, Rai M.Emerging of mycotic infection in patients infected with Mycobacterium tuberculosis. World J Med Sci 2008; 3: 74–78. [Google Scholar]